Abstract
Listeria monocytogenes-based vaccines for HER-2/neu are capable of breaking tolerance in FVB/N rat HER-2/neu transgenic mice. The growth of implanted NT-2 tumors, derived from a spontaneously occurring tumor in the FVB/N HER-2/neu transgenic mouse, was significantly slower in these mice following vaccination with a series of L. monocytogenes-based vaccines for HER-2/neu. Mechanisms of T cell tolerance that exist in these transgenic mice include the absence of functional high avidity anti-HER-2/neu CD8+ T cells and the presence of CD4+CD25+ regulatory T cells. The in vivo depletion of these regulatory T cells resulted in the slowing in growth of tumors even without the treatment of mice with an anti-HER-2/neu vaccine. The average avidities of responsive CD8+ T cells to six of the nine epitopes in HER-2/neu we examined, four of which were identified in this study, are shown here to be of a lower average avidity in the transgenic mice versus wild type FVB/N mice. In contrast, the average avidity of CD8+ T cells to three epitopes that showed the lowest avidity in the wild-type mice did not differ between wild type and transgenic mice. This study demonstrates the ability of L. monocytogenes-based vaccines to impact upon tolerance to HER-2/neu in FVB/N HER-2/neu transgenic mice and further defines some of the aspects of tolerance in these mice.
Keywords: Listeria monocytogenes, CD8+ T cell epitopes, HER-2/neu, Tolerance, Vaccines
Introduction
A drawback to many cancer immunotherapy studies in animal models is the lack of applicability of these models to human cancers due to tolerance mechanisms, which limit patients’ immune responses to their cancers [1–3]. Tolerance allows for the growth of tumors, which have gained the ability to divide very rapidly while being resistant to death, because they are ignored by the immune system.
There are several mechanisms that lead to the induction of tolerance. First, there is the deletion of self-reactive cells in the thymus during T cell development [4, 5]. The majority of cells that could target self-antigens, in particular the high avidity T cells that target self-antigens undergo negative selection in the thymus to prevent the occurrence of autoimmune disease. Another major mechanism of tolerance induction is the action of CD4+CD25+ T cells, or regulatory T cells [6, 7]. These cells non-specifically induce tolerance by inhibiting the action of cells, such as killer CD8+ T cells in their vicinity. Anergy is yet another tolerance mechanism that allows tumor cells to evade the immune system [1, 8]. In this case, CD8+ T cells engage specific-peptide MHC complexes on tumor cells, but then are rendered unresponsive due to a lack of co-stimulation (the second signal) that would lead to full activation [1].
In order for a cancer immunotherapeutic to be effective, it will have to overcome these tolerance mechanisms and activate self-reactive T cells to target the tumor. Studying novel cancer immunotherapies in a transplantable mouse model is a first approach to evaluating the effectiveness of a therapy. However, if wild type mice are used as recipients, there may be little or no tolerance unless a self-antigen is expressed by the tumor. A better test of cancer immunotherapies may be to test them in tolerance models, such as transgenic mouse models for cancer [9, 10]. Effective treatments in animals that are tolerant to the tumor antigen will theoretically have a greater chance of being effective in human clinical trials.
HER-2/neu is a self-antigen that is overexpressed in 15–40% of human breast cancers [11, 12]. As the tumor is capable of growing in people with intact immune systems, this suggests that they may be tolerant to the antigen. In addition, the immune response that patients do generate against these tumors is not effective in preventing the tumor from developing and growing [13–15]. As the over expression of HER-2/neu in tumors is associated with the poor prognosis of patients [16], it is an especially important antigen to target with specific immunotherapies. The evaluation of therapies targeting HER-2/neu has been aided by the development of mice transgenic for the rat HER-2/neu proto-oncogene [17–20].
Although there are several transgenic mouse models, the one used in these studies is the HER-2/neu transgenic mouse on the FVB/N background [17, 18]. These mice express the rat HER-2/neu proto-oncogene under the control of the mouse mammary tumor virus (MMTV) promoter [17, 18]. As such, the rat HER-2/neu should be expressed in the thymus during development and reactive cells deleted through negative selection. This model has been very useful in immunotherapeutic studies because it closely resembles the ontogeny and progression of human breast cancer. Focal mammary tumors develop stochastically in >90% of the female transgenic mice after a latency of about 4–6 months and metastatic disease in the lung occurs in many of the tumor bearing mice. In addition, although the mouse HER-2/neu protein differs from the rat by <6% of amino acid residues, there is evidence that rat HER-2/neu is immunogenic in the mouse [21]. This implies that the rat HER-2/neu expressing tumors commonly used to test immune strategies may be immunogenic in wild type mice. Thus rat HER-2/neu transgenic mice are a more stringent model to test the ability of immunotherapy to break tolerance against tumor antigens, such as HER-2/neu, which are expressed at low levels in normal tissue.
We have previously described a series of Listeria monocytogenes-based vaccines that express overlapping fragments of HER-2/neu, which are equally capable of inducing stasis in the growth of transplanted NT-2 tumors in wild type FVB/N mice [22]. The NT-2 tumor cell line was developed from a spontaneously occurring mammary tumor in the FVB/N rat HER-2/neu transgenic mouse [23]. Three of these vaccines target the extracellular domain [Lm-LLO (Listeriolysin O) -EC1, -EC2, and -EC3] and two the intracellular domain (Lm-LLO-IC1 and -IC2) of rat HER-2/neu.
Wild type FVB/N mice are not specifically tolerant to the rat HER-2/neu gene product. However, the mouse and rat HER-2/neu genes are greater than 90% homologous [24], so some degree of tolerance is expected. Although these sequences are extremely homologous, there are differences in the degree of homology in the fragments targeted by the different vaccine constructs. Lm-LLO-IC1 expresses a HER-2/neu fragment that contains the highly conserved kinase domain, which is 98% homologous to the mouse protein sequence, and this sequence shows the highest level of homology among the five vaccine constructs. Both the EC1 and EC2 regions are 94% homologous with the corresponding mouse regions and the EC3 and IC2 regions are 93% homologous. In all of the vaccines excluding Lm-LLO-IC1, the amino acid differences are spread throughout the entire fragment, while in IC1, the differences flank the kinase domain with only one non-homologous amino acid in the kinase domain making the kinase domain 99.6% homologous. Thus, in order to impact on the growth of implanted tumors in the transgenic mice, the vaccines will need to stimulate an immune response that can overcome several levels of tolerance. In addition to the tolerance of the mice to rat HER-2/neu, the similarity of the rat and the mouse proteins implies that the immune response may also have to overcome tolerance to the mouse protein.
Here, we show that Lm-LLO-HER-2/neu vaccination can significantly delay the growth of transplanted NT-2 tumors in FVB/N HER-2/neu transgenic mice. In addition, the CD8+ T cells specific for several HER-2/neu epitopes in each of the five Lm-LLO-HER-2/neu vaccines are of a lower avidity in the transgenic mice as compared to the wild type mice. In order to measure the average avidities of the polyclonal CD8+ T cells arising in wild type versus transgenic mice vaccinated with each of the five Lm-LLO-HER-2/neu vaccines we identified several novel epitopes in the HER-2/neu for the FVB mouse. This brings the number of known epitopes in HER-2/neu to 13 ([27, 31] Singh and Paterson, manuscript submitted and this study).
Materials and methods
Mice
FVB/N HER-2/neu transgenic mice were housed and bred at the Veterans’ Administration Hospital affiliated with the University of Pennsylvania. Six to 8 week old wild type female FVB/N mice were purchased from Charles River Laboratories (Wilmington, MA). All experiments on mice were approved by and done in accordance with regulations by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania and the Veterans’ Administration.
Listeria vaccine strains
The L. monocytogenes strains used for these studies are Lm-LLO-EC1, Lm-LLO-EC2, Lm-LLO-EC3, Lm-LLO-IC1, and Lm-LLO-IC2 all of which contain a fragment of rat HER-2/neu fused to a truncated form of the listerial hly gene in an episomal expression system that has been previously described by our lab [25, 26]. Their construction has been described previously [22]. The amino acid sequences contained in each HER-2/neu fragment are 20–326 in Lm-LLO-EC1, 303–501 in Lm-LLO-EC2, 479–655 in Lm-LLO-EC3, 690–1,081 in Lm-LLO-IC1, and 1,020–1,260 in Lm-LLO-IC2 [22]. A similarly constructed L. monocytogenes-based vaccine for NY-ESO-1 was kindly provided by Dr. Paulo Maciag, University of Pennsylvania, and was used as a control for antigen specificity in some experiments. Bacteria were grown in brain heart infusion medium (BD, Sparks, MD) with 50 μg/ml chloramphenicol and frozen in 1 ml aliquots at −80°C. To inject mice, the vaccines were thawed and washed twice with sterile PBS prior to being resuspended in PBS.
Cell lines
The NT-2 tumor cell line was developed from a spontaneously occurring mammary tumor in an FVB/N HER-2/neu transgenic mouse [27]. These cells constitutively express low levels of rat HER-2/neu and were injected into transgenic mice to form solid tumors. They were also used as a feeder cell line as a source of antigen for the restimulation of splenocytes in CTL assays. NT-2 cells were grown in RPMI 1640 medium supplemented with 20% FCS, 10.2 ml HEPES, 2 mM l-glutamine, 100 μM nonessential amino acids, 1 mM sodium pyruvate, 50 U/ml penicillin G, 50 μg/ml streptomycin, 20 μg/ml insulin, and 2 μg/ml gentamycin. For the CTL assays, the NIH 3T3 cells (3T3-wt) were cultured in DMEM supplemented with 10% FCS, 2 mM l-glutamine, 100 μM nonessential amino acids, 1 mM sodium pyruvate, 50 U/ml penicillin G, and 50 μg/ml streptomycin. All cells were grown at 37°C with 5% CO2.
Tumor regression
About 6–8 week old female FVB/N HER-2/neu transgenic mice were given 5 × 105 NT-2 tumor cells s.c. on the right flank on day 0. Mice were vaccinated with 0.1 LD50 [22] of each vaccine or 200 μl PBS i.p. on days 7, 14, 21, 28, and 35. One week post-tumor inoculation the tumors reached a palpable size at 4–5 mm. Each treatment group consisted of eight mice.
For the tumor regression experiment in which CD25+ T cells were depleted, groups of six 6–8 week old female FVB/N HER-2/neu transgenic mice were given NT-2 tumors s.c. in the right flank on day 0. The mice were then treated with either the PC-61 anti-CD25 antibody (Bio Express, West Lebanon, NH) or a control antibody on days 4 and 6 post tumor inoculation. Mice were treated with 1 mg of antibody per mouse i.v. The control depletion antibody was an isotype-matched rat antibody, GL117.41 that produces an anti-E. coli β-galactosidase. The Lm-LLO-EC2 vaccine was given on days 7, 14, 21, 28, and 35.
Tumors were measured every 2 days with calipers spanning the shortest and longest surface diameters. Plots show the mean of these two measurements as the tumor size in millimeters versus time. Mice were sacrificed when mean tumor diameter reached 20 mm and tumor measurements for each time point are shown only for the surviving mice.
For the tumor regression assay in which, Listeria-based vaccines were co-injected with the anti-HER-2/neu monoclonal antibody, eight 6–8 week old female FVB/N HER-2/neu transgenic mice were given NT-2 tumors s.c. on the right flank on day 0. Mice were injected with 100 μg of the 7.21.2 anti-HER-2/neu antibody per mouse per week [28, 29] for a total of five injections. The antibody injections were given at the same time as the vaccine injections on days 7, 14, 21, 28, and 35. The control antibody was an isotype matched antibody purified from ascites and the 7.21.2 antibody was produced from a hybridoma cell line grown in a bioreactor (BD, San Jose, CA). Both antibodies were affinity purified on a Protein G Sepharose column (Amersham Biosciences, Uppsala, Sweden). Groups are shown as an average tumor size at each time point.
Peptides
Peptides were synthesized as custom peptides by EZBiolab, Inc. (Westfield, IN). The sequences are as follows: PDSLRDLSVF (420–429), PYNYLSTEV (302–310), LFRNPHQALL (489–498), PGPTQCVNCS (528–537), PNQAQMRIL (712–720), GSGAFGTVYK (732–741), AFGTVYKGI (735–743), PYVSRLLGI (785–793), and LQRYSEDPTL (1,114–1,123). The peptides were all produced in a desalted form at a greater than 70% purity.
CTL assays
Six week old female HER-2/neu transgenic mice or wild type FVB/N mice were vaccinated with 0.1 LD50 of one of the Listeria-based vaccines or 200 μl PBS. In order to identify novel epitopes, spleens were harvested 9 days later and splenocytes were cultured in vitro for 4 days with irradiated NT-2 tumor cells (20,000 rads) at a ratio of 10 splenocytes to 1 tumor cell with 20 U/ml IC-2. Splenocytes were then harvested and used in a standard CTL assay with 3T3 target cells loaded with the target peptides as previously described [22, 30] at a peptide concentration of 1 μg/ml. Each ratio was set up in triplicate. Total lysis of chromium loaded target cells was stimulated by the addition of 2% Triton X. The percent specific lysis was calculated as follows: % = 100 [(experimental lysis − spontaneous lysis)/(total lysis − spontaneous lysis)].
For the peptide titration CTL assays, mice were again treated once with 0.1 LD50 of a particular vaccine of with PBS and 21 days later spleens were harvested and splenocytes were cultured in vitro for 6 days with irradiated NT-2 tumors cells (20,000 rads) at a ratio of 10 splenocytes to 1 tumor cell with 20 U/ml IL-2. Spleens were harvested at 21 days based on previous studies using this time frame to undertake studies of T cell avidity [30]. The concentrations of peptides used to load onto target cells were 1, 0.1, 0.01, 1, and 0.1 μg/ml. Each ratio was set up in triplicate. Total lysis of chromium loaded target cells was stimulated by the addition of 2% Triton X. The percent specific lysis was calculated as follows: % = 100 [(experimental lysis−spontaneous lysis)/(total lysis−spontaneous lysis)]. Results are shown at an E:T ratio of 200:1.
Statistical analysis
The Student’s t test was done and significance of P < 0.05 is shown.
Results
NT-2 tumor growth slows after Lm-LLO-HER-2/neu vaccination of FVB/N HER-2/neu transgenic mice
Treatment of wild type NT-2 tumor bearing FVB/N mice with the L. monocytogenes-based HER-2/neu vaccines resulted in stasis in tumor growth for greater than 100 days, and the eventual regression of a subset of these tumors [22]. These mice are not considered to be tolerant to the rat HER-2/neu protein and as such were expected to mount an immune response targeting the implanted tumors. However, due to the high degree of similarity between the rat and mouse HER-2/neu proteins, they should exhibit some tolerance to rat HER-2/neu. Prior to beginning a tumor regression study in the FVB/N HER-2/neu transgenic mice, we undertook a tumor load study to determine the minimum number of tumor cells needed to lead to the growth of palpable tumors of 4–5 mm 1 week after tumor implantation. As expected, these mice are tolerant to HER-2/neu, and tumors can be established by fewer tumor cells in the transgenic mouse versus the wild type FVB/N mouse (5 × 105 versus 1 × 106).
Transgenic mice were implanted with 5 × 105 NT-2 tumor cells s.c. on the right flank and when the tumors were palpable on day 7, the Lm-LLO-HER-2/neu vaccinations were given. Instead of the three vaccinations that were given to the wild type mice, five vaccinations, one every week beginning on day 7, were given to the transgenic mice. This was done in order to give a stronger boost and to enhance the generation of antigen-specific T cells since these mice are tolerant to rat HER-2/neu. Treatment with each of the five Lm-LLO-HER-2/neu vaccines leads to much slower tumor growth than the control treated tumors (Fig. 1). The control tumors grow out to 20 mm by day 50 when the mice are euthanized. In contrast, the tumors in the HER-2/neu vaccinated groups do not grow beyond 10 mm on average. No tumor measurements could be made after day 50 because each mouse in the Lm-LLO-HER-2/neu vaccinated groups scratched away her tumor and thus the size of the tumors was lost to assessment. This was a very striking development as all of these mice removed their tumors within the space of 2 days. This experiment was repeated three times with the same consequences. We believe that immune cell infiltrates in these tumors must make them very itchy to the mice since the PBS and control vaccinated mice did not scratch even the larger tumors that they carried. We have, as yet, not fully characterized what these cells are: we did not observe this phenomenon when we performed similar experiments in the wild type FVB mouse [22]. The majority of the mice were euthanized after scratching away their tumors as the scratching resulted in chronic open wounds. The few mice for which the open wounds healed rapidly survived long term (observed for greater than 4 months) with no evidence of further tumor growth (unpublished observations).
Fig. 1.
NT-2 tumor growth is significantly slower after Lm-LLO-HER-2/neu vaccination. NT-2 tumor cells were implanted s.c. on the flank of female HER-2/neu transgenic mice. The tumors in control mice when vaccinated with PBS or Lm-LLO-NYESO-1 rapidly grew out by day 45. The tumors in all of the HER-2/neu vaccinated mice grow much more slowly and on average do not grow beyond 10 mm. There were no tumor measurements after day 50 because each of the HER-2/neu vaccinated mice scratched away her tumor. All of the Lm-LLO-HER-2/neu vaccines are significantly different (P < 0.0001) from the control vaccines (PBS and the Lm-LLO-NYESO-1 vaccines) on day 42 based on the Student’s t test
Identification of new epitopes in the HER-2/neu fragments of EC2, EC3, IC1 and IC2
HER-2/neu epitopes have been identified in the EC2 fragment [27] and in the EC1 fragment [31]. We have also recently identified seven epitopes in the IC1 fragment (Singh and Paterson, manuscript submitted). Since we wish to compare the avidity of CD8+ T cells induced by the vaccines in the wild type versus the HER-2/neu transgenic mouse, it was necessary to identify at least one CD8+ T cell epitope for all of the fragments expressed by the Listeria vaccine.
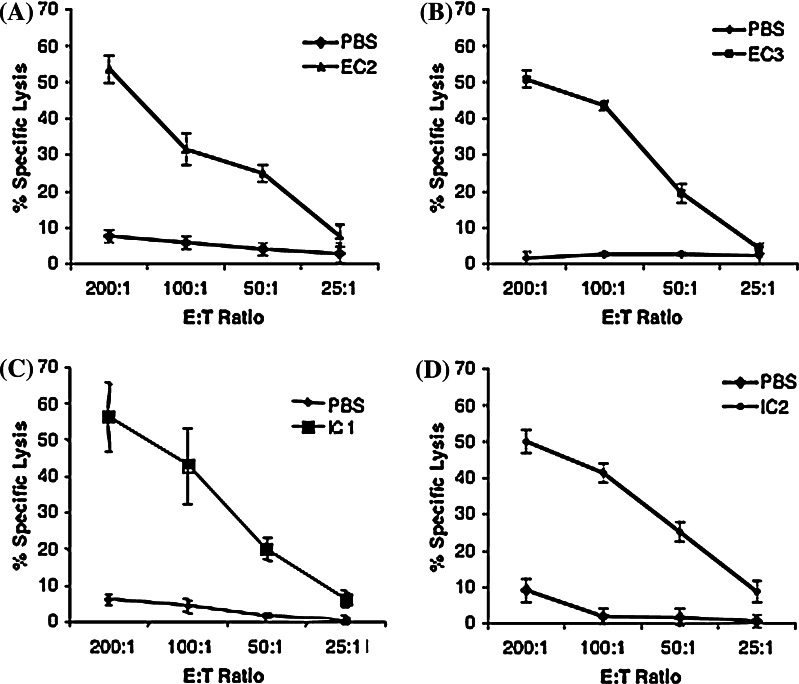
We used the strategy that we have previously described in which splenocytes from wild type FVB/N mice immunized with each of the five vaccines were used as effector cells in 51Cr release CTL assays [22, 31]. First we used pools of overlapping 20-mer peptides to pulse target cells for CTL assays to narrow down the regions of HER-2/neu contained within each vaccine that contain potential epitopes. For positive pools we then tested individual 20-mer peptides in the pools to identify the 20-mer that contained a CD8+ T cell epitope. We then sought to identify the epitope in each of these 20-mers. As we have previously discussed [31], there are no predictive algorithms for the FVB/N mouse, which is on the H-2q background. However, Hansen and associates have shown that the H-2Dq and H-2Lq genes are very similar to the H-2d genes [32, 33]. Using the “d” haplotype as a guide we analyzed the sequence of positive 20-mers using the Rankpep algorithm (http://www.mif.dfci.harvard.edu/Tools/rankpep.html) and identified 9- and 10-mers that were likely to bind to d, and thus to q, for each of the identified 20-mers. These were tested in CTL assays to identify individual epitopes (Fig. 2).
Fig. 2.
Identification of novel CD8+ T cell epitopes in the FVB/N HER-2/neu transgenic mouse. Novel epitopes in each of the Listeria monocytogenes vaccines have been identified through CTL analyses. Peptides were used at a concentration of 1 μg/ml each pulsed onto 3T3-wt target cells. The E:T ratios used were 200:1, 100:1, 50:1, and 25:1, with splenocytes from PBS or Lm-LLO-HER-2/neu vaccinated mice. The epitopes are as follows: a the EC2 epitope LFRNPHQALL, b the EC3 epitope PGPTQCVNCS, c the IC1 epitope PNQAQMRIL, d the IC2 epitope LQRYSEDPTL
Following this, we went on to identify the MHC class I restriction of each of the new epitopes and previously identified epitopes as we have previously described [31]. The epitopes and their MHC class I restrictions are as follows. The EC2 epitope LFRNPHQALL (489–498) is H-2Kq, the EC3 epitope PGPTQCVNCS (528–537) is H-2Dq, and the IC2 epitope LQRYSEDPTL (1,114–1,123) is H-2Dq. The IC1 epitopes PNQAQMRIL (712–720) and GSGAFGTVYK (732–741) are H-2Dq whereas the AFGTVYKGI (735–743) and PYVSRLLGI (785–793) are H-2Kq (data not shown). It should be noted that we have not attempted to identify all of the epitopes in the HER-2/neu molecule since our aim was to identify at least one. Indeed, we believe that there may be many others.
CD8+ T cells are of lower avidity in FVB/N HER-2/neu transgenic versus wild type mice
High avidity T cells to self-antigens are generally negatively selected during thymic development to prevent the development of autoimmunity [34]. We compared the avidity of the T cells for each of the epitopes through a series of peptide titration CTL analyses. A measure of the avidity of CD8+ T cells can be obtained by titrating down the concentration of the peptide used to pulse target cells and observing at what concentration of peptide the lysis of the target cells is halved compared to the maximum lysis obtained in the presence of excess peptide [30].
We next tested the avidities of the eight HER-2/neu epitopes that we have mapped previously (31, Singh and Paterson, manuscript submitted) and in this paper. Since previous studies in the FVB/N HER-2/neu transgenic mouse have focused on the epitope, PDSLRDLSVF [35], we also sought to determine the avidity of T cells specific for this epitope in order to compare this to the epitopes we have mapped. The average avidity of the PDSLRDLSVF specific T cells from Lm-LLO-EC2 vaccinated transgenic mice is just over one log lower than those induced in the Lm-LLO-EC2 vaccinated wild type mice (Fig. 3; Table 1).
Fig. 3.
Some of the CD8+ T cells in the FVB/N HER-2/neu transgenic mouse are of lower avidity than the cells from wild type FVB/N mice. CTL analysis of each of the epitopes using decreasing concentrations of the peptide pulsed onto 3T3-wt target cells. The concentrations used were 10−6, 10−7, 10−8, 10−9, 10−10 M. Wild type and transgenic mice are directly compared at a 200:1 E:T ratio. a PDSLRDLSVF, b PYNYLSTEV, c LFRNPHQALL, d PGPTQCVNCS, e PNQAQMRIL, f GSGAFGTVYK, g AFGTVYKGI, h PYVSRLLGI, and i LQRYSEDPTL
Table 1.
Avidity of HER-2/neu specific CD8+ T cells in the FVB/N HER-2/neu transgenic mice versus wild type FVB/N mice
| Vaccine | Epitope | AA region | MHC class I | Wild type mice (nM) | Transgenic mice (nM) | Log difference |
|---|---|---|---|---|---|---|
| Lm-LLO-EC1 | PYNYLSTEV | 302–310 | H-2Kq | 1 | 8 | 0.9 |
| Lm-LLO-EC2 | PDSLRDLSVF | 420–429 | H-2 Dq | 0.5 | 10 | 1.3 |
| LFRNPHQALL | 489–498 | H-2Kq | 5 | 7 | 0.15 | |
| Lm-LLO-EC3 | PGPTQCVNCS | 528–537 | H-2Dq | 1 | 30 | 1.48 |
| Lm-LLO-IC1 | PNQAQMRIL | 712–720 | H-2Dq | 0.5 | 20 | 1.6 |
| GSGAFGTVYK | 732–741 | H-2Dq | 1 | 10 | 1 | |
| AFGTVYKGI | 735–743 | H-2Kq | 5 | 8 | 0.2 | |
| PYVSRLLGI | 785–793 | H-2Kq | 8 | 8 | 0 | |
| Lm-LLO-IC2 | LQRYSEDPTL | 1,114–1,123 | H-2Dq | 1 | 10 | 1 |
Each epitope is shown with the peptide concentration at which the percent specific lysis drops to a level 50% lower than initially seen at a high peptide concentration of 1,000 nM. The epitopes are shown with the corresponding vaccine, the amino acid region that delineates each epitope, the MHC class I restriction, and the log difference of the CD8+ T cells that respond to each epitope in the wild type versus the transgenic mouse
Our findings are summarized in Table 1, and reported as the peptide concentration at which the percent specific lysis drops 50% for each epitope (Table 1). Two of the epitopes we have identified in EC3: PGPTQCVNCS and IC1: PNQAQMRIL induce T cells with similar avidity differences in wild type versus transgenic mice as the PDSLRDLSVF epitope, i.e., 1.3–1.6 logs lower in the transgenic mice. Three of the other epitopes, EC1: PYNYLSTEV, IC1: GSGAFGTVYK, and IC2: LQRYSEDPTL induce T cells that are 1 log lower in avidity in the transgenic mice compared to wild type mice. However, the CD8+ T cells specific for the EC2 epitope LFRNPHQALL, and the IC1 epitopes AFGTVYKGI and PYVSRLLGI are of essentially similar avidities in both the wild type and the transgenic mouse (Table 1).
CD4+CD25+ T cells limit the anti-tumor efficacy of the Lm-LLO-HER-2/neu vaccinations
Table 1 indicates that high avidity CD8+ T cells are not detected in the HER-2/neu transgenic mice after Lm-LLO-HER-2/neu vaccination for all of the identified epitopes (Table 1). We believe that these cells are not detected because they have been deleted during thymic development, but it is possible that they are present but not responding due to the presence of CD4+CD25+ regulatory T cells which suppress their function. CD8+ T cell responses to the EC2 epitope, PDSLRDLSVF, in the HER-2/neu transgenic mouse have been shown to be dampened by the presence of CD4+CD25+ regulatory T cells [35]. To examine the effect of regulatory T cells on the function of anti-HER-2/neu T cells induced by a Listeria vaccine, we set up an in vivo depletion of CD4+CD25+ T cells in conjunction with a tumor regression experiment in FVB/N HER-2/neu transgenic mice using the Lm-LLO-EC2 vaccine. Regulatory T cells were depleted by the iv injection of the anti-CD25 antibody, PC61, 4 and 6 days after tumor implantation and then confirmed 4 days after the second antibody injection through FACs analysis of spleen and peripheral blood. When no antibody is used 2.28% of the CD4+ cells are also CD25+, and similarly when an isotype matched control depletion antibody is used, 2.6% of the CD4+ cells are also CD25+ (data not shown). After treatment with the anti-CD25 antibody 0.18% of the CD4+ cells are CD25+, demonstrating that the CD4+CD25+ cells have been depleted and so can no longer be detected (data not shown).
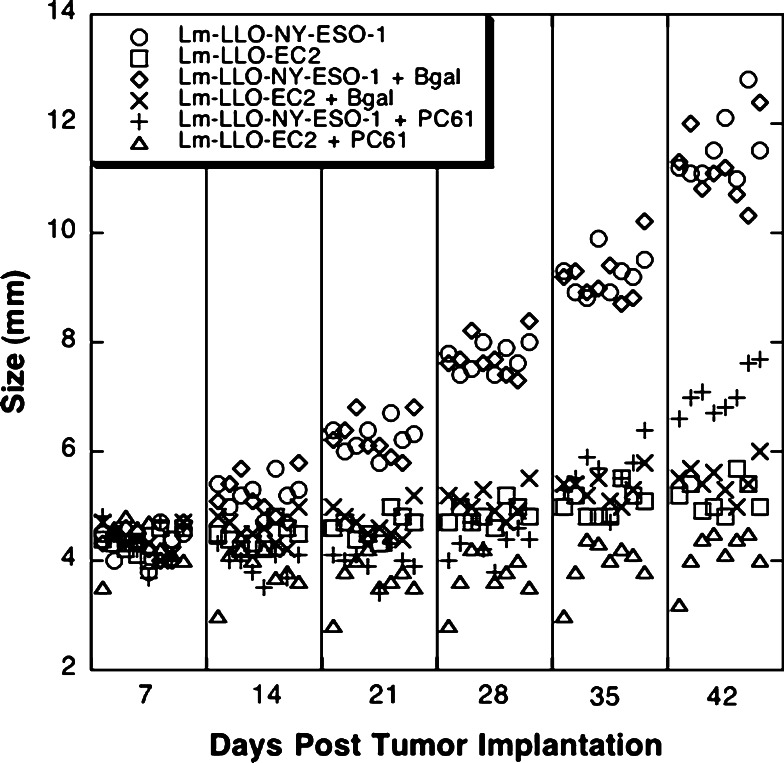
Depletion of regulatory T cells slows down the growth of tumors in control Lm-LLO-NYESO-1-treated and Lm-LLO-EC2 treated mice (Fig. 4). Injection of the irrelevant antibody had no effect on the growth of the tumors, as the growth of these tumors was the same as the growth of the tumors treated with no antibody (Fig. 4). The improvement in Lm-LLO-EC2 vaccine efficacy on depletion of CD25+ cells appears marginal, but it is a significant improvement (P < 0.0001 on day 42). However, depletion of CD25+ T cells slowed NT-2 growth, much more dramatically, in control-vaccinated mice (P < 0.0001), indicating that Lm-LLO-EC2 can partially overcome tolerance mediated by Tregs.
Fig. 4.
Regression of NT-2 tumor cells in FVB/N HER-2/neu transgenic mice following depletion of CD4+CD25+ regulatory T cells. Mice were given tumors and then injected with no antibody, a control antibody, or a CD25 depletion antibody and then observed for the ability to induce the regression of tumors following depletion of regulatory T cells. Both the Lm-LLO-NYESO-1 + PC61 and the Lm-LLO-EC2 + PC61 groups are significantly different (P < 0.0001) from the Lm-LLO-NYESO-1 + β-gal and the Lm-LLO-EC2 + β-gal group on day 42 based on the Student’s t test
Treatment with an anti-HER-2/neu specific monoclonal antibody controls NT-2 tumor growth in FVB/N HER-2/neu transgenic mice
Patients with cancers that over express HER-2/neu exhibit immune tolerance regardless of the fact that they have a detectable humoral immune response [13–15, 36, 37]. One of the most effective treatments in recent years for HER-2/neu over expressing cancers has been the development of Herceptin® (trastuzumab), a monoclonal antibody that targets HER-2/neu expressed on cancerous cells [38]. Herceptin has been shown to be extremely effective post-surgery and chemotherapy in women with late-stage recurring cancers. It is currently being tested in women with earlier stages of cancer and has also shown some initial effectiveness in these cases [38, 39].
As none of the L. monocytogenes-based vaccines for HER-2/neu induce a detectable antibody response in either wild type or transgenic mice (data not shown), we co-administered an anti-HER-2/neu specific monoclonal antibody, 7.21.2 [40] with the vaccine to tumor-bearing mice to determine whether supplementing the low avidity CD8+ T cells with humoral immunity could improve the therapeutic outcome. Antibody was administered weekly from day 7 to day 35 after tumor challenge. The administration of an irrelevant isotype matched antibody had no effect on the growth of the tumors and resulted in mice vaccinated with the HER-2/neu vaccines scratching away their tumors by day 49 post-tumor injection (Fig. 5a). However, the injection of the anti-HER-2/neu specific antibody had no discernable effect on the growth of the tumors in mice that were simultaneously treated with the Listeria-based HER-2/neu vaccines until day 42 when the differences in tumor growth are significantly improved with the addition of the anti-HER-2/neu specific antibody (P < 0.01) (Fig. 5b). Interestingly, the control mice treated with the anti-HER-2/neu antibody were able to control tumor growth until about 3 weeks after the last dose of antibody was administered after which the tumors rapidly grew out (Fig. 5b).
Fig. 5.
An anti-HER-2/neu specific monoclonal antibody is capable of controlling NT-2 tumor growth in FVB/N HER-2/neu transgenic mice. Mice were given tumors and injected with both the Listeria-based vaccines and either a control (a) or the 7.21.2 anti-HER-2/neu antibody (b) on days 7, 14, 21, 28, and 35. Groups are shown as an average tumor size at each time point. Both the PBS and Lm-LLO-NYESO-1, anti-HER-2/neu antibody groups are significantly better at day 42 than the PBS and Lm-LLO-NYESO-1 control antibody groups (P < 0.0001). Each of the Lm-LLO-HER-2/neu vaccine groups co-administered with the anti-HER-2/neu specific antibody is also significantly better at day 42 than the vaccine groups with the control antibody (P < 0.01)
Discussion
Tolerance in the FVB/N HER-2/neu transgenic mouse is mediated at least in part by CD4+CD25+ regulatory T cells, as evidenced by the fact that when these cells are depleted, mice treated with a control vaccine show a dramatic slowing in the growth of their tumors (Fig. 4). Other groups have previously shown that mice can have large numbers of regulatory T cells, which can limit the effectiveness of cancer immunotherapies [35]. Recently it has been shown that the depletion of regulatory T cells also slows down the growth of autochthonous HER-2/neu tumors in HER-2/neu transgenic mice, supporting our study that tolerance in these mice is mediated at least in part by CD4+CD25+ regulatory T cells [41]. The regulatory T cells that are present, and that limit immune responses to tumor antigens can either always be present in the mouse or in some cases may in fact be induced by an anti-tumor immunotherapy [35, 42]. In a tolerance model such as the one described here, an immunotherapeutic approach must induce an immune response that is strong enough to overcome the presence of the regulatory T cells, which are in place to prevent the immune system from targeting self. However, there are clearly other mechanisms of tolerance involved in limiting the immune response to HER-2/neu as the depletion of these cells did not lead to the complete regression of the NT-2 tumors in Lm-LLO-EC2 vaccinated mice.
In addition to the presence of regulatory T cells limiting the anti-tumor response to HER-2/neu, it is likely that high avidity T cells responsive to HER-2/neu have either been deleted or anergized. Previous studies have shown that the CD8+ T cell repertoire present in the HER-2/neu transgenic mice is of low avidity [43], although these cells do have anti-tumor capabilities. Similarly, we see that the specific epitopes that induce higher avidity CD8+ T cells in the wild type mice after Lm-LLO-HER-2/neu vaccination (PYNYSTLEV, PDSLRDLSVF, PGPTQCVNCS, PNQAQMRIL, GSGAFGTVYK, and LQRYSEDPTL) are not present in the transgenic mice following vaccination. This suggests that they have been deleted by central tolerance. However, CD8+ T cells to the epitopes LFRNPHQALL, AFGTVYKGI, and PYVSRLLGI generated by Lm-LLO-HER-2/neu vaccination are of only moderate affinity in the wild type mice and appear not to be deleted in the transgenic mice (Table 1). Thus, in the transgenic mice the CD8+ T cells induced by Lm-LLO-HER-2/neu vaccination to these epitopes appear to have a higher avidity than the T cells induced to some of the epitopes (PGPTQCVNCS and PNQAQMRIL) that are of higher avidity in the wild-type mice.
There are two sources of HER-2/neu in the transgenic mouse, endogenous mouse HER-2/neu and transgenic rat HER-2/neu whereas only mouse HER-2/neu is present in the wild type mouse. Curiously, there are no sequence differences between HER-2/neu in the mouse or the rat for any of the epitopes shown in Table 1, which suggests that the high avidity T cells specific for these epitopes should have been deleted in the thymi of both the wild type and the transgenic mice. That they emerge in the wild type mice suggests that mouse HER-2/neu is not very tolerogenic, possibly because of its limited tissue expression. This does appear to be the case for high avidity CD8+ T cells to some of the epitopes in the transgenic mice. It is possible that these cells are being deleted in the transgenic mice due to a higher expression of HER-2/neu driven by the strong MMTV promoter [17, 18], but this has not, as yet, been fully elucidated.
As patients with HER-2/neu over expressing cancers often produce antibodies targeting HER-2/neu, we sought to determine whether the FVB/N HER-2/neu transgenic mouse is capable of producing HER-2/neu specific antibodies in response to the Lm-LLO-HER-2/neu vaccines. B cells that are capable of producing HER-2/neu specific antibodies appear to be either not present, or non-functional in this transgenic mouse model (data not shown). When exogenous anti-HER-2/neu antibody is added, the control groups are able to control tumor growth, until the antibody is no longer administered, at which point the tumors grow out (Fig. 5b). This phenomenon has previously been seen with HER-2/neu over expressing tumors [40]. Our results contrast the results obtained by Wolpoe et al. [28] where the addition of anti-HER-2/neu antibodies increased the survival of mice when given in addition to a HER-2/neu specific vaccine therapy. There are several differences between Wolpoe’s study and ours. Wolpoe et al. did not directly measure tumor growth. Instead they measured survival times of the mice and observed modest improvements when the antibodies were administered. We are unable to measure survival time of the mice in the transgenic mice because of their propensity to scratch away their tumors after which they are lost to assessment. However, we do see an increase of about 2 weeks in the time period during which we can measure tumors in the vaccinated mice that received the anti-HER-2/neu antibody treatment (56 days) compared to control antibodies (42 days). In addition, in agreement with other studies [28, 40] antibody treatment prolongs the survival of control-vaccinated mice (Fig. 5).
The Lm-LLO-HER-2/neu vaccines are capable of breaking tolerance in the FVB/N HER-2/neu transgenic mouse as seen by the slowing in growth of transplanted tumors in these mice (Fig. 1). Immunotherapies developed by other groups have also been shown to impact on the growth of tumors in transgenic mice, although these studies were often done by challenging mice with a tumor following prophylactic treatment with an immunotherapy [28, 44, 45]. A major difference in the outcomes of immunotherapies developed by other groups and the Lm-LLO-HER-2/neu vaccines is the scratching away of tumors by the transgenic mice after Lm-LLO-HER-2/neu vaccination. Delayed-type hypersensitivity (DTH) reactions have been seen in cancer patients, but these responses are generally limited to the site that the treatment was injected [46, 47]. If what we are observing is a DTH response, it is not occurring at the site of injection and it is occurring about 2 weeks after the fifth and final vaccine injection. The response is specific to the site of the tumor, as the Lm-LLO-HER-2/neu mice do not disfigure themselves in any way other than to scratch and bite at the tumors until they are completely gone. Mice that received no vaccine or control vaccines do not display this behavior. Once the tumors are gone, the mice are generally euthanized as open wounds can get infected, although a few of the mice have healed and have re-grown skin and hair where the tumor used to be (unpublished observations). As this is the case it is clear that there is something specifically infiltrating these tumors and that once the tumors have been removed, some mice are capable of recovering fully. We are currently exploring this phenomenon to determine the source of discomfort for the mice, which we may then be able to address with adjunct therapy.
In the development of novel immunotherapies targeting HER-2/neu it is necessary to keep in mind the fact that the T cells that are present, to be stimulated in response to immunization, are of a lower avidity. In order to stimulate these low avidity cells, a very strong stimulus is necessary, as they are not responsive to low antigen concentrations [30]. Stimulating a larger population of low avidity cells instead of those responsive to a single epitope is likely to be a more effective method of inducing a potent anti-tumor immune response [48, 49]. In addition sub-dominant epitopes that induce low avidity populations in the wild type mice may be a better target for immunotherapy than immunodominant epitopes since we show here that they do not appear to be depleted in the transgenic mouse. There is also evidence that altering the sequence of the immunodominant epitope (using mimotopes) stimulates a better anti-tumor immune response than simply using the native epitope sequence [50–52]. These altered epitopes will bind differently to the MHC, most likely with a lower affinity, leading to the stimulation of a different subset of T cells.
Lm-LLO-HER-2/neu vaccination leads to the slowing in growth of implanted tumors in FVB/N HER-2/neu transgenic mice even though the immune response against these tumors is inhibited by the lack of high avidity T cells and the presence of regulatory T cells. The ultimate test of the anti-tumor efficacy of the Lm-LLO-HER-2/neu vaccines will be to determine if they can prevent or delay the onset of spontaneous tumors in FVB/N HER-2/neu transgenic mice.
Acknowledgments
We would like to thank Dr. Mark Greene (University of Pennsylvania, Philadelphia, PA) for the generous donation of the 7.21.2 hybridoma cell line and Dr. Gail Massey (University of Pennsylvania, Philadelphia, PA) for her assistance in culturing this cell line. We would also like to thank. Dr. Paulo Maciag (University of Pennsylvania, Philadelphia, PA) for allowing us to use Lm-LLO-NY-ESO-1 prior to publication and Dr. Zhen-Kun Pan (University of Pennsylvania, Philadelphia, PA) for her assistance with the mouse work. Yvonne Paterson wishes to disclose that she has a financial interest in Advaxis, Inc., a vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria or listerial products as vaccines.
This work was supported by Department of Defense Grant W81XWH-04-1-0338 (R.S.) and NIH R01CA109253 (Y.P.).
References
- 1.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay S, Mehrotra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG. Effect of CD4+CD25+ and CD4+CD25- T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol. 2006;176:984. doi: 10.4049/jimmunol.176.2.984. [DOI] [PubMed] [Google Scholar]
- 3.Wang RF, Peng G, Wang HY. Regulatory T cells and Toll-like receptors in tumor immunity. Semin Immunol. 2006;18:136. doi: 10.1016/j.smim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzsen GF, Hofgaard PO, Schenck K, Bogen B. Clonal deletion of thymocytes as a tumor escape mechanism. Int J Cancer. 1998;78:216. doi: 10.1002/(SICI)1097-0215(19981005)78:2<216::AID-IJC16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.De Visser KE, Schumacher TN, Kruisbeek AM. CD8+ T cell tolerance and cancer immunotherapy. J Immunother. 2003;26:1. doi: 10.1097/00002371-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia M, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 8.Schirrmacher V, Feuerer M, Beckhove P, Ahlert T, Umansky V. T cell memory, anergy and immunotherapy in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:201. doi: 10.1023/A:1020308104613. [DOI] [PubMed] [Google Scholar]
- 9.Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12:1410. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 10.Esposito V, Palescandolo E, Spugnini EP, Montesarchio V, De Luca A, Cardillo I, Cortese G, Baldi A, Chirianni A. Evaluation of antitumoral properties of the protease inhibitor indinavir in a murine model of hepatocarcinoma. Clin Cancer Res. 2006;12:2634. doi: 10.1158/1078-0432.CCR-05-2188. [DOI] [PubMed] [Google Scholar]
- 11.Knutson K, Schiffman LK, Rinn K, Disis ML. Immunotherapeutic approaches for the treatment of breast cancer. J Mammary Gland Biol Neoplasia. 1999;4:353. doi: 10.1023/A:1018714300217. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML, Cheever MA. HER-2/neu protein: a target for antigen-specific immunotherapy of human cancer. Adv Cancer Res. 1997;71:343. doi: 10.1016/S0065-230X(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 13.Coronella JA, Telleman P, Kingsbury GA, Truong TD, Hays S, Junghans RP. Evidence for an antigen-driven humoral immune response in medullary ductal breast cancer. Cancer Res. 2001;61:7889. [PubMed] [Google Scholar]
- 14.Peoples GE, Smith RC, Linehan DC, Yoshino I, Goedegebuure PS, Eberlein TJ. Shared T cell epitopes in epithelial tumors. Cell Immunol. 1995;164:279. doi: 10.1006/cimm.1995.1171. [DOI] [PubMed] [Google Scholar]
- 15.Tuttle TM, Anderson BW, Thompson WE, Lee JE, Sahin A, Smith TL, Grabstein KH, Wharton JT, Ioannides CG, Murray JL. Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer patients. Clin Cancer Res. 1998;4:2015. [PubMed] [Google Scholar]
- 16.Lenahan C, Dennis C, Isakovich NV, Pories SE. Breast cancer: what’s HER-2/neu got to do with it? Curr Surg. 2005;62:459. doi: 10.1016/j.cursur.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Muller WJ. Expression of activated oncogenes in the murine mammary gland: transgenic models for human breast cancer. Cancer Metastasis Rev. 1991;10:217. doi: 10.1007/BF00050793. [DOI] [PubMed] [Google Scholar]
- 18.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Cavallo F, Pupa SM, Rossi I, Colombo MP, Ricci C, Astolfi A, Musiani P, Forni G, Lollini PL. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194:1195. doi: 10.1084/jem.194.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cefai D, Morrison BW, Sckell A, Favre L, Balli M, Leunig M, Gimmi CD. Targeting HER-2/neu for active-specific immunotherapy in a mouse model of spontaneous breast cancer. Int J Cancer. 1999;83:393. doi: 10.1002/(SICI)1097-0215(19991029)83:3<393::AID-IJC16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Nagata Y, Furugen R, Hiasa A, Ikeda H, Ohta N, Furukawa K, Nakamura H, Kanematsu T, Shiku H. Peptides derived from a wild-type murine proto-oncogene c-erbB-2/HER2/neu can induce CTL and tumor suppression in syngeneic hosts. J Immunol. 1997;159:1336. [PubMed] [Google Scholar]
- 22.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 23.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, Muller WJ, Dixon KH, Jaffee EM. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569. [PubMed] [Google Scholar]
- 24.Gallo P, Dharmapuri S, Nuzzo M, Maldini D, Iezzi M, Cavallo F, Musiani P, Forni G, Monaci P. Xenogeneic immunization in mice using HER2 DNA delivered by an adenoviral vector. Int J Cancer. 2005;113:67. doi: 10.1002/ijc.20536. [DOI] [PubMed] [Google Scholar]
- 25.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 26.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 27.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, Jaffee EM. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 28.Wolpoe ME, Lutz ER, Ercolini AM, Murata S, Ivie SE, Garrett ES, Emens LA, Jaffee EM, Reilly RT. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J Immunol. 2003;171:2161. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 29.Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2:273. [PubMed] [Google Scholar]
- 30.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643. [PubMed] [Google Scholar]
- 31.Singh R, Paterson Y. Vaccination strategy determines the emergence and dominance of CD8+ T-cell epitopes in a FVB/N rat HER-2/neu mouse model of breast cancer. Cancer Res. 2006;66:7748. doi: 10.1158/0008-5472.CAN-05-4469. [DOI] [PubMed] [Google Scholar]
- 32.Rubocki RJ, Lee DR, Lie WR, Myers NB, Hansen TH. Molecular evidence that the H-2D and H-2L genes arose by duplication. Differences between the evolution of the class I genes in mice and humans. J Exp Med. 1990;171:2043. doi: 10.1084/jem.171.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DR, Rubocki RJ, Lie WR, Hansen TH. The murine MHC class I genes, H-2Dq and H-2Lq, are strikingly homologous to each other, H-2Ld, and two genes reported to encode tumor-specific antigens. J Exp Med. 1988;168:1719. doi: 10.1084/jem.168.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kattman SJ, Lukin KR, Oh JZ, Berg RE, Staerz UD. Maturational stage-dependent thymocyte responses to TCR engagement. Eur J Immunol. 2005;35:2051. doi: 10.1002/eji.200425293. [DOI] [PubMed] [Google Scholar]
- 35.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8 (+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16. [PubMed] [Google Scholar]
- 37.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 38.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744. [PubMed] [Google Scholar]
- 39.Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, Pauletti G, Pegram MD, Slamon DJ. Docetaxel, Cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24:1831. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 40.Drebin JA, Link VC, Greene MI. Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene. 1988;2:387. [PubMed] [Google Scholar]
- 41.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, Wei WZ, Cavallo F. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 42.Hussain SF, Paterson Y. CD4+CD25+ regulatory T cells that secrete TGFbeta and IL-10 are preferentially induced by a vaccine vector. J Immunother. 2004;27:339. doi: 10.1097/00002371-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34:752. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Berencsi K, Basak S, Somasundaram R, Ricciardi RP, Gonczol E, Zaloudik J, Linnenbach A, Maruyama H, Miniou P, Herlyn D. Human colorectal cancer (CRC) antigen CO17-1A/GA733 encoded by adenovirus inhibits growth of established CRC cells in mice. J Immunol. 1997;159:763. [PubMed] [Google Scholar]
- 45.Lo Iacono M, Cavallo F, Quaglino E, Rolla S, Iezzi M, Pupa SM, De Giovanni C, Lollini PL, Musiani P, Forni G, Calogero RA. A limited autoimmunity to p185neu elicited by DNA and allogeneic cell vaccine hampers the progression of preneoplastic lesions in HER-2/NEU transgenic mice. Int J Immunopathol Pharmacol. 2005;18:351. doi: 10.1177/039463200501800217. [DOI] [PubMed] [Google Scholar]
- 46.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, Day R, Troetschel M, Finn OJ. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zbar AP, Thomas H, Wilkinson RW, Wadhwa M, Syrigos KN, Ross EL, Dilger P, Allen-Mersh TG, Kmiot WA, Epenetos AA, Snary D, Bodmer WF. Immune responses in advanced colorectal cancer following repeated intradermal vaccination with the anti-CEA murine monoclonal antibody, PR1A3: results of a phase I study. Int J Colorectal Dis. 2005;20:403. doi: 10.1007/s00384-004-0726-x. [DOI] [PubMed] [Google Scholar]
- 48.Lustgarten J, Dominguez AL, Pinilla C. Identification of cross-reactive peptides using combinatorial libraries circumvents tolerance against Her-2/neu-immunodominant epitope. J Immunol. 2006;176:1796. doi: 10.4049/jimmunol.176.3.1796. [DOI] [PubMed] [Google Scholar]
- 49.Duraiswamy J, Bharadwaj M, Tellam J, Connolly G, Cooper L, Moss D, Thomson S, Yotnda P, Khanna R. Induction of therapeutic T-cell responses to subdominant tumor-associated viral oncogene after immunization with replication-incompetent polyepitope adenovirus vaccine. Cancer Res. 2004;64:1483. doi: 10.1158/0008-5472.CAN-03-2196. [DOI] [PubMed] [Google Scholar]
- 50.Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC–peptide–TCR complex. Immunity. 2000;13:529. doi: 10.1016/S1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 51.Tumenjargal S, Gellrich S, Linnemann T, Muche JM, Lukowsky A, Audring H, Wiesmuller KH, Sterry W, Walden P. Anti-tumor immune responses and tumor regression induced with mimotopes of a tumor-associated T cell epitope. Eur J Immunol. 2003;33:3175. doi: 10.1002/eji.200324244. [DOI] [PubMed] [Google Scholar]
- 52.Luo W, Ko E, Hsu JC, Wang X, Ferrone S. Targeting melanoma cells with human high molecular weight-melanoma associated antigen-specific antibodies elicited by a peptide mimotope: functional effects. J Immunol. 2006;176:6046. doi: 10.4049/jimmunol.176.10.6046. [DOI] [PubMed] [Google Scholar]