Abstract
Knowledge of the interactions between MHC-unrestricted cytotoxic effector cells and solid tumour cells is essential for introducing more effective NK cell-based immunotherapy protocols into clinical practise. Here, to begin to obtain an overview of the possible universe of molecules that could be involved in the interactions between immune effector cells and melanoma, we analyse the surface expression of adhesion and costimulatory molecules and of ligands for NK-activating receptors on a large panel of cell lines from the “European Searchable Tumour Cell Line and Data Bank” (ESTDAB, http://www.ebi.ac.uk/ipd/estdab/) and discuss their potential role in the immune response against this tumour. We show that most melanoma cell lines express not only adhesion molecules that are likely to favour their interaction with cells of the immune system, but also their interaction with endothelial cells potentially increasing their invasiveness and metastatic capacity. A high percentage of melanoma cell lines also express ligands for the NK-activating receptor NKG2D; whereas, the majority express MICA/B molecules, ULBP expression, however, was rarely found. In addition to these molecules, we also found that CD155 (poliovirus receptor, PVR) is expressed by the majority of melanoma cell lines, whereas CD112 (Nectin-2) expression was rare. These molecules are DNAM-1 ligands, a costimulatory molecule involved in NK cell-mediated cytotoxicity and cytokine production that also mediates costimulatory signals for triggering naïve T cell differentiation. The phenotypical characterisation of adhesion molecules and ligands for receptors involved in cell cytotoxicity on a large series of melanoma cell lines will contribute to the identification of markers useful for the development of new immunotherapy strategies.
Keywords: Melanoma, ESTDAB, NK cells, Cell-mediated cytotoxicity, MICA/B, Adhesion, Activating receptor, Cancer, Tumour
Introduction
Melanoma is considered the most lethal form of skin cancer, because it tends to spread early and rapidly progresses to a disseminated metastatic stage. Because melanoma is generally poorly responsive to chemotherapy, research has focussed on developing new cell-based immunotherapies for this cancer. Thus, identification of parameters relevant for cell-mediated cytotoxicity against melanoma may bring us a step closer to an effective treatment of these patients.
NK cells and CD8 T cells are major players in cell-mediated cytotoxicity against tumours and frequently share adhesion and activating receptors. In the earlier phases of lymphocyte activation, the formation of a conjugate between cytotoxic cells and tumour cells requires direct cell-to-cell interaction and the formation of an immunological synapse providing a microenvironment for the release of cytotoxic granules [8, 13, 14]. During the effector phase, cytotoxicity can be modulated by inhibitory signals, such as those mediated by MHC class I-specific inhibitory receptors (e.g. KIR, ILT, CD94/NK2A) initially described in NK cells and afterwards found in subsets of T cells [22, 75, 79].
Tumours evade T cell recognition by many different mechanisms, in particular down-regulation of HLA expression [5, 51, 69], low expression of co-stimulatory molecules [30, 45] and loss of cell adhesion molecules. In addition, several types of dysfunctional antigen-specific T cells have been described both in vivo [4, 55] and after in vitro expansion of CD8 T cells [20]. In human melanoma cell lines, several alterations of HLA class I molecules have been described by Garrido et al. [5, 51, 69]. Total or partial loss of MHC class I molecules is a frequent event in human solid tumours of different origins, and constitutes an important hurdle for T cell based immunotherapy [5, 52]. In contrast to T cells, NK cells recognise melanoma cells with low MHC expression more efficiently [61], and their activation depends on a complex balance between inhibitory and activating signals [12, 75, 80]. Accumulating evidences support a crucial contribution of NK cells to the immunosurveillance of tumours (for review see Waldhauer and Steinle [87]). In the absence of inhibitory signals (e.g. in the case of MHC class I-negative cells), tumour cells can be susceptible to NK-mediated lysis by recognizing ligands for activating receptors [14, 15, 53].
Although MHC class I loss makes tumour cells more susceptible to NK cell-mediated cytotoxicity, they have also developed mechanisms to avoid such killing [12, 19, 53]. Thus, downregulation or shedding of ligands for NK cell-activating receptors has been reported in tumour cells [38, 71, 85, 86]. Therefore, the study of the basis of NK cell cytotoxicity of solid tumour cells is of interest for assessing the possibility of introducing NK cell-based immunotherapy protocols [50].
Here, we describe the analysis of adhesion and costimulatory molecules and the expression of ligands for NK-activating receptors on a large panel of melanoma cell lines and discuss their role in the immune response against melanoma. Although it can be argued whether cell lines faithfully reflect the properties of the cells of the originating tumour, nonetheless, regarding their expression of NK inhibiting and activating ligands they offer a range of phenotypes also found on tumour cells in situ and thus provide valuable models for detailed investigation of tumour cell–NK cell interactions not possible when using fresh tumour samples. The phenotype of each individual melanoma cell line was established as part of the European project “European Searchable Tumour Cell Line and Data Bank” (ESTDAB) and can be searched on-line (see http://www.ebi.ac.uk/ipd/estdab/). The 5th Framework Program project “Outcome and Impact of Specific Treatment in European Research on Melanoma” (OISTER) also aimed to characterise melanoma cell lines together with their relevant clinical information. The expression of HLA class I antigens, ligands for NK cell inhibitory receptors, on this panel of cell lines has been previously reported [51]. The analysis of the expression of ligands for adhesion and activating NK receptors involved in cell recognition and killing will help to clarify the mechanisms involved in melanoma susceptibility or resistance to NK cells.
Analysis of adhesion molecules expressed in melanoma cell lines
Intercellular interactions through cell surface molecules on effector cells and their ligands on tumour cells are required to form stable conjugates to construct the “immunological synapse” essential for cell activation. Normal melanocytes express few adhesion molecules, but melanoma cells show an increased expression of these molecules. As melanoma progresses the repertoire of adhesion molecules changes and the acquisition of cell adhesion molecules during the process of tumour progression is suggested to contribute to the development of metastasis in melanoma [35, 56]. Using the ESTDAB cell bank, we have analysed the expression of adhesion molecules of the immunoglobulin superfamily (CD54 and CD58) and integrin superfamily (CD49d and CD11b) and also CD56 and CD57 molecules that have previously been described as mediators of cellular adhesion.
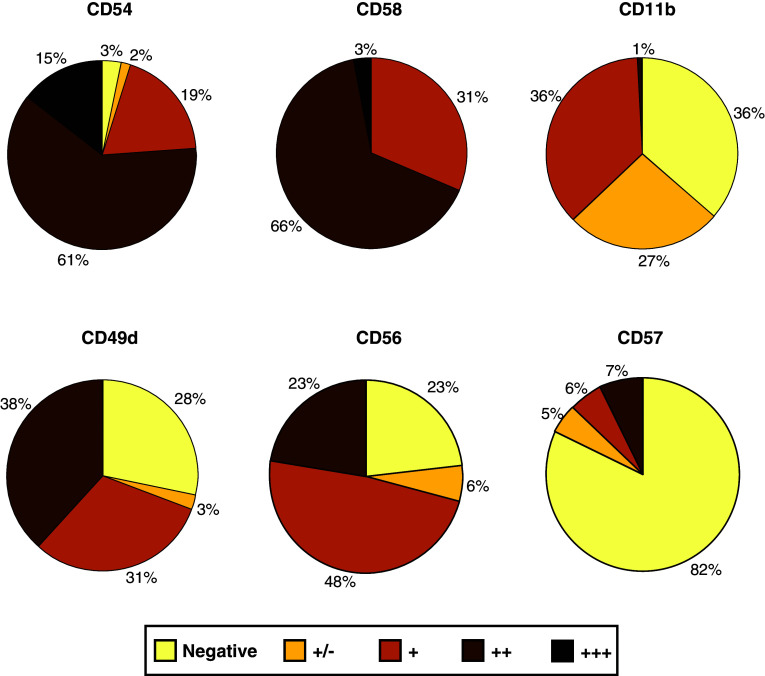
As shown in Fig. 1, most melanoma cell lines (95%) expressed CD54 (ICAM-1) at different levels of intensity. The CD54/LFA-1 (CD11a) interaction has been shown to play a crucial role in enhancing anti-tumour immune responses by its participation in effector–target conjugate formation. The expression of CD54 is required for lysis of melanoma cell lines by specific CTLs [42] and CD54high melanoma cells are more susceptible to NK cell-cytotoxicity than those with low CD54 expression [66], suggesting that CD54 expression on melanoma should associate with a better prognosis. However, high levels of CD54 are frequently found in metastatic or invasive melanoma cells [25] and CD54 expression correlates to a worse prognosis in primary melanomas [58]. Furthermore, it has been demonstrated that low expression of CD54 by melanoma cells associates with a longer overall survival following immunotherapy [67]. This apparent discrepancy may be explained by the fact that shedding of CD54 by melanoma cells has been described [35], and soluble CD54 can bind to its ligand on effector cells blocking effector–target interactions, contributing to tumour escape from immunity [2, 3, 10]. Furthermore, the analysis of CD54 polymorphism has shown an association of the R241 allele with high levels of soluble CD54 and with high relative risk of melanoma, supporting the possible significance of soluble CD54 in melanoma progression [83]. On the other hand, CD54 is also involved in the adhesion of tumour cells to the vascular epithelium, and it has been suggested that these interactions may promote the development of metastases [35].
Fig. 1.
Percentage of melanoma cell lines expressing the adhesion molecule indicated. Surface expression was determined by flow cytometry using a FACScalibur and specific mAbs. Intensity of staining of CD54, CD58, CD11b, CD49d, CD56 and CD57 (n = 124). Normalised scores were calculated by dividing the mean fluorescent channel (MFC) by its negative control. Negative <1.5; weakly positive (±) >1.5; positive (+) >2, strongly positive (++) >10 and very strong (+++) >100
CD58 (LFA-3) is a ligand of the cell surface receptor CD2, expressed on NK and T lymphocytes. In our study, all melanoma cell lines analysed expressed CD58 at variable intensity (Fig. 1). An increased CD58 expression on melanoma cells can modify their lysis susceptibility by melanoma-specific cytotoxic T lymphocyte (CTL) clones and co-stimulate cytokine production by these cells. It has been shown that a minimal expression of CD58 is required for optimal activation and full functionality of melanoma-specific CTL clones [42]. In particular, increasing CD58 density on melanoma cells enhances cytokine production by melanoma-specific CTL clones, indicating that CD58 expression is critical for the efficiency of specific immune reactions against melanoma cells [43]. In contrast to CD54, the expression of CD58 is not induced by cytokines such as TNF-α or IFN-γ [3].
As previously reported by Altomonte et al. [3], CD11a was not detected on the panel of melanoma cell lines analysed in this study (data not shown). However, our results showed that 37% of melanoma cell lines expressed detectable CD11b levels (Fig. 1). Although its role in melanoma cell interactions with lymphocytes or monocytes has not been defined so far, CD11b mediates cellular adhesion to endothelial cells through CD54 and its expression on melanoma cells may be relevant to their metastatic potential.
High levels of CD56 expression were observed on 71% of melanoma cell lines (Fig. 1). It is a NK-associated molecule commonly used for distinguishing cytotoxic or secretory NK subsets (CD56dim and CD56bright, respectively) [29]. It can be induced on CD8 T cells and it is a marker for T cells with a high cytotoxic potential [33]. Although CD56 is not a triggering molecule in NK cells, recent results suggest that it plays an important role in activating CD56+ CD8 CTL [44]. CD56 is also expressed on neural cells, where it is considered to be an adhesion molecule (neural cell adhesion molecule, N-CAM) playing a crucial role in neuronal development [27]. CD56 has been shown to be involved in homotypic adhesion between tumour cells and CD56+ cells of the immune system [36, 88]. It is expressed on a variety of tumours as Ewing’s sarcoma [60], small cell carcinoma [57], neuroblastoma [90], ovary tumours [84, 90] and melanoma [1, 31]. Although the functional implications of CD56 expression on tumour cells are not fully defined, the overexpression of CD56 by tumour cells leads to decreased NK cell adhesion and inhibition of lysis by NK cells [39]. CD56 is also involved in tumour cell binding to endothelial cells [92]. These interactions could be related to enhanced malignancy of CD56+ melanomas [1, 31].
Analysis of CD57 expression by flow cytometry showed that 12.9% melanoma cell lines were positive (Fig. 1). The CD57 molecule has been implicated in cell adhesion and cell migration [82]. It can be expressed in a wide range of tumours, including uveal and cutaneous melanoma and immunohistochemical studies have linked CD57 expression to their metastatic behaviour [78, 81]. In vitro assays using melanoma cell lines expressing CD57 have demonstrated an active role for this molecule in melanoma invasiveness and migration [21], supporting the functional relevance of CD57 expression in melanoma.
CD49d, the alpha chain of VLA-4 beta-1 integrin, was detected on 69% of melanoma cell lines (Fig. 1). This molecule plays a role in cell–cell interactions and cell adhesion to the extracellular matrix. It has been suggested that VLA-4 expressed on melanoma cells could allow the melanoma cells to migrate from the vascular system to tissues by enhancing melanoma cell tethering, adhesion to endothelial cells and establishing metastases [40, 46]. This supports a potential role of this integrin in the invasive and metastatic capacity of melanoma.
Altogether, it can be hypothesised that adhesion molecule expression by melanoma cells can act as a two-edged sword that could both favour recognition and elimination by immune effector cells and also their interaction with endothelial cells allowing tumour cells to cross the endothelial barrier.
Analysis of ligands for activating receptors involved in NK cell-mediated cytotoxicity
Ligands for NKG2D on melanoma cell lines
The important human NK-activating receptor NKG2D is a homodimeric C-type lectin-like receptor that has been shown to interact with several MHC class I-related molecules. Thus, human NKG2D ligands (NKG2DLs) include the stress-inducible surface glycoproteins MICA and MICB, and the UL16-binding proteins (ULBPs). The latter form a multigene family with at least six functional members. NKG2DL can be expressed on tumour and tumour cell lines of different origins and are also upregulated after viral infection. In humans, NKG2D is constitutively expressed on NK cells and CD8 T cells, as well as in gamma–delta T cells and NKT cells. Activation through NKG2D directly leads to triggering of NK cell-mediated cytotoxicity, whereas on T cells, it acts as a costimulatory molecule [9, 14, 59, 68, 93].
It has been shown that NKG2DL expression stimulates anti-tumour activity of NK cells [6, 7, 9, 16, 18, 26]. On the basis of these previous studies, a large panel of melanoma cell lines were analysed showing that 85% expressed at least one ligand for NKG2D (Fig. 2a). Detailed analysis revealed that most cell lines expressed MICA/B molecules (80%), but ULBP expression was less widespread (ULBP1, 15%; ULBP2, 25%; ULBP3, 20% of melanoma cell lines analysed).
Fig. 2.
Percentage of melanoma cell lines expressing the ligands for NKG2D and DNAM-1. a Analysis of NKG2D ligands (MICA/B and ULBP-1, 2 and 3). b Analysis of DNAM-1 ligands (CD155 and CD112). The study was performed by FACS and the scores normalised as indicated in Fig. 1 legend
The high level of expression of NKG2DL on melanoma cell lines suggests that NKG2D–NKG2DL interactions are likely to represent an important mechanism in NK cell recognition of melanoma cells. Therefore, we addressed the functional significance of NKG2DL expression on melanoma cell lines with regard to recognition by NK cells, by using the NK cell line NKL as model effectors. NKL expresses high levels of NKG2D, but only marginal levels of the NCR activating receptors (NKp30, NKp44, and NKp46). Analysis of melanoma susceptibility to NKL-mediated lysis using cell lines selected by their expression of NKG2DL, together with mAb directed against MICA/B or NKG2D, supports the role of NKG2D–NKG2DL on NK susceptibility of melanoma cell lines. As shown in Fig. 3a and b, NKL cytotoxicity towards the cell lines ESTDAB-075 and ESTDAB-081 (MICA+ ULBP+) was partially inhibited by anti-MICA/B and strongly by anti-NKG2D mAb. In a similar manner, cytotoxicity against ESTDAB-167 (MICAnegative ULBP2+) was not affected by anti-MICA/B, but the addition of anti-NKG2D mAb strongly blocked NKL-mediated lysis. This indicates that the recognition of ULBP2 was involved in the killing (Fig. 3c). In contrast, ESTDAB-067, expressing low levels of NKG2DL, was only marginally killed by NKL and this was not altered by the addition of mAb against MICA/B or NKG2D (Fig. 3d). As a control, we used the standard NK target, EBV transformed cell line 721.221, which does not express NKG2DL, but was highly susceptible to NKL lysis by an NKG2D independent mechanism. As expected, the addition of anti-MICA/B or anti-NKG2D mAb did not inhibit cytotoxicity (Fig. 3e) indicating that receptor–ligand interactions other than NKG2D–NKG2DL are involved in the lysis of this cell line. Killing of these cell lines was not affected by the addition of the appropriate isotype control (not shown). Together these results thus indicate that NKG2DL expression renders melanoma cells susceptible to NK cell-mediated cellular cytotoxicity and that NKL lysis of NKG2DL+ melanoma cell lines was critically dependent on MICA/B or ULBP interactions with NKG2D. This has been described previously for several cell lines of various tissue origins including melanoma [9, 63] and more extensively for leukaemia cells [70].
Fig. 3.
Analysis of melanoma susceptibility to NKG2D-dependent NKL cell cytotoxicity. Specific lysis of melanoma cell lines by NKL cell line was analysed by blocking experiments with mAb anti-MICA/B or anti-NKG2D mAb. The EBV transformed cell line 721.221, a standard NK target that does not express NKG2DLs, was used as control. Data shown are representative of three to five independent experiments at an E:T ratio of 20:1
It has been shown that cells expressing NKG2D infiltrate melanoma tumours and that a high percentage of metastatic melanoma lesions have lower levels of NKG2DL compared with the primary tumour that was positive for these molecules [48, 49]. These results suggest both a role for NKG2D-mediated activation in anti-melanoma response and that immunosurveillance by NKG2D positive NK and CD8 T cells can be subverted in vivo by the downregulation of MICA/B, although extensive correlative studies should be performed to confirm this possibility.
One of the mechanisms used by tumour cells to evade NKG2D-mediated recognition is the release of NKG2DL, which has been observed in a variety of human tumour entities and is thought to interfere with NKG2D-mediated tumour immunity in several ways (reviewed by Salih et al. [72]). Furthermore, increased levels of soluble NKG2DL, either MICA/B or ULBP2, have also been found in serum of patients with different malignant conditions [37, 38, 71, 86], indicating that established tumours can escape NKG2D-mediated tumour immunosurveillance by releasing NKG2DL. The shedding of both MICA and ULBP2 involves the activation of metalloproteases (MPs) [34, 70, 73, 86] likely belonging to the ADAM family of proteases [85].
Persistent NKG2DL expression induces a pronounced down-regulation of surface NKG2D on NK and activated CD8 T cells and a severe impairment of NKG2D-mediated cytotoxicity in vitro and in vivo [91]. Thus, the release of NKG2DL by tumour cells induces the downregulation of NKG2D on cytotoxic lymphocytes, thereby contributing to the escape of MICA/B or ULBP-positive tumour cells in vivo. The shedding of NKG2DL by tumour cells as a possible mechanism to escape immunosurveillance and the demonstration of the involvement of MPs in this process are of particular interest, since MP inhibitors are clinically available. Therefore, therapeutic blockade of MPs offers the possibility of interfering with this mechanism and subsequently increasing melanoma immunogenicity and susceptibility to NK and CD8 T cell cytotoxicity as suggested by Waldhauer et al. [85]. In a similar manner, several immunoregulatory molecules that can be released by tumour cells can also interfere with NKG2D–NKG2DL interactions. For example, it has been shown that TGF-β can transcriptionally inhibit the expression of NKG2D on effector cells [23] and also MICA, ULBP2 and ULBP4 expression [28]. In a recent study, it has been demonstrated that MIF can also contribute to the immune escape of ovarian carcinoma by transcriptionally down-regulating NKG2D in vitro and in vivo, thus impairing NK cell cytotoxicity towards the tumour cells [41].
Taken together, the results discussed earlier support the idea that the expression of NKG2DL on melanoma cells promotes anti-melanoma immunosurveillance by activating natural killer cells and, likely, by costimulating CD8 T cells, but NKG2DL shedding constitutes a major countermechanism of tumour cells to subvert NKG2D-mediated immunosurveillance. The possibility of interfering with NKG2DL shedding using MP inhibitors opens new therapeutic avenues to be explored.
Expression of ligands for DNAX accessory molecule-1 (DNAM-1)
DNAX accessory molecule-1 (DNAM-1) was originally identified as an adhesion molecule constitutively expressed on the majority of peripheral blood T lymphocytes [74]. DNAM-1 is also expressed by virtually all human NK cells and cross-linking DNAM-1 transduces activating signals resulting in enhancement of cytotoxicity and cytokine production by T and NK cells. It has recently been demonstrated that DNAM-1 induces NK cell activation through interactions with CD155 (poliovirus receptor, PVR) and CD112 (Nectin-2), two closely related molecules belonging to the Nectin family [11, 77]. Gilfillan et al. [ 32] show in mice lacking DNAM-1 that CD8 T cells require DNAM-1 for co-stimulation when recognizing antigen presented by nonprofessional antigen-presenting cells and that NK cells require DNAM-1-mediated signals for the elimination of tumour cells resistant to NK cell-mediated cytotoxicity due to low expression of other NK cell-activating ligands. The expression of DNAM-1 ligands in certain tumours is involved in cell-mediated cytotoxicity by NK and T cells [24, 62, 64, 76]. Here we show that DNAM-1 ligands are frequently expressed by melanoma cell lines (Fig. 2b). In particular, CD155 is expressed by the majority, but in contrast, CD112 was found on only 26% of the lines (Fig. 2b). These results suggest that these molecules may represent major ligands for triggering NK-mediated cytotoxicity and cytokine secretion against human tumour cells.
Expression of ligands for other activating NK receptors
The NK stimulating receptor 2B4 (CD244) interact with his ligand CD48, which is broadly expressed by cells of the haematological lineage [47]. Our results show that CD48 was not expressed by any of the melanoma cell lines studied (n = 75). Other NK-activating receptor is NKp80 that interacts with the C-type lectin-like receptors AICL, which is expressed on myeloid cells, such as monocytes and macrophages. NKp80–AICL interactions can promote lysis of a malignant myeloid cell line [89]. However, to our knowledge, the expression of this marker on solid tumours has not been studied.
A recent study has analysed the expression of NCR ligands on a small panel of tumour cell lines of different origins [17]. NCR are activating NK receptors that are expressed by resting (NKp30 and NKp46) or activated (NKp30, NKp46 and NKp44) NK cells [53]. Although the ligands for NCRs on tumour cells are still unknown, the use of NCR chimeric proteins has allowed the demonstration that NKp30 and NKp44 ligands, but not NKp46 ligands are expressed in a high percentage of cell lines from solid tumours, such as pancreatic and breast carcinoma, including a melanoma cell line [17]. Whereas NK cells use NCRs as activating receptors inducing killing of target cells [53, 54], it has also been shown that, on the contrary, tumour cells can induce apoptosis of NK cells by engaging the NCRs [65]. These findings indicate that NCRs are not only triggering molecules essential for antitumor activity, but also surface receptors that can be involved in NK cell death.
Concluding remarks
Melanoma cells can be recognized by NK cells through the NKG2D-activating receptor, but their lysis may require different thresholds of effector cell activation depending on the level and number of ligands for activating receptors that they express. Participation of different activating receptors may act in a synergistic manner favouring the elimination of melanoma cells. Thus, the redundancy of ligands for activating receptors will be advantageous for tumour immunosurveillance. It remains to be determined whether the activation threshold for NKG2D can be lowered by simultaneous engagement of other activating or costimulatory receptors within the immunological synapse.
Identification of ligands for NK-associated receptors and co-stimulatory molecules expressed on melanoma cells can be used as indicators of susceptibility to NK-mediated lysis. Further insights into the functional significance of the coexpression of different ligands for NKRs on melanoma cells and how different NKRs participate in melanoma recognition and lysis are required. For the use of NK cells in immunotherapy against melanoma, predicting NK cell-mediated therapeutic efficacy could be approached based on phenotypic analysis of ligand expression.
Acknowledgments
Work in the laboratories of R.T., R.S. and G.P. was partially supported by grants SAF2003/05184 and SAF2006/03687 (to R.T.) from the Spanish Ministry of Education and Science, FIS PI061320 (to R.S.) from the Spanish Ministry of Health, 03/2 and 3PR05A012, GRU07044 and GRU08077 (to R.T.) from Junta de Extremadura, cofinanced by the European Regional Development Fund (FEDER) and DFG-SFB685-B4 (to G.P.). The establishment of the database and cell bank was supported by the European Commission (contract QLRICT-2001-01325) (see http://www.ebi.ac.uk/ipd/estdab/). This work was also supported by contracts QLRT-2001-00668 (Outcome and Impact of Specific Treatment in European Research on Melanoma, OISTER), QLK6-CT2002-02283 (T cells in Ageing, T-CIA) from the 5th Framework Program of the European Union and 503306 from the 6th FP (European Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumor immunology, ENACT). J.G.C. received a post-doctoral fellowship associated to the 5th Framework Programme, contract QLRT-2001-00668 (OISTER) and B.S.C and S.M. are pre-doctoral fellows from Junta de Extremadura. Special thanks are due to M.R. Gonzalez and J.J. Gordillo for their technical assistance in cell culture and flow cytometry.
Footnotes
This paper is a Focussed Research Review from the meeting which took place during 28–29th May 2008 in Nottingham, UK, celebrating the contribution of Prof. I.A. “Tony” Dodi (29.1.2008) to the EU project “Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumour immunology (ENACT)”.
References
- 1.Abbott JJ, Amirkhan RH, Hoang MP. Malignant melanoma with a rhabdoid phenotype: histologic, immunohistochemical, and ultrastructural study of a case and review of the literature. Arch Pathol Lab Med. 2004;128:686–688. doi: 10.5858/2004-128-686-MMWARP. [DOI] [PubMed] [Google Scholar]
- 2.Altomonte M, Colizzi F, Esposito G, Maio M. Circulating intercellular adhesion molecule 1 as a marker of disease progression in cutaneous melanoma. N Engl J Med. 1992;327:959. doi: 10.1056/NEJM199209243271314. [DOI] [PubMed] [Google Scholar]
- 3.Altomonte M, Gloghini A, Bertola G, Gasparollo A, Carbone A, Ferrone S, Maio M. Differential expression of cell adhesion molecules CD54/CD11a and CD58/CD2 by human melanoma cells and functional role in their interaction with cytotoxic cells. Cancer Res. 1993;53:3343–3348. [PubMed] [Google Scholar]
- 4.Anichini A, Vegetti C, Mortarini R. The paradox of T-cell-mediated antitumor immunity in spite of poor clinical outcome in human melanoma. Cancer Immunol Immunother. 2004;53:855–864. doi: 10.1007/s00262-004-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 6.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, Steinle A, Salih HR. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 7.Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, Lauer UM, Bitzer M, Salih HR. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res. 2008;14:3520–3528. doi: 10.1158/1078-0432.CCR-07-4744. [DOI] [PubMed] [Google Scholar]
- 8.Backstrom E, Kristensson K, Ljunggren HG. Activation of natural killer cells: underlying molecular mechanisms revealed. Scand J Immunol. 2004;60:14–22. doi: 10.1111/j.0300-9475.2004.01475.x. [DOI] [PubMed] [Google Scholar]
- 9.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 10.Becker JC, Dummer R, Hartmann AA, Burg G, Schmidt RE. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. Functional consequences on cell-mediated cytotoxicity. J Immunol. 1991;147:4398–4401. [PubMed] [Google Scholar]
- 11.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottino C, Moretta L, Moretta A. NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol. 2006;298:175–182. doi: 10.1007/3-540-27743-9_9. [DOI] [PubMed] [Google Scholar]
- 13.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busche A, Goldmann T, Naumann U, Steinle A, Brandau S. Natural killer cell-mediated rejection of experimental human lung cancer by genetic overexpression of major histocompatibility complex class I chain-related gene A. Hum Gene Ther. 2006;17:135–146. doi: 10.1089/hum.2006.17.135. [DOI] [PubMed] [Google Scholar]
- 17.Byrd A, Hoffmann SC, Jarahian M, Momburg F, Watzl C. Expression analysis of the ligands for the natural killer cell receptors NKp30 and NKp44. PLoS ONE. 2007;2:e1339. doi: 10.1371/journal.pone.0001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, Catalano L, Tassone P, Rotoli B, Venuta S. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 19.Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 20.Casado JG, Delarosa O, Pawelec G, Peralbo E, Duran E, Barahona F, Solana R, Tarazona R. Correlation of effector function with phenotype and cell division after in vitro differentiation of naive MART-1-specific CD8+ T cells. Int Immunol. 2009;21:53–62. doi: 10.1093/intimm/dxn123. [DOI] [PubMed] [Google Scholar]
- 21.Casado JG, Delgado E, Patsavoudi E, Duran E, Sanchez-Correa B, Morgado S, Solana R, Tarazona R. Functional implications of HNK-1 expression on invasive behaviour of melanoma cells. Tumour Biol. 2008;29:304–310. doi: 10.1159/000156707. [DOI] [PubMed] [Google Scholar]
- 22.Casado JG, Soto R, Delarosa O, Peralbo E, Carmen Munoz-Villanueva M, Rioja L, Pena J, Solana R, Tarazona R. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–1171. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castriconi R, Cantoni C, Della CM, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, Moretta L, Bottino C, Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 25.Collins KA, White WL. Intercellular adhesion molecule 1 (ICAM-1) and bcl-2 are differentially expressed in early evolving malignant melanoma. Am J Dermatopathol. 1995;17:429–438. doi: 10.1097/00000372-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 27.Ditlevsen DK, Povlsen GK, Berezin V, Bock E. NCAM-induced intracellular signaling revisited. J Neurosci Res. 2008;86:727–743. doi: 10.1002/jnr.21551. [DOI] [PubMed] [Google Scholar]
- 28.Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 29.Farag SS, VanDeusen JB, Fehniger TA, Caligiuri MA. Biology and clinical impact of human natural killer cells. Int J Hematol. 2003;78:7–17. doi: 10.1007/BF02983234. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara K, Higashi T, Nouso K, Nakatsukasa H, Kobayashi Y, Uemura M, Nakamura S, Sato S, Hanafusa T, Yumoto Y, Naito I, Shiratori Y. Decreased expression of B7 costimulatory molecules and major histocompatibility complex class-I in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:1121–1127. doi: 10.1111/j.1440-1746.2004.03467.x. [DOI] [PubMed] [Google Scholar]
- 31.Gao Z, Stanek A, Chen S. A metastatic melanoma with an unusual immunophenotypic profile. Am J Dermatopathol. 2007;29:169–171. doi: 10.1097/DAD.0b013e31802e49a3. [DOI] [PubMed] [Google Scholar]
- 32.Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gritzapis AD, Dimitroulopoulos D, Paraskevas E, Baxevanis CN, Papamichail M. Large-scale expansion of CD3(+) CD56(+) lymphocytes capable of lysing autologous tumor cells with cytokine-rich supernatants. Cancer Immunol Immunother. 2002;51:440–448. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 35.Haass NK, Smalley KS, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 36.Hinsby AM, Berezin V, Bock E. Molecular mechanisms of NCAM function. Front Biosci. 2004;9:2227–2244. doi: 10.2741/1393. [DOI] [PubMed] [Google Scholar]
- 37.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 38.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother. 2006;55:1584–1589. doi: 10.1007/s00262-006-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarahian M, Watzl C, Issa Y, Altevogt P, Momburg F. Blockade of natural killer cell-mediated lysis by NCAM140 expressed on tumor cells. Int J Cancer. 2007;120:2625–2634. doi: 10.1002/ijc.22579. [DOI] [PubMed] [Google Scholar]
- 40.Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/A:1006304806799. [DOI] [PubMed] [Google Scholar]
- 41.Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, Weller M, Bucala R, Dietl J, Wischhusen J. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol. 2008;180:7338–7348. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labarriere N, Diez E, Pandolfino MC, Viret C, Guilloux Y, Le Guiner S, Fonteneau JF, Dreno B, Jotereau F. Optimal T cell activation by melanoma cells depends on a minimal level of antigen transcription. J Immunol. 1997;158:1238–1245. [PubMed] [Google Scholar]
- 43.Le Guiner S, Le Drean E, Labarriere N, Fonteneau JF, Viret C, Diez E, Jotereau F. LFA-3 co-stimulates cytokine secretion by cytotoxic T lymphocytes by providing a TCR-independent activation signal. Eur J Immunol. 1998;28:1322–1331. doi: 10.1002/(SICI)1521-4141(199804)28:04<1322::AID-IMMU1322>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 44.Lemster BH, Michel JJ, Montag DT, Paat JJ, Studenski SA, Newman AB, Vallejo AN. Induction of CD56 and TCR-independent activation of T cells with aging. J Immunol. 2008;180:1979–1990. doi: 10.4049/jimmunol.180.3.1979. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Yang Y, Inoue H, Mori M, Akiyoshi T. The expression of costimulatory molecules CD80 and CD86 in human carcinoma cell lines: its regulation by interferon gamma and interleukin-10. Cancer Immunol Immunother. 1996;43:213–219. doi: 10.1007/s002620050324. [DOI] [PubMed] [Google Scholar]
- 46.Liang S, Dong C. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am J Physiol Cell Physiol. 2008;295:C701–C707. doi: 10.1152/ajpcell.00245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 48.Maccalli C, Nonaka D, Piris A, Pende D, Rivoltini L, Castelli C, Parmiani G. NKG2D-mediated antitumor activity by tumor-infiltrating lymphocytes and antigen-specific T-cell clones isolated from melanoma patients. Clin Cancer Res. 2007;13:7459–7468. doi: 10.1158/1078-0432.CCR-07-1166. [DOI] [PubMed] [Google Scholar]
- 49.Maccalli C, Scaramuzza S, Parmiani G (2009) TNK cells (NKG2D(+) CD8 (+) or CD4 (+) T lymphocytes) in the control of human tumors. Cancer Immunol Immunother (in press) [DOI] [PMC free article] [PubMed]
- 50.Malmberg KJ, Bryceson YT, Carlsten M, Andersson S, Bjorklund A, Bjorkstrom NK, Baumann BC, Fauriat C, Alici E, Dilber MS, Ljunggren HG. NK cell-mediated targeting of human cancer and possibilities for new means of immunotherapy. Cancer Immunol Immunother. 2008;57:1541–1552. doi: 10.1007/s00262-008-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendez R, Rodriguez T, Del CA, Monge E, Maleno I, Aptsiauri N, Jimenez P, Pedrinaci S, Pawelec G, Ruiz-Cabello F, Garrido F. Characterization of HLA class I altered phenotypes in a panel of human melanoma cell lines. Cancer Immunol Immunother. 2008;57:719–729. doi: 10.1007/s00262-007-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendez R, Ruiz-Cabello F, Rodriguez T, Del CA, Paschen A, Schadendorf D, Garrido F. Identification of different tumor escape mechanisms in several metastases from a melanoma patient undergoing immunotherapy. Cancer Immunol Immunother. 2007;56:88–94. doi: 10.1007/s00262-006-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Human natural killer cells: molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett. 2005;100:7–13. doi: 10.1016/j.imlet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F, Cascinelli N, Parmiani G, Anichini A. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535–2545. [PubMed] [Google Scholar]
- 56.Moschos SJ, Drogowski LM, Reppert SL, Kirkwood JM. Integrins and cancer. Oncology (Williston Park) 2007;21:13–20. [PubMed] [Google Scholar]
- 57.Murray N, Salgia R, Fossella FV. Targeted molecules in small cell lung cancer. Semin Oncol. 2004;31:106–111. doi: 10.1053/j.seminoncol.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 58.Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, Giannarelli D, Temponi M, Ferrone S. Clinical significance of alpha(v)beta3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res. 1997;57:1554–1560. [PubMed] [Google Scholar]
- 59.Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 60.Olsen SH, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol. 2006;19:659–668. doi: 10.1038/modpathol.3800569. [DOI] [PubMed] [Google Scholar]
- 61.Pende D, Accame L, Pareti L, Mazzocchi A, Moretta A, Parmiani G, Moretta L. The susceptibility to natural killer cell-mediated lysis of HLA class I-positive melanomas reflects the expression of insufficient amounts of different HLA class I alleles. Eur J Immunol. 1998;28:2384–2394. doi: 10.1002/(SICI)1521-4141(199808)28:08<2384::AID-IMMU2384>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 62.Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, Spaggiari GM, Dondero A, Carnemolla B, Reymond N, Mingari MC, Lopez M, Moretta L, Moretta A. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42:463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 63.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 64.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 65.Poggi A, Massaro AM, Negrini S, Contini P, Zocchi MR. Tumor-induced apoptosis of human IL-2-activated NK cells: role of natural cytotoxicity receptors. J Immunol. 2005;174:2653–2660. doi: 10.4049/jimmunol.174.5.2653. [DOI] [PubMed] [Google Scholar]
- 66.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proc Natl Acad Sci USA. 1997;94:13140–13145. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quereux G, Pandolfino MC, Knol AC, Khammari A, Volteau C, Nguyen JM, Dreno B. Tissue prognostic markers for adoptive immunotherapy in melanoma. Eur J Dermatol. 2007;17:295–301. doi: 10.1684/ejd.2007.0203. [DOI] [PubMed] [Google Scholar]
- 68.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9 V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez T, Mendez R, Roberts CH, Ruiz-Cabello F, Dodi IA, Lopez Nevot MA, Paco L, Maleno I, Marsh SG, Pawelec G, Garrido F. High frequency of homozygosity of the HLA region in melanoma cell lines reveals a pattern compatible with extensive loss of heterozygosity. Cancer Immunol Immunother. 2005;54:141–148. doi: 10.1007/s00262-004-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 71.Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol. 2006;67:188–195. doi: 10.1016/j.humimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci. 2008;13:3448–3456. doi: 10.2741/2939. [DOI] [PubMed] [Google Scholar]
- 73.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 74.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/S1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 75.Solana R, Casado JG, Delgado E, Delarosa O, Marin J, Duran E, Pawelec G, Tarazona R. Lymphocyte activation in response to melanoma: interaction of NK-associated receptors and their ligands. Cancer Immunol Immunother. 2007;56:101–109. doi: 10.1007/s00262-006-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, Ohkochi N, Honda S, Shibuya A. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- 77.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 78.Tang NE, Luyten GP, Mooy CM, Naus NC, de Jong PT, Luider TM. HNK-1 antigens on uveal and cutaneous melanoma cell lines. Melanoma Res. 1996;6:411–418. doi: 10.1097/00008390-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Tarazona R, Casado JG, Soto R, Delarosa O, Peralbo E, Rioja L, Pena J, Solana R. Expression of NK-associated receptors on cytotoxic T cells from melanoma patients: a two-edged sword? Cancer Immunol Immunother. 2004;53:911–924. doi: 10.1007/s00262-004-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarazona R, Delarosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/S0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 81.Thies A, Schachner M, Berger J, Moll I, Schulze HJ, Brunner G, Schumacher U. The developmentally regulated neural crest-associated glycotope HNK-1 predicts metastasis in cutaneous malignant melanoma. J Pathol. 2004;203:933–939. doi: 10.1002/path.1595. [DOI] [PubMed] [Google Scholar]
- 82.Uusitalo M, Kivela T. The HNK-1 carbohydrate epitope in the eye: basic science and functional implications. Prog Retin Eye Res. 2001;20:1–28. doi: 10.1016/S1350-9462(00)00018-5. [DOI] [PubMed] [Google Scholar]
- 83.Vinceti M, Pellacani G, Casali B, Malagoli C, Nicoli D, Farnetti E, Bassissi S, Bergomi M, Seidenari S. High risk of cutaneous melanoma amongst carriers of the intercellular adhesion molecule-1 R241 allele. Melanoma Res. 2006;16:93–96. doi: 10.1097/01.cmr.0000198450.19204.dd. [DOI] [PubMed] [Google Scholar]
- 84.Volker HU, Engert S, Cramer A, Schmidt M, Kammerer U, Muller-Hermelink HK, Gattenlohner S. Expression of CD56 isoforms in primary and relapsed adult granulosa cell tumors of the ovary. Diagn Pathol. 2008;3:29. doi: 10.1186/1746-1596-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 86.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006;66:2520–2526. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- 87.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 88.Walmod PS, Kolkova K, Berezin V, Bock E. Zippers make signals: NCAM-mediated molecular interactions and signal transduction. Neurochem Res. 2004;29:2015–2035. doi: 10.1007/s11064-004-6875-z. [DOI] [PubMed] [Google Scholar]
- 89.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80–AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 90.Wick MR. Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol. 2000;17:194–203. [PubMed] [Google Scholar]
- 91.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 92.Zocchi MR, Vidal M, Poggi A. Involvement of CD56/N-CAM molecule in the adhesion of human solid tumor cell lines to endothelial cells. Exp Cell Res. 1993;204:130–135. doi: 10.1006/excr.1993.1017. [DOI] [PubMed] [Google Scholar]
- 93.Zwirner NW, Fuertes MB, Girart MV, Domaica CI, Rossi LE. Cytokine-driven regulation of NK cell functions in tumor immunity: role of the MICA-NKG2D system. Cytokine Growth Factor Rev. 2007;18:159–170. doi: 10.1016/j.cytogfr.2007.01.013. [DOI] [PubMed] [Google Scholar]