Abstract
Tumor cell death potentially engages with the immune system. However, the efficacy of anti-tumor chemotherapy may be limited by tumor-driven immunosuppression, e.g., through CD25+ regulatory T cells. We addressed this question in a mouse model of mesothelioma by depleting or reconstituting CD25+ regulatory T cells in combination with two different chemotherapeutic drugs. We found that the efficacy of cyclophosphamide to eradicate established tumors, which has been linked to regulatory T cell depletion, was negated by adoptive transfer of CD25+ regulatory T cells. Analysis of post-chemotherapy regulatory T cell populations revealed that cyclophosphamide depleted cycling (Ki-67hi) T cells, including foxp3+ regulatory CD4+ T cells. Ki-67hi CD4+ T cells expressed increased levels of two markers, TNFR2 and ICOS, that have been associated with a maximally suppressive phenotype according to recently published studies. This suggest that cyclophosphamide depletes a population of maximally suppressive regulatory T cells, which may explain its superior anti-tumor efficacy in our model. Our data suggest that regulatory T cell depletion could be used to improve the efficacy of anti-cancer chemotherapy regimens. Indeed, we observed that the drug gemcitabine, which does not deplete cycling regulatory T cells, eradicates established tumors in mice only when CD25+ CD4+ T cells are concurrently depleted. Cyclophosphamide could be used to achieve regulatory T cell depletion in combination with chemotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-008-0628-9) contains supplementary material, which is available to authorized users.
Keywords: Tumor immunity, Regulatory CD4+ T cells, Chemotherapy, Mesothelioma, Gemcitabine, Cyclophosphamide
Introduction
Cancer is rarely cured by cytotoxic chemotherapy alone. However, chemotherapy can set the stage for the generation of effective anti-tumor immune responses by immunotherapy or vaccination [9, 17, 22, 33, 45, 48]. For example, combination of the cytotoxic drug gemcitabine (GEM) with agonistic anti-CD40 antibodies resulted in curative responses in a mouse model of mesothelioma, whereas neither single therapy could achieve this [33]. The successful combination of immunotherapy with chemotherapy suggests that chemotherapy alone does not sufficiently engage with the immune system to generate effective anti-tumor immune responses. One possible explanation for this is that chemotherapeutic drugs do not break tumor-driven immunosuppression. Suppression of anti-tumor immune responses is emerging as a cardinal feature of tumor immune-editing [5, 36]. Foxp3-expressing CD25+ regulatory CD4+ T cells are key players that shape such suppressive immune responses [11], but other cell types, e.g., IL-10 producing Tr1 cells [37], other less-well characterized CD4+ T cells [8] and IL-13 producing type II NKT cells [4, 44] have been shown to play a role as well. The concept that tumors drive immuno-suppression has implications for therapy as the specific depletion of suppressive cells could generate productive anti-tumor responses.
The cytotoxic drug cyclophosphamide (CY) has received considerable attention because of its immuno-stimulatory properties. In the early 1980 s, it was shown that CY depleted suppressor T cells and thereby rescued anti-tumor effector T cells [3]. Although the concept of suppressor T cells was controversial at the time, the discovery of CD25+ CD4+ regulatory T cells [11] sparked a renewed interest in the link between CY and loss of immuno-suppression. Several studies have now demonstrated that CY is indeed associated with a loss of immuno-suppressive functions and that this loss is caused by a selective depletion of regulatory T cells [14, 24]. A causal link between CY, NO production by Gr1+ CD11b+ myeloid suppressor cells and the selective depletion of proliferating T cells has provided a cellular mechanism for this phenomenon [2, 34].
Since there is emerging evidence that regulatory T cells play a role in mesothelioma [18, 29, 38], we used the AB1-HA model of murine mesothelioma [25, 26] to investigate the link between chemotherapy and CD25+ regulatory T cells. AB1-HA tumor cells were generated by transfection of the asbestos-induced AB1 tumor cell line [25] with the influenza virus HA-gene [26]. The objective of the present study was to determine whether the efficacy of gemcitabine can be improved by regulatory T cell depletion, and, conversely, whether the anti-tumor effects of cyclophosphamide can be explained by its impact on regulatory T cells. In both cases, we found that the outcome of chemotherapy was critically dependent on CD25+ CD4+ regulatory T cells. Phenotypic characterization of post-chemotherapy regulatory T cell populations revealed that CY, but not GEM, depleted a population of Ki-67hi cycling T cells. The Ki-67hi population that was depleted was characterized by the expression of ICOS and TNFR2. These markers have been associated with a maximally suppressive phenotype [6, 19, 41]. To our knowledge, this study is the first to report (1) that the anti-tumor efficacy of chemotherapy is enhanced by regulatory T cell depletion, either by immunotherapy (anti-CD25 antibodies) or by using CY and (2) that a discrete population of KI-67hi ICOShi TNFR2hi regulatory T cells exists that is enriched in the tumor and that is depleted by cyclophosphamide.
Materials and methods
Reagents and antibodies
Cyclophosphamide and maphosphamide were purchased from Sigma–Aldrich and Baxter Oncology (Halle, Germany), respectively. Gemcitabine was obtained from the Sir Charles Gairdner Hospital pharmacy. CFSE was from Molecular Probes. The following conjugated antibodies were used: TCRβ-AF488 (H57-597, eBioscience), CD3ε-FITC (145-2C11, eBioscience), CD4-PECy7 and CD4-PE (RM4-5, eBioscience), CD8α-PECy5 (5H10, Caltag), CD25-PE and CD25-APC-AF750 (PC-61, eBioscience), Ki-67-FITC (B56, BD), foxp3-AF647 (150D, Biolegend), ICOS-PECy5 (7E.17G9, eBioscience), TNFR2-PE (TR75-32, BD Biosciences), CD62L-APC (MEL-14, eBioscience) and iNOS-FITC (Clone 6, BD Biosciences). Flow cytometry was performed using BD FACSCalibur and FACSCanto II instruments and analyzed using Flowjo software (TreeStar).
Mice
BALB/c (H-2d) wild-type and nude mice were purchased from the Animal Resources Centre (Canning Vale, Western Australia) and maintained under specific pathogen-free conditions. All experiments used female mice between 6 and 8 weeks of age. Animal experimentation was conducted according to University of Western Australia Animal Ethics Committee approvals following the NH&MRC Code of Practice.
Chemotherapy
Cyclophosphamide (CY) was administered as a single intraperitoneal injection of 150 mg/kg (3 mg in 150 μl in a 20 g mouse) when tumors became palpable (day 9 for immunocompetent BALB/c mice, day 7 for athymic nude mice). Occasionally and as indicated in the text, CY injections were done at later timepoints. In initial studies, the optimal CY dose was emperically determined as the minimum dose resulting in 80–100% cure rate (150 mg/ml). Lower dose (15–50 mg/kg) resulted in dramatically decreased efficacy, whereas higher doses (200 mg/kg) were 100% curative. Gemcitabine treatment was done at an optimized dose and schedule, as published previously: 5 injections, every third day (q3dx5) at 100 mg/kg per dose, starting when tumors became palpable (day 9 for immunocompetent BALB/c mice, day 7 for athymic nude mice) [33].
Tumor cell culture and inoculation
Generation of the BALB/c-derived mouse mesothelioma cell line AB1 and transfection with the gene encoding influenza HA (AB1-HA) has been previously described [26]. Cell lines were maintained in RPMI 1640 (Invitrogen Life Technologies) supplemented with 20 mM HEPES, 0.05 mM 2-ME, 60 μg/ml penicillin (CSL), 50 μg/ml gentamicin (West), and 5% FCS (Invitrogen Life Technologies). Tumor cells (1 × 106 in 100 μl of PBS) were injected s.c. into the right flank of recipient mice and subsequent tumor growth monitored by taking two perpendicular diameter measurements using microcalipers. Tumor size is then operationally defined as the product of the two measurements. Mice were euthanased when tumors reached 10 × 10 mm as per Animal Ethics guidelines.
Cell depletion studies
CD4, CD8α and CD25+ T cell depletion was performed using purified GK1.5, YTS.169 and PC61 mAbs, respectively, prepared by Dr Kathy Davern, Monoclonal Antibody Facility (Western Australian Institute for Medical Research, Perth, Australia). For CD4 and CD8 depletion, mice received an initial dose of 200 μg i.v., 1 day before CY treatment, followed by a second dose of 150 μg administered i.p. on the day of treatment and then 150 μg i.p. every second day thereafter for a total of six doses. CD4 and CD8 depletion (>95%) was verified during treatment by FACS analysis of peripheral blood using Abs specific for TCR-β, CD4, and CD8α. CD25 depletion was done using two injections of antibody (200 μg/injection) on the day before treatment and 10 days later.
Preparation and staining of cells
For flow cytometry analysis, tumors, spleens, and lymph nodes were removed from mice and placed into ice-cold PBS containing 1% FCS (v/v). The axillary and inguinal nodes were pooled for the tumor flank (draining lymph nodes) and for the contralateral flank (nondraining lymph nodes). Homogenized tissues were resuspended in FACS buffer (PBS with 1% BSA (w/v), and 0.01% NaN3 (w/v)) along with appropriate dilutions of Abs or isotype controls. After 30 min, cells were washed and resuspended in 2% paraformaldehyde/PBS (v/v) (Sigma–Aldrich) before FACS analysis. For intracellular staining (Ki-67-FITC, foxp3-AF647) cells were permeabilized and fixed according to the instructions of the manufacturer (BD Biosciences and Biolegend).
Purification and adoptive transfer of CD25+ CD4+ T cells
CD25+ CD4+ T cells were purified from the spleens and lymph nodes of tumor-bearing mice using magnetic beads (Regulatory T cell purification kit, Miltenyi). The phenotypes of CD25high and CD25low cells were tested by staining purified cell populations for Foxp3 expression. Cells were counted and transferred into tumor-bearing and cyclophosphamide-treated mice by intravenous injection of 107 cells.
Statistics
Data was statistically evaluated using Prism software (GraphPad). Survival data are presented as Kaplan–Meyer plots and were analyzed the using log-rank test. Growth curves were compared using a two-tailed paired t test, with pairs defined by time point. All other variables were compared using a two-tailed Mann–Whitney U test. Significant differences were defined as P < 0.05.
Results
Cyclophosphamide but not gemcitabine cures murine mesothelioma: responses are CD8 T cell dependent
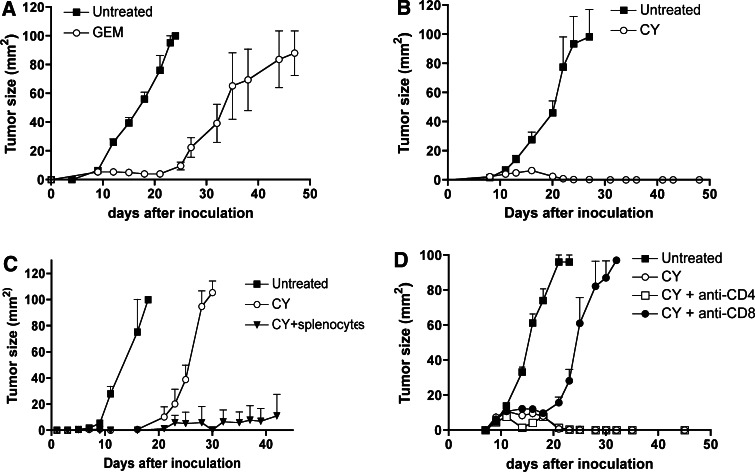
Treatment of established AB1-HA tumors with gemcitabine (GEM) (q3dx5, starting at day 9 after tumor cell inoculation) led to tumor regression but did not cure mice (Fig. 1a), consistent with previously published data [33]. This non-curative anti-tumor response was in part CD8 T cell dependent (Mahendran et al., manuscript in preparation). However, we have previously shown that curative responses were achieved when GEM was combined with anti-CD40 antibodies [33], which suggests that chemotherapy alone does not sufficiently engage the immune system. As recent evidence suggests that T cell responses against AB1-HA tumors are controlled by CD25+ CD4+ regulatory T cells [29, 38], we hypothesized that regulatory T cells could be the limiting factor in generating post-gemcitabine immune responses. Because cyclophosphamide (CY) has been linked to the selective depletion of CD4+ CD25+ regulatory T cells (Tregs) [3, 10, 14, 24, 43], we evaluated the effects of this drug in our model. Note that CY is also a cytotoxic drug and that it kills AB1-HA tumor cells (Electronic supplementary material (ESM) Fig. 1, available on-line). Thus, CY provides us with a tool to combine a cytotoxic drug with regulatory T cell depletion. AB1-HA tumor-bearing mice were treated with CY (150 mg/kg) when tumors became palpable (day 9–10 after tumor cell inoculation). We observed that a single dose of CY cured 100% of the mice (Fig. 1b). CY-cured mice invariably resisted rechallenge with AB1-HA tumors, suggesting that the curative response had generated tumor-specific immunological memory. We then wanted to determine whether the immune system was necessary to achieve cures. To test this, we evaluated the effects of CY in athymic nude mice. Because CY did not result in any curative responses in nude mice (Fig. 1c), we concluded that T cells played a key role in converting transient anti-tumor effects into cures. The transient tumor regression observed in nude mice (Fig. 1c) was the direct result of drug cytotoxicity as NK cell depletion had no effect (data not shown). Further proof that the curative effects of CY were immune mediated was obtained from an adoptive transfer experiment. Transfer of 107 splenocytes from immunocompentent BALB/c mice previously cured by CY-treament (‘AB1-HA survivors’) into AB1-HA tumor-bearing nude mice (CY at day 7 post tumor cell inoculation and i.v. splenocyte transfer at day 8) dramatically prolonged the anti-tumor response (Fig. 1c). Splenocyte transfer without CY had no effect on tumor growth (data not shown). The curative responses in immunocompetent mice were dependent on CD8 T cells as revealed by depletion of specific T cell subsets using monoclonal antibodies (Fig. 1d). CD4 depletion had no impact on the response (Fig. 1d).
Fig. 1.
In vivo antitumor effects of GEM and CY. 106 tumor cells were inoculated subcutaneously and mice (BALB/c) were treated when tumors became palpable (day 9 after tumor cell inoculation). a Treatment of immunocompetent BALB/c mice with 100 mg/kg GEM, q3dx5, starting on day 9 after tumor cell inoculation. b Treatment of immunocompetent BALB/c mice with single dose 150 mg/kg cyclophosphamide, day 9 after tumor cell inoculation. c Treatment of AB1-HA tumor-bearing athymic nu/nu mice with single dose 150 mg/kg cyclophosphamide, day 7 after tumor cell inoculation. Selected groups received 107 splenocytes from AB1-HA survivors (AB1-HA-cured BALB/c mice, through CY treatment) by i.v. injection at day 8 post inoculation. d Cyclophosphamide efficacy depends on CD8 T cells. T cell subsets were depleted in AB1-HA tumor-bearing BALB/c mice as described that Materials and Methods. All experiments shown involved five mice and were representative of two to three experiments
Chemotherapy is effective when combined with CD25 depletion
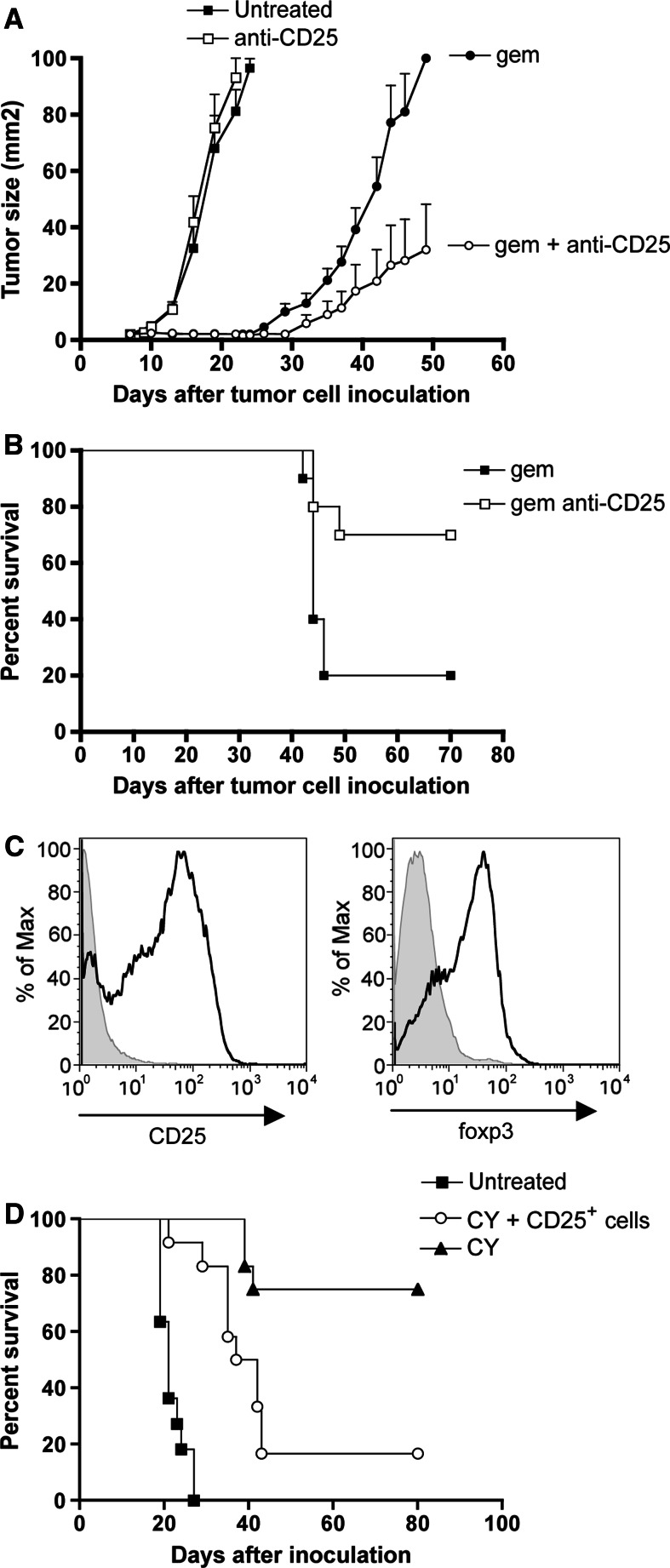
The strong anti-tumor effect of CY suggests that regulatory T cell depletion is essential to achieve cures in the context of cytotoxic chemotherapy. This suggests that the anti-tumor efficacy of GEM could be improved by the concurrent depletion of regulatory T cells. Thus, tumor-bearing mice were treated with GEM at the previously reported schedule of q3dx5 [33], starting at day 7 post inoculation, and were injected with anti-CD25 antibody on days 7 and 17 after tumor cell inoculation. This antibody treatment led to >97% depletion of CD25+ CD4+ T cells within three days after the first injection (ESM Fig.2, available on-line). Control groups received either chemotherapy or anti-CD25 antibodies as a single therapy. CD25 depletion without chemotherapy had no effect at all on tumor growth (Fig. 2a). Importantly, depletion of CD25+ cells significantly improved the efficacy of GEM (P < 0.01, GEM + anti-CD25 compared to GEM alone) as observed in tumor growth rates (Fig. 2a), and resulted in a significant survival benefit (Fig. 2b, P < 0.05). Note that GEM + anti-CD25 appears to be less effective than CY alone (compare Figs. 1b, 2). This may reflect incomplete depletion of regulatory T cells by the PC61 antibody, as reported in the literature [35, 39, 40].
Fig. 2.
CD25 regulatory T cell depletion and reconstitution. a Growth curves of tumors in untreated, PC61-treated, GEM-treated and GEM+PC61-treated mice. Effects of CD25 (PC61) depletion in GEM-treated (open vs. solid circles) and in untreated (open vs. solid squares) mice. GEM and PC61 treatments were done as described in Materials and Methods. Data shown are from two experiments involving 12 mice. (b) Kaplan–Meier survival data of CD25 depletion in GEM-treated mice. (c) Phenotypic characterization of purified CD25+ and foxp3+ cells for adoptive transfer. Cells shown are CD4+ cells. (d) Kaplan-Meier survival curve of tumor-bearing mice treated with saline (solid squares), CY (solid triangles) or CY+Tregs (open circles). CY was injected at day 9 post inoculation and regulatory T cells were transferred at day 12. Data shown are from two experiments involving 12 mice
We then performed the reciprocal experiment. Assuming that the success of chemotherapy depended on depletion of regulatory T cells, we reasoned that adoptive transfer of purified CD25+ regulatory T cells into CY-treated mice would negate the curative property of the drug. CD25+ CD4+ T cells were isolated from AB1-HA tumor bearing mice using magnetic beads and their identity was confirmed by foxp3 staining (Fig. 2c). To evaluate the effect of regulatory T cell reconstitution, we set up a transfer experiment. Recipient mice were first inoculated with AB1-HA tumor cells. Untreated control mice all had 10 × 10 mm tumors between days 19–27 after inoculation (Fig. 2d). Chemotherapy alone, with CY injected at day 9 after inoculation, resulted in a 75% cure rate (Fig. 2d). Transfer of 107 purified CD25+ CD4+ T cells into tumor-bearing mice at day 3 after CY injection (day 12 after inoculation) completely inhibited the curative response induced of CY: tumors developed rapidly in 10/12 of the CD25+ T cell recipients (Fig. 2d), essentially reducing the efficacy of CY to that observed in athymic nude mice or CD8-depleted mice (Fig. 1c, d). Thus, adoptive transfer of CD25+ CD4+ T cells significantly diminished the survival benefit of CY (P < 0.005, comparison of CY with CY+Tregs). The combined data demonstrate that CD25+ regulatory T cells limit antitumor CD8 T cell responses, implying that the antitumor CD8+ effector T cells are sensitive to suppression from CD25+ CD4+ regulatory T cells.
Cyclophosphamide but not gemcitabine depletes proliferating T cells
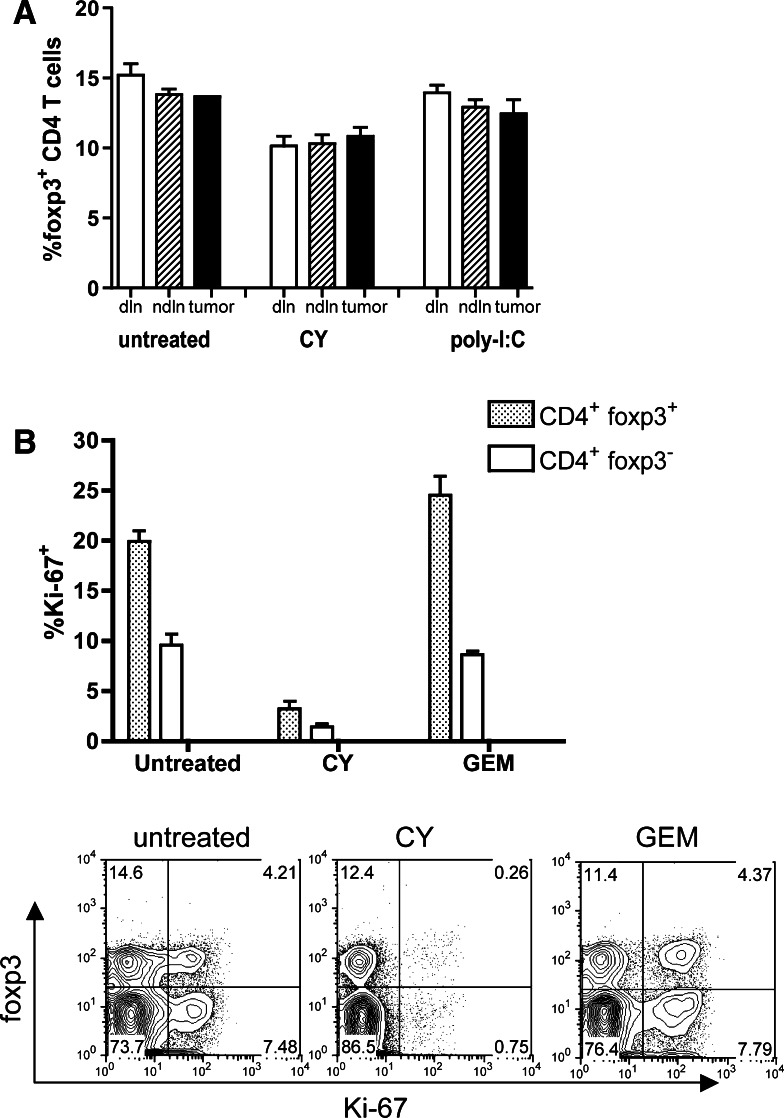
Having established that the presence or absence of CD25+ regulatory T cells makes a critical difference in the post-chemotherapy anti-tumor immune response, we first tested the hypothesis that CY preferentially depletes regulatory T cells. We observed a modest decrease in the frequencies of foxp3+ regulatory T cells in the spleens and lymph nodes of CY-treated mice (~15% decrease) (Fig. 3a), compared to untreated mice and to mice treated with poly-I:C-immunotherapy. As poly-I:C treatment leads to tumor regression [7], this latter group was included to control for the impact of reduced tumor burden on regulatory T cells.
Fig. 3.
Impact of CY and GEM on CD25+ CD4+ regulatory T cells. a Regulatory T cell frequencies expressed as percentage foxp3+ cells in the total CD4+ T cell population in draining lymph nodes (dln), non-draining lymph nodes (ndln) and the tumor. P < 0.005 for draining lymph nodes and P < 0.01 for non-draining lymph nodes when CY-treated mice are compared to untreated controls. Poly-I:C treated mice [7] were used to control for the immune impact of tumor resolution. b CY but not GEM depletes cycling Ki-67+ CD4+ T cells. Lymphocytes from draining lymph nodes were stained for CD4, foxp3 and Ki-67. Cells shown were gated for CD4+ cells. Data shown are from four mice and were repeated twice. Representative dot plots are shown in the lower panel
Because the CY-induced decrease in regulatory T cell frequencies was modest we asked how regulatory T cell phenotypes differed between CY and GEM-treated tumor-bearing mice. Both CY and GEM are lymphodepleting drugs and cause a 75–90% loss in splenic cellularity (not shown and [32]. We used the cell cycle marker Ki-67 to assess the proliferative status of CD4+ T cells [13]. Ki-67 staining experiments revealed that CY specifically depleted cycling Ki-67hi CD4+ T cells (Fig. 3b). Importantly, both foxp3pos (regulatory) T cells and foxp3neg (non-Treg) T cells were targeted (Fig. 3b). GEM treatment was not associated with a loss of Ki-67hi T cells (Fig. 3b). Since GEM is a cytotoxic and lymphodepleting drug [32], it is likely that the difference between CY and GEM is one of kinetics, i.e., both drugs target cycling cells, but T cell proliferation recovers more rapidly after GEM treatment.
Ki-67+ effector suppressor T cells are TNFR2hi and express ICOS
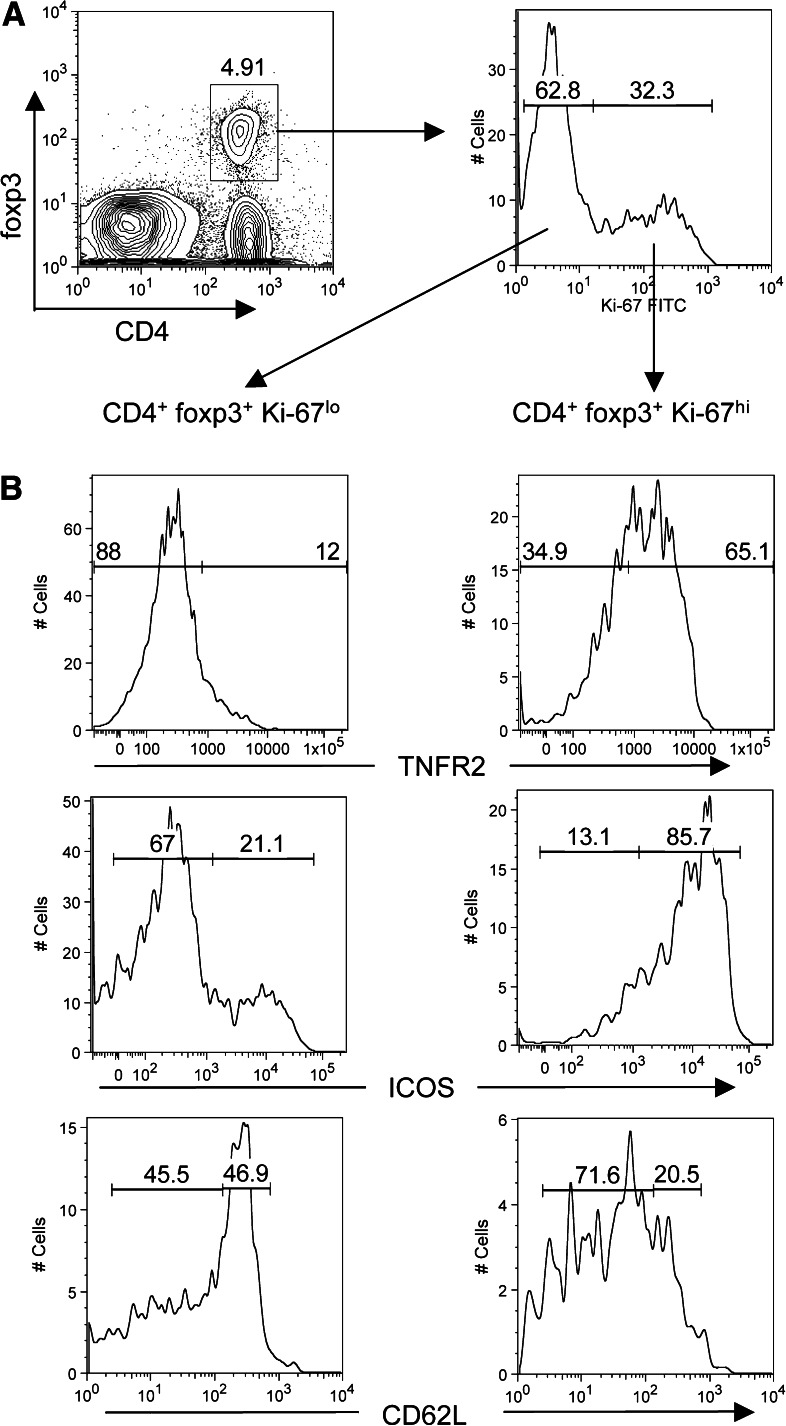
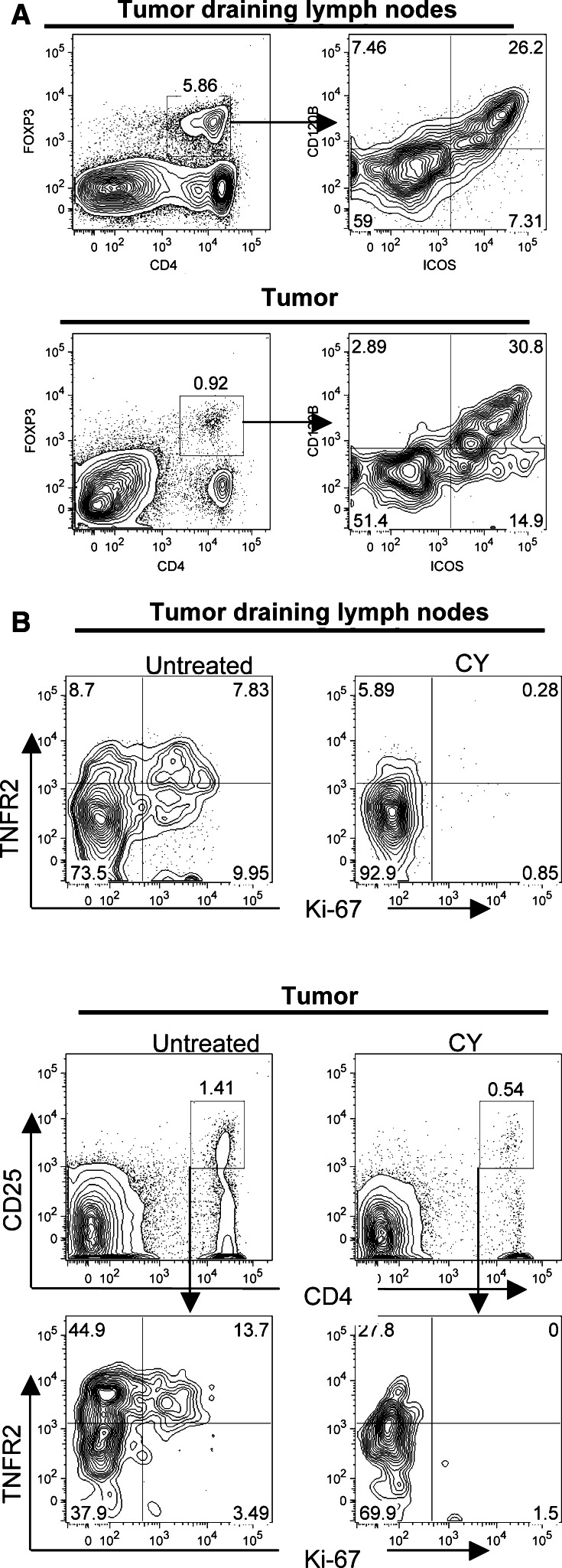
We hypothesized that the pool of Ki-67hi T cells depleted by CY would harbor the effector suppressor cells, such that their removal would facilitate anti-tumor CD8 T cell responses. To address this issue, we asked whether Ki-67hi regulatory cells were phenotypically distinct from non-cycling (Ki-67lo) cells using the markers TNFR2 (CD120B), CD62L and ICOS. It was very recently demonstrated that TNFR2 identifies a maximally suppressive subset of foxp3+ regulatory T cells [6]. These TNFR2hi cells are CD62Llo [6]. ICOS is a B7 family member that has also been implicated in suppressive function of CD25+ regulatory cells [19, 21, 23, 41].
Regulatory T cells from tumor draining lymph nodes were identified by foxp3 staining (Fig.4a, left panel) and the phenotypes of Ki-67lo and Ki-67hi foxp3+ CD4+ T cells were compared. We found that Ki-67hi regulatory CD4+ T cells in the draining lymph nodes of tumor-bearing mice expressed higher levels of TNFR2 and ICOS when compared to the Ki-67lo pool (Fig. 4b). Furthermore, CD62L (L-selectin) expression was downregulated in cycling regulatory T cells (Fig. 4b). TNFR2 and ICOS were expressed by the same population of cells (Fig. 5a), indicating that a single pool of Ki-67hi TNFR2hi ICOShi regulatory CD4+ T cells existed. Also, tumor-infiltrating regulatory T cells expressed high levels of both TNFR2 and ICOS (Fig. 5a). Because CY depleted Ki-67hi cells, these results predicted that CY-treatment would result in the selective loss of maximally suppressive TNFR2hi regulatory T cells. To test this, we analyzed TNFR2 expression on regulatory cells after CY treatment or in saline treated control mice, both in the lymph nodes and in the tumor. Indeed, CY treatment resulted in the selective loss of TNFR2hi regulatory T cells, principally because these are the Ki-67hi cycling cells (Fig. 5b). This was observed both in the tumor-draining lymph nodes and in the tumor itself (Fig. 5b). Note that CY also reduced the frequency of TNFR2+ Ki-67lo regulatory T cells, which may be explained by the fact that Ki-67 expression is transient [27]. Thus, depletion of activated Ki67hi cells also reduces the pool of activated regulatory T cells that have recently exited cell cycle. In summary, CY specifically depletes a population of effector suppressor T cells that express Ki-67, ICOS and TNFR2.
Fig. 4.
Regulatory T cell phenotypes. Expression of TNFR2, ICOS and CD62L on Ki-67lo and Ki-67hi foxp3+ CD4+ T cells. a Regulatory T cells from tumor-draining lymph nodes were identified by foxp3 staining (left panel) and analyzed for Ki-67 expression in foxp3+ CD4+ gated cells (right panel). b Expression patterns of TNFR2, ICOS and CD62L are shown in Ki-67lo (left panels) and Ki-67hi (right panels) foxp3+ CD4+ T cells
Fig. 5.
a Single pool of ICOS+ TNFR2+ foxp3+ regulatory T cells. Lymphocytes were harvested from tumor-draining lymph nodes and tumors in untreated BALB/c mice and stained for CD4, foxp3, ICOS and TNFR2 (CD120B). Cells shown in the right panels are gated on CD4+ foxp3+ cells. b Impact of CY on TNFR2 expression. Gated CD25+ CD4+ T cells from tumor-draining lymph nodes (upper panels) and from the tumor (lower panels) were analyzed for expression of TNFR2 and Ki-67. TNFR2+ cells are predominantly Ki-67hi and are depleted after CY treatment
Discussion
The data presented herein show that the success of chemotherapy can hinge on the status of CD25+ CD4+ regulatory T cells. Thus, drugs with equivalent cytotoxicity can have profoundly different outcomes. We show that cyclophosphamide cures mice because it depletes a defined population of effector suppressor T cells and that gemcitabine-induced tumor regression can be converted into a curative response by simultaneous depletion of these cells. The observation that the efficacy of gemcitabine can be improved by depleting regulatory T cells is novel and builds on our previous work showing a strong synergy between GEM and immunotherapy (anti-CD40 antibodies) [33]. Thus, gemcitabine, and potentially other chemotherapies, create immune priming conditions that are counteracted by active tumor-driven immune suppression. When interpreted in this light, our data indicate that cyclophosphamide is effective because it acts as a chemotherapeutic drug (it kills tumor cells) and because it depletes a subpopulation of regulatory T cells. CD25-depletion as a single therapy was completely ineffective in our model, indicating that the cytotoxic aspect of CY (e.g., tumor debulking, increased apoptosis, tumor cell sensitization) was essential. This view of cyclophosphamide differs from the current views expressed in the literature, in which the Treg-depleting capacities of the drug have received most attention [10, 14, 43]. For example, Ercolini et al showed that the therapeutic benefit of cyclophosphamide was entirely attributable to regulatory T cell depletion since identical results were obtained with CD25+ T cell depletion [10].
A second novel observation made in the present study is that CY depletes all Ki-67hi T cells. Importantly, this includes both foxp3pos and foxp3neg CD4+ T cells. Therefore, our data challenge the view that CY is effective because it specifically depletes regulatory T cells per se [3, 10, 14, 24, 43]. Although we observed a modest decrease in the frequencies of Tregs after CY treatment, the impact on the Ki-67hi subset was much more dramatic. Thus, we postulate that CY abrogates functional suppression because it depletes those regulatory T cells that are maximally suppressive, i.e., in this case the cycling cells. Note that Jaffee and coworkers also showed that CY targets cycling regulatory T cells [10]. However, these authors did not extend this conclusion to other non-Treg T cells. Because CY also depletes cycling foxp3neg effector CD4+ T cells, our current data predict that the net outcome of CY-immunomodulation depends on the balance between immune-suppressive regulatory T cell responses and non-Treg effector responses. Taken together, we think this helps to explain the CY paradox: CY activates anti-tumor T cell responses because the tumor-induced immune response (before therapy) is dominated by cycling regulatory T cells, which are then depleted by CY. In contrast, CY can have a net immune-suppressive role in autoimmune models because it can deplete cycling non-Treg effector cells in this setting [16]. Depletion of all Ki-67hi T cells seems paradoxical as CY-induced cures also depend on T cells. A possible solution is provided by our recent observation that such curative responses are TRAIL-dependent, suggesting that tumor cell sensitization rather then effector cell expansion is important (van der Most, manuscript in preparation). We conclude that proliferation is an essential property of the maximally suppressive regulatory T cell subset but is not an essential property of effector CD8 T cells, consistent with previously reported data [30].
An important question is how CY depletes proliferating Ki-67hi T cells. A comparison between CY and GEM suggests that depletion of Ki-67hi T cells is not simply a function of drug cytotoxicity. Both drugs target proliferating cells and should result in a loss of Ki-67hi T cells. The most likely explanation for the difference between CY and GEM is that the kinetics of T cell depletion are different: whereas CY triggers a long-lasting cell cycle arrest, T cell proliferation recovers faster after GEM treatment. It has been reported that a CY-induced T cell cycle arrest is causally linked to an expansion of NO producing CD11b+ Gr1+ myeloid-derived suppressor cells (MDSC) [2, 34]. MDSC and NO have a demonstrated capacity to blunt T cell proliferation [2, 28, 46, 47]. Indeed, we have preliminary data showing an upregulation of iNOS expression in the tumor after CY treatment (Van der Most, manuscript in preparation). In this view, the lack of a long-term effect of GEM on cycling T cells is not surprising since it has been reported that GEM specifically depletes Gr1+ CD11b+ MDSC [42].
It is becoming clear that phenotypically different populations of regulatory T cells constitute functionally distinct compartments. For example, expression of ICOS [41] and CD101 [12] have been linked to enhanced suppressive function. Very recently, TNFR2 (CD120B) has been identified as a marker for a subset of maximally suppressive regulatory T cells [6]. We now report that the TNFR2 and ICOS expression patterns are linked to Ki-67 expression in foxp3pos cells, i.e., to cellular proliferation. We propose that a subset of cycling TNFR2hi ICOShi regulatory T cells constitute the effector suppressor population, based on the reported ‘maximally suppressive’ phenotype of TNFR2hi regulatory T cells [6]. We found that tumor-infiltrating regulatory T cells have this phenotype, consistent with recent findings that human melanoma-infiltrating CD4+ CD25+ foxp3+ T cells expressed high levels of ICOS and were functionally different from ICOSlo regulatory T cells in the periphery [41]. The identification of a single subpopulation of Ki-67+ ICOS+ TNFR2+ regulatory T cells is novel and adds to the rapidly expanding field of regulatory T cell phenotypic characterization [6, 19, 20, 41]. Our combined data challenge the notion that regulatory T cells are ‘anergic’ and suggest instead that the cycling Ki-67hi cells are the active suppressors that respond to antigen by increasing their suppressive effector functions.
Although several questions remain to be answered, our findings provide excellent opportunities for translation since it may be relatively straightforward to combine current chemotherapy regimens with regulatory T cell depletion. GEM, usually in combination with platinum compounds is used as a second-line treatment for mesothelioma [31], whereas CY has limited efficacy [1]. However, a translatable possibility is to use low-dose CY as a tool to deplete regulatory T cells in GEM/CY combination treatment, as it was recently shown that CY also affects regulatory T cell function in humans [15].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material (PPT 2524 kb)
Acknowledgments
This work was supported in part by the National Health and Medical Research Council of Australia.
References
- 1.Andersen MK, Krarup-Hansen A, Martensson G, Winther-Nielsen H, Thylen A, Damgaard K, Olling S, Wallin J. Ifosfamide in malignant mesothelioma: a phase II study. Lung Cancer. 1999;24:39–43. doi: 10.1016/S0169-5002(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 2.Angulo I, Gomez de las Heras F, Garcia-Bustos JF, Gargallo D, Munoz-Fernandez MA, Fresno M. Nitric oxide-producing CD11b+Ly-6G(Gr-1)+CD31(ER-MP12)+ cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95:212–220. [PubMed] [Google Scholar]
- 3.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- 4.Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 5.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie AJ, van der Most RG, Broomfield SA, Prosser AC, Tovey MG, Robinson BW. Targeting the effector site with IFN-alphabeta-inducing TLR ligands reactivates tumor-resident CD8 T cell responses to eradicate established solid tumors. J Immunol. 2008;180:1535–1544. doi: 10.4049/jimmunol.180.3.1535. [DOI] [PubMed] [Google Scholar]
- 8.den Boer AT, van Mierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE. CD4+ T cells are able to promote tumor growth through inhibition of tumor-specific CD8+ T-cell responses in tumor-bearing hosts. Cancer Res. 2005;65:6984–6989. doi: 10.1158/0008-5472.CAN-04-3344. [DOI] [PubMed] [Google Scholar]
- 9.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 10.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez I, Zeiser R, Karsunky H, Kambham N, Beilhack A, Soderstrom K, Negrin RS, Engleman E. CD101 surface expression discriminates potency among murine FoxP3+ regulatory T cells. J Immunol. 2007;179:2808–2814. doi: 10.4049/jimmunol.179.5.2808. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki–67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 14.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonsette RE. Compared benefit of approved and experimental immunosuppressive therapeutic approaches in multiple sclerosis. Expert Opin Pharmacother. 2007;8:1103–1116. doi: 10.1517/14656566.8.8.1103. [DOI] [PubMed] [Google Scholar]
- 17.Haynes NM, van der Most RG, Lake RA, Smyth MJ (2008) Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol [DOI] [PubMed]
- 18.Hegmans JP, Hemmes A, Hammad H, Boon L, Hoogsteden HC, Lambrecht BN. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur Respir J. 2006;27:1086–1095. doi: 10.1183/09031936.06.00135305. [DOI] [PubMed] [Google Scholar]
- 19.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 21.Kohyama M, Sugahara D, Sugiyama S, Yagita H, Okumura K, Hozumi N. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc Natl Acad Sci USA. 2004;101:4192–4197. doi: 10.1073/pnas.0400214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 23.Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 25.Manning LS, Whitaker D, Murch AR, Garlepp MJ, Davis MR, Musk AW, Robinson BW. Establishment and characterization of five human malignant mesothelioma cell lines derived from pleural effusions. Int J Cancer. 1991;47:285–290. doi: 10.1002/ijc.2910470219. [DOI] [PubMed] [Google Scholar]
- 26.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59:1071–1079. [PubMed] [Google Scholar]
- 27.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 29.Needham DJ, Lee JX, Beilharz MW. Intra-tumoural regulatory T cells: a potential new target in cancer immunotherapy. Biochem Biophys Res Commun. 2006;343:684–691. doi: 10.1016/j.bbrc.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 30.North RJ, Awwad M. Elimination of cycling CD4+ suppressor T cells with an anti-mitotic drug releases non-cycling CD8+ T cells to cause regression of an advanced lymphoma. Immunology. 1990;71:90–95. [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak AK, Byrne MJ, Millward MJ, Alvarez JM, Robinson BW. Current chemotherapeutic treatment of malignant pleural mesothelioma. Expert Opin Pharmacother. 2004;5:2441–2449. doi: 10.1517/14656566.5.12.2441. [DOI] [PubMed] [Google Scholar]
- 32.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 33.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 34.Pelaez B, Campillo JA, Lopez-Asenjo JA, Subiza JL. Cyclophosphamide induces the development of early myeloid cells suppressing tumor cell growth by a nitric oxide-dependent mechanism. J Immunol. 2001;166:6608–6615. doi: 10.4049/jimmunol.166.11.6608. [DOI] [PubMed] [Google Scholar]
- 35.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 38.Rudge G, Barrett SP, Scott B, van Driel IR. Infiltration of a mesothelioma by IFN-gamma-producing cells and tumor rejection after depletion of regulatory T cells. J Immunol. 2007;178:4089–4096. doi: 10.4049/jimmunol.178.7.4089. [DOI] [PubMed] [Google Scholar]
- 39.Simon AK, Jones E, Richards H, Wright K, Betts G, Godkin A, Screaton G, Gallimore A. Regulatory T cells inhibit Fas ligand-induced innate and adaptive tumour immunity. Eur J Immunol. 2007;37:758–767. doi: 10.1002/eji.200636593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci USA. 2005;102:17418–17423. doi: 10.1073/pnas.0507454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on Human Melanoma-Infiltrating CD4+CD25highFoxp3+ T Regulatory Cells: Implications and Impact on Tumor-Mediated Immune Suppression. J Immunol. 2008;180:2967–2980. doi: 10.4049/jimmunol.180.5.2967. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 43.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jese G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 44.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G (2008) Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol [DOI] [PubMed]
- 46.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 47.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 48.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material (PPT 2524 kb)