Abstract
Background: The greater omentum is frequently involved in the course of gastrointestinal and ovarian tumors. Therefore, common practice in surgical treatment for especially gastric and ovarian cancer includes removal of the greater omentum. Paradoxically, many immune cells, such as macrophages that accumulate in so-called milky spots, reside within the omentum and are cytotoxic against tumor cells ex vivo. Consequently, omental macrophages might play an important role in killing tumor cells, and may hereby prevent development into local peritoneal recurrences. In the present study, we therefore evaluated the role of the omentum and the clinical relevance of omentectomy in minimal residual disease (MRD). Methods: Tumor cell dissemination patterns on the omentum in a rat model were examined using DiI-labelled CC531s tumor cells. Additionally, intra peritoneal (i.p.) tumor load was investigated in rats that underwent omentectomy or sham laparotomy followed by i.p. injection of CC531s cells on day 21, which represented MRD. Results: At 4 h post injection, tumor cells predominantly adhered on milky spots. Number of cells thereafter declined rapidly suggesting initial tumor killing functions in these specific immune aggregates. Despite initial reduction observed in milky spots, numbers of tumor cells however increased at fatty tissue stripes that border the omentum. This indicated proliferation at these locations, which corresponded to macroscopic observations of the omenta on day 21 after tumor cell injection. Omentectomy resulted in reduced intra-abdominal tumor load, which was completely attributable to the absence of the omentum, as tumor development did not differ on other sites. Even in the MRD group microscopic clusters of tumor cells located in the omentum eventually developed into macroscopic nodules.Conclusion: Since the ability of omental milky spots is, even in MRD, insufficient to prevent intra abdominal tumor outgrowth, omentectomy, which reduces tumor load, is recommended in surgical treatment of intra abdominal tumors that are prone to disseminate intraperitoneally.
Keywords: Omentum, Metastases, Rat model, Macrophage, Milky spots, Minimal residual disease
Introduction
The greater omentum is frequently involved in intra abdominal dissemination of human malignancies, either in primary spreading or as site of recurrent cancer after surgical treatment. Omental disease can be observed in several types of intra abdominal malignancies [1–4]. In ovarian cancer the omentum is most frequently involved at the time of diagnosis, which often implies late stage disease [2]. Standard treatment practice for epithelial ovarian cancer includes the removal of the (infracolic) omentum as part of the surgical treatment in more advanced stages and to allow adequate staging in perceptible early stage disease according to the guidelines of the International Federation of Gynecology and Obstetrics [1]. Likewise, peritoneal seeding involving the omentum is a frequently observed route of dissemination in advanced colorectal malignancies (including peritoneal carcinomatosis) [5]. Based on these observations and retrospective data, it has become common practice to co-resect the omentum in surgical treatment of ovarian and advanced colorectal cancer [1, 4, 6].
Paradoxically, many immune cells, such as macrophages, reside within the omentum [7, 8]. In addition, large aggregates of macrophages and lymphocytes, which comprise so-called milky spots, have been shown to represent a site for leukocyte proliferation and play a role as source of peritoneal macrophages [9, 10]. These immune cells may have a major function in host defense against foreign particles and bacteria. Furthermore, it has been established that omental macrophages exhibit cytotoxicity against tumor cells ex vivo that was enhanced after in vivo immune stimulation with GM-CSF [11]. Therefore, omental macrophages might play an important role in clearing minimal residual disease (MRD) or free peritoneal (peroperatively spilled) tumor cells, which are present after resection for gastrointestinal and ovarian cancer. Hence, omentectomy may theoretically impair anti-tumor immune responses. Evidence is now accumulating that presence of MRD significantly correlates with the incidence of recurrent peritoneal disease and worse patient outcome after intentionally curative surgery [4, 5, 12–14].
Previously, we and others have demonstrated that intraperitoneal (i.p.) injection of tumor cells in rodents resulted in preferential tumor load on the omentum [15, 16]. However, in these experiments large numbers of tumor cells were inoculated, which resulted in massive intra abdominal tumor outgrowth [15, 17]. It has now been demonstrated that macrophages are not able to efficiently kill tumor cells at low effector-to-target (ET) ratios (Ref. 11 and data not shown). As such, macrophages were presumably not capable of killing the high numbers of tumor cells, which were used in the previous in vivo studies [15, 18]. Additionally, it has been shown that detection of MRD does not result in recurrent disease in all patients [12]. As in the previous described studies all rodents developed tumors, the high tumor cell dose that was used probably did not reflect clinical MRD [5, 13, 15].
In the current study, we therefore used the i.p. CC531s rat model to investigate the role of the omentum, and its immune defense mechanisms, as well as the clinical relevance of omentectomy in MRD.
Materials and methods
Animals
Male Wag/Rij rats (180–200 g) were obtained from Charles River, Maastricht, The Netherlands. Rats were kept under standard laboratory conditions and had free access to standard laboratory food and water. All study protocols were approved by the Committee for Animal Research of the VU University Medical Center, Amsterdam, The Netherlands, according to national guidelines.
Tumor cell line
The CC531s is a well-defined weakly immunogenic adenocarcinoma cell line, originating from the colon of Wag/Rij rats exposed to methylazoxymethanol [19]. CC531s cells were cultured under standard incubator conditions in RPMI 1640 (Invitrogen, Paisley, Scotland) supplemented with 10% heat-inactivated fetal calf serum (Gibco, Irvine, Scotland), penicillin (100 U/ml), streptomycin (100 μg/ml) and l-glutamine (2 mmol/l). Cells were prepared by enzymatic detachment from culture flasks with the use of trypsin-EDTA solution (7 min at 37°C), centrifuged for 5 min at 500 g, counted and resuspended in RPMI. Viability, as determined with trypan blue exclusion, always exceeded 95%.
Tumor cell attachment on the omentum
The CC531s were incubated in complete RPMI 1640 in a concentration of 5×106 cells/ml with 50 μg DiI (Sigma-Aldrich, St Louis, US) for 30 min at 37°C. Cells were washed three times with Hanks’ balanced salt solution (HBSS) and 2×105 CC531s cells (in 0.5 ml HBSS) were injected i.p. Animals (n=3/group) were killed after four, 24 and 72 h. Omenta were excised, stretched on microscope glasses and air-dried for 24 h before further processing.
Immunohistochemistry
For immunohistochemistry, omenta were fixed in anhydrous acetone, air-dried and incubated with PBS containing 0.5% BSA (PBSA) and 10% normal goat serum for 15 min. Omenta were incubated for 60 min with primary mouse-α-CC531 antibody (Ab) (CC52, 20 μg/ml) [20] in PBSA, washed three times in PBS and further incubated for 45 min with secondary Alexa-488-labelled goat-α-mouse Ab (1:400) (Molecular Probes Inc., Eugene, USA) in PBSA. Washing of omenta in PBS (3 times) was followed by 60-min incubation with biotin-labelled ED1 (1:250) [21] (monocyte/macrophage marker) (4 μg/ml, Serotec, Oxford, UK) or biotin-labelled ED2 (1:100) [21] (mature macrophage marker) (4 μg/ml, Serotec) in PBSA and subsequent washing with PBS (3 times). After incubation of the omenta with either Alexa-594 (1:400) (Molecular Probes) or Alexa-488-labelled streptavidin (1:400) (Molecular Probes) in PBSA for 45 min, omenta were washed in PBS (3 times) and mounted in Vecta mount (Vector laboratories, Burlingham, CA, USA). Immunohistochemical staining was directly scored and images were captured with a Leica fluorescent microscope equipped with a CCD camera.
Study design
Escalating dose-effect on tumor load
In order to establish the minimal tumor cell dosage required to initiate tumor growth, rats were injected with CC531s cells at day 0 in the following doses (4/group): 2×106, 5×105, 2×105, 5×104, 1×104, 2×103. Animals were killed 21 days after tumor cell injection, and i.p. tumor load was semiquantatively scored according to an index (ranging from zero to five) previously described by Steller et al. [22]. Briefly, no tumor was indicated by zero, a total tumor diameter per site of <5 mm indicated by 1, >5, <10 mm by 2, >10 and <20 by 3, >20 and <30 by 4,and >30 mm by 5 (maximum). Two independent observers blindly evaluated the following sites: injection site (subcutaneous), parietal peritoneum, mesentery, liver surface and hilum, spleen, omentum majus, kidney and gonadal fat. Scores of all peritoneal sites were added, resulting in total tumor score per rat.
Omentectomy versus sham operation
Rats underwent either an omentectomy or a sham operation. Operations were performed under isoflurane anesthesia and aseptic conditions. The greater omentum was reached via a left paramedian transverse incision in the upper abdomen and subsequently excised from the hilum of the spleen (preserving the splenic artery), the greater gastric curvature including the epiploic arcade and from the medial connection with pancreatic tissue. Sham operated animals underwent a similar incision, without removal of the omentum. After haemostasis, the abdomen was closed in two layers using Ethilon stitches. Operation time was 15 min in both groups. No animal died as a result of the operative procedure. After a 21 days recovery period, animals were injected with either 1×104 or 5×105 CC531s cells i.p. (n=9/group). Animals were sacrificed 21 days after tumor cell administration. Additionally, two groups of animals (n=5/group) received 1×104 CC531s cells and were killed on day 35 and 42, respectively, in order to investigate outgrowth of low-dose CC531s after a longer period of time.
Statistical analysis
Non-parametric Mann–Whitney tests were used to compare two groups. Statistical significance was defined as P<0.05.
Results
Visualization of tumor cells on the omentum at early time points
To investigate the region of the omentum involved in tumor cell attachment and outgrowth, we injected 2×105 DiI-labelled CC531s (DiI-CC531s) i.p. and removed omenta at several early time points. In vitro, no differences in adhesion abilities and proliferation rates were shown between DiI-CC531s cells and non-labelled CC531s cells. DiI-CC531s displayed high signal intensity after 4 days of culture (data not shown). Due to the type and dimensions of the tissue (dried stretched whole omentum with fatty tissue regions), which precluded exact quantification, attached tumor cells were scored semi-quantitatively (Table 1). Four hours after injection DiI-lCC531s cells were particularly concentrated in milky spot areas (mainly consisting of macrophage aggregates) (Fig. 1a). In addition, many tumor cells clusters were found in fatty tissue regions of the omentum and to a lesser extent on non-milky spot areas in thin transparent regions (Table 1). At 24 h post injection, numbers of DiI-CC531s cells declined in milky spot areas (Fig. 1b), while in fatty tissue stripes large numbers of clusters were observed. After 72 h, numbers of CC531s cells further decreased in milky spot areas and no proliferating tumor cells were observed in milky spots (Fig. 1c). In contrast, large cell clusters were detected in fatty tissue stripes, which suggest that tumor cells proliferated in these areas (Fig. 1d). Additionally, some clusters that were increased in size and cell number compared to previous time points were found in thin transparent non-milky spot regions. Tumor spread in animals that had been injected with 5×105 CC531s cells showed the same pattern as observed in the 2×105 CC531s dose group. However, at 72 h post-injection few proliferating tumor cell clusters were found in milky spots (data not shown).
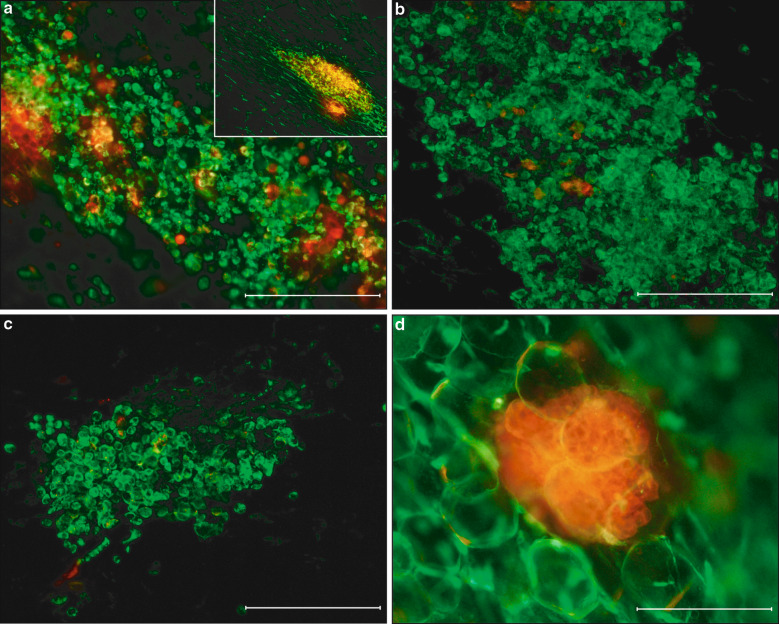
Fig. 1.
Visualization of tumor cell adhesion on the omentum. a Image of whole omentum stretched on glass showing milky spot macrophages (ED1; green) and large numbers of DiI-CC531s (red) cells concentrated in milky spot areas 4 h after i.p. injection (an overview in the insert). b After 24 h numbers of DiI-CC531s (red) cells in milky spots decreased (macrophages ED1; green). c Only very low numbers of tumor cells were observed in milky spots after 72 h. d However, tumor cell clusters (red) in fatty tissue stripes were increased in size at 72 h post injection compared to 4 h post injection, suggesting proliferation. The very large green cells are fat cells. All bars represent 50 μm
Table 1.
Semi-quantatitive analysis of sequential observations of DiI-CC531s tumor cell attachment on the omentum after injection of 2×105 cells i.p
| Time point | Milky spots | Transparent region | Fatty tissue stripe | |||
|---|---|---|---|---|---|---|
| Presence | Proliferation | Presence | Proliferation | Presence | Proliferation | |
| 4 h | ++ | n.a. | ++ | n.a. | ++ | n.a. |
| 24 h | ++ | n.a. | +** | n.a. | ++ | n.a. |
| 72 h | +/* | – | +** | + | ++ | + |
− negative, + moderate numbers, ++ high numbers, n.a. not applicable, * very scarce single cells, ** only large clusters (>10 tumor cells), no single cells
Additionally, we investigated tumor dissemination in the mesentery, since presence of milky spot-like structures has been described in a study using i.p. carbon particles in mice [23]. Stainings with ED1 and ED2 demonstrated the presence of many single (spindle-shaped) macrophages. However, only very few small aggregates (10–30 cells) were observed, which were not supplied by convoluted small blood vessels. As milky spots are defined as large clusters of macrophages and lymphocytes, which are supplied by blood vessels, these data support that the mesentery does not contain genuine milky spots. As this is characteristically seen in milky spots, it suggests that established milky spots are not present. Some DiI-CC531s cells (mostly single or small cell clusters) were observed on the mesentery after four and 72 h post-injection, although in much lower quantities than on the omentum. No clear interactions with macrophages were found (data not shown).
Escalating dose-effect on tumor load
To further explore the role of the omentum in a MRD setting, we injected escalating numbers of CC531s cells i.p. in rats to determine the minimum dose required for tumor development. Injections with dosages ranging from 1×104 to 5×105 CC531s cells resulted in well quantifiable tumor load at day 21 (Fig. 2). No tumor load was observed after i.p. injection of 2×103 CC531s cells, while inoculation of 1×104 CC531s cells resulted in a small number of tumor deposits on the omentum and liver hilum (at day 21). Significant larger tumor load was found when higher tumor cell numbers were injected. Large confluent tumors and omental cake were observed in the group that received 2×106 CC531s cells, precluding exact quantification due to maximal scoring of the index scale. The omentum, liver hilum, gonadal fat and diaphragm were preferential sites for metastasis formation (data not shown).
Fig. 2.
Tumor dose–tumor load relation. Injections with dosages ranging from 1×104 to 5×105 CC531s cells resulted in well quantifiable tumor load at day 21. Tumor load was semi-quantitatively scored according to a previously established index scale (see Materials and methods). No tumor load was observed after i.p. injection of 2×103 CC531s cells, while large confluent tumors and omental cake were observed in the group that received 2×106 CC531s cells, precluding exact quantification as the maximum was reached on the index scale. Mean ± SD are depicted
The effect of omentectomy on intra-abdominal metastases formation
No animal experienced a complication or died as a result of the omentectomy or sham laparotomy. Since i.p. administration of <1×104 CC531s cells did not result in tumor outgrowth, we used 1×104 cells as dose to investigate the effect of omentectomy on MRD development. Furthermore, i.p. inoculation of dosages >5×105 CC531s tumor cells resulted in massive confluent tumor outgrowth, while tumor load resulting from injection of 5×105 CC531s tumor cells could adequately be quantified, so this dose was used as positive control. In all groups tumor cells were injected on day 21 after surgery.
Cumulative tumor load was significantly larger in the sham group compared with the omentectomy group (15.1 vs. 10.1, P=0.001) in rats that received 5×105 CC531s cells (Fig. 3a). The omentum (in sham-operated animals), liver hilum and gonadal fat pads were sites of preferential tumor outgrowth in the high dose groups (Fig. 3). No macroscopic tumor deposits were found on the capsule of the liver, kidney and serosa of the intestines. To investigate whether tumor outgrowth on abdominal sites was enhanced because of omentectomy, we also compared tumor scores of these sites (tumor load excluding the omentum) between the omentectomy and control group. However, no difference was found (Fig. 3a).
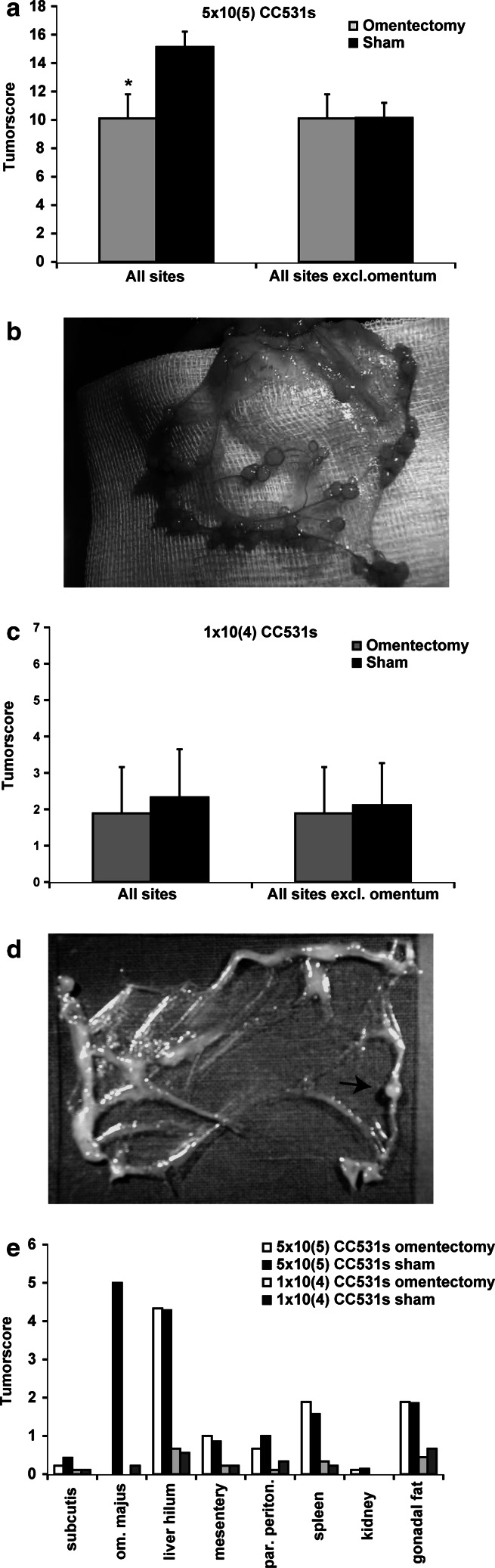
Fig. 3.
Omentectomy and tumor load. a Tumor load, quantified 3 weeks after i.p. injection with 5×105 CC531s cells. Sham operated group had a significantly higher total tumor load than omentectomy group, when all abdominal scoring sites were included. However, after excluding the omentum, no difference in tumor development was seen on other abdominal sites. Mean ± SD are depicted. *P=0.001. b Macroscopic image of a representative omentum (n=9), 3 weeks after i.p. injection with 5×105 CC531s cells. Tumor load displayed a nodular growth pattern, mainly located along fatty tissue stripes that regularly form the margins of the omentum. c Tumor load as scored 3 weeks after i.p. administration of 1×104 CC531s cells, showing no significant differences between both groups. Mean ± SD are depicted. d Macroscopic image of an omentum, 3 weeks after i.p. injection with 1×104 CC531s cells (n=9), showing fatty tissue stripes bordering thin transparent regions. One small tumor nodule is visible located in fatty tissue (arrow). e Distribution of i.p. tumor load in sham and omentectomy animals 3 weeks after CC531s injections (n=9/group). Tumor load was semi-quantitatively scored according to a previously established index scale (for a, c and e) (see Materials and methods)
In animals that received 1×104 CC531s, no difference in macroscopic tumor load was observed between the omentectomy and the control group (Fig. 3c). In two sham-operated rats a single small tumor nodule was macroscopically observed on the omentum, while none were found on the omentum in the remaining seven animals (Fig. 3d). Tumors were mainly found on the liver hilum (Fig. 3e). Tumor load on peritoneal sites other than the omentum did not differ between the two low dose groups (Fig. 3c).
Evaluation of tumor development in the low dose group
Even though no metastases were observed macroscopically on omenta in rats receiving 1×104 tumor cells, presence of micrometastases was investigated. On day 21, omenta were excised, dried and stained for CC531s tumor cells with the use of immunofluorescence. In all omenta 10–20 clusters containing 20 to >200 tumor cells were found. Contact between tumor cells and macrophages was seen in double stainings with ED2 (mature macrophages) markers (Fig. 4a) and ED1 (pan-macrophage) (Fig. 4b). However, no tumor cells or clusters of tumor cells were observed in specific milky spot areas (Fig. 4c).
Fig. 4.
Long-term tumor development in the low dose group. a immunofluorescence staining with α-CC531 Ab CC52 (green) and α-ED2 (mature macrophages; red) of a representative omentum (n=9), showing a large cluster of tumor cells surrounded by macrophages 21 days after injection of 1×104 CC531s cells i.p. b Omentum, double-stained with α-CC531 Ab CC52 (green) and α-ED1 (macrophages; red) from the sham operated group injected with 1×104 CC531s cells (n=9); 10–20 of these clusters of tumor cells were found microscopically after 21 days. c A representative double staining with α-ED1 (macrophages; red) and α-CC531 Ab CC52 (green), revealing that no tumor cells were present in milky spots at 21 days post injection. d Total tumor load as scored on respectively, day 21, 35 and 42 after i.p. injection of 1×104 CC531s (n=5/group). Tumor load was semi-quantitatively scored according to a previously established index scale (see Materials and methods). All bars represent 200 μm
To investigate whether these tumor cell clusters eventually will grow out or get killed by macrophages, we also investigated tumor development at later time points. Rats were sacrificed at day 35 and 42, respectively. Immunohistochemical analyses of the inflammatory infiltrate in these tumors showed ED1-positive macrophages scattered throughout the tumors, while mature ED2-positive macrophages were confined to the peripheral zone of the tumors (data not shown). Both CD8- and CD4-positive cells were observed to infiltrate CC531s tumors. However, as total tumor load increased over time the omental immune defense was not capable of eliminating (all) intra abdominal MRD (Fig. 4d).
Discussion
The peritoneum, including the omentum, is a relative common site of either recurrent disease or primary seeding in both gastrointestinal and ovarian cancer. Previously, several experimental studies have reported that cancer cells seeded in the peritoneal cavity preferentially grow on the omentum [15, 23, 24]. However, the omentum and especially milky spots that are composed of large aggregates of macrophages and lymphocytes have important functions in peritoneal defense [11, 25]. Moreover, peritoneal and omental macrophages have previously been shown cytotoxic against tumor cells ex vivo [11]. Therefore, omentectomy could theoretically impair peritoneal anti-tumor defense against exfoliated tumor cells, especially in MRD. This paradox prompted us to further study tumor cell dissemination on the omentum and the effects of omentectomy on i.p. tumor growth.
Previously, milky spots have been reported to represent preferential sites of intra abdominal tumor cell adhesion [15, 23, 18, 26]. In the present study, we found tumor cells mainly adhered on milky spots 4 h after injection. This preferred attachment may be explained by the fact that intercellular gaps and pores (stomata) are present on the surface of milky spots that expose underlying connective tissue matrix, which is proposed to represent a preferential location for tumor cell adhesion [27–29]. Alternatively, mesothelial cells lining milky spots showed higher levels of cellular adhesion molecules such as intercellular adhesion molecule-1 than other omental regions, which may contribute to enhanced adhesion as well [27]. However, 72 h after injection a sharp decline in numbers of tumor cells was seen in specific milky spot areas, suggesting that tumor cells have been eliminated by the abundantly present macrophages.
Shortly after injection, large quantities of CC531s cells were observed on fatty stripes of the omentum. Previous electron microscopic analysis showed a cell layer with microvilli similar to that seen at milky spots on the surface of these fatty tissue regions, which may explain the comparable enhanced tumor cell attachment [27]. In contrast to milky spot areas, tumor cell clusters located in fatty tissue noticeably increased in size, supporting that CC531s cells are able to grow out, which is possibly due to the absence of large amounts of macrophages. Macroscopic tumor outgrowth correlated with microscopic observations as tumor nodules were mainly found on fatty streaks, regularly bordering the rat omentum.
Removal of the omentum or omentectomy resulted in reduced intra-abdominal tumor load. The effect was attributable to the absence of the omentum as site of tumor implantation, indicating that presence of the omentum or absence thereof does not influence tumor growth on abdominal sites other than the omentum. Although it is a common procedure in the surgical treatment of ovarian cancers, very little experimental data exist about the effects of omentectomy in tumor models. Previously, it was shown that removal of omental tissue reduced the ability of spilled tumor cells to form local tumors after a bowel anastomosis, indicating a supporting role of the omentum in tumor formation at wound (healing) sites [30]. In this experimental model, tumor cells were however administered during intra abdominal surgery, which strongly enhances outgrowth of tumors [29–32] To rule out possible effects of surgery in our model, (while exclusively focusing on the role of the omentum) omentectomy or sham laparotomy was followed by a 3 weeks recovery period. Indeed, no tumors were observed in previously traumatized areas such as the laparotomy wound or greater curvature of the stomach indicating that the prior surgical trauma did not play a role in adhesion processes or outgrowth of cells by the time of injection. Furthermore, very little data are available from clinical studies addressing the role of omentectomy in intra abdominal cancers. In a recent randomized phase III trial, it was shown that patients with early stage ovarian cancer that were optimally staged (including omentectomy) had statistically better survival than patients who were non-optimally staged (e.g. leaving the omentum in situ).[33] However, adjuvant chemotherapy improved survival in non-optimally staged patients but did not influence prognosis in optimally staged patients, which indicates that MRD in e.g. the omentum affects outcome in ovarian cancer. Thus, this study indirectly supports the value of omentectomy in abdominal tumors prone to disseminate intraperitoneally [33].
From preceding in vitro studies, it was shown that macrophages were not able to efficiently kill tumor cells at low ET ratios (Ref. 11 and data not shown). As such, macrophages are presumably not capable of killing the high numbers of tumor cells, which were used in previous in vivo studies. No tumor cells were found in milky spots (in the 1×104 CC531s dose), which supported the killing capacity of macrophages at high E:T ratios. However, long-term observation in the MRD group displayed microscopic clusters of tumor cells on the omentum (but not in milky spots) of which some developed into macroscopic nodules. Consequently, macrophages are even in the MRD model not capable of eliminating all tumor cells present on omental sites other than milky spots.
In conclusion, a sharp decline in tumor cell presence in milky spot macrophages was observed following i.p. injection, supporting a killing function by macrophages. However, even in MRD, the ability of omental milky spots to prevent peritoneal tumor outgrowth remains insufficient. As omentectomy reduced total (macroscopic and microscopic) i.p. tumor load, removal of the omentum is recommended in the surgical treatment of intra-abdominal malignancies with high risk of peritoneal dissemination, such as ovarian cancer and recurrences of gastro-intestinal origin.
Abbreviations
- Ab
Antibody
- MRD
Minimal residual disease
- i.p.
Intraperitoneal
- GM-CSF
Granulocyte macrophage-colony stimulating factor
References
- 1.Benedet JL, Bender H, Jones H, III, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO committee on gynecologic oncology. Int J Gynaecol Obstet. 2000;70:209–262. doi: 10.1016/S0020-7292(00)90001-8. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg JJ, Demopoulos RI, Bigelow B. The evaluation of the omentum in ovarian cancer. Gynecol Oncol. 1986;24:327–330. doi: 10.1016/0090-8258(86)90309-4. [DOI] [PubMed] [Google Scholar]
- 3.Deraco M, Santoro N, Carraro O, Inglese MG, Rebuffoni G, Guadagni S, Somers DC, Vaglini M. Peritoneal carcinomatosis: feature of dissemination. A review. Tumori. 1999;85:1–5. doi: 10.1177/030089169908500101. [DOI] [PubMed] [Google Scholar]
- 4.Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76. doi: 10.1007/s101200200012. [DOI] [PubMed] [Google Scholar]
- 5.Bosch B, Guller U, Schnider A, Maurer R, Harder F, Metzger U, Marti WR. Perioperative detection of disseminated tumour cells is an independent prognostic factor in patients with colorectal cancer. Br J Surg. 2003;90:882–888. doi: 10.1002/bjs.4129. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara A, Sawai K, Sakakura C, Shirasu M, Ohgaki M, Yamasaki J, Togawa T, Takahashi T. Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology. 1998;45:1922–1929. [PubMed] [Google Scholar]
- 7.Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 8.Beelen RH. Role of omental milky spots in the local immune response. Lancet. 1992;339:689. doi: 10.1016/0140-6736(92)90857-Y. [DOI] [PubMed] [Google Scholar]
- 9.Wijffels JF, Hendrickx RJ, Steenbergen JJ, Eestermans IL, Beelen RH. Milky spots in the mouse omentum may play an important role in the origin of peritoneal macrophages. Res Immunol. 1992;143:401–409. doi: 10.1016/S0923-2494(05)80072-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhu H, Naito M, Umezu H, Moriyama H, Takatsuka H, Takahashi K, Shultz LD. Macrophage differentiation and expression of macrophage colony-stimulating factor in murine milky spots and omentum after macrophage elimination. J Leukoc Biol. 1997;61:436–444. doi: 10.1002/jlb.61.4.436. [DOI] [PubMed] [Google Scholar]
- 11.Koenen HJ, Smit MJ, Simmelink MM, Schuurman B, Beelen RH, Meijer S. Effect of intraperitoneal administration of granulocyte/macrophage-colony-stimulating factor in rats on omental milky-spot composition and tumoricidal activity in vivo and in vitro . Cancer Immunol Immunother. 1996;42:310–316. doi: 10.1007/s002620050288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schott A, Vogel I, Krueger U, Kalthoff H, Schreiber HW, Schmiegel W, Henne-Bruns D, Kremer B, Juhl H. Isolated tumor cells are frequently detectable in the peritoneal cavity of gastric and colorectal cancer patients and serve as a new prognostic marker. Ann Surg. 1998;227:372–379. doi: 10.1097/00000658-199803000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanellos I, Demetriades H, Zintzaras E, Mandrali A, Mantzoros I, Betsis D. Incidence and prognostic value of positive peritoneal cytology in colorectal cancer. Dis Colon Rectum. 2003;46:535–539. doi: 10.1007/s10350-004-6595-0. [DOI] [PubMed] [Google Scholar]
- 14.Lennon AM, Mulcahy HE, Hyland JM, Lowry C, White A, Fennelly D, Murphy JJ, O’Donoghue DP, Sheahan K. Peritoneal involvement in stage II colon cancer. Am J Clin Pathol. 2003;119:108–113. doi: 10.1309/J6BD-TWM2-M792-TN2V. [DOI] [PubMed] [Google Scholar]
- 15.Lopes Cardozo AM, Gupta A, Koppe MJ, Meijer S, van Leeuwen PA, Beelen RJ, Bleichrodt RP. Metastatic pattern of CC531 colon carcinoma cells in the abdominal cavity: an experimental model of peritoneal carcinomatosis in rats. Eur J Surg Oncol. 2001;27:359–363. doi: 10.1053/ejso.2001.1117. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki Y, Nakanishi H, Kodera Y, Ito S, Yamamura Y, Kato T, Hibi K, Akiyama S, Nakao A, Tatematsu M. TNF-alpha promotes progression of peritoneal metastasis as demonstrated using a green fluorescence protein (GFP)-tagged human gastric cancer cell line. Clin Exp Metastasis. 2004;21:39–47. doi: 10.1023/B:CLIN.0000017181.01474.35. [DOI] [PubMed] [Google Scholar]
- 17.Weese JL, Ottery FD, Emoto SE. Does omentectomy prevent malignant small bowel obstruction? Clin Exp Metastasis. 1988;6:319–324. doi: 10.1007/BF01753578. [DOI] [PubMed] [Google Scholar]
- 18.Krist LF, Kerremans M, Broekhuis-Fluitsma DM, Eestermans IL, Meyer S, Beelen RH. Milky spots in the greater omentum are predominant sites of local tumour cell proliferation and accumulation in the peritoneal cavity. Cancer Immunol Immunother. 1998;47:205–212. doi: 10.1007/s002620050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marquet RL, Westbroek DL, Jeekel J. Interferon treatment of a transplantable rat colon adenocarcinoma: importance of tumor site. Int J Cancer. 1984;33:689–692. doi: 10.1002/ijc.2910330521. [DOI] [PubMed] [Google Scholar]
- 20.Kuppen PJ, Eggermont AM, Smits KM, van Eendenburg JD, Lazeroms SP, van de Velde CJ, Fleuren GJ. The development and purification of a bispecific antibody for lymphokine-activated killer cell targeting against the rat colon carcinoma CC531. Cancer Immunol Immunother. 1993;36:403–408. doi: 10.1007/BF01742257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berg TK, Dopp EA, Dijkstra CD. Rat macrophages: membrane glycoproteins in differentiation and function. Immunol Rev. 2001;184:45–57. doi: 10.1034/j.1600-065x.2001.1840105.x. [DOI] [PubMed] [Google Scholar]
- 22.Steller EP, Ottow RT, Eggermont AM, Marquet RL, Sugarbaker PH. Local conditions in the host influence immunotherapy with interleukin-2 and LAK cells. Cancer Detect Prev. 1988;12:81–90. [PubMed] [Google Scholar]
- 23.Hagiwara A, Takahashi T, Sawai K, Taniguchi H, Shimotsuma M, Okano S, Sakakura C, Tsujimoto H, Osaki K, Sasaki S. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993;53:687–692. [PubMed] [Google Scholar]
- 24.Tsujimoto H, Hagiwara A, Shimotsuma M, Sakakura C, Osaki K, Sasaki S, Ohyama T, Ohgaki M, Imanishi T, Yamazaki J, Takahashi T. Role of milky spots as selective implantation sites for malignant cells in peritoneal dissemination in mice. J Cancer Res Clin Oncol. 1996;122:590–595. doi: 10.1007/BF01221190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agalar F, Sayek I, Cakmakci M, Hascelik G, Abbasoglu O. Effect of omentectomy on peritoneal defence mechanisms in rats. Eur J Surg. 1997;163:605–609. [PubMed] [Google Scholar]
- 26.Tsujimoto H, Takhashi T, Hagiwara A, Shimotsuma M, Sakakura C, Osaki K, Sasaki S, Shirasu M, Sakakibara T, Ohyama T. Site-specific implantation in the milky spots of malignant cells in peritoneal dissemination: immunohistochemical observation in mice inoculated intraperitoneally with bromodeoxyuridine-labelled cells. Br J Cancer. 1995;71:468–472. doi: 10.1038/bjc.1995.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui L, Johkura K, Liang Y, Teng R, Ogiwara N, Okouchi Y, Asanuma K, Sasaki K. Biodefense function of omental milky spots through cell adhesion molecules and leukocyte proliferation. Cell Tissue Res. 2002;310:321–330. doi: 10.1007/s00441-002-0636-6. [DOI] [PubMed] [Google Scholar]
- 28.van Rossen ME, Hofland LJ, van den Tol MP, van Koetsveld PM, Jeekel J, Marquet RL, van Eijck CH. Effect of inflammatory cytokines and growth factors on tumour cell adhesion to the peritoneum. J Pathol. 2001;193:530–537. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH805>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.van den Tol PM, van Rossen EE, van Eijck CH, Bonthuis F, Marquet RL, Jeekel H. Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg. 1998;227:242–248. doi: 10.1097/00000658-199802000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrance RJ, Loizidou M, Cooper AJ, Alexander P, Taylor I. Importance of the omentum in the development of intra-abdominal metastases. Br J Surg. 1991;78:117–119. doi: 10.1002/bjs.1800780135. [DOI] [PubMed] [Google Scholar]
- 31.van den Tol MP, Haverlag R, van Rossen ME, Bonthuis F, Marquet RL, Jeekel J. Glove powder promotes adhesion formation and facilitates tumour cell adhesion and growth. Br J Surg. 2001;88:1258–1263. doi: 10.1046/j.0007-1323.2001.01846.x. [DOI] [PubMed] [Google Scholar]
- 32.Ten Raa S, Oosterling SJ, Van der Kaaij N, van den Tol MP, Beelen RHJ, Meijer S, Van Eijck CHJ, Van der Sijp JRM, Van Egmond M, Jeekel J. Surgery promotes implantation of disseminated tumor cells, but does not increase growth of tumor cell clusters. J Surg Oncol. 2005;92:124–129. doi: 10.1002/jso.20273. [DOI] [PubMed] [Google Scholar]
- 33.Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone G, Giurgea L, Timmers P, Coens C, Pecorelli S. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: european organisation for research and treatment of cancer-adjuvant chemotherapy in ovarian neoplasm trial. J Natl Cancer Inst. 2003;95:113–125. doi: 10.1093/jnci/95.2.113. [DOI] [PubMed] [Google Scholar]