Abstract
T cell targeting immunotherapy is now considered in acute myelogenous leukemia (AML), and local recruitment of antileukemic T cells to the AML microcompartment will then be essential. This process is probably influenced by both intravascular as well as extravascular levels of T cell chemotactic chemokines. We observed that native human AML cells usually showed constitutive secretion of the chemotactic chemokines CXCL10 and CCL5, whereas CCL17 was only released for a subset of patients and at relatively low levels. Coculture of AML cells with nonleukemic stromal cells (i.e., fibroblasts, osteoblasts) increased CXCL10 and CCL17 levels whereas CCL5 levels were not altered. However, a wide variation between patients in both CXCL10 and CCL5 levels persisted even in the presence of the stromal cells. Neutralization of CXCL10 and CCL5 inhibited T cell migration in the presence of native human AML cells. Furthermore, serum CCL17 and CXCL10 levels varied between AML patients and were determined by disease status (both chemokines) as well as patient age, chemotherapy and complicating infections (only CCL17). Thus, extravascular as well as intravascular levels of T cell chemotactic chemokines show a considerable variation between patients that may be important for T cell recruitment and the effects of antileukemic T cell reactivity in local AML compartments.
Keywords: Acute myelogenous leukemia, CXCL10, CCL5, CCL17
Introduction
Acute myelogenous leukemia (AML) is an aggressive disorder characterized by accumulation of immature malignant cells in the bone marrow [1]. The overall long-term disease-free survival after optimal chemotherapy is less than 50% [1]. New therapeutic strategies are therefore considered, including T cell targeting immunotherapy [2–5]. Leukemia-reactive T cells may then recognize differentiation-associated epitopes or specific peptides encoded by AML-associated genetic abnormalities [2, 4]. However, immunotherapy seems most effective in patients with a low AML cell burden [4], an observation consistent with the hypothesis that effective antileukemic reactivity requires local T cell recruitment and a high in vivo ratio of effector T cells to target AML cells.
Systemic serum levels together with extravascular release in the AML microenvironment of T cell chemotactic chemokines will determine the functional chemotactic gradients for circulating AML-reactive cells [5–7]: (i) local chemokine release creates tissue gradients and extravascular chemokines are in addition presented as binding molecules on the luminal surface of the endothelial cells; (ii) serum and endothelial-bound chemokines compete for binding sites on circulating cells. The chemokines CCL5 (RANTES) [6, 7], CCL17 (TARC) [8] and CXCL10 (IP-10) [9, 10] can all increase T cell chemotaxis and may thus be important for the local recruitment of AML-reactive T cells. In the present study we therefore investigated their local release, by native human AML cells (including modulation by various bone marrow stromal cells), and chemokine serum levels before therapy and following intensive chemotherapy when patients have a low AML cell burden but still a remaining system of circulating and functional T cells [11].
Materials and methods
Acute leukemia patients
The studies were approved by the local Ethics Committee and cells collected after informed consent.
AML cell donors
The study included 74 consecutive patients (72 Caucasians, 29 females and 45 males; median age 63 years, range 29–84 years) with high peripheral blood blast counts [12]. Forty-nine patients had de novo AML; the remaining minority had AML relapse (three patients), chronic myeloid leukemia in blast phase (two patients) and AML secondary to chemotherapy or primary myelodysplasia (4 and 16 patients, respectively). The patients showed the following FAB classification: M0 7 patients, M1 18 patients, M2 22 patients, M3 1 patient, M4 14 patients, M5 11 patients and M6 1 patient. Cytogenetic analysis was performed for 54 patients and the abnormalities classified as described by Wheatley et al. [13]: 28 patients had normal chromosomes whereas two patients had low-risk, nine patients high-risk and 16 patients intermediate-risk abnormalities. Sixty-six patients were tested for genetic Flt3 abnormalities [12]; 23 patients had internal tandem duplications and eight patients D835 mutations. Thirty-five patients received induction therapy; all these patients received cytarabine 200 mg/m2 days 1–7 together with anthracycline (daunorubicin 45 mg/m2 or idarubicin 12 mg/m2 days 1–3) and complete hematological remission after one induction cycle was then achieved for 18 patients.
Acute lymphoblastic leukemia (ALL) cell donors
We investigated eight consecutive patients (four males and four females, median age 26 years, range 21–82 years). One patient had T-ALL whereas the others had B cell disease: pro-B two patients, common-B three patients and mature-B two patients [14].
Serum samples collected from untreated AML patients
The samples were collected from two groups of consecutive patients: (i) 20 Russian patients younger than 50 years of age; and (ii) 38 consecutive Norwegian patients, 14 younger and 24 older than 50 years. These results were compared with Russian and Norwegian healthy controls. Control samples were collected at the same time as the corresponding patient samples and matched for sex and age (age difference ≤5 years between the patient and the corresponding control, all Russian controls being <50 years of age).
Serum samples collected during therapy
We included 21 consecutive acute leukemia patients (18 AML and three ALL patients, median age 40 years, range 15–69 years) who received intensive induction (16 chemotherapy cycles) or consolidation chemotherapy (18 cycles). These patients were compared with a group of 22 healthy individuals (median age 38 years, range 21–64 years). All patients received cytarabine-based chemotherapy: (i) cytarabine 200 mg/m2 days 1–5 + daunorubicin 45 mg/m2 days 1–3 (9 induction cycles in 9 different AML patients); (ii) cytarabine 200 mg/m2 days 1–5 + etoposide 110 mg/m2 days 1–5 + amsakrine 150 mg/m2 days 1–5 (three induction cycles in three AML patients); (iii) cytarabine 1 g/m2 days 1–5 + mitoxanthrone 12 mg/m2 days 1–3 (two induction cycles in one ALL and one AML patient); (iv) cytarabine 3 g/m2 days 1–4 + etoposide 100 mg/m2 days 1–5 (two induction cycles in two ALL patients); (v) cytarabine 3 g/m2 days 1, 3 and 5 (one consolidation cycle in one AML patient); (vi) cytarabine 100 mg/m2 days 1–5 + amsakrine 150 mg/m2 days 1–3 + etoposide 110 mg/m2 days 1–3 (11 consolidation cycles in eight different AML patients); (vii) cytarabine 200 mg/m2 days 1–5 + mitoxanthrone 12 mg/m2 days 1–3 (five consolidation cycles in five AML patients); (viii) cytarabine 200 mg/m2 days 1–7 + daunorubicin 50 mg/m2 days 1–3 + thioguanine 150 mg/m2 days 1–6 (one consolidation cycle in one ALL patient).
Serum samples were collected three times weekly from the beginning of chemotherapy and during the period of treatment-induced cytopenia, i.e., peripheral blood leukocyte counts <0.5×109/l and dependency on platelet transfusions to maintain peripheral blood platelet counts >10–20×109/l. Patients reached cytopenia within 9 days after the beginning of chemotherapy. Although, we investigated samples derived at four different time intervals (before therapy and after 1, 8–10 and 13–15 days of cytopenia) during 34 chemotherapy cycles, at each time point less than 34 samples were available due to either: (i) death during cytopenia; (ii) early reconstitution; (iii) new induction therapy due to regrowth of leukemia; or (iv) technical reasons. Eight patients with untreated leukemia were cytopenic at the beginning of therapy, and for these patients we analysed serum samples collected 13–15 and 18–20 days after the start of chemotherapy (corresponding to the day 8–10 and 13–15 samples, respectively). Residual leukemia was not detected for any patient by light microscopy of bone marrow samples collected 14 days after induction therapy or prior to consolidation treatment.
Preparation of leukemia blasts
Leukemic peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation (Ficoll-Hypaque; NyCoMed, Oslo, Norway; specific density 1.077) and stored in liquid nitrogen [12, 15, 16].
Nonleukemic cells
Human osteosarcoma and fibroblast cell lines
The osteosarcoma cell lines Cal72 (Deutsche Sammlung von Zellkulturen und Mikroorganismen; Braunschweig, Germany) and SJSA1 (American Type Culture Collection, ATCC, Vanassas, VA; ATCC no. CRL2098) have a phenotype close to normal osteoblasts [15, 17, 18]. These cells and the HFL1 fibroblast line (ATCC no. CCL-153) can support leukemic hematopoiesis [15, 17–19].
Normal human cells
Normal osteoblasts, microvascular endothelial cells (>95% purity) and bone marrow stromal cells were delivered in frozen vials (Clonetics, BioWhittacker; Walkersville, MA, USA) and stored in liquid nitrogen. The osteoblasts and endothelial cells were highly enriched populations whereas the stromal cells were a heterogeneous population of fibroblasts, reticulum cells, endothelial cells, macrophages and fat cells [14]. Normal PBMC were prepared by gradient separation [20] and thrombocytes were sampled from concentrates prepared for clinical use.
In vitro culture of native human AML blasts
Reagents
The Stem Span SFEMTM medium (referred to as StemSpanTM; Stem Cell Technologies; Vancouver, BC, Canada) supplemented with 10% heat-inactivated fetal calf serum (FCS; BioWhitacker) and 100 μg/ml of gentamicin was used in all coculture experiments except for cultures including normal osteoblasts and bone marrow stromal cells that were prepared in the Osteoblast Growth Medium with FCS and gentamicin (Clonetics). Recombinant human chemokines (CXCL10, CCL5, CCL17; Peprotech, Rocky Hill, NJ, USA) were used at 20 ng/ml. Recombinant human (rh) IL1 receptor antagonist (IL1RA, R&D Systems, Abingdon, UK) was used at 50 ng/ml. Neutralizing mouse IgG1 antibodies specific for human cytokines (R&D Systems) were used at 10 μg/ml; at this concentration anti-granulocyte-macrophage colony-stimulating factor (GM-CSF, antibody 3209.01), anti-CCL5 (antibody 21445) and anti-CXCL10 (antibody 33036) neutralized >95% of the biological activity of rhGM-CSF 0.5 ng/ml, rhCCL5 10 ng/ml and rhCXCL10 300 ng/ml, respectively (manufacturer’s information). Control cultures were prepared with normal mouse IgG1 (R&D Systems).
Suspension cultures of AML blasts alone
AML blasts (106 cells/ml) were cultured alone in 24-well plates (see below) for 2 days before supernatants were harvested and chemokine levels determined. Proliferation in suspension cultures was assayed by 3H-thymidine incorporation after 7 days of culture as described previously [12].
Coculture of leukemia blasts separated from nonleukemic cells by a semipermeable membrane
As described in detail previously [15, 19, 21], cultures were prepared in transwell culture plates (Transwell 3401; Costar, Cambridge, MA, USA) where nonleukemic cells in the lower large compartment were separated from the leukemia cells in the upper smaller chamber by a semipermeable membrane with a pore diameter of 0.4 μm. Nonleukemic cells (104 cells in 1 ml) were incubated for 3 days before leukemia cells (106 cells in 0.5 ml) were added. Cocultures were thereafter incubated for an additional 7 days before supernatants were harvested.
Coculture of leukemia cells in direct contact with HFL1 fibroblasts
These cultures were prepared in 24 well culture plates (Costar 3524) [12, 18]. HFL1 fibroblasts (104 cells in 1 ml) were incubated for 3 days before leukemia blasts (106 cells in 0.5 ml) were added; cultures were thereafter incubated for an additional 7 days before supernatants were harvested [15, 21].
Analysis of chemokine protein levels in culture supernatants
Chemokine levels were determined by ELISA analyses (Quantikine ELISA kits; R&D Systems) that were performed strictly according to the manufacturer’s instructions [12]. Minimum detectable levels were CCL5 <2.0 pg/ml, CCL17 <7 pg/ml and CXCL10 <1.7 pg/ml.
Analysis of chemokine mRNA levels
CXCL10- and CCL5-specific mRNA levels were quantitated by a colorimetric assay (R&D Systems). Cell lysates were prepared and samples analysed strictly according to the manufacturers recommendations. The lysate corresponded to 4×106 cells/ml and the results are presented as the mRNA concentration (amol/ml). Alternatively, relative mRNA levels were analysed by microarray techniques as described in detail previously [22, 23].
Chemotaxis assay
Native human AML blasts (1×106 cells) were incubated in the lower chamber and normal PBMC (2×106 cells) in the upper chamber of transwell plates with a pore size of 3 μm (Costar 3504; 1.5 ml Stem SpanTM medium per well). After 18 h of incubation the cells in the lower chamber were counted and the number of CD3+ (monoclonal antibody 345765), CD4+ (antibody 348809) and CD8+ (antibody 345772) lymphocytes determined by flow cytometry (all antibodies were conjugated monoclonal mouse IgG1antibodies supplied from Becton-Dickinson, San Jose, CA, USA) [5].
In vitro culture of nonleukemic cells
Cultures were prepared in 24 well culture plates (Costar 3524). Microvascular endothelial cells (cultured in EBM medium supplemented with EGM-2MV single quots; Clonetics), osteoblastic sarcoma cell lines (Cal72, SJSA-1), fibroblast cell lines (HFL1, Hs27), normal osteoblasts and bone marrow stromal cells (all cultured in Stem SpanTM medium) were seeded at 104 cells in 1.5 ml medium per well and supernatants harvested after 7 days when the cells had reached 0.4–1.0×106/well [21]. Normal PBMC were seeded at 106 cells/ml (X-vivo 10 medium, BioWhitacker) and incubated for 2 days in the medium or together with lipopolysaccharide (LPS) or streptococcal lipoteichoic acid (LTA) before supernatants were harvested [20]. Platelets were stored for 2 days before chemokine levels were determined.
Presentation of the data
Cytokine levels in coculture experiments were transformed to logarithmic values before statistical comparisons. The Wilcoxon’s test for paired samples and the Mann–Whitney’s test for independent samples were used for statistical comparisons, whereas the Kendall’s test was used for correlation analysis. Differences were regarded as statistically significant when P<0.05. P-values were corrected for the number of comparisons.
Results
Native human AML blasts show constitutive release of CXCL10, CCL5 and CCL17
AML blasts derived from 74 consecutive patients were cultured in serum-free Stem SpanTM medium for 48 h before chemokine levels in the supernatants were determined. The constitutive release showed a wide variation for all three chemokines (Table 1, upper part). CXCL10 and CCL5 release was detected for most patients, whereas CCL17 release was observed only for a minor subset. Most patients released at least two chemokines, and CXCL10 levels were usually higher than CCL5 and CCL17 levels. CXCL10 and CCL5 levels were significantly correlated (Kendall’s test, P<0.0005), whereas CCL17 levels showed no correlation with CXCL10/CCL5. Furthermore, no differences were observed in CXCL10/CCL5/CCL17 levels when comparing (i) different age groups or de novo versus secondary AML; (ii) patients with morphological signs of monocytic (AML FAB-type M4/M5; n=25) and granulocytic differentiation (AML-M2/M3; n=23) and patients without morphological differentiation (AML-M0/M1; n=25); (iii) patients with high (>50% positive cells; 29 patients), intermediate (20–50%; 22 patients) and low (<20%; 23 patients) expression of the stem cell marker CD34; (iv) patients with normal and abnormal karyotype; (v) patients with and without genetic Flt3 abnormalities; and (vi) patients with and without remission after the first induction chemotherapy cycle. Furthermore, serum levels and in vitro release of CXCL10 and CCL17 could be compared for 28 patients (see below), but no significant correlations were observed (data not shown).
Table 1.
Release of CXCL10, CCL5 and CCL17 protein by native human AML blasts: (i) constitutive release of CXCL10, CCL5 and CCL17 by AML cells cultured alone and (ii) levels of CXCL10 and CCL17 when AML cells were cocultured with nonleukemic bone marrow stromal cells in transwell cultures
| Experimental model | Nonleukemic cells chemokine | In transwell cultures | Detectable chemokine levels (pg/ml) | |||
|---|---|---|---|---|---|---|
| Patient fraction | Median | Variation range | P-value | |||
| Constitutive release for AML cells cultured alone | CXCL10 (n=74) | – | 63/74 | 636 | 5.9–17,400 | |
| CCL5 (n=74) | 72/74 | 131.3 | 2.4–2288 | |||
| CCL17 (n=74) | 32/74 | 135.1 | 16.7–3707 | |||
| AML cells cocultured with nonleukemic cells | ||||||
| Fibroblasts (HFL1) | CXCL10 (n=25) | None | 20/25 | 478 | 12.4–5,909 | |
| ↑HFL1 fibroblasts | 22/25 | 840 | 2.7–5909 | <0.001 | ||
| CCL17 (n=25) | None | 21/25 | 292 | 10.0–3250 | ||
| ↑HFL1 fibroblasts | 22/25 | 910 | 10.5–3980 | <0.001 | ||
| Osteoblasts (Cal72, SJSA1) | CXCL10 (n=38) | None | 32/38 | 1850 | 10.8–2840 | |
| ↑Cal72 osteoblasts | 34/38 | 2300 | 2.9–2880 | 0.0052 | ||
| ↑SJSA-1 osteoblasts | 36/38 | 2340 | 3.2–3355 | <0.002 | ||
| CCL17 (n=15) | None | 10/15 | 279 | 16.5–2,900 | ||
| ↑Cal72 osteoblasts | 11/15 | 310 | 48–2,860 | 0.042 | ||
| ↑SJSA-1 osteoblasts | 11/15 | 345 | 9.8–2,900 | 0.042 | ||
| Normal stromal cells | CXCL10 (n=15) | None | 4/15 | 34.5 | 4.5–350 | |
| ↑Normal bone marrow stromal cells | 6/15 | 41.4 | 5.1–375 | 0.016 | ||
| ↑Normal osteoblasts | 6/15 | 39.5 | 6.3–350 | 0.016 | ||
AML cells were cultured alone in 24 well tissue culture plates (1×106 cells/ml and 2 days of culture, the constitutive release results) or in coculture with various nonleukemic stromal cells (1×106 AML cells/1.5 ml, 7 days of culture). The cocultures were prepared in transwell plates and chemokine levels determined after 7 days. Patient numbers are given in parenthesis in the chemokine column. The results are presented as (i) the fraction of patients with detectable chemokine levels; and (ii) the median and range for those patients with detectable levels. For AML cultures the concentrations in the supernatants are given, for cocultures the incremental concentrations are given (i.e., the level in cocultures minus the levels for nonleukemic cells cultured alone). The Wilcoxon’s test for paired samples was used for statistical analysis, and patients without detectable levels were not included in the analyses
The effect of exogenous CXCL10 and CCL5 on the cytokine-dependent (Flt3L+GM-CSF+SCF) proliferation of AML cells was examined for 66 consecutive patients (3H-thymidine incorporation). Altered proliferation was observed only for a minority of patients (increased proliferation for 10, decreased for 11 patients) and showed no correlation with CXCL10/CCL5 levels. CCL17 did not affect proliferation for any out of the 17 investigated patients (data not shown).
When investigating AML cells derived from 40 consecutive patients detectable mRNA levels were observed for most patients both for CXCL10 (undetectable levels 13/40, median 14 amol/ml, range <9–37 amol/ml) and CCL5 (undetectable 8/40; median 5.4 amol/ml, range <3.5–26 amol/ml) when using the colorimetric method. A wide variation in mRNA levels was also observed when investigating 16 randomly selected patients in microarray studies (data not shown). CCL5 and CXCL10 mRNA levels showed no significant correlation with any method, and mRNA and protein levels were not correlated either (data not shown).
Chemokine release by nonleukemic cells
We investigated CXCL10 and CCL5 release by various nonleukemic cells. Firstly, in vitro cultured normal bone marrow stromal cells (three donors), osteoblasts (two donors) and microvascular endothelial cells (dermal and lung cells from two different donors) showed undetectable CXCL10 (<1.7 pg/ml) and CCL5 levels (<2 pg/ml). Secondly, HFL1 and Hs27 fibroblasts both showed low CCL5 release (<15 pg/ml, three experiments) and only Hs27 showed detectable CXCL10 release (110–126 pg/ml). The osteoblastic sarcoma lines Cal72 and SJSA-1 did not release detectable CXCL10 or CCL5. Thirdly, normal PBMC showed spontaneous CCL5 release (seven individuals tested, median level 1,100 pg/ml, range 410–2,150 pg/ml) that was increased by LTA (median 6,750 pg/ml, range 1,250–10,200 pg/ml) and LPS (median 6,800 pg/ml, range 3,750–10,340 pg/ml), whereas spontaneous and LTA/LPS stimulated CXCL10 release corresponded to <200 pg/ml. Finally, platelet CCL5 release during ex vivo storage was <230 pg/106 platelets (five donors tested).
CCL17 and CXCL10 protein levels, but not CCL5 levels, are increased during coculture of native human AML cells with fibroblasts, osteoblasts and bone marrow stromal cells
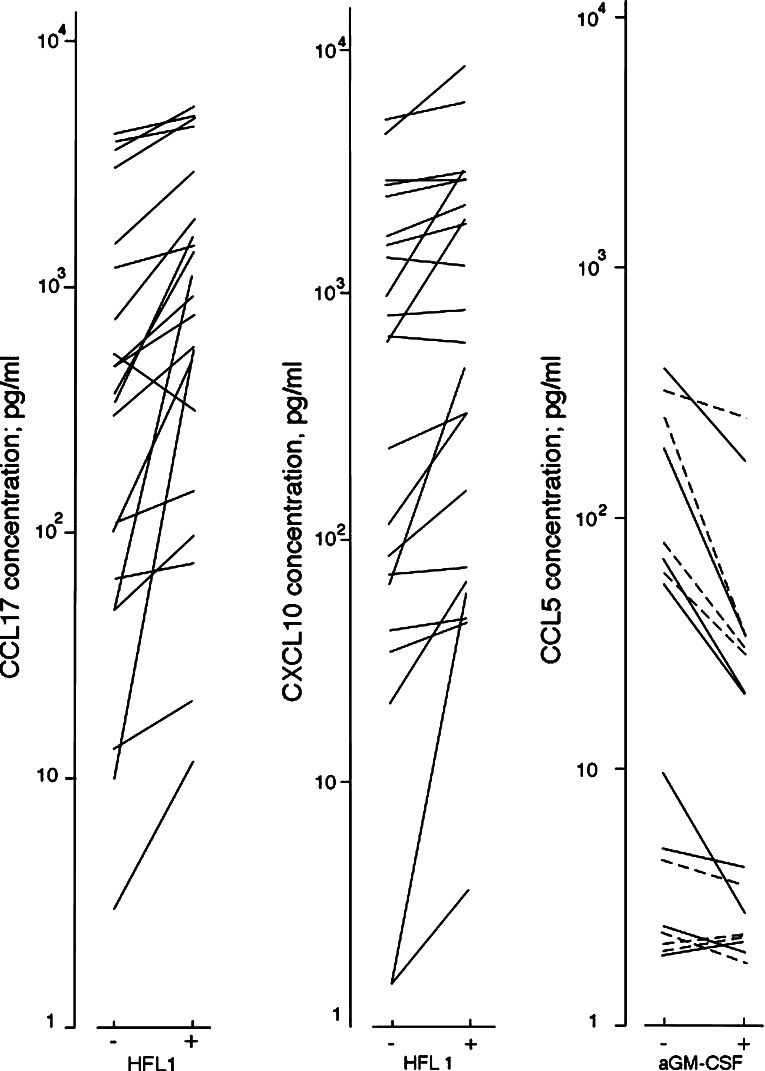
Chemokine levels were determined in transwell cocultures with fibroblasts and AML cells derived from 20 patients showing constitutive CCL17 release and five randomly selected patients (Fig. 1; Table 1 lower part). Increased CCL17 and CXCL10 levels were observed in cocultures compared with AML cell cultures, and increased CXCL10 levels were detected for all the five randomly selected patients. CCL5 levels were not altered during coculture (n=56). CXCL10, but not CCL5, levels were further increased by adding exogenous GM-CSF+SCF+Flt3L+IL3 (P=0.001).
Fig. 1.
Left part: CCL17 levels (pg/ml) in culture supernatants when native human AML blasts were cultured together in transwell cultures with HFL1 fibroblasts. Cells were cocultured for 7 days in transwell cultures before CCL17 levels were determined. The AML blasts derived from 25 patients were examined, and the figure shows the results for the 21 patients with either constitutive CCL17 secretion (20 patients) or detectable CCL17 reached during coculture (1 additional patient). CCL17 could not be detected in the supernatants for fibroblasts cultured alone (<7 pg/ml), and detectable levels were not observed during coculture for the last four AML patients. Middle: CXCL10 levels in culture supernatants during coculture in transwell cultures of native human AML blasts with HFL1 fibroblasts (25 patients examined). All results are presented as incremental concentrations (pg/ml) in the supernatants, i.e., concentrations for AML blast cultures or cocultures minus the levels in control cultures containing nonleukemic cells alone. The figure shows the results only for those patients that showed detectable CXCL10 levels either in AML cultures (i.e., constitutive CXCL10 release) or only in the cocultures with HFL1 fibroblasts (20 and 2 patients, respectively), whereas the results for patients with undetectable levels for both AML cells cultured alone and together with nonleukemic cells are not included in the figure. Right: The effect of cytokine inhibition/neutralization on CCL5 levels during coculture of native human AML blasts with nonleukemic cells. Transwell cultures were prepared without (−) and with (+) GM-CSF neutralizing antibodies. AML cells derived from 11 patients were cocultured with HFL1 fibroblasts (—) and Cal72 sarcoma cells (- - -) and CCL5 levels (pg/ml) determined in the supernatants after 7 days of coculture. The figure presents the results only for the eight patients with constitutive chemokine release or detectable chemokine levels in cocultures. All results are presented as incremental concentrations in the supernatants, i.e., concentrations for AML blast cultures or cocultures minus the levels in corresponding controls
AML blasts derived from 38 consecutive patients were cocultured with the osteoblastic sarcoma cells SJSA-1 and Cal72 in transwell cultures. Increased CXCL10 levels were observed in the cocultures (Table 1, lower part) whereas CCL5 levels were not altered. CCL17 levels were only examined for 15 consecutive patients; increased levels were then observed for cocultures with SJSA-1 and Cal72 osteoblasts (Table 1).
CXCL10 and CCL5 levels were determined when AML blasts derived from 15 consecutive patients were cultured in direct contact with the nonleukemic cells. CXCL10 levels were significantly increased in cocultures containing HFL1, SJSA-1 and Cal72 cells (P<0.0005 for all, data not shown), whereas CCL5 levels were not altered.
Chemokine levels were determined when AML blasts derived from 15 consecutive patients (Table 1, lower part) were cocultured with normal osteoblasts and bone marrow stromal cells in transwell cultures. Significantly increased CXCL10 levels were observed in the presence of both osteoblasts and stromal cells, whereas CCL5 levels were not significantly altered.
AML blasts derived from 17 consecutive patients were cultured alone and together with nonleukemic cells (HFL1, Cal72, SJSA-1 cells) in transwell cultures for 48 h before mRNA levels were determined. The levels of CXCL10 (median 5.4 amol/ml, range <2.2–9.6 amol) and CCL5 (median 5.1 amol, range <2.0–15.2) mRNA varied between patients for AML cells cultured alone and the levels were not altered in cocultures (data not shown).
GM-CSF neutralization decreases CCL5 but not CXCL10 protein levels during coculture of native human AML cells with HFL1 fibroblasts and Cal72 osteoblasts
CXCL10 and CCL5 levels were determined when AML blasts derived from 11 randomly selected patients were cultured in transwell cultures together with HFL1 and Cal72 cells. When comparing the overall results neither IL1RA nor anti-GM-CSF altered CXCL10 levels, whereas anti-GM-CSF decreased CCL5 levels in the presence of HFL1 fibroblasts (Fig. 1, Wilcoxon’s test, P=0.008) and Cal72 cells (P=0.02).
CXCL10 and CCL5 levels are not increased during coculture of ALL and osteoblastic cells
ALL blasts derived from eight consecutive patients were cocultured in transwell cultures with SJSA-1 and Cal72 sarcoma cells. ALL blasts showed undetectable CXCL10 and low CCL5 levels (<20 pg/ml) when cultured alone, and the levels were not altered in cocultures (data not shown).
CXCL10+CCL5 neutralization inhibits T cell chemotaxis in the presence of AML cells
AML cells derived from nine randomly selected patients were tested in the chemotaxis assay (Table 2). The overall results demonstrated that (i) migration of T cells towards the lower AML cell-containing chamber varied between patients; (ii) T cell migration was not a random process and a decreased CD4:CD8 ratio was observed for T cells detected in the lower chamber compared with normal PBMC with a ratio >1.5; (iii) combined neutralization of CXCL10 and CCL5 reduced transmembrane migration of CD3+ T cells corresponding to 9–34%, the only exception being patient 3 with AML-M6; and (iv) neutralization of CXCL10 or CCL5 alone did not inhibit migration (data not shown).
Table 2.
T cell chemotaxis in the presence of native human AML cells: effects of CCL5 and CXCL10 neutralization
| Patient | Constitutive AML cell release | Effect of CXCL10 + CCL5 neutralization | |||
|---|---|---|---|---|---|
| CXCL10 level | CCL5 level | Antibodies | Concentration of CD3+ lymphocytes | CD4:CD8 ratio | |
| 1. | nd | 5.0 | Controls | 24.2 | 0.73 |
| Anti-CXCL10 + anti-CCL5 | 21.2 (87%) | 0.68 | |||
| 2. | 157 | 23.4 | Controls | 11.9 | 0.58 |
| Anti-CXCL10 + anti-CCL5 | 7.8 (66%) | 0.63 | |||
| 3. | <1.7 | 18 | Controls | 8.7 | 0.61 |
| Anti-CXCL10 + anti-CCL5 | 10.5 (120%) | 1.05 | |||
| 4. | 174 | 11.1 | Controls | 11.0 | 0.82 |
| Anti-CXCL10 + anti-CCL5 | 10.5 (95%) | 0.77 | |||
| 5. | 24,906 | 947 | Controls | 4.6 | 0.58 |
| Anti-CXCL10 + anti-CCL5 | 3.7 (81%) | 0.63 | |||
| 6. | >15,000 | 1,530 | Controls | 15.0 | 0.98 |
| Anti-CXCL10 + anti-CCL5 | 13.0 (86%) | 0.99 | |||
| 7. | 313 | 74 | Controls | 12.0 | 1.61 |
| Anti-CXCL10 + anti-CCL5 | 9.7 (81%) | 1.46 | |||
| 8. | <1.7 | 124 | Controls | 5.1 | 0.25 |
| Anti-CXCL10 + anti-CCL5 | 5.0 (98%) | 0.26 | |||
| 9. | 13.6 | <2.0 | Controls | 8.4 | 1.00 |
| Anti-CXCL10 + anti-CCL5 | 5.8 (69%) | 0.56 | |||
AML cells (1×106 cells) were incubated in the lower chamber and normal PBMC (2×106) cells in the upper chamber in the chemotaxis assay. The absolute cell number and the percentages of CD3+, CD4+ and CD8+ cells were determined for the lower chamber after 18 hours. The cell numbers are presented as the number of cells x 10-4. CXCL10/CCL5 levels were determined after culture of native human AML blasts for 48 hours in serum-free Stem SpanTM medium (2×106 cells in 2 ml medium per well), these results are presented as pg/ml
CCL17 and CXCL10 serum levels are altered in patients with untreated AML
CCL17 serum levels were decreased for 20 consecutive Russian patients younger than 50 years compared with healthy controls (Table 3). We also investigated CCL17 serum levels for 38 consecutive Norwegian patients and the levels were significantly decreased both for the 14 younger and for the 24 patients older than 50 years of age (Table 3). Decreased platelet counts were observed for Russian (median 39×109/l, variation range 29–167), young Norwegian (median 48×109/l, range12–99) and elderly Norwegian patients (median 41×109/l, range 11–134), whereas the controls had normal platelet counts (140–400×109/l). CCL17 serum levels in patients showed no correlation with peripheral blood platelet counts (data not shown).
Table 3.
CCL17 and CXCL10 serum levels in AML patients: studies of patients with untreated AML and patients in complete hematological remission that were tested before consolidation therapy
| DISEASE STATUS | |||
|---|---|---|---|
| Chemokine | Patient group | Serum levela | P-value |
| UNTREATED AMLb | |||
| CCL17 | Russian patients <50 yrs of age | ↓41 pg/ml (7–2,500 pg/ml) | 0.02 |
| Healthy controls <50 years of age | 254 pg/ml (29–853 pg/ml) | ||
| Norwegian patients <50 years of age | ↓28 pg/ml (7–285 pg/ml) | <0.0005 | |
| Healthy controls <50 years of age | 332 pg/ml (75–707 pg/ml) | ||
| Norwegian patients >50 years of age | ↓24 pg/ml (7–185 pg/ml) | <0.0005 | |
| Healthy controls >50 years of age | 502 pg/ml (116–760 pg/ml) | ||
| CXCL10 | Russian patients <50 years of age | ↑162 pg/ml (36–500 pg/ml) | <0.0005 |
| Healthy controls <50 years of age | 61 pg/ml (24–500 pg/ml) | ||
| Norwegian patients <50 years of age | ↑123 pg/ml (47–820 pg/ml) | 0.035 | |
| Healthy controls <50 years of age | 74 pg/ml (48–142 pg/ml) | ||
| Norwegian patients >50 years of age | 123 pg/ml (37–830 pg/ml) | n.s. | |
| Healthy controls >50 years of age | 123 pg/ml (32–227 pg/ml) | ||
| AML IN REMISSIONc | |||
| CCL17 | Patients | ↑966 pg/ml (61–3203 pg/ml) | 0.003 |
| Healthy controls | 365 pg/ml (37–1734 pg/ml) | ||
| CXCL10 | Patients | ↑286 pg/ml (43–1243 pg/ml) | <0.0005 |
| Healthy controls | 91 pg/ml (44–320 pg/ml) | ||
aSerum levels were determined by the ELISA analysis and the Mann–Whitney test used for statistical comparisons. The concentrations are presented as the median concentrations and the variation ranges are given in parenthesis
bWe examined two groups of consecutive patients with untreated AML and their corresponding healthy controls. Twenty Russian and 38 Norwegian patients were examined together with the same number of Russians and Norwegian healthy controls matched for age and sex
cWe investigated 14 Norwegian patients in complete hematological remission (17 samples). Samples were collected immediately before consolidation chemotherapy, and four patients were examined before two different chemotherapy cycles. The controls were 17 healthy individuals matched for age and sex
CXCL10 serum levels were significantly increased for the young Russian (Table 3; P<0.0005) and Norwegian patients (P=0.035), whereas CXCL10 levels were not altered for the elderly Norwegian patients.
CCL17 but not CXCL10 serum levels are decreased following intensive chemotherapy
Serum samples were obtained from a consecutive group of acute leukemia patients who received intensive chemotherapy for AML (30 cycles) or ALL (4 cycles). All therapy cycles included cytarabine, and residual bone marrow disease was not detected for any patient by light microscopy prior to consolidation therapy or 14 days after the start of induction treatment. Fourteen AML patients were in hematological remission with normal platelet counts and received consolidation therapy (17 cycles), and the pretherapy CXCL10 (Table 3, P<0.0005) and CCL17 serum levels (P=0.003) were significantly increased compared with the healthy controls.
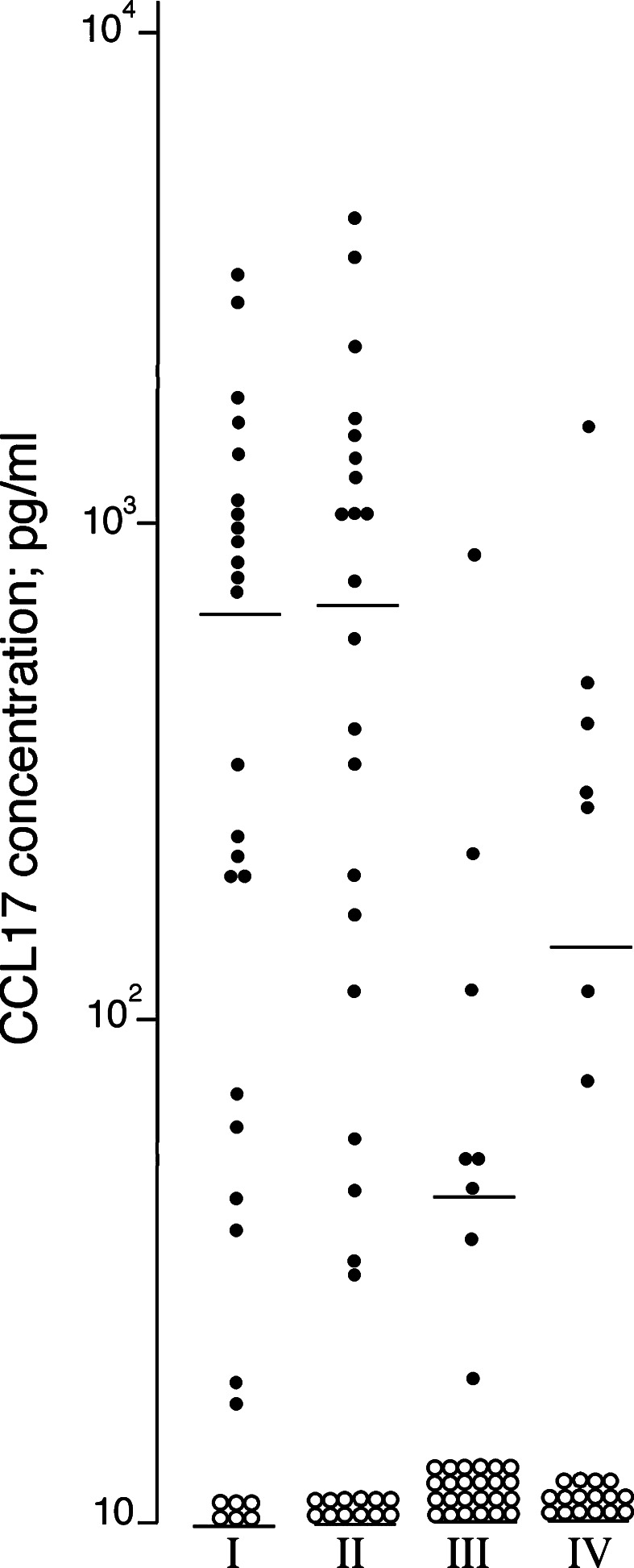
When comparing serum levels before therapy and during chemotherapy-induced cytopenia (first day of cytopenia, after 8–10 and 13–15 days of cytopenia) we observed (Fig. 2): (i) CCL17 levels on day 1 did not differ from pretherapy levels (n=29); (ii) CCL17 levels after 8–10 days were significantly lower than both pretherapy levels (n=28, Wilcoxon’s test, P< 0.0005), levels on day 1 (n=32, P=0.001) and levels on day 13–15 (n=23, P=0.01). In contrast, CXCL10 levels showed no significant variations. The results for induction and consolidation cycles did not differ. Differences were statistically significant also when AML patients were analysed separately, and decreased levels were observed for the ALL patients. Furthermore, during cytopenia all patients were dependent on platelet transfusions; platelet counts showed relatively small variations between 10 and 28×109/l compared with the larger variation in CCL17 levels.
Fig. 2.
CCL17 levels (pg/ml) in serum samples collected from acute leukemia patients (I) before chemotherapy, n=29; (II) at the beginning of severe treatment-induced cytopenia, n=33; (III) after 8–10 days of cytopenia, n=32; and (IV) after 13–15 days of cytopenia, n=23. The mean concentration at each time is indicated. Open symbols represent undetectable CCL17 levels (<7 pg/ml), and for these samples the detection limit was used in the statistical calculations
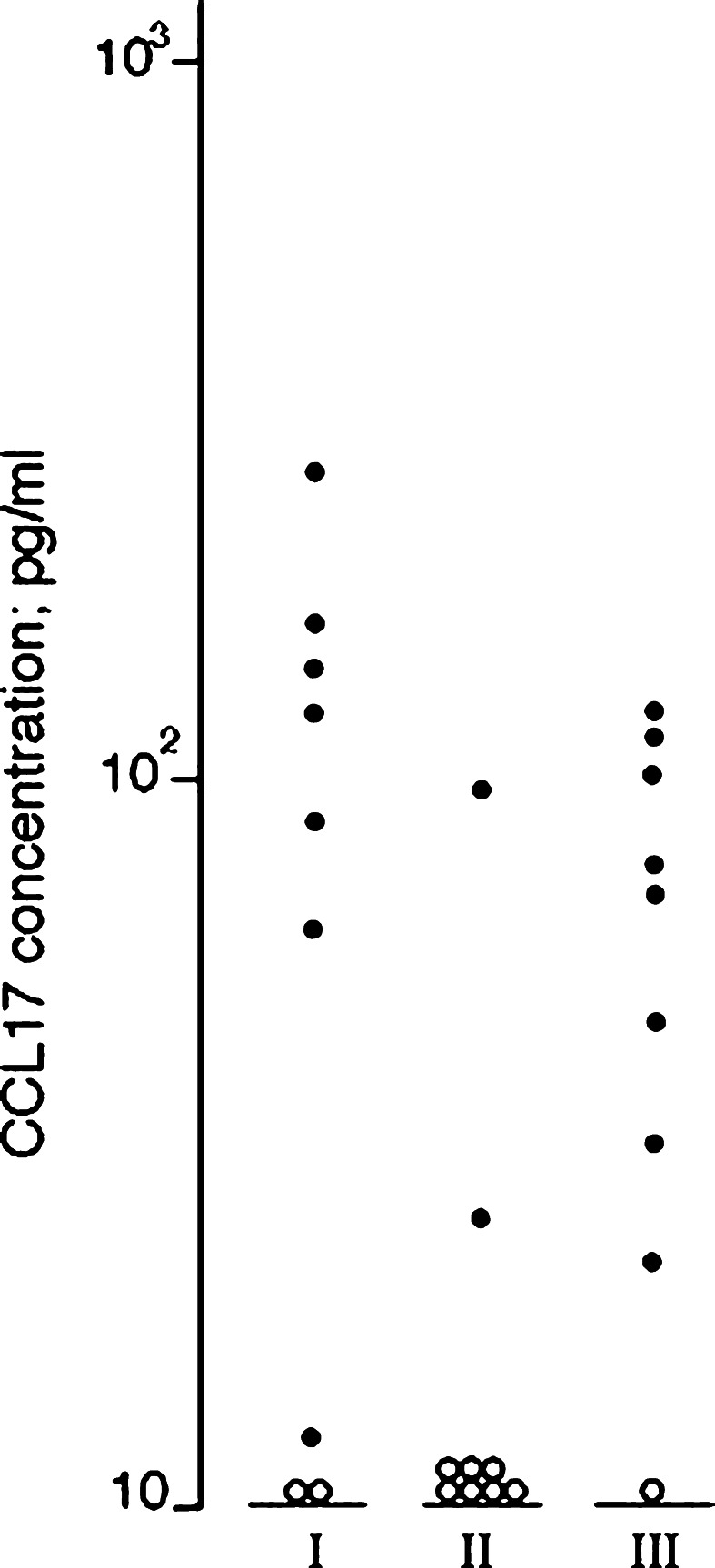
Serum CCL17 levels were investigated for nine AML patients who developed complicating bacterial infections after 2–13 days of cytopenia. CCL17 levels decreased during infections (Fig. 3; Wilcoxon’s test, P=0.012); the levels thereafter increased during clinical improvement due to antibiotic therapy (P=0.028). CXCL10 levels were not altered during infections. The blood platelet counts showed no significant differences; the counts varied between 8 and 29×109/l before infections and during infections the counts were 17–32×109/l due to a higher threshold for prophylactic platelet transfusion in febrile patients (10 vs. 20×109/l).
Fig. 3.
CCL17 serum levels (pg/ml) for AML patients with severe chemotherapy-induced cytopenia and complicating bacterial infections. Samples were collected from nine patients (I) less than 3 days before infections when patients were afebrile and without clinical signs of infections; (II) during bacterial infections when patients were febrile with clinical signs of complicating infections and serum C-reactive protein (CRP) levels >50 mg/l; and (III) after clinical improvement when patients were afebrile with serum CRP levels <50 mg/l. Open symbols represent undetectable CCL17 levels (<7 pg/ml)
Discussion
The effect of immunotherapy in AML will depend on both specific recognition and local recruitment of circulating T cells to the AML cells’ microenvironment. This recruitment is probably affected by several parameters including: (i) the AML cells’ release of T cell chemotactic chemokines and their presentation as binding molecules on the luminal surface of neighboring endothelial cells; and (ii) free serum chemokines that compete with the surface molecules for receptor binding [5–10]. In this study we therefore characterized AML cell release and serum levels of T cell chemotactic chemokines, and for our in vitro studies we then used experimental strategies that have been characterized in detail previously [12, 15, 16, 19–25].
The T cell chemotactic chemokines CXCL10, CCL5 and CCL17 could be released by native human AML cells (Table 1), and the levels showed no correlation with age, leukemic cell differentiation, AML-associated genetic abnormalities or response to induction therapy. CXCL10 and CCL5 were more often released and usually at higher levels than CCL17. The release of CXCL10 and CCL5 was therefore characterized more in detail. Firstly, exogenous CXCL10 and CCL5 affected AML blast proliferation only for a minority of patients independent of the endogenous chemokine levels, suggesting that CXCL10/CCL5 levels are mainly determined by differences in chemokine release and not by differences in chemokine consumption by proliferating AML cells. Secondly, AML cells usually showed a relatively high release of both chemokines compared with bone marrow stromal cells, but the AML cell release was often lower than the release by activated immunocompetent cells. Thirdly, the constitutive release of CXCL10 and CCL5 showed a wide variation between AML patients, and the wide variation persisted even after the coculture of AML cells with nonleukemic stromal cells. These protein levels showed no correlations with variations in CXCL10- and CCL5-specific mRNA levels for freshly isolated or cocultured cells. Taken together our mRNA results thereby suggest that in vitro release of CXCL10 and CCL5 by native AML cells is mainly regulated by post-transcriptional mechanisms. Finally, the levels of CXCL10 and CCL17 (but not CCL5) were increased when AML cells were cocultured with various nonleukemic cells. This increase was dependent on other soluble mediators than IL1 and GM-CSF (only CCL5 release seems to depend on GM-CSF) and was not counteracted by direct cell–cell contact.
Normal T cells migrated in the direction of AML cells when tested in our transwell chemotaxis model. The migration usually involved CD8+ more than CD4+ T cells and could be inhibited by combining anti-CXCL10 and anti-CCL5. These observations suggest that CCL5 and CXCL10 release by the AML cells can affect local T cell migration.
Activated platelets release large amounts of CLL5 [26] and serum levels mainly reflect ex vivo release during sample preparation. Although platelets also contain CCL17 [26], differences in platelet levels/release could not explain (i) the variation in CCL17 serum levels between patients with untreated AML; (ii) the decreased levels during cytopenia/febrile neutropenia; and (iii) the increased levels before consolidation therapy. Taken together these observations suggest that the variations in CCL17 levels, like the variations in CXCL10 levels, reflect true in vivo variations. The variations are probably caused by indirect disease- or therapy-induced effects on nonleukemic cells, e.g. decreased levels caused by therapy- or AML-associated immune defects [11], and increased levels caused by AML-induced abnormal activation of the remaining immunocompetent cells [27]. A direct effect of AML cell release on the serum levels seems less likely because the AML cells’ constitutive release and corresponding serum levels showed no correlation.
To summarize, T cell chemotaxis is influenced by both serum levels and local tissue release of T cell chemotactic chemokines, e.g. CXCL10, CCL5 and CCL17. Serum levels of chemotactic chemokines vary in AML patients and are determined by (i) patient age; (ii) disease/treatment status; and (iii) complicating infections. Furthermore, AML cells show a wide variation in their constitutive chemokine release, but our in vitro studies of T cell migration suggest that CXCL10 and CCL5 can affect T cell migration both for patient with high and low constitutive release.
Acknowledgements
The work was supported by the Norwegian Cancer Society and Olaf Ruunshaugens Foundation. The technical assistance of Line Wergeland and Kristin Paulsen is gratefully acknowledged.
References
- 1.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Bruserud Ø. Acute myelogenous leukemia blasts as accessory cells during T lymphocyte activation: possible implications for future therapeutic strategies. Leukemia. 1999;13:1175–1187. doi: 10.1038/sj/leu/2401452. [DOI] [PubMed] [Google Scholar]
- 3.Bruserud Ø, Gjertsen BT. New strategies for the treatment of acute myelogenous leukemia: differentiation induction–present use and future possibilities. Stem Cells. 2000;18:157–165. doi: 10.1634/stemcells.18-3-157. [DOI] [PubMed] [Google Scholar]
- 4.Bruserud Ø, Wendelbo Ø. Biological treatment of acute myelogenous leukemia: how should T cell targeting immunotherapy be combined with intensive chemotherapy. Exp Opin Biol Ther. 2001;1:1005–1016. doi: 10.1517/14712598.1.6.1005. [DOI] [PubMed] [Google Scholar]
- 5.Bruserud Ø, Ulvestad E. Acute myelogenous leukemia blasts as accessory cells during in vitro T lymphocyte activation. Cell Immunol. 2000;206:35–50. doi: 10.1006/cimm.2000.1725. [DOI] [PubMed] [Google Scholar]
- 6.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 7.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2001;38:881–885. doi: 10.1016/S0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 8.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Neville LF, Mathiak G, Bagasra Ø. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth F R. 1997;8:207–219. doi: 10.1016/S1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 11.Wendelbo Ø, Nesthus I, Sjo M, Paulsen K, Ernst P, Bruserud Ø. Functional characterization of T lymphocytes derived from patients with acute myelogenous leukemia and chemotherapy-induced leukopenia. Cancer Immunol Immun. 2004;53:740–747. doi: 10.1007/s00262-004-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruserud Ø, Hovland R, Wergeland L, Huang T-s, Gjertsen BT. Flt3-mediated signalling in human acute myelogenous leukemia (AML) blasts: a functional characterization of the effects of Flt3-ligand in AML cell populations with and without Flt3 abnormalities. Haematologica. 2003;88:416–428. [PubMed] [Google Scholar]
- 13.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukemia derived from the MRC AML 10 trial. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 14.Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. Leukemia. 1995;9:1783–1786. [PubMed] [Google Scholar]
- 15.Bruserud Ø, Ryningen A, Wergeland L, Glenjen NI, Gjertsen BT. Osteoblasts increase proliferation and release of proangiogenic interleukin 8 by native human acute myelogenous leukemia blasts. Haematologica. 2004;89:391–402. [PubMed] [Google Scholar]
- 16.Bruserud Ø, Gjertsen BT, Foss B, Huang T-s. New strategies in the treatment of acute myelogenous leukemia (AML): in vitro culture of AML cells–the present use in experimental studies and the possible importance for future therapeutic strategies. Stem Cells. 2001;19:1–11. doi: 10.1634/stemcells.19-1-1. [DOI] [PubMed] [Google Scholar]
- 17.Bruserud Ø, Tronstad KJ, Berge R. In vitro culture of human osteosarcoma cell lines: a comparison of functional characteristics for cell lines cultured in medium without and with fetal calf serum. J Cancer Res Clin Oncol. 2005;741:24–34. doi: 10.1007/s00432-004-0650-z. [DOI] [PubMed] [Google Scholar]
- 18.Rochet N, Leroy P, Far DF, Ollier L, Loubat A, Rossi B. CAL72: a human osteosarcoma cell line with unique effects on hematopoietic cells. Eur J Haematol. 2003;70:43–52. doi: 10.1034/j.1600-0609.2003.02766.x. [DOI] [PubMed] [Google Scholar]
- 19.Ryningen A, Wergeland L, Glenjen NI, Gjertsen BT, Bruserud Ø. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leukemia Res. 2005;292:185–196. doi: 10.1016/j.leukres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Bruserud Ø, Wendelbo Ø, Paulsen K. Lipoteichoic acid derived from Enterococcus faecalis modulates the functional characteristics of both normal peripheral blood leukocytes and native human acute myelogenous leukemia blasts. Eur J Haematol. 2004;73:340–350. doi: 10.1111/j.1600-0609.2004.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Glenjen NI, Ersvær E, Ryningen A, Bruserud Ø. In vitro effects of native human acute myelogenous leukemia blasts on fibroblasts and osteoblasts. Int J Cancer. 2004;111:858–867. doi: 10.1002/ijc.20353. [DOI] [PubMed] [Google Scholar]
- 22.Øyan AM, Bo TH, Jonassen I, et al. CD34 expression in native human acute myelogenous leukemia blasts: Differences in CD34 membrane molecule expression are associated with different gene expression profiles. Cytometry B Clin Cytom. 2005;64:18–27. doi: 10.1002/cyto.b.20044. [DOI] [PubMed] [Google Scholar]
- 23.Gjertsen BT, Øyan AM, Marzolf B, et al. Analysis of acute myelogenous leukemia: preparation of samples for genomic and proteomic analyses. J Hematother Stem Cell Res. 2002;11:469–481. doi: 10.1089/15258160260090933. [DOI] [PubMed] [Google Scholar]
- 24.Bruserud Ø, Gjertsen BT, von Volkman HL. In vitro culture of human acute myelogenous leukemia (AML) cells in serum-free media: studies of native AML blasts and AML cell lines. J Hematother Stem Cell Res. 2000;9:923–932. doi: 10.1089/152581600750062372. [DOI] [PubMed] [Google Scholar]
- 25.Bruserud Ø, Glenjen NI. Coculture of native human acute myelogenous leukemia blasts with fibroblasts and osteoblasts results in an increase of vascular endothelial growth factor levels. Eur J Haematol. 2005;74:24–34. doi: 10.1111/j.1600-0609.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 26.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 27.Panoskaltsis N, Reid CD, Knight SC. Quantification and cytokine production of circulating lymphoid and myeloid cells in acute myelogenous leukaemia. Leukemia. 2003;17:716–730. doi: 10.1038/sj.leu.2402835. [DOI] [PubMed] [Google Scholar]