Abstract
Tumor-associated antigens resulting from aberrant glycosylation, such as the SialylTn carbohydrate antigen, are frequently over-expressed on cancer cells and provide potential targets for cancer vaccination. Immunization of Rhesus monkeys with SialylTn coupled to a highly immunogenic carrier molecule and formulated on aluminum hydroxide induced a strong immune response against the carrier protein but only a moderate IgM immune response against the SialylTn carbohydrate antigen. Co-formulation with QS-21 adjuvant dramatically enhanced the anti-SialylTn immune response and resulted in a SialylTn-specific IgG switch. The kinetics of the carbohydrate-specific IgG response correlated with a temporary release of cytokines such as IFNγ, IL-2, IL-1β, TNFα and GM-CSF which was measurable in the immune serum by xMAP Multiplex technology. Furthermore, tumor cell killing by activated natural killer cells was induced. These data demonstrate that immunization with a tumor-associated carbohydrate antigen in a highly immunogenic formulation results in a temporary release of type 1 cytokines which may be required for the induction of a specific IgG immune response against the carbohydrate antigen as well as for activation of effector cells against tumor cells.
Keywords: SialylTn, Carbohydrate-specific immune response, Type 1 cytokines, QS-21, NK cell activation, Anti-tumor cytotoxicity
Introduction
Cancer is often associated with an increasingly impaired immune response against the tumor. Escape mechanisms of the tumor such as loss of antigen expression [22], reduced MHC expression [35], absence of co-stimulatory molecules [24], suppression of anti-tumor immune responses by CD4 + CD25 + T regulatory (Treg) cells [1], and alterations in the cytokine profile [40]—often skewed toward a Th2 response [2, 30]—affect the ability of the immune system to mount an effective response against the growing tumor. On the other hand, tumor-associated antigens can be recognized by T-cells or antibodies resulting in tumor cell destruction [13, 36, 37]. Therefore, the identification of appropriate target antigen(s) and a Th1/Th2 balanced cytokine profile may be essential to increase the therapeutic efficacy of cancer immune therapy [19, 32]. First hints of efficacy of this approach were already provided by the application of Coley’s toxin—a bacterial extract administered directly into the tumor—which resulted in marked alterations in cytokine levels and dramatic anti-tumor responses, but also significant toxicities in patients [23]. Application of recombinant cytokines also has been found to be associated with severe side effects and therapeutic effects have been shown in only few cases so far [12, 27, 33]. This limited efficacy may be due to the limitation in simulating correctly the biological paracrine function of cytokines in the context of antigen uptake and presentation [28]. Furthermore, the synchronized, often synergistic, action of cytokine patterns rather than single cytokines may be required for optimal induction of an immune response [15]. Proof of principle for the paracrine action of cytokines has been demonstrated using cytokine gene-modified tumor cells [3, 6]. However, for clinical application molecularly defined synthetic vaccines, which are amenable to a large-scale pharmaceutical manufacturing process, are warranted. Defined tumor-associated antigens, such as carbohydrate and mucin antigens resulting from aberrant glycosylation of tumor cells, may provide specific targets for immune therapy [20, 21, 29, 41]. The SialylTn carbohydrate antigen is expressed in more than 80% of cancers of breast, colorectal, prostate and ovarian origin [10, 42] and has been shown to correlate with a more aggressive tumor phenotype resulting in poor prognosis [11, 39], and antibodies against the SialylTn epitope have been found to correlate with improved survival of metastatic adenocarcinoma patients [21].
To increase the immunogenicity of the non-immunogenic carbohydrate antigen, SialylTn has been coupled to a highly immunogenic carrier protein, mAb17-1A [4], and formulated onto aluminum hydroxide [17]. Application of this vaccine formulation (designated IGN402) to Rhesus monkeys generated a strong immune response against the xenogeneic carrier protein but only a moderate IgM immune response against the SialylTn carbohydrate antigen. In contrast, co-formulation of the vaccine with a strong adjuvant, QS-21, dramatically enhanced the anti-SialylTn immune response and resulted in production of SialylTn-specific IgG antibodies [18]. The induction of carbohydrate-specific IgG antibodies implicates the involvement of carrier-induced T-cell help against the per se T-cell independent carbohydrate antigen. To investigate the mechanism contributing to the induction of the carbohydrate-specific immune response, cytokines were measured in the serum during the time course of this vaccination study using xMAP technology. Noteworthy, systemically measurable levels of cytokines such as IFNγ, IL-2, IL-1β, TNFα and GM-CSF were found to be temporarily released into the serum after boost immunizations. In contrast, no cytokines were detectable in pre-serum (PS) or in serum after primary immunization. A tight correlation between the kinetics of cytokine release and the induction of the carbohydrate-specific IgG response was found. Concurrently with the induction of measurable levels of cytokines, activation of natural killer (NK) cell mediated cytotoxicity against tumor cells was found following boost immunizations.
Methods and materials
Coupling of SialylTn carbohydrate to mAb17-1A
The SialylTn carbohydrate antigen was coupled to the mAb17-1A (murine IgG2a) protein carrier at a molar ratio of 18:1 by reacting 10 mg of nitrophenylated spacered SialylTn, Neu5Acα2-6GalNAcα-O(CH2)3NHCO(CH2)4COO-(p-NO2C6H4) (MW 819 g/mol, Lectinity, Finland), with 100 mg of mAb17-1A. Briefly, 100 mg of mAb17-1A (10 mg/ml) were dialyzed twice at 4°C for 20 h against 700 ml coupling buffer (0.1 M NaPO4, 0.15 M NaCl, pH 8.5) using a Slide-A-Lyzer dialysis cassette MWCO 10 K (Pierce). The concentration of mAb17-1A was determined by Size Exclusion Chromatography (SEC). In parallel, 10 mg of Neu5Acα2-6GalNAcα-O(CH2)3NHCO(CH2)4COO-(p-NO2C6H4) were dissolved in 300 μl DMF and added to the ice cold mAb17-1A. The reaction mixture was incubated rotating at +4°C. The kinetic of the reaction was monitored by the size of the coupling product as analyzed by SEC. After 28 h, the reaction mixture was dialyzed against formulation buffer (1 mM NaPO4, 0.86% NaCl, pH 6) using Slide-A-Lyzer dialysis Cassette 3.5 K (Pierce) at +4°C for 20 h. For comparison, uncoupled mAb17-1A was processed in parallel. SialylTn–mAb17-1A coupling products were analyzed by SEC, LDS-PAGE, Western blot, isoelectric focusing, and Resorcinol assay as described [17, 18].
IGN402 vaccine formulations
Five hundred micrograms of SialylTn–mAb17-1A conjugate was adsorbed onto 1.67 mg aluminum hydroxide in 0.5 ml formulation buffer (1 mM NaPO4, 0.86% NaCl, pH 6). The vaccine was formulated either without additional adjuvant or co-formulated with 100 μg QS-21 adjuvant (Antigenics Inc., Lexington, MA, USA).
LAL assay and pyrogenicity test in rabbits
Levels of endotoxin in the vaccine formulations were determined by Limulus Amebocyte Lysate—Endochrome™ assay (Charles River, MA, USA) according to the manufacturer. None of the final vaccine formulations contained detectable amounts of endotoxin.
The formulated vaccines were tested for pyrogenicity by i.v. application in rabbits. The formulations used for this study were negative regarding pyrogenicity testing with the sum of individual temperature rise recorded in three rabbits being +0°C (w/o additional adjuvant) and +0.3°C for vaccines co-formulated with QS-21 adjuvant.
Rhesus monkey immunization study
Safety, tolerability and immunogenicity of multiple subcutaneous injections of IGN402 were evaluated in vaccination studies in Rhesus monkeys. All animal studies were performed under controlled and documented conditions in accordance with animal health care standards at Biotest Ltd, Konarovice, Czech Republic. Per group, four healthy adult Rhesus monkeys (age and sex matched, group I: #152, #258, #292, #330 and group II: #38, #269, #308, #382, without or with QS-21, respectively) were vaccinated on days (d) 1, 15, 29 and 57 by subcutaneous injection and re-boosted on d226. Blood samples were taken before (d −7 and d −3) and after immunization (d15, 22, 29, 43, 57, 71, 85, 99) and d226 (before re-boost) and d240 (2 weeks after re-boost) for serum analytic.
In a second Rhesus monkey study animals—four animals per group (#31, #48, #76, #318)—were vaccinated with four initial immunizations of IGN402 co-formulated with QS-21 on d1, 21, 49 and 76. Heparinized blood samples were taken before (d1) and after immunization (d38, 60 and 86) for preparation of peripheral blood mononuclear cell (PBMC).
All immunizations were well tolerated by all animals with no signs of systemic or local toxicity related to immunization.
ELISA for immune reactivity against mAb17-1A
Pre-sera and immune sera (IS) of Rhesus monkeys, i.e., sera obtained before and after immunization, respectively, were analyzed to determine the induced immune response against mAb17-1A by ELISA. Briefly, ELISA plates (F96 Maxisorp, NUNC) were coated with 10 μg/ml mAb17-1A. Wells were blocked with 10% FCS in PBS for 1 h at 37°C and samples pre-diluted in PBS with 2% FCS were incubated for 1.5 h at 37°C. A positive control serum (derived from a Rhesus monkey immunized with mAb17-1A formulated onto alhydrogel) with known reactivity against mAb17-1A was tested in parallel and used for normalization of OD values between different ELISA plates. For detection plates were incubated with a 1:2,000 diluted sheep anti-human IgG-(γ-chain)-HRP conjugate (Chemicon, CA, USA) for 30 min at 37°C. Staining with substrate OPD (10 mg OPD dissolved in 25 ml staining buffer containing 10 μl 30% H2O2) was stopped by adding 50 μl H2SO4 (30%) and the color intensity was measured at OD492/620. The titer was defined as reciprocal serum dilution yielding an absorbance of OD = 1.0 on a titration curve. Curve fitting was done using GraphPad Prism version 4.0.
SialylTn-PAA ELISA
Pre-serum and IS of Rhesus monkeys were tested for reactivity against the synthetic SialylTn carbohydrate antigen by SialylTn-PAA ELISA. Briefly, ELISA plates (F96 Maxisorp, NUNC) were coated with 10 μg/ml SialylTn-PAA (Lectinity, Finland). Wells were blocked with PBS containing 10% FCS for 1 h at 37°C. Serum samples were pre-diluted in PBS containing 2% FCS and 5% glucose, and incubated for 2 h at 37°C. A positive control serum with known reactivity against SialylTn was used for normalization between different ELISA plates. For detection plates were incubated with 1:2,000 diluted mouse anti-human IgM-HRP conjugate (Southern Biotechnology, AL, USA) or 1:2,000 diluted sheep anti-human IgG-(γ-chain)-HRP conjugate (Chemicon, CA, USA), respectively, for 30 min at 37°C. Staining with substrate OPD (10 mg OPD dissolved in 25 ml staining buffer containing 10 μl 30% H2O2) was stopped by adding 50 μl H2SO4 (30%) and the color intensity was measured at OD492/620. Titers were defined as reciprocal of serum dilutions yielding an absorbance of OD = 1.0 and 0.5 for IgM and IgG, respectively. Curve fitting was done using GraphPad Prism version 4.0.
Cytokine release in serum (xMAP Multiplex)
Cytokines of PS or IS were analyzed by xMAP Multiplex technology (Luminex, TX, USA). Using the Beadlyte Human Multi-Cytokine Detection System 3 (Upstate, Dundee, UK) according to the manufacturer’s protocol the following cytokines were measured: IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12(p70), TNFα, IFNγ and GM-CSF. Briefly, 25 μl sample was incubated with 25 μl assay buffer for 20 min at 25°C per well of a 96-well ELISA filter plate. The mixture of anti-human cytokine antibodies coupled to beads was added, followed by addition of biotinylated anti-cytokine antibodies and incubated 1.5 h at 25°C in the dark as described by the manufacturer. After incubation, the liquid was removed and streptavidin–phycoerythrin was added. Subsequently, the beads were washed with assay buffer and the emitted fluorescent signal was quantitated using a Luminex100 reader (settings—50 events per bead, 50 μl sample size, gate—7,500–13,000 MFI).
In vitro activation of human NK cell by IS
Human PBMCs were isolated from buffy coats of healthy donors (obtained from the Austrian Red Cross) on a Ficoll density gradient. The NK cell enriched fraction was obtained from the non-adherent PBMC fraction (after removal of adherent cells by 1 h plastic adherence) and by negative sorting on an AutoMACS™ (Miltenyi, Germany) using antibodies against CD3, CD14, CD34 and CD19. The NK-enriched population was characterized by FACS analysis using CD56/CD16 as marker and consisted of at least 60% NK cells (data not shown).
The NK-enriched cell fraction was incubated with PS or IS (at a final dilution of 1:5), and in the presence or absence of supplements of indicated amounts of hIL-2, for 48 h. The medium was replaced with fresh RPMI 1640 containing 10% FCS and incubation was continued for another 24 h. After washing, the cells were re-suspended in fresh medium and used as effector cells in a 4 h 51Cr-release lysis assay against labeled KATOIII tumor target cells at effector to target (E:T) ratios of 10:1 and 1:1, respectively. Release of 51Cr from lysed target cells into the supernatant of the samples (“Cs”) was measured using a γ-counter (Cobra 5005, Canberra-Packard, Australia). Spontaneous release (“Sr”) and maximum release (100%, “Mr”) were measured after incubation of target cells with medium alone or with detergent (2% SDS), respectively. Cytotoxicity was calculated using the formula 100% × (Cs − Sr)/(Mr − Sr).
Ex vivo measurement of activation of non-adherent PBMCs derived from Rhesus monkeys before and after immunization
Peripheral blood mononuclear cells were isolated from heparinized blood derived from Rhesus monkeys before or after immunization, respectively, on a Ficoll density gradient. After washing, the non-adherent PBMC fraction was obtained by removal of adherent cells following 2 h plastic adherence. Non-adherent PBMCs were incubated in RPMI 1640 supplemented with 10% FCS for 2 h and subsequently used as effector cells in an overnight 51Cr-release lysis assay with labeled KATOIII target cells at the E:T ratios of 60:1, 30:1, 10:1 and 3:1. Cytotoxicity was calculated as described above.
Results
Immunization of Rhesus monkeys with SialylTn–mAb17-1A in the presence or absence of QS-21 adjuvant
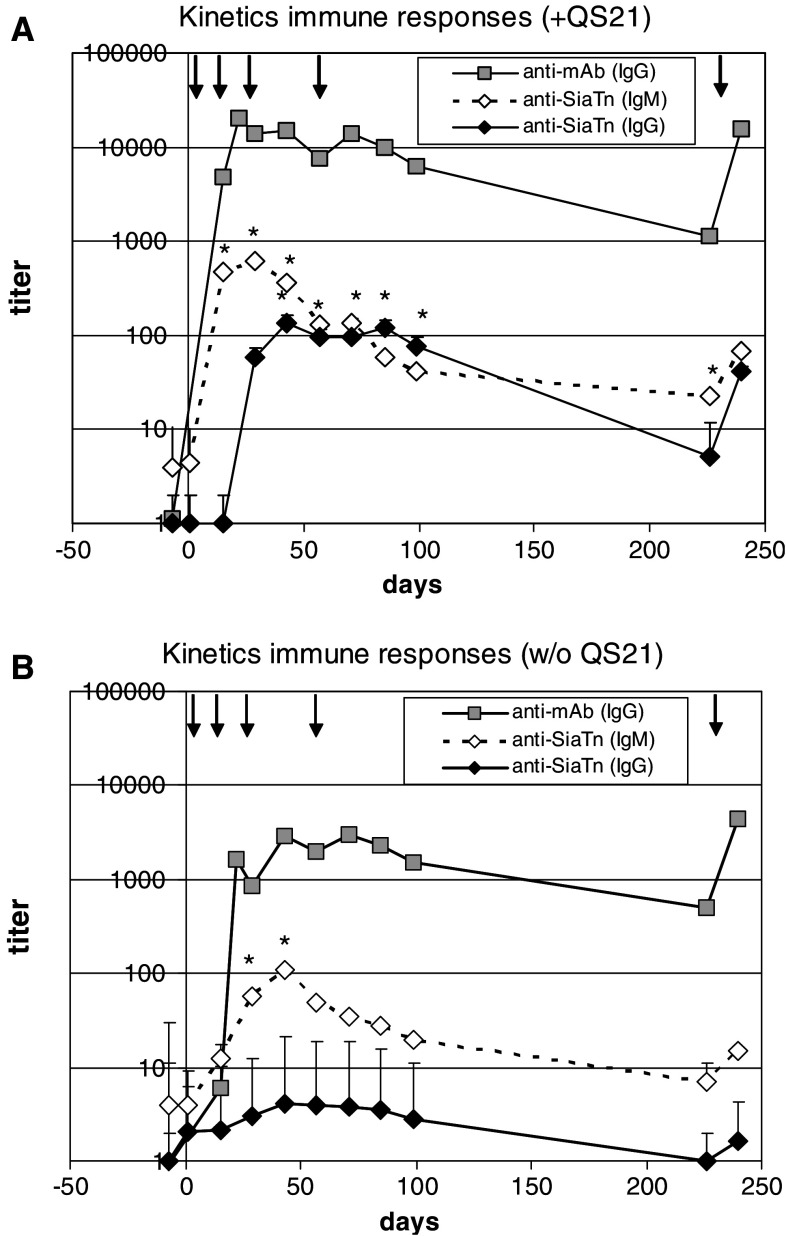
SialylTn–mAb17-1A conjugate was formulated onto alhydrogel, either with or without QS-21 adjuvant. Application of both vaccine formulations to Rhesus monkeys was well tolerated by all animals. The immune responses against the SialylTn carbohydrate antigen (IgG, IgM) and the mAb17-1A carrier protein (IgG) were analyzed by the respective ELISA. The summarized data of the anti-SialylTn immune response and the immune response against the mAb17-1A carrier molecule are shown in Fig. 1. Both vaccines induced high IgG titers against the carrier molecule. In the presence of QS-21, the immune response against the carrier showed a slightly more rapid onset (significant titers at d15 with QS-21 vs. d22 without QS-21) and approximately 5–10-fold higher peak titers as compared to the vaccine without QS-21. A similar kinetic was found for the IgM response against SialylTn, with a more rapid onset and approximately tenfold higher IgM titers in the presence of QS-21 adjuvant. The most dramatic effect of QS-21 adjuvant was the induction of a pronounced SialylTn-specific IgG response. In three out of four animals, a SialylTn-specific IgG response was measurable starting from d29, i.e., 2 weeks after the second immunization. In one animal (#308), the SialylTn-specific IgG response was found only after the fifth immunization. As expected, there was a 1–2 weeks delay in the onset of the anti-SialylTn IgG response compared to anti-SialylTn IgM response and IgG response against the carrier (Fig. 1a). In contrast, no SialylTn-specific IgG was induced in the group immunized without QS-21 adjuvant.
Fig. 1.
Immune responses against carrier protein and SialylTn after immunization with SialylTn–mAb17-1A vaccine with or without co-formulation with QS-21. Rhesus monkeys (four animals per group) were immunized with SialylTn–mAb17-1A vaccine with (a) or without (b) QS-21 adjuvant at d1, 15, 29, 57 and re-boosted at d226. PS and IS were analyzed for immune response by ELISA. The kinetics of the immune responses, i.e., antibody titers (geomean and scatter factor) against SialylTn (IgG, IgM) and mAb17-1A (IgG) are shown. Statistics: *P < 0.05 vs. PS (one-tailed, paired t-test). Arrows show immunization time points
Specificity of anti-SialylTn response and anti-carrier response
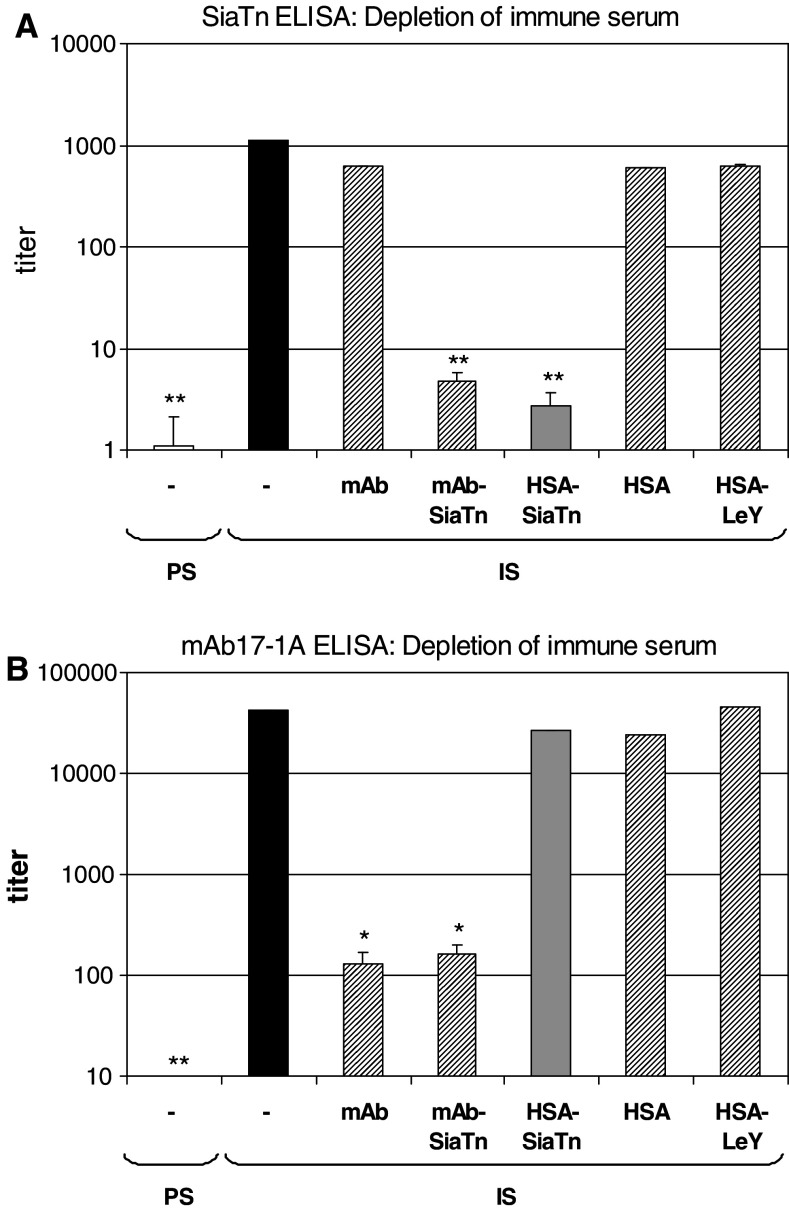
Specificity of the anti-SialylTn response induced by SialylTn–mAb17-1A immunizations of Rhesus monkeys was confirmed by depletion experiments: IS was incubated with Sepharose beads coupled with either SialylTn–mAb17-1A, mAb17-1A, SialylTn–HSA, HSA or LeY–HSA, respectively. PS or IS or depleted IS were analyzed for binding to SialylTn in a SialylTn-PAA ELISA. The induced immune response measurable in the IS was significantly decreased by depletion with SialylTn–mAb17-1A or SialylTn–HSA Sepharose beads, but not with mAb17-1A, or HSA, or HSA–LeY Sepharose beads where HSA was coupled to the unrelated LeY carbohydrate antigen and used as control (Fig. 2a).
Fig. 2.
Specificity of induced immune responses. The specificity of the immune responses against the SialylTn carbohydrate antigen (a) and mAb17-1A carrier protein (b) was confirmed by depletion experiments. PS or IS, or IS depleted by incubation with Sepharose beads coupled to mAb17-1A (mAb), SialylTn–mAb17-1A, SialylTn–HSA, HSA or LeY–HSA, respectively, were measured for antibody titers against SialylTn (a) or mAb17-1A (b) by ELISA. Summarized data (geomean and scatter factor) of four Rhesus monkeys are shown. Statistics: *P < 0.05 vs. IS and **P < 0.01 vs. IS (one-tailed, paired t-test)
The specificity of the anti-carrier (i.e., anti-mAb17-1A) immune response induced by SialylTn–mAb17-1A immunizations of Rhesus monkeys was confirmed by depletion experiments: IS was incubated with Sepharose beads coupled with either SialylTn–mAb17-1A, mAb17-1A, SialylTn–HSA, HSA or LeY–HSA, respectively. Untreated PS or IS or depleted IS were analyzed for binding to mAb17-1A by ELISA. The induced immune response measurable in the IS was found to be significantly decreased by depletion with mAb17-1A or SialylTn–mAb17-1A Sepharose beads, but not with HSA–SialylTn, or HSA or HSA–LeY Sepharose beads (Fig. 2b).
Cytokine release in serum after repeated vaccination
The serum cytokine profile during the time course of the immunizations was measured using xMAP Multiplex technology (Fig. 3). Apart from detectable levels of the chemokine IL-8, no significant cytokine levels were found in the PS (i.e., before immunization) of healthy Rhesus monkeys. Also following the first immunization no significant cytokine levels were measured. Starting from d22, i.e., 1 week following the second immunization, low amounts of IFNγ could be detected. Moreover, following the third and fourth immunizations (d43 and 71, respectively) significant levels of IFNγ, IL-8, IL-1β, TNFα, IL-2, GM-CSF and IL-4, were measured in the serum of animals immunized with the vaccine plus QS-21 adjuvant (Fig. 3a, c). Low levels of IL-6 and IL-12 were also found in some of the animals, but IL-10 was not measured during any vaccination regime. In contrast, immunization with the vaccine without QS-21 resulted in marginal cytokine release only, with some IFNγ and IL-8 measurable after the third and fourth immunizations. Interestingly, following a re-boost (fifth) immunization, half a year later, in two out of four animals immunized with the vaccine without QS-21 significant cytokine levels were found, although cytokine levels were generally lower than in the QS-21 group (Fig. 3b, d vs. Fig. 3a, c).
Fig. 3.
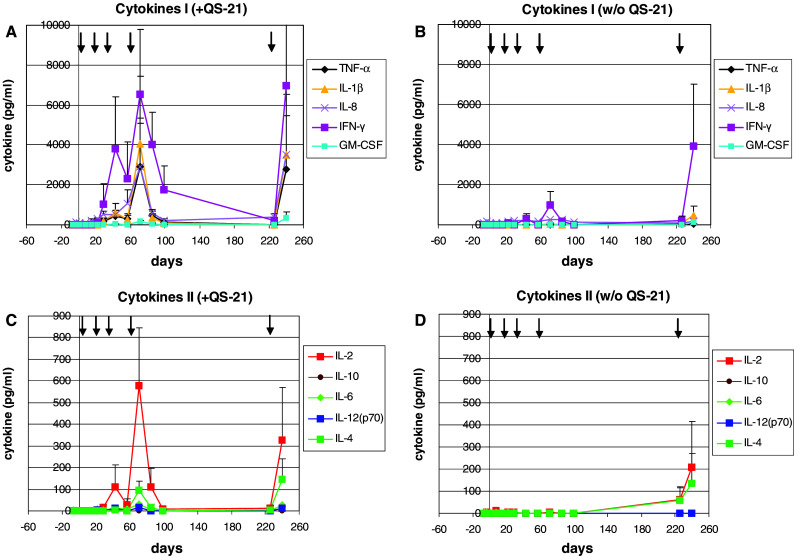
Cytokine release after immunization with SialylTn–mAb17-1A vaccine with or without QS-21 adjuvant. Rhesus monkeys (four animals per group) were immunized with SialylTn–mAb17-1A vaccine with (a, c) or without (b, d) QS-21 adjuvant at d1, 15, 29, 57 and re-boosted at d226. PS and IS were analyzed for cytokine release by xMAP technology (Luminex). Cytokine levels (pg/ml) measured in serum are shown (mean and SD). Arrows show immunization time points
Correlation of time kinetics of SialylTn-specific IgG response and cytokine release
Comparing the kinetics for induction of immune responses against carrier molecule (IgG) or the SialylTn carbohydrate antigen (IgM, IgG) (Fig. 1) with the kinetics for cytokine release (Fig. 3) suggested a timely correlation between IgG switch induction against SialylTn and the cytokine release measurable in serum. For more detailed analysis, for each animal the kinetics of the immune responses against the carrier and the SialylTn carbohydrate antigen, respectively, and the kinetics of the cytokine release were superimposed on the same time scale (Fig. 4). The absolute values for IgG titers (against carrier and SialylTn) were normalized by a multiplication factor as indicated to fit the scale for the cytokine levels (pg/ml). Neither induced SialylTn-specific IgG antibodies nor measurable cytokines were found during the first two immunizations. In contrast, after the third and fourth or fifth immunizations along side with high titer IgG responses against the carrier molecule also detectable IgG responses against the SialylTn carbohydrate antigen were found. Noteworthy, the induction of the anti-SialylTn IgG response coincided with the temporary cytokine release measurable in the IS. A particularly consistent correlation in timing was evident for the SialylTn-specific IgG switch induction and the release of IL-2, IL-1β and IFNγ (Fig. 4a–d). In one animal of this group, #308, the IgG switch against SialylTn was induced only after the fifth re-boost immunization, again correlating with a pronounced cytokine release found after the fifth re-boost immunization (Fig. 4c).
Fig. 4.
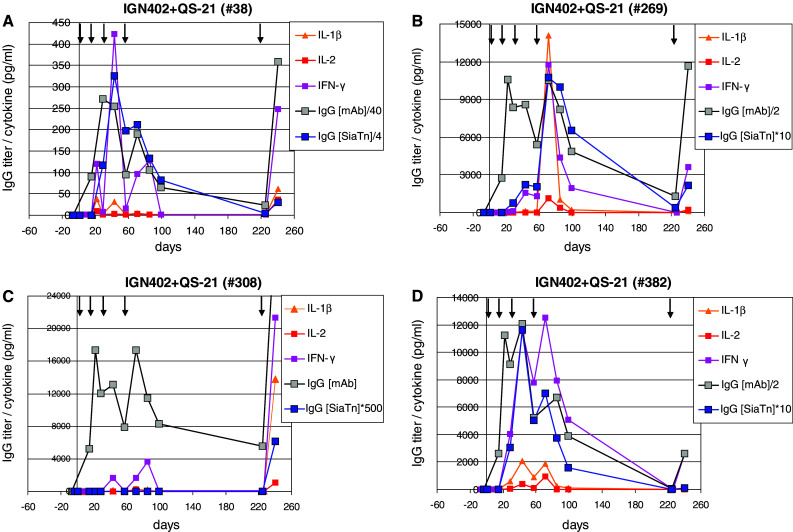
Kinetics of IgG immune response against SialylTn coincides with a temporary cytokine release in serum. Rhesus monkeys were immunized with SialylTn–mAb17-1A vaccine co-formulated with 100 μg QS-21 adjuvant at d1, 15, 29, 57, and re-boosted at 24 weeks after last immunization at d226. PS (d −7, d1) and IS (d15, 22, 29, 43, 57, 71, 85, 99, 226 and 240) were analyzed for reactivity against SialylTn (IgG) or mAb17-1A carrier (IgG) by ELISA and cytokine release in the serum was measured by xMAP Multiplex technology (Luminex). Antibody titers and cytokine levels (pg/ml serum) of each individual Rhesus monkey (a, b, c, d, respectively) are shown. The indicated multiplication factor was used to align antibody titer values with the cytokine levels (pg/ml)—figure scales are adjusted to cytokine levels. Arrows show immunization time points
In vitro activation of human NK activity by pre-incubation with IS
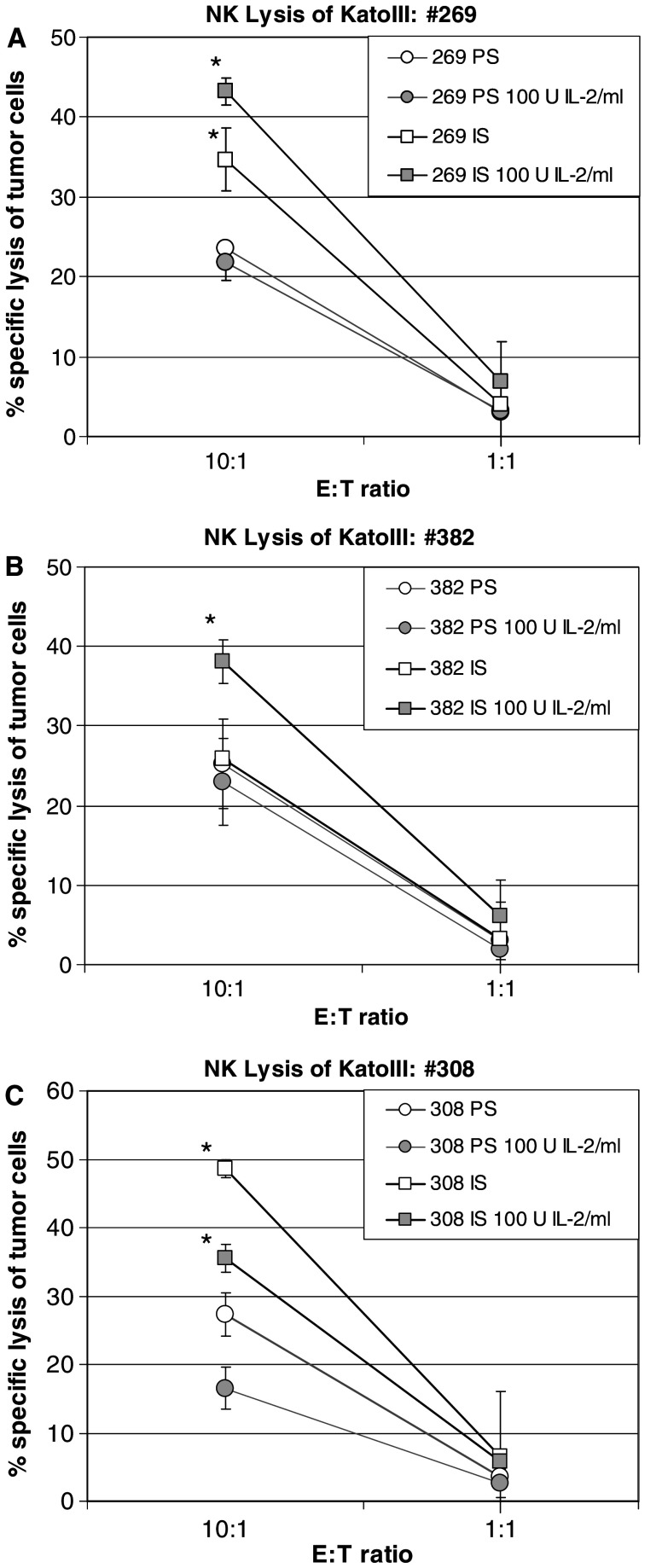
To elucidate whether the induced cytokines may have triggered further biological effects, the potential activation of NK cells was investigated. NK cells are known to kill target cells in a MHC independent manner and without re-exposure to antigen. NK cells have been shown to be activated by cytokines such as IL-1, IFNγ and IL-2 [25] and can be induced by high dose IL-2 to obtain feature of lymphokine-activated killer (LAK) cells [26]. Our preliminary experiments using enriched human NK cells showed efficient lysis of the NK sensitive target cells K562, but only marginal efficacy against the NK-resistant DAUDI cells or KATOIII tumor cells. In contrast, pre-incubation of the NK cells with high doses of recombinant IL-2 (1,000 U/ml) resulted in pronounced lysis of DAUDI cells and KATOIII cells indicating the generation of LAK cells (data not shown). To test the effect of released cytokines on NK effector functions, the NK-enriched cell fraction of human PBMCs was incubated for 2 days with 1:5 diluted PS or IS derived from Rhesus monkeys following repeated boost immunizations (at time points of the highest measured cytokine levels). In order to compensate for potential inhibitory factors present in the Rhesus monkey serum, PS and IS were used either alone or supplemented with a moderate IL-2 dose (100 U/ml) which by itself was not sufficient to induce sufficient activation of NK cells to lyse DAUDI or KATOIII tumor cells (data not shown). Incubation of effector cells with IS enhanced the lytic activity of NK-enriched human PBMCs against KATOIII target cells in three out of four animals (Fig. 5). No effect (specific lysis less than 10%, data not shown) was seen in animal #38 which showed also the lowest cytokine release in the IS (compare scales in Fig. 4).
Fig. 5.
Natural killer cell mediated lysis of KATOIII tumor cells after in vitro activation of human NK cells by IS of immunized Rhesus monkeys. Rhesus monkeys were immunized with SialylTn–mAb17-1A vaccine co-formulated with QS-21. NK cells derived from PBMCs (obtained after negative sorting by CD3/CD14/CD34/CD19) were incubated with PS or IS (at peak cytokine levels) for 48 h. After removal of medium, washed cells were incubated for additional 24 h. Activated NK cells were used as effector cells in a 4-h 51Cr-release lysis assay against labeled KATOIII target cells. Data of three individual animals, each measured in independent triplicates (mean and SD shown), are presented (panels a–c). Statistics: *P < 0.05 vs. respective PS control (one-tailed, paired t-test)
Rhesus monkey derived PBMCs show enhanced cytolytic activity against tumor cells after immunization
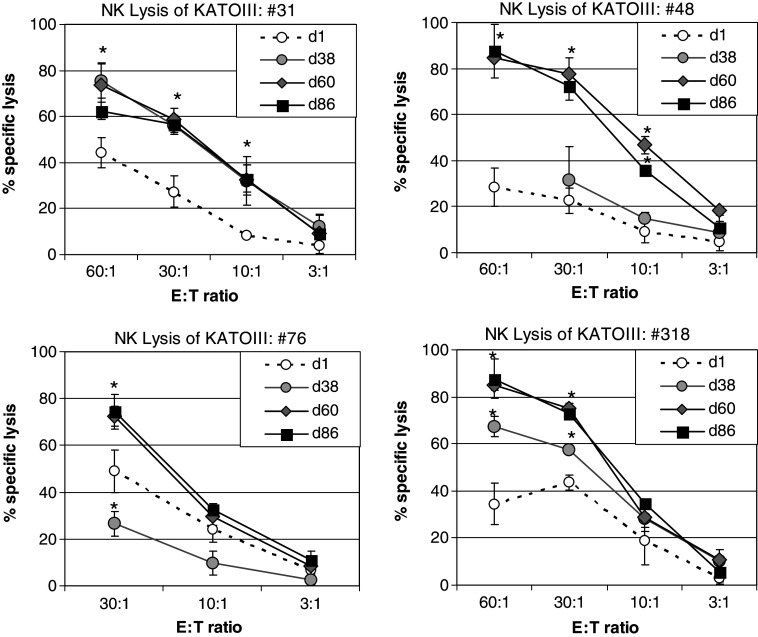
In order to test whether the in vitro activation of NK-enriched PBMC by pre-incubation with IS (Fig. 5) is reflecting also a stimulation of cellular cytotoxicity in vivo, PBMCs derived from Rhesus monkey before and after immunization, respectively, were tested for their cytolytic effect on 51Cr labeled KATOIII tumor cells at different E:T ratios. Before being used as effector cells the PBMCs were depleted of monocytes by plastic adherence and any Rhesus monkey derived serum was removed by repeated washing steps. The data show that in all four tested Rhesus monkeys the cytolytic activity of their PBMCs was enhanced following repeated immunization of the animals compared to the time points before immunization, i.e., d1 (Fig. 6).
Fig. 6.
Lysis of KATOIII tumor cells by Rhesus monkey derived PBMCs after repeated-boost immunizations. Rhesus monkeys were immunized with SialylTn–mAb17-1A vaccine co-formulated with QS-21. PBMCs were isolated from heparinized blood derived from Rhesus monkeys before or after immunization, respectively, on a Ficoll density gradient. Following removal of adherent cells by 2 h plastic adherence and repeated washing steps, the non-adherent PBMCs were used as effector cells in an overnight 51Cr-release lysis assay against labeled KATOIII target cells. Data of four individual animals (#31, #48, #76, #318), each measured in independent triplicates (mean and SD shown), are presented. Statistics: *P < 0.05 vs. PS (d1) (one-tailed, paired t-test)
Discussion
The immunogenicity of a carbohydrate–protein conjugate vaccine, IGN402, consisting of SialylTn carbohydrate epitopes chemically coupled to an immunogenic protein carrier, and formulated with or without QS-21 adjuvant, was tested in Rhesus monkeys. As described previously [17, 18], our strategy is to couple tumor-associated carbohydrate epitopes to a highly immunogenic murine antibody with intrinsic anti-tumor activity in order to (a) use an immunogenic carrier protein to increase the immunogenicity of the carbohydrate antigen and to (b) capitalize on the anti-tumor immune response induced by the carrier molecule itself [4, 5]. IGN402 is a first candidate of this type of conjugate vaccines consisting of SialylTn carbohydrate epitopes chemically coupled to mAb17-1A. The murine 17-1A antibody—a monoclonal antibody recognizing EpCAM [31]—adsorbed onto aluminum hydroxide has been used as vaccine antigen in the cancer vaccine candidate IGN101 and has recently been reported to prolong survival in metastatic colorectal cancer patients [34].
Application of IGN402 without QS-21 induced a strong immune response against the carrier protein but only a moderate immune response against the SialylTn carbohydrate antigen which was mainly of IgM type. In contrast, co-formulation of IGN402 with QS-21 dramatically enhanced the anti-SialylTn immune response and resulted in production of SialylTn-specific IgG antibodies, and measurable ADCC activity against SialylTn positive OVCAR-3 tumor cells [17]. Measurement of significant amounts of SialylTn-specific IgG implicates that the carrier-induced T-cell activation also provided help for the per se T-cell independent carbohydrate antigen. To address the question how the immune response induced by the immunogenic protein carrier molecule is translated into T-cell help for the carbohydrate-specific immune response, the cytokine profile in the serum was measured. PS and IS of the immunized animals were analyzed using xMAP Multiplex technology. Whereas no significant cytokine levels were found in the PS of Rhesus monkeys, a temporary release of IFNγ and other cytokines was detected in the IS starting 1 week after the second immunization and—more pronounced—after the third and fourth immunizations. Noteworthy, this cytokine release pattern was not found after the primary immunization but obviously was related to the repeated-boost immunization. In particular, following third and fourth immunizations (d43 and 71, respectively) significant cytokine levels including IFNγ, IL-8, IL-1β, TNFα, IL-2, GM-CSF and IL-4, and lower levels of IL-6 and IL-12 were measured in the serum of animals immunized with the vaccine co-formulated with QS-21. Importantly, IL-10 which is a negative regulator of T-cell activity was not detected in serum. To our knowledge this is the first report describing a systemic cytokine pattern temporarily released during the time course of a vaccination study in non-human primates. The observed cytokine pattern indicates that mainly type 1 cytokines such as IFNγ (TNFα), IL-2 and pro-inflammatory cytokines such as IL-1β and TNFα as well as the pro-inflammatory chemokine IL-8 are released. Moderate levels of released IL-4 suggest that also the Th2 pathway may partially be induced as well. Compared to the QS-21 co-formulated vaccine, immunization with the vaccine in the absence of QS-21 showed a much less pronounced cytokine release pattern, with some IFNγ release and low IL-8 release measurable after the third or fourth immunization.
An important finding of this study is the tight correlation in timing between cytokine release and the immunoglobulin class switch leading to carbohydrate-specific IgG antibodies. While peak IgG titers against the carrier protein were reached shortly after the second immunization, a significant IgG response against the carbohydrate was measurable mainly after third or further immunizations. Cytokines were detectable at earliest 1 week after second immunization coinciding with the pronounced IgG boost response against the carrier protein and with the induction of the first detectable SialylTn-specific IgG in the QS-21 group. We therefore hypothesize that this temporary systemic cytokine release—obviously induced by the strong anti-carrier immune response—subsequently drives also the immune response against the co-presented carbohydrate antigen. Co-localization and the exact timely connection of the induced cytokine release with the presentation of the carbohydrate antigen may help to trigger the induction of the IgG response against the structurally unrelated (to the protein carrier) carbohydrate antigen. While additional mechanisms for the generation of the carbohydrate-specific immune responses, such as co-processing and co-presentation of carbohydrate–peptide sequences on MHC of antigen-presenting cells to the T-cells, may be involved as well [8, 38], the present data indicate that the temporary induction of a type-1 cytokine profile by repeated-boost immunizations can trigger an efficient immune response against carbohydrate tumor-associated antigens. Furthermore, the presented data indicate that besides connecting the carrier-induced immune response and the anti-carbohydrate immune response, the released cytokines have the potential to activate a variety of immune effector cells, such as NK cells or even trigger the differentiation from NK into LAK cells in vivo. Noteworthy, the vaccine induced cytokine profile showed some variation between the individual monkeys: IFNγ was found to be the most prominent and consistently released cytokine, while IL-2 and IL-1β were found at varying amounts. A more homogenous cytokine pattern may be achieved by optimizing the immunization scheme and further optimizing the doses of both, conjugate vaccine and adjuvant.
The vaccination approach described in this study combines the advantages of non-specific immune stimulation, i.e., cytokines, with a specific immune response against a tumor-associated carbohydrate antigen. The synchronized release of type 1 cytokines elicited by the anti-carrier immune response is more likely to mimic the adequate cytokine pattern which is necessary also for the induction of the carbohydrate-specific immune response, as compared to systemic application of single cytokines. The phenomenon of co-stimulation of an immune response against a target antigen by induction of a strong immune response against another antigen is analogous to some approaches which are currently under investigation in cancer immunotherapy, such as the graft versus leukemia effect associated with a graft-versus-host-reaction (GvHR). A moderate GvHR, i.e., induced by partial mismatches between the donor bone marrow transplant and the recipient, has been found to correlate with lower probability of relapse in myeloid leukemia patients, and was found to be associated with a Th1 response [7]. Furthermore, vaccination with allogenic tumor cells has been shown to efficiently induce systemic immune responses against the wild-type tumor. This effect has been observed not only with cells transfected with genes for stimulatory cytokines [14, 16] but also when used as non-transfected, irradiated cells [9] indicating that this effect may have been induced by a concomitant cytokine release during the strong immune response against the allogenic MHC.
In conclusion, the present study indicates that molecularly defined synthetic vaccines eliciting a specific immune response against defined target antigen(s) together with a synchronized cytokine release may be promising candidates for cancer vaccine development.
Acknowledgment
We thank Dr C.R. Kensil from Antigenics Inc., Lexington, MA, USA, for kindly providing the QS-21 adjuvant and for critical review of the manuscript.
References
- 1.Antony PA, Restivo NP. CD4 + CD25 + T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerici M, Shearer GM, Clerici E. Cytokine dysregulation in invasive cervical carcinoma and other human neoplasias: time to consider the Th1/Th2 paradigm. J Natl Cancer Inst. 1998;90:261–263. doi: 10.1093/jnci/90.4.261. [DOI] [PubMed] [Google Scholar]
- 3.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagerberg J, Frodin JE, Wigzell H, Mellstedt H. Induction of an immune network cascade in cancer patients treated with monoclonal antibodies (ab1). I. May induction of ab1-reactive T cells and anti-anti-idiotypic antibodies (ab3) lead to tumor regression after mAb therapy? Cancer Immunol Immunother. 1993;37:264–270. doi: 10.1007/BF01518521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagerberg J, Ragnhammar P, Liljefors M, Hjelm AL, Mellstedt H, Frodin JE. Humoral anti-idiotypic and anti-anti-idiotypic immune response in cancer patients treated with monoclonal antibody 17-1A. Cancer Immunol Immunother. 1996;42:81–87. doi: 10.1007/s002620050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon ER, Pardoll DM, Itaya T, et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 7.Guo H, Qiao Z, Zhu L, Wang H, Su L, Lu Y, Cui Y, Jiang B, Zhu Q, Xu L. Th1/Th2 cytokine profiles and their relationship to clinical features in patients following nonmyeloablative allogeneic stem cell transplantation. Am J Hematol. 2004;75:78–83. doi: 10.1002/ajh.10443. [DOI] [PubMed] [Google Scholar]
- 8.Haurum JS, Holer IB, Arsequell G, Neisig A, Valencia G, Zeuthen J, Neefjes J, Elliott T. Presentation of cytosolic glycosylated peptides by human class I major histocompatibility complex molecules in vivo. J Exp Med. 1999;190:145–150. doi: 10.1084/jem.190.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsueh EC, Essner R, Froshag LJ, Ye X, Morton DL. Active immunotherapy by reinduction with a polyvalent allogeneic cell vaccine correlates with improved survival in recurrent metastatic melanoma. Ann Surg Oncol. 2001;9:486–492. doi: 10.1007/BF02557273. [DOI] [PubMed] [Google Scholar]
- 10.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 11.Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Sialosyl-Tn: a novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::AID-CNCR2820660919>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805. doi: 10.1002/(SICI)1097-0142(19980815)83:4<797::AID-CNCR25>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayaga J, Souberbielle BE, Sheikh N, et al. Antitumor activity against B16F10 melanoma with a GM-CSF-secreting allogeneic tumor cell vaccine. Gene Ther. 1999;6:1475–1481. doi: 10.1038/sj.gt.3300961. [DOI] [PubMed] [Google Scholar]
- 15.Kircheis R, Küpcü Z, Wallner G, Wagner E. Cytokine gene-modified tumor cells for prophylactic and therapeutic vaccination: IL-2, IFNγ, or combination IL-2 + IFNγ. Cytokines Cell Mol Ther. 1998;4:95–103. [PubMed] [Google Scholar]
- 16.Kircheis R, Küpcü Z, Wallner G, Rössler V, Schweighoffer T, Wagner E. Interleukin-2 gene-modified allogeneic melanoma cell vaccine can induce cross-protection against syngeneic tumors in mice. Cancer Gene Ther. 2000;7:870–878. doi: 10.1038/sj.cgt.7700183. [DOI] [PubMed] [Google Scholar]
- 17.Kircheis R, Vondru P, Nechansky A, Ohler R, Loibner H, Himmler G, Mudde GC. SialylTn-mAb17-1A carbohydrate–protein conjugate vaccine: effect of coupling density and presentation of SialylTn. Bioconjug Chem. 2005;16(6):1519–1528. doi: 10.1021/bc050157m. [DOI] [PubMed] [Google Scholar]
- 18.Kircheis R, Vondru P, Zinöcker I, Häring D, Nechansky A, Loibner H, Mudde GC. Immunization of Rhesus monkeys with the conjugate vaccine IGN402 induces an IgG immune response against carbohydrate and protein antigens, and cancer cells. Vaccine. 2006;24:2349–2357. doi: 10.1016/j.vaccine.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;5:365–371. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingston PO, Wong GYC, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Helling F, Ritter G, Oettgen HF, Old LJ. Improved survival in AJCC stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 21.MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin-associated SialylTn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 23.Nauts H, Fowler G, Bogatko F. A review of the influence of bacterial infection and of bacterial products (Coley’s toxin) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand. 1953;144:S1–S103. [PubMed] [Google Scholar]
- 24.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumor location, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 25.Ortaldo JR, Herberman RB. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- 26.Ortaldo JR, Mason A, Overton R. Lymphokine-activated killer cells: analysis of progenitors and effectors. J Exp Med. 1986;164:1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panelli MC, White R, Foster M, Martin B, Wang E, Smith K, Marincola FM. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Translat Med. 2004;2:17–31. doi: 10.1186/1479-5876-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Ann Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 29.Ragupathi G, Koide F, Santhyan N, Kagan E, Spassova M, Bornmann W, Gregor P, Reis CA, Clausen H, Danishefsky SJ, Livingston PO. A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancer. Cancer Immunol Immunother. 2003;52:608–616. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rayman P, Wesa AK, Richmond AL, Das T, Biswas K, Raval G, Storkus WJ, Tannenbaum C, Novick A, Bukowski R, Finke J. Effect of renal cell carcinomas on the development of type-1 T-cell responses. Clin Cancer Res. 2004;10:6360S–6366S. doi: 10.1158/1078-0432.CCR-050011. [DOI] [PubMed] [Google Scholar]
- 31.Riethmuller G, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmaier H, Hirsche H, Pichlmayr R, et al. Randomized trial of monoclonal antibody for adjuvant therapy of resected Dukes’ C colorectal carcinoma. German Cancer Aid 17-1A Study group. Lancet. 1994;343:1172–1174. doi: 10.1016/S0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/S0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 33.Schiller JH, Pugh M, Kirkwood JM, Karp D, Larson M, Borden E. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin Cancer Res. 1996;2:29–36. [PubMed] [Google Scholar]
- 34.Settaf A, Salzberg M, Groiss F, Eller N, Schuster M, Himmler G, Kundi M, Eckert H, Loibner H (2004) A randomized placebo-controlled phase II study with the cancer vaccine IGN101 in patients with epithelial cancers. iSBTc 19th Annual Meeting, San Francisco, November, 4–7
- 35.Travers PJ, Arklie JL, Trowsdale J, Patillo RA, Bodmer WF. Lack of expression of HLA-ABC antigens in choriocarcinoma and other human tumor cell lines. Natl Cancer Inst Monogr. 1982;60:175–180. [PubMed] [Google Scholar]
- 36.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, DePlaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 37.Watson SA, Michaeli D, Grimes S, Morris TM, Robinson G, Varro A, Justin TA, Hardcastle JD. Gastrimmune raises antibodies that neutralize amidated and glycine-extended gastrin-17 and inhibit the growth of colon cancer. Cancer Res. 1996;56:880–885. [PubMed] [Google Scholar]
- 38.Werdelin O, Meldal M, Jensen T. Processing of glycans on glycoprotein and glycopeptide antigens in antigen-presenting cells. Proc Natl Acad Sci USA. 2002;99:9611–9613. doi: 10.1073/pnas.152345899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werther JL, Rivera-MacMurray S, Bruckner H, Tatematsu M, Itzkowitz SH. Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. Br J Cancer. 1994;69:613–616. doi: 10.1038/bjc.1994.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamura M, Modlin RL, Ohmen JD, Moy RL. Local expression of anti-inflammatory cytokines in cancer. J Clin Invest. 1993;91:1005–1010. doi: 10.1172/JCI116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Walberg LA, Helling F, Ragupathi G, Adluri S, Lloyd KO, Livingston PO. Augmenting the immunogenicity of synthetic MUC-1 vaccines in mice. Cancer Res. 1996;55:3364–3368. [PubMed] [Google Scholar]
- 42.Zhang S, Zhang SH, Cordon-Cardo C, Reuter VE, Singgal AK, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry. II. Blood group-related antigens. Int J Cancer. 1997;73:50–56. doi: 10.1002/(SICI)1097-0215(19970926)73:1<50::AID-IJC9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]