Abstract
This study was designed to determine the characteristics of tumour cell-derived microvesicles (TMV) and their interactions with human monocytes. TMV were shed spontaneously by three different human cancer cell lines but their release was significantly increased upon activation of the cells with phorbol 12-myristate 13-acetate (PMA). TMV showed the presence of several surface determinants of tumour cells, e.g. HLA class I, CD29, CD44v7/8, CD51, chemokine receptors (CCR6, CX3CR1), extracellular matrix metalloproteinase inducer (EMMPRIN), epithelial cell adhesion molecule (EpCAM), but their level of expression differed from that on cells they originated from. TMV also carried mRNA for growth factors: vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), interleukin-8 (IL-8) and surface determinants (CD44H). TMV were localized at the monocytes surface following their short exposure to TMV, while at later times intracellularly. TMV transferred CCR6 and CD44v7/8 to monocytes, exerted antiapoptotic effect on monocytes and activated AKT kinase (Protein Kinase B). Thus, TMV interact with monocytes, alter their immunophenotype and biological activity. This implicates the novel mechanism by which tumour infiltrating macrophages may be affected by tumour cells not only by a direct cell to cell contact, soluble factors but also by TMV.
Keywords: Tumour-derived microvesicles, Monocytes, Transfer, CCR6, mRNA
Introduction
Many cell types including leukocytes, platelets, and endothelial cells during growth, activation or apoptosis are shedding small circular membrane fragments called microparticles or microvesicles [15, 23, 24, 32, 33]. The increased level of microvesicles (MV) in plasma is observed in different disease states, like unstable angina, diabetes, sepsis, thromboembolic complications and cancer [14, 19, 28]. The biological activity of MV is associated with the contents of poorly characterized membrane proteins and lipids, especially sphingolipids [18]. MV are also shed by tumour cells in vitro and in vivo and these tumour-derived MV (TMV) from melanoma, breast, lung and pancreatic tumour cell lines express several surface molecules: CD44, CD63, CD95L, CD147 (extracellular matrix metalloproteinase inducer, EMMPRIN), β1, α3, α5 integrins, HLA class I [2, 9, 10, 12, 23, 36, 37]. They also contain cytoplasm and actin cytoskeleton [23]. It is still unclear whether MV derived from normal and malignant cells posses similar biological activities. However, tumour cell lines with highly metastatic potential release a greater amount of TMV than cells with low metastatic ability [1, 6].
In the local tumour microenviroment tumour infiltrating macrophages (TIM) interact both with cancer, stromal cells and extracellular matrix. These interactions result in the production by TIM both protumour and antitumour mediators [21, 29]. In the tumour bed freshly arriving monocytes, giving rise to TIM, may also be exposed to MV released by tumour cells, platelets, endothelial cells, which modulate cellular interactions [11, 16, 31]. Platelet-derived MV (PMV) can transfer tissue factor to monocytes, which results in thrombotic events [33]. However, to our knowledge no information is available on the interactions of TMV with human monocytes/macrophages.
The present study was designed to characterize immunophenotype of TMV obtained from different human cancer cell lines and to determine whether TMV may transfer some of tumour cell determinants and/or mRNA to monocytes. This paper shows that TMV carry several surface determinants expressed by tumour cells and deliver them to monocytes. TMV protect monocytes from spontaneous apoptosis in vitro and activate signaling pathways, like AKT-PI3K (AKT kinase-phosphatidylinositide 3′-OH kinase). This suggests that TMV may be one of the players in the macrophage–tumour cell interactions.
Materials and methods
TMV isolation
TMV were obtained from the following human cell lines: HPC-4 (pancreatic adenocarcinoma, TMVHPC), DeTa (colorectal adenocarcinoma, TMVDeTa) and A549 (lung carcinoma, TMVA549) previously described [26, 27]. Cells were cultured by bi-weekly passages in RPMI 1640 (Sigma, St Louis, MO, USA) with 5% FCS (foetal calf serum, Biochrom, Berlin, Germany) centrifuged at 50,000g. Cell lines were regularly tested for Mycoplasma sp. contamination by using PCR-ELISA kit according to the manufacturer’s procedure (Roche, Mannheim, Germany). Supernatants from well-grown cell cultures were collected, centrifuged at 2,000g for 20 min to remove cell debris and centrifuged again at 50,000g (RC28S, Sorvall, Newton, CT, USA) for 1 h at 4°C. Pellets were washed several times to remove FCS and finally resuspended in serum-free medium. Quantification of TMV proteins was evaluated by the Bradford method (BioRad, Hercules, CA, USA). TMV were tested for endotoxin contamination by the Limulus test according to the manufacturer’s instruction (Charles River Laboratories, Inc., Wilmington, MA, USA) and stored at −20°C.
Determination of size and number of TMV by flow cytometry and transmission electron microscopy
The size of TMV was determined by flow cytometry (FACS Canto, BD Biosciences Immunocytometry Systems, San Jose, CA, USA). The instrument was rinsed with particle-free FACS Rinse Solution (BD Bioscience, San Diego, CA, USA) for 15 min to eliminate the background. The beads of different size: 1, 2.5 or 6 μm (Molecular Probes, Eugene, OR, USA) were used as the size markers and analysis was performed using a log scale for FSC and SSC parameters. Tumour cells were cultured for 4 h in serum-free medium alone or in the presence of phorbol 12-myristate 13-acetate (PMA, 100 ng/ml, Sigma), supernatants were collected, spun at 2,000g for 20 min and the number of TMV was analysed by flow cytometry during 20 s of acquisition in a medium flow option. In addition, HPC-4 cells stimulated with PMA were used for transmission electron microscopy to confirm TMV size and shape. Briefly, cells were pre-fixed in 2% glutaraldehyde in phosphate buffer at pH 7,3 and post-fixed in 1% buffered osmium tetroxide. After dehydration in graded series of ethanol, the samples were embedded in low viscosity medium, thin sectioned and stained with 2% uranyl acetate and lead citrate. The micrographs were taken with a Philips EM 300 electron microscope (Philips, Eindhoven, The Netherlands) operating at 80 KV.
Isolation of monocytes
Human peripheral blood mononuclear cells were isolated from EDTA-blood of healthy donors by the standard Ficoll/Isopaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. Monocytes were separated from mononuclear cells by counter-flow centrifugal elutriation with a JE-5.0 elutriation system equipped with a 5 ml Sanderson separation chamber (Beckman, Palo Alto, CA, USA) as previously described [26]. Monocytes were suspended in RPMI 1640 culture medium supplemented with L-glutamine (Sigma) with gentamycin (25 μg/ml).
Immunophenotyping of TMV
The following monoclonal antibodies (mAbs) were used: FITC-labelled anti: HLA class I, CD29, CD44H, (BD Biosciences Pharmingen, San Diego, CA, USA), CD51, CD58 (Immunotech, Marseille, France), CD44v6, CD44v7/8 (Bender Medsystem, Vienna, Austria), TNFR1, TNFR2 (R&D Systems Minneapolis, MN, USA) EMMPRIN (CD147 clone 8D6, Santa Cruz Biotechnology, Santa Cruz, CA, USA), EpCAM (epithelial cell adhesion molecule, clone Ber-EP4, Dako Cytomation, Glostrup, Denmark), and PE-labelled anti: CCR3, CXCR1, CXCR2, CXCR4 (R&D Systems), CX3CR1 (MBL, Nagoya Japan), CCR6, CD95 (BD Biosciences Pharmingen), with appropriate isotype controls. Tumour cells or TMV were incubated with mAbs for 30 min at 4°C, washed (TMV were not washed) and then analysed in FACSCalibur flow cytometer (BD Biosciences Immunocytometry Systems).
Transfer of TMV to monocytes
TMV were incubated for 5 min with red PKH26 dye (Sigma) according to the manufacturer’s instruction. After that TMV were washed with 1% bovine serum albumin (BSA) and several times in serum-free RPMI 1640 medium. Monocytes (1×106 /ml) were incubated with PKH26-labelled TMV (30 μg/ml) for 30 min to 24 h at 37°C in serum-free medium. Binding of PKH26-labelled TMV to monocytes was determined by flow cytometry analysis of red fluorescence intensity and the percentage of positive cells. Vital dye crystal violet was used for quenching extracellular fluorescence [38]. Transfer of CCR6, CD44v7/8 to monocytes was analysed after incubation for 15 min to 3 h with TMV by staining the cells with appropriate mAbs. In parallel, confocal microscopy analysis was performed to visualize localization of TMV in monocytes. Monocytes incubated with PKH26-labelled TMV (30 min, 1 h, 24 h) were seated on Super Frost Plus microscopy slides (Menzel-Glaser, Germany), fixed with 4% paraformaldehyde, washed and covered with fluorescent mounting medium (Dako Cytomation). Analysis was performed with the use of DM RXA2 TCS SL confocal laser-scanning microscope using 63x/1.4–0.6 (HCX PL APO) oil objective and confocal software (Leica, Microsystems). The PKH26 fluorescence was excited with He/Ne laser emitting line at 543 nm. The exposure settings and gain were identical for each image. The background noise of each confocal image was reduced by average two scans/line and four frames/image.
Determination of chemotaxis
Chemotactic assay was performed in Costar Transwell 24-well plates with 8 μm pore filter (Costar Corning, Cambridge, MA, USA) as described before [4]. Monocytes were incubated in the medium supplemented with 0,5% BSA alone or with TMVHPC(30 μg/ml) for 1 h. After that monocytes (1×106 /ml) were washed and distributed to the upper chamber of transwell. As TMVHPC carry CCR6, macrophage inflammatory protein-3α (MIP-3α, 100 ng/ml, PeproTech, Inc. Rocky Hill, NJ, USA) was used as a chemoatractant. After 4 h cells from lower chamber of transwell were scored using FACSCalibur. The cells were gated according to their FSC/SSC parameters and counted during a 20 s acqusition at a high-flow rate. Data are expressed as the percentage of the cells input corrected by the percentage of cells, which migrated spontaneously to the medium alone.
Determination of IL-8-, VEGF-, HGF-, CD44-and β-actin mRNA expression by real time PCR
The total RNA was extracted from TMV or tumour cell lines by the single-step isolation method using TRI-ZOL reagent (Gibco-BRL, Grand Island, NY, USA) according to the manufacturer’s protocol. The first strand cDNA was obtained from the total RNA samples (2 μg) with Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Sigma) and oligo-dT (Sigma) primer as specified by the manufacturer’s protocol. The quantitative polymerase chain reactions (PCR) for interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), CD44H and β-actin were performed using the LightCycler system (Roche Diagnostics, Mannheim, Germany) with the following primer pairs: IL-8: 3′TTA GCA CTC CTT GGC AAA ACT G 5′CTG GCC GTG GCT CTC TTG VEGF: 3′GGT CTC GAT TGG ATG GCA GTA G 5′CAC CCA TGG CAG AAG GAG GA HGF: 3′TCC TTG ACC TTG GAT GCA TTC 5′CTC ACA CCC GCT GGG AGT AC CD44H: 3′TTA TCA ATA CCA TTA ACC AGG 5′GTG TCC ATC TGA TTC AGS TCC β-actin: 3′CGA TCC ACA CGG ATG ATC TG 5′GGA TGC AGA AGG AGA TCA CTG
The thermal profile for IL-8, VEGF, HGF, and β-actin was the following: initial denaturation at 95°C for 10 min, and then 45 cycles of 95°C for 10 s, and 60°C for 30 s. For CD44H the initial denaturation was the same, and then 45 cycles of 95°C for 0 s, 55°C for 10 s and 72°C for 15 s. The melting curve analysis was performed to verify the specificity of the amplified products.
Effect of TMV on survival of monocytes
Survival of monocytes was determined using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS) performed according to the manufacturer’s description (Promega, Madison, WI, USA). Briefly, monocytes suspended (1×105/100 μl) in serum-free RPMI 1640 medium with or without TMV (30 μg/ml) were cultured for 48 h in 96 well microplates. At the end of culture 20 μl of CellTiter 96 Aqueous One Solution reagent was added, microplates were incubated for 1 h at 37°C and then absorbance at 490 nm was measured using the microplate reader ELx800NB (Bio-tek Instruments, Inc., Winooski, VT, USA). Apoptosis was determined by flow cytometry using mAb against the active form of caspase-3 according to the manufacturer’s protocol (BD Pharmingen). The expression of caspase-3 in monocytes cultured in serum free medium alone or with TMV for 3 and 24 h was compared. For determination of annexin V binding monocytes were incubated alone or with TMV for 3 or 24 h in serum free medium in low attachment tubes. Annexin V binding was performed according to the manufacturer’s protocol (BD Pharmingen).
Western Blotting
Monocytes were stimulated with TMV (30 μg/ml) for 15–60 min at 37°C and then lysed in M-Per lysing buffer containing protease and phosphatase inhibitors (Sigma). The extracted proteins were loaded on 4% loading gel, separated in 12% SDS gel and transferred to the polyvinylidene fluoride membrane (Immune-blot PVDF, 2 μm, BioRad, Hercules, CA, USA). Phosphorylation of AKT (Ser 473) and p44/42 MAPK (Mitogen Activated Protein Kinase Thr202/Tyr204) protein were detected using rabbit polyclonal anti-phospho-AKT antibody (clone#9106) or mouse anti phospho-p44/42 MAPK mAb (clone# 9271) (Cell Signaling, Beverly, MA, USA) with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG as a secondary Abs (Santa Cruz Biotech, Santa Cruz, CA, USA). The equivalence of loading was evaluated by treating the blots with stripping buffer (Restore Western Blot Stripping Buffer, Pierce, Rockford, IL, USA) and incubated with appropriate polyclonal Abs: anti p44/42 MAPK and anti AKT (all from Cell Signaling). The membranes were developed with the SuperSignal West Pico Chemiluminescent Substrate (Pierce), dried and subsequently exposed to HyperFilm (Amersham Life Science, Little Chalfont, UK).
Statistical analysis
Statistical analysis was performed by paired Student’s-t test using Excell software. Differences were considered significant at p < 0.05.
Results
Determination of size and number of TMV released by tumour cells
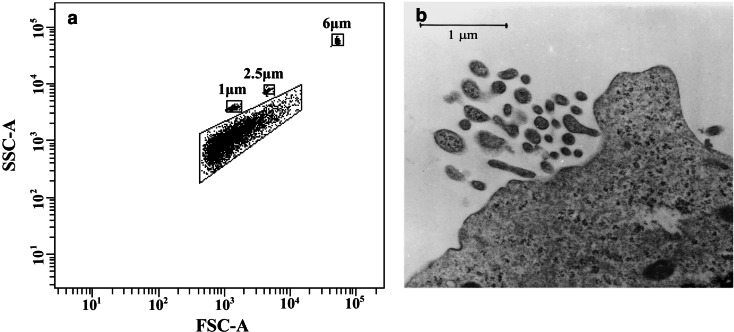
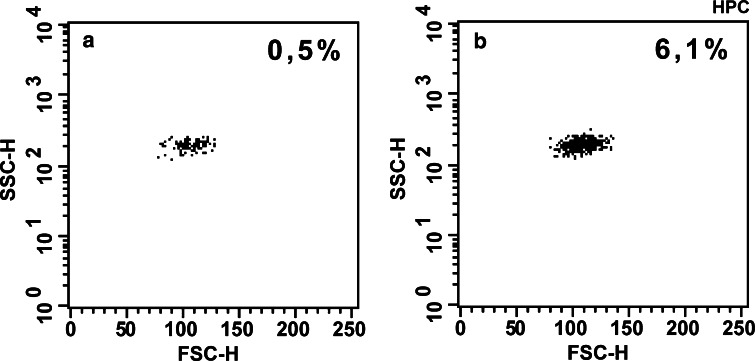
TMV size was determined by flow cytometry and electron microscopy. In FACS analysis using 1, 2.5 and 6 μm beads as a size internal standards, the major part of TMV were observed below FSC signal corresponding to 1 μm beads (Fig. 1a), suggesting ≤1 μm size of TMV. In transmission electron microscopy TMV were visible as circular or villi like structures with the size below 1 μm (Fig. 1b), and were located closely to the cell surface. A slightly bigger size of TMV as determined by FACS may be related to heterogeneity in shape of TMV as well as different three-dimentional orientation of these particles in the flow chamber during analysis. The number of events recorded in the supernatants during 20 s of acquisition in FACS analysis was 23,485±1,541 for PMA-stimulated and 7,577±793 for unstimulated tumour cell cultures indicating significantly higher release of TMV by activated cells.
Fig. 1.
Determination of TMVHPC size. a Flow cytometry analysis: beads (1, 2.5 and 6 μm) were used as an internal standard. b Transmission electron microscopy of TMV released by HPC-4 cells activated by PMA
Expression of surface determinants by tumour cells and TMV
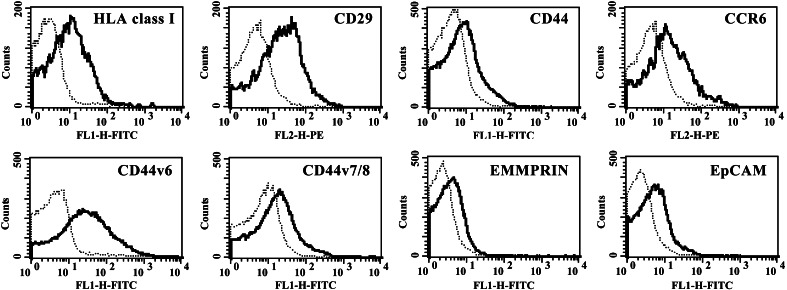
A flow cytometry analysis of surface determinants on tumour cells and TMV isolated from them was performed following staining with various mAbs (Table 1, Fig. 2). More than 20% of TMVHPC-4 and A549 and more than 5% TMVDeTa showed the expression of CD29 and HLA class I while the expression of chemokine receptors: CCR6, CX3CR1, TNF receptors (TNFR1, TNFR2), EpCAM, EMMPRIN was lower. TMV also showed low (below 3% of TMV) expression of CCR3, CD58 and CD95. Surprisingly, CD51 (except TMVA549), CXCR1, CXCR2 were not detected on TMV although they were present on respective tumour cells. On the other hand, TMV expressed a higher percentage of CD44 variants (v6 and v7/8) than tumour cells. Interestingly enough, TMV showed a lower expression of CD44H molecule than tumour cells. These data indicated that several, but not all, determinants expressed by tumour cells are present on TMV and their level of expression does not always correlate with that on tumour cells.
Table 1.
Expression of surface markers on tumour cell lines (HPC-4, DeTa, A549) and TMV released from them. Results summarize the data obtained from five independent experiments and are presented as % of positive cells or TMV, as defined by FACS analysis
| HPC-4 | TMVHPC | DeTa | TMVDeTa | A549 | TMVA549 | |
|---|---|---|---|---|---|---|
| HLA class I | +++* | ++ | ± | + | ++ | + |
| CD29 | +++ | ++ | + | + | +++ | ++ |
| CD44H | +++ | + | ± | − | ++ | ± |
| CD44v7/8 | + | + | + | ++ | + | + |
| CCR3 | + | ± | ± | − | − | − |
| CCR6 | + | + | + | + | + | + |
| CXCR1 | ± | − | + | − | − | − |
| CXCR2 | + | − | ± | − | − | − |
| CXCR4 | − | − | ± | + | − | − |
| CX3CR1 | + | + | + | + | ± | + |
| CD95 | ++ | ± | − | − | +++ | + |
| CD51 | +++ | − | − | − | +++ | ++ |
| CD58 | +++ | ± | + | − | ++ | − |
| TNFR1 | + | + | ± | − | ± | − |
| TNFR2 | + | + | + | + | − | − |
*±<3%; +<20%; ++<50%; +++>50%; −no expression
Fig. 2.
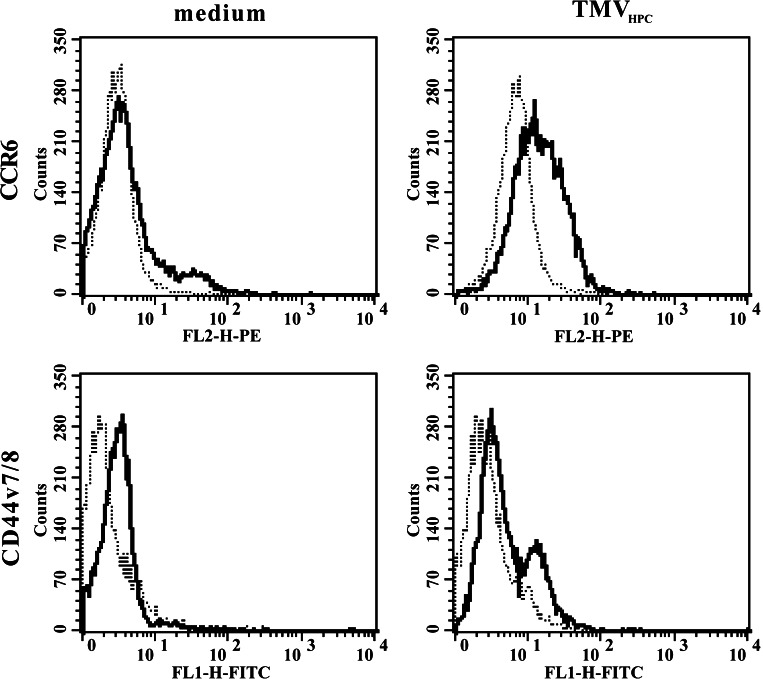
Immunophenotypic characteristics of TMVHPC. Histograms from representative experiments showing the expression of some studied determinants are presented. Dotted lines represent isotype control
Expression of IL-8-, VEGF-, HGF- and CD44H-mRNA
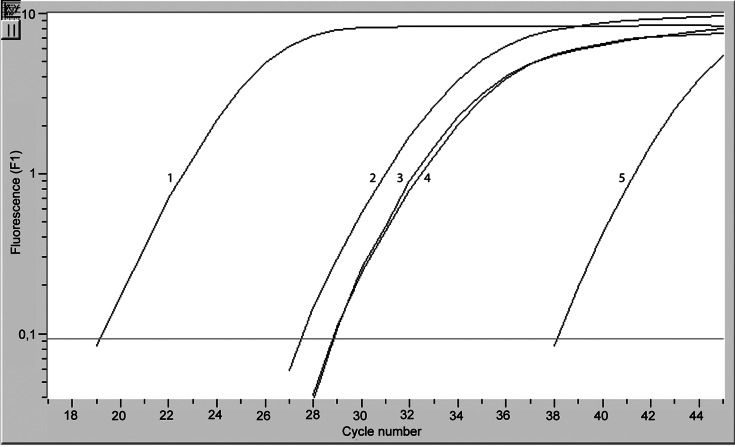
Since surface determinants were observed on TMV, and other evidence indicates that they may contain cytokeratins, fibronectins and other cytoplasic compounds [23] we asked whether TMV may contain mRNA transcribed from some genes of tumour cells. TMV released from all cell lines contained IL-8 (app. 27 cycle), VEGF, HGF (28 cycles) and substantial amount of β actin-mRNA (19 cycle). The negative control was around 38th cycle (Fig. 3). TMV also expressed CD44H-mRNA (app. 33 cycle, data not shown). Also cell lines contained mRNA for these transcripts (amplification about 10 cycles earlier than for TMV, data not shown). Hence TMV carry not only surface determinants but also mRNAs.
Fig. 3.
Expression of β actin (1), IL-8 (2), HGF (3) and VEGF (4)-mRNA by TMVHPC detected by “real time” PCR. Negative control (5) for β actin is shown (all controls were of similar range). One representative experiment of three performed is shown
Interaction of TMV with monocytes
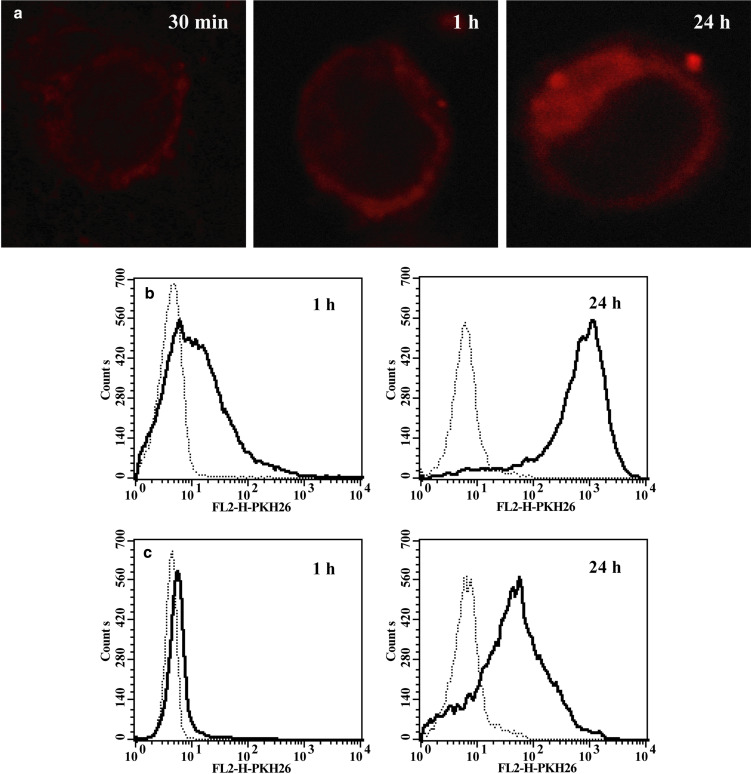
Then we asked whether TMV can adhere to monocytes, i.e. are present on their surface, or are also engulfed, i.e. localized intracellularly. To determine localization of TMV in monocytes confocal microscopy analysis was employed. A strong red fluorescence in cytoplasm was observed in monocytes incubated with PKH26-labelled TMV for 24 h (Fig. 4a). After 30 min–1 h PKH26-labelled TMV were localized closely to the cell membrane, but their precise localization (intracellular or extracellular) was difficult to establish. To answer the latter, flow cytometry was used and the extracellular fluorescence was quenched with crystal violet. A strong red fluorescence was observed following incubation of monocytes with PKH26-labelled TMV for 1 h (56±17% of positive cells, MFI 72.8) (Fig. 4b) that was significantly decreased following quenching (9±7%, MFI 9.15) (Fig. 4c). However, when monocytes were cultured with PKH26 labelled TMV for 24 h approximately 90±6% of cells showed MFI 1160.0 (Fig. 4b) which was decreased to 57±17% of cells and MFI 319,6 by crystal violet quenching (Fig. 4c). This suggested that by that time most of TMV were localized intracellularly. Hence, it was concluded that TMV not only adhere to the surface of monocytes but are also engulfed probably via phagocytosis, as at 4°C engulfment was significantly reduced (data not shown).
Fig. 4.
Transfer of PKH26 labelled TMVHPC to monocytes. a Confocal microscopy of monocytes exposed to TMVHPC for 30 min, 1 h and 24 h. b Flow cytometry of monocytes exposed to TMVHPC for 1 h and 24 h in the absence and presence of crystal violet. c Monocytes were incubated either in the medium (dotted line) or with TMV (30 μg/ml) (bold line)
Next, we determined whether monocytes may gain some determinants from TMV. Since monocytes express many determinants that are present on TMV, we studied the expression of CCR6 and CD44v7/8 that are absent on monocytes, and demonstrated that these determinants were transferred from TMV onto monocytes (Fig. 5). The transfer of CCR6 to monocytes by TMV from all lines used was observed already after 30 min (app. on 4% of cells), and its expression after 2 h was detected on app. 29% of cells. Also, after 2 h of incubation transfer of CD44v7/8 by TMV was observed as app. 5% of monocytes were found to be positive. To establish whether transferred CCR6 was biologically active chemotaxis of monocytes preincubated with TMVHPC to MIP-3α was studied. Control monocytes showed no chemotaxis while cells pretreated with TMVHPC migrated to MIP-3α (app. 6% of cells input, Fig. 6), which was roughly in keeping with the number of CCR6+ cells.
Fig. 5.
Expression of CCR6 and CD44v7/8 by monocytes (bold lines) following their incubation for 2 h either in the medium or with TMVHPC (30 μg/ml). Dotted lines represent isotype control. The representative experiment of three performed is shown
Fig. 6.
Chemotaxis of monocytes preincubated in the medium (a) or with TMVHPC (b) to MIP-3α (100 ng/ml). Data are expressed as the percentage of the cells input corrected by the percentage of cells, which migrated spontaneously to the medium alone. The representative experiment of four performed is shown
TMV regulate survival of monocytes in vitro and activate AKT kinase
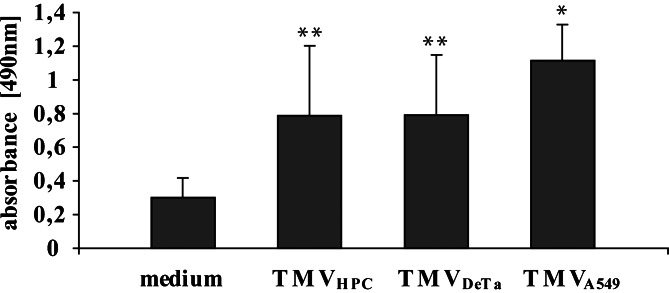
Our previous data indicated that PMV modulate survival and proliferation of human hematopoietic cells [4]. It is also known that monocytes in vitro undergo spontaneous apoptosis, in particular under serum free conditions [5]. To determine the effect of TMV on viability of monocytes, modified MTS reduction test was used. TMV significantly enhanced survival of monocytes cultured in serum free medium (Fig. 7). In keeping, the increased binding of annexin V by control monocytes seen at 24 h was significantly inhibited when monocytes were incubated with TMV (Fig. 8a). Furthermore, monocytes incubated with TMV for 24 h, but not for 3 h, showed the decreased expression of active caspase-3 as compared to unstimulated monocytes (Fig. 8b). It correlated with the level of AKT phosphorylation, which peak occurred at 30 min, after which a decrease was observed (Fig. 9). Results of the representative experiment show that all tested TMV activated AKT but not MAPK (data not shown). Taken together it indicates that TMV enhance survival of monocytes in vitro probably through prevention of their spontaneous apoptosis.
Fig. 7.
The effect of TMV on monocytes survival defined by MTS assay. Monocytes were cultured in serum free medium for 48 h in the presence or absence of TMV (30 μg/ml). Mean (OD at 490 nm) ± SD of six independent experiments is shown. * p<0.05; ** p<0.005
Fig. 8.
The effect of TMVHPC on spontaneous apoptosis of monocytes as measured by annexin-V binding (a) or the presence of active form of caspase-3 (b). Monocytes were incubated for 3 h or 24 h in the serum free medium (dotted line) or with TMVHPC (bold line). Representative experiment of three performed is shown. TMVHPC–treated monocytes at 24 h show less annexin V binding and lower expression of caspase-3
Fig. 9.
Phosphorylation of AKT in unstimulated monocytes (line 1) or monocytes stimulated for 30 min with TMVHPC (line 2), TMVDeTa (line 3) and TMVA549 (line 4)
Discussion
The present data show that TMV: (1) are constitutively released by tumour cells and serve as a vehicle for several tumour determinants, (2) carry mRNA of tumour cells, (3) adhere to and are engulfed by monocytes, (4) modulate monocyte survival, and (5) activate some signalling pathways in monocytes that regulate their survival in vitro.
This paper demonstrates that TMV are spontaneously released by three different cancer cell lines. However, PMA activation of cells led to a significantly higher release, which is in keeping with other recent observation [36]. Electron microscopy and flow cytometry analysis indicated that the size of TMV was around and below 1 μm, which is similar to TMV from MCF-7 cell line [35].
Previous reports indicated that invading melanoma cells release significant amount of TMV expressing surface receptors like CD44 and β1 integrin [12]. Moreover, expression of α3, α5 integrin chains, HLA class I, carcinoembryonic antigen (CEA), EMMPRIN and cytokeratins was described on various TMV [9, 10]. This study presents phenotypic analysis of TMV released from three different cancer cell lines and confirms the occurrence of EMMPRIN on TMV [36]. TMV showed the strong expression of HLA class I and CD29. However, not all surface determinants present on tumour cells were expressed on TMV. This refers to CD51 on TMVHPC, CXCR1 on TMVHPC and DeTa and CD58 on TMVDeTa and A549. Moreover, lower expression of CD44H and CCR3 on TMVHPC, CD95 on TMVHPC, A549 than on respective cells was found. Our data are in accordance with other observations indicating a higher expression of folate receptors, c-erb-B2 and globo-H antigen on breast carcinoma cell lines than on TMV obtained from them [9]. Surprisingly, CD44v6 and v7/8 expression was higher on TMV than on tumour cells. These results are in keeping with the suggestion that MV play a role in “gain and loose” of cell surface receptor expression [13].
To our knowledge the present paper provides the first evidence that TMV may carry mRNA for chemokines (IL-8), growth factors (VEGF, HGF), surface determinants (CD44H) and β actin. Not surprisingly, the level of β actin-mRNA expression was higher than for IL-8, HGF, VEGF and CD44H, but it was significantly lower than in intact cells. It is difficult to estimate whether it is biologically meaningful because of a lack of appropriate standards. We have no direct proof that mRNA was transferred to monocytes but the evidence for TMV engulfment makes it likely. It is an open question whether such mRNA may be translated in monocytes but it should be taken under consideration that TMV may act as transport system for proteins and nucleic acids operating both distantly, e.g. in the circulation and locally, e.g. in the tumour bed.
The present study shows that TMV adhere to and are engulfed by monocytes. This is based on confocal microscopy and further confirmed with flow cytometry analysis of monocytes incubated with PKH26-labelled TMVHPC. Already, at 1 h incubation app. 56% of monocytes showed PKH26 red fluorescence, which was quenched to app. 9% by crystal violet indicating that most of TMV were localized at the cell surface. After overnight incubation, app. 90% of monocytes showed fluorescence, which was only partly quenched (to app. 56%) by crystal violet suggesting that TMV were present intracellurarly. Moreover, engulfment of TMV was significantly reduced when incubation was performed in 4°C suggesting the role of phagocytosis.
This study also demonstrates that TMV act as vehicle delivering to monocytes some of the molecules (CD44v7/8, CCR6) they carry. It is likely, that enhanced expression of CD44v7/8 on monocytes was due to their transfer, as expression of CD44v7 did not change following monocytes activation [34]. Our previous observation indicated that expression of CD44v7/8 on monocytes is enhanced following their coculture with tumour cells [25]. Hence, the question arises whether it may be associated with TMV transfer of these determinants to monocytes. Upregulated expression of CD44v on monocytes cultured in vitro is associated with an increased binding of hyaluronan (HA) [20, 40]. Hence, the transfer of CD44v to monocytes by TMV may enhance their ability to react with HA and other extracellular matrix compounds. It has been recently shown that CD44v7 supports survival of activated T cells by interfering with the activation-induced cell death [22], which is in keeping with our observation that CD44v7/8+ TMVHPC inhibit apoptosis of monocytes. Furthermore, blocking of CD44v7 reduces production of proinflammatory cytokines by B cells and interactions between CD4 lymphocytes and monocytes [34]. A gain of CCR6 by monocytes may result in their responsiveness to MIP-3α. However, it is unclear whether incorporated CCR6 is biologically active as little chemotaxis of monocytes incubated with TMV to MIP-3α was observed but it should be noted that only a small population of monocytes became CCR6+. Furthermore, a caution is needed in the interpretation of such data as TMV rapidly activate monocytes that results in enhanced adherence, which decreases their mobility.
In keeping, TMV as a source of biologically active proteins and lipids may support survival of cells, via antiapoptotic mechanism. This is based on findings that TMV significantly decreased spontaneous apoptosis of monocytes in vitro as measured by the inhibition of annexin V binding and active caspase-3 expression. It is rather surprising finding since contact with tumour cells leads to apoptosis of monocytes [25]. Moreover, this is in contrast to the report indicating that TMV induce apoptosis of lymphocytes [2]. This effect may be due to lack of Fas–FasL interactions because TMV used in the present studies did not expressed FasL. Hence, it may indicate that TMV contain and deliver to the culture microenviroment some biologically active substances that influence monocyte survival. It may be consisted with the other data that phagocytosis of apoptotic cells, which release MV, by macrophages induces their cytokine-independent survival [30] and that PMV enhance hemopoietic cells survival [4].
Finally, in TMV-stimulated monocytes AKT phosphorylation was observed, which provides molecular evidence that TMV may modulate the balance between survival and apoptosis. AKT is known as a critical regulator of cell survival, which blocks apoptosis induced by UV radiation [39], matrix detachment [3], removal of growth factors or DNA damage [7, 8]. The lack of MAPK phosphorylation may not be surprising as both p42 and p44 MAPK play a crucial role in the regulation of cell proliferation. Moreover, it was shown that apoptotic but not necrotic cells promote survival and inhibit proliferation of murine macrophages via simultaneous activation of AKT and inhibition of the p44/42 MAPK [30].
In conclusion, the present data indicate that TMV carry and transfer some surface determinants from tumour cells to monocytes. TMV may also act as a vehicle for transport of mRNA from tumour cells. Transfer of TMV to monocytes enhances their survival in vitro by the inhibition of spontaneous apoptosis. Hence, immunophenotype and some biological activities of monocytes may be altered by cancer cells not necessarily by a direct cell to cell contact or soluble factors but also via TMV. The role of TMV as a vehicle for EMMPRIN involved in tumour–stromal interaction has recently been described [36]. Present data suggest that transfer of EMMPRIN to monocytes may be responsible for the induction of MMP-9 secretion that occurs in monocytes exposed to TMV (Baj-Krzyworzeka et al. manuscript in preparation).
Acknowledgements
This study was supported by the State Committee for Scientific Research (grant no. 3P05A 057 22 and PBZ-KBN-091/P05/2003). We thank Ms B. Hajto, I.Ruggiero and M. Wołoszyn for skillful technical assistance.
References
- 1.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, Dainiak N. Biologically active Fas antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood. 1998;91:3862–3874. [PubMed] [Google Scholar]
- 2.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachelder RE, Wendt MA, Fujita N, Tsuruo T, Mercurio AM. The cleavage of Akt/protein kinase B by death receptor signaling is an important event in detachment induced apoptosis. J Biol Chem. 2001;276:34702–34707. doi: 10.1074/jbc.M102806200. [DOI] [PubMed] [Google Scholar]
- 4.Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30:450–459. doi: 10.1016/S0301-472X(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 5.Baran J, Weglarczyk K, Mysiak M, Guzik K, Ernst M, Flad HD, Pryjma J. Fas (CD95)-Fas ligand interactions are responsible for monocyte apoptosis occurring as a result of phagocytosis and killing of Staphylococcus aureus. Infect Immun. 2001;69:1287–1297. doi: 10.1128/IAI.69.3.1287-1297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barz D, Goppelt M, Szamel M, Schirrmacher V, Resch K. Characterization of cellular and extracellular plasma membrane vesicles from a non-metastasizing lymphoma (Eb) and its metastasizing variant (ESb) Biochim Biophys Acta. 1985;814:77–84. doi: 10.1016/0005-2736(85)90421-3. [DOI] [PubMed] [Google Scholar]
- 7.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 8.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–1927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 9.Dolo V, Adobati E, Canevari S, Picone MA, Vittorelli ML. Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clin Exp Metastasis. 1995;13:277–286. doi: 10.1007/BF00133483. [DOI] [PubMed] [Google Scholar]
- 10.Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, Nagase H, Canevari S, Pavan A, Vittorelli ML. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58:4468–4474. [PubMed] [Google Scholar]
- 11.Forlow SB, McEver RP, Nollert MU. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood. 2000;95:1317–1323. [PubMed] [Google Scholar]
- 12.Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matric reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- 13.Fritzsching B, Schwer B, Kartenbeck J, Pedal A, Horejsi V, Ott M. Release and intercellular transfer of cell surface CD81 via microparticles. J Immunol. 2002;169:5531–5537. doi: 10.4049/jimmunol.169.10.5531. [DOI] [PubMed] [Google Scholar]
- 14.Fujimi S, Ogura H, Tanaka H, Koh T, Hosotsubo H, Nakamori Y, Kuwagata Y, Shimazu T, Sugimoto H. Activated polymorphonuclear leukocytes enhance production of leukocyte micropraticles with increased adhesion molecules in patients with sepsis. J Trauma. 2002;52:443–448. doi: 10.1097/00005373-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 15.George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–840. [PubMed] [Google Scholar]
- 16.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–1135. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 18.Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 19.Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, Kim HK, Ryu KW, Bae JM, Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–191. doi: 10.1016/S0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 20.Levesque MC, Haynes BF. In vitro culture of human peripheral blood monocytes induces hyaluronan binding and up-regulates monocyte variant CD44 isoform expression. J Immunol. 1996;156:1557–1565. [PubMed] [Google Scholar]
- 21.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 22.Marhaba R, Bourouba M, Zoller M. CD44v7 interferes with activation – induced cell death by up-regulation of anti apoptotic gene expression. J Leukoc Biol. 2003;74:135–148. doi: 10.1189/jlb.1202615. [DOI] [PubMed] [Google Scholar]
- 23.Mayer C, Maaser K, Daryab N, Zanker KS, Brocker EB, Friedl P. Release of cell fragments by invading melanoma cells. Eur J Cell Biol. 2004;83:709–715. doi: 10.1078/0171-9335-00394. [DOI] [PubMed] [Google Scholar]
- 24.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 25.Mytar B, Siedlar M, Woloszyn M, Collizzi V, Zembala M. Cross-talk between human monocytes and cancer cells during reactive oxygen intermediates generation the essential role of hyaluronan. Int J Cancer. 2001;94:727–732. doi: 10.1002/ijc.1530. [DOI] [PubMed] [Google Scholar]
- 26.Mytar B, Baran J, Gawlicka M, Ruggiero I, Zembala M. Immunophenotypic changes and induction of apoptosis of monocytes and tumour cells during their interactions in vitro. Anticaner Res. 2002;22:2789–2796. [PubMed] [Google Scholar]
- 27.Mytar B, Woloszyn M, Szatanek R, Baj-Krzyworzeka M, SiedlarM Ruggiero I, Wieckiewicz J, Zembala M. Tumor cell-induced deactivation of human monocytes. J Leukoc Biol. 2003;74:1094–1101. doi: 10.1189/jlb.0403140. [DOI] [PubMed] [Google Scholar]
- 28.Omoto S, Nomura S, Shouzu a, Nishikawa M, Fukuhara S, Iwasaka T. Detection of monocyte-derived macroparticles in patients with Type II diabetes mellitus. Diabetologia. 2002;45:550–555. doi: 10.1007/s00125-001-0772-7. [DOI] [PubMed] [Google Scholar]
- 29.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 30.Reddy SM, Hsiao KH, Abernethy VE, Fan H, Longacre A, Lieberthal W, Rauch J, Koh JS, Levine JS. Phagocytosis of apoptotic cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J Immunol. 2002;169:702–713. doi: 10.4049/jimmunol.169.2.702. [DOI] [PubMed] [Google Scholar]
- 31.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–3970. doi: 10.1182/blood.V99.11.3962. [DOI] [PubMed] [Google Scholar]
- 32.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 33.Scholz T, Temmler U, Krause S, Heptinstall S, Losche W. Transfer of tissue factor from platelets to monocytes: role of platelet-derived microvesicles and CD62P. Thromb Haemost. 2002;66:1033–1038. [PubMed] [Google Scholar]
- 34.Seiter S, Schmidt DS, Zoller M. The CD44 variant isoforms CD44v6 and CD44v7 are expressed by distinct leukocyte subpopulations and exert non-overlapping functional activities. Int Immunol. 2000;12:37–49. doi: 10.1093/intimm/12.1.37. [DOI] [PubMed] [Google Scholar]
- 35.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 36.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;29:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signaling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Amersfoort ES, Van Strijp JA. Evaluation of a flow cytometric fluorescence quenching assay of phagocytosis of sensitized sheep erythrocytes by polymorphonuclear leukocytes. Cytometry. 1994;17:294–301. doi: 10.1002/cyto.990170404. [DOI] [PubMed] [Google Scholar]
- 39.Wang HQ, Quan T, He T, Franke TF, Voorhees JJ, Fisher GJ. Epidermal growth factor receptor dependent, NFkB independent activation of the phosphatidyloinositol 3 kinase/Akt pathway inhibits ultraviolet irradiation induced caspase −3, −8, and 9 in human keratinocytes. J Biol Chem. 2003;278:45737–45745. doi: 10.1074/jbc.M300574200. [DOI] [PubMed] [Google Scholar]
- 40.Weiss JM, Renkl AC, Ahrens T, Moll J, Mai BH, Denfeld RW, Schopf E, Ponta H, Herrlich P, Simon JC. Activation-dependent modulation of hyaluronate-receptor expression and of hyaluronate-avidity by human monocytes. J Invest Dermatol. 1998;111:227–232. doi: 10.1046/j.1523-1747.1998.00286.x. [DOI] [PubMed] [Google Scholar]