Abstract
Dendritic cell (DC) vaccines have emerged as a promising strategy to induce antitumoral cytotoxic T cells for the immunotherapy of cancer. The maturation state of DC is of critical importance for the success of vaccination, but the most effective mode of maturation is still a matter of debate. Whereas immature DC carry the risk of inducing tolerance, extensive stimulation of DC may lead to DC unresponsiveness and exhaustion. In this study, we investigated how short-term versus long-term DC activation with a Toll-like receptor 9 agonist influences DC phenotype and function. Murine DC were generated in the presence of the hematopoietic factor Flt3L (FL-DC) to obtain both myeloid and plasmacytoid DC subsets. Short activation of FL-DC for as little as 4 h induced fully functional DC that rapidly secreted IL-12p70 and IFN-α, expressed high levels of costimulatory and MHC molecules and efficiently presented antigen to CD4 and CD8 T cells. Furthermore, short-term activated FL-DC overcame immune suppression by regulatory T cells and acquired high migratory potential toward the chemokine CCL21 necessary for DC recruitment to lymph nodes. In addition, vaccination with short-term activated DC induced a strong cytotoxic T-cell response in vivo and led to the eradication of tumors. Thus, short-term activation of DC generates fully functional DC for tumor immunotherapy. These results may guide the design of new protocols for DC generation in order to develop more efficient DC-based tumor vaccines.
Keywords: Dendritic cells, Tumor, Immunotherapy, Toll-like receptor, Cancer vaccine
Introduction
In the field of cancer immunotherapy, dendritic cells (DC) have emerged as a powerful tool to initiate T-cell responses, induce immunological memory and break immunological tolerance to tumors [46, 49]. The ability of DC-based vaccines to stimulate cytotoxic T lymphocytes (CTL) and to polarize T helper cells towards a Th1 profile highlights their potential in the immunotherapy of cancer. However, despite rapid progress in the field of DC biology, clinical responses to DC vaccination have yet to meet expectations [9, 53]. Several questions remain to be resolved in order to improve the efficacy of DC vaccines, such as the development of effective strategies for DC maturation and the appropriate usage of DC subtypes. In particular, fms-like tyrosine kinase 3 ligand (Flt3L)-generated DC are a promising alternative to conventional monocyte-derived DC generated with GM-CSF and IL-4. In tumor patients, the in vivo administration of the hematopoietic growth factor Flt3L mobilizes DC into blood, allowing the isolation of large numbers of DC that efficiently prime immune responses to cancer antigens [6, 35]. In contrast to conventional monocyte-DC derived from myeloid precursors, Flt3L-generated DC comprise different DC subsets including myeloid DC (mDC), that efficiently present antigen, and plasmacytoid DC (pDC), that produce high amounts of type-I interferon, a major Th1-promoting cytokine type [35].
Maturation and activation of DC are essential in order to elicit the protective T-cell responses required for tumor immunotherapy [5, 7, 8]. Activated DC produce proinflammatory cytokines, upregulate surface expression of MHC and co-stimulatory molecules and migrate from peripheral tissues to lymph nodes. There, DC present antigen to T cells in order to initiate an antigen-specific immune response [13]. In the majority of published clinical trials to date, DC are matured with a cytokine cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2 [23]. This protocol induces a mature DC phenotype with upregulation of co-stimulatory molecules that supports clonal expansion of CD4 T cells. However, recent findings show that this activation by inflammatory cytokines may not promote T-cell differentiation towards a Th1 phenotype in vivo [48]. Furthermore, cytokine-activated DC can induce immunosuppressive regulatory T (Treg) cells [32]. In contrast, DC activated by agonists of Toll-like receptors (TLR), which are centrally involved in the initiation of innate and adaptive immune responses, induce efficient Th1 responses both in vitro and in vivo [21, 42].
Although the importance of DC maturation for tumor immunotherapy is now widely accepted, the timing of DC activation remains to be investigated in detail. In most clinical trials, maturation protocols require extensive in vitro activation of DC for 48 h. However, it has been shown that this long activation time leads to exhaustion of cytokine production, in particular for the key Th1-polarizing cytokine IL-12p70 [17, 24, 28]. Furthermore, DC activated in this manner become refractory to further stimulation and may induce Th2 responses [24, 28]. Although short activation times could possibly prevent DC exhaustion, little is known about the type of immune response induced in vivo by DC activated through short TLR stimulation [33].
In this study, we compare DC activated for a short versus a prolonged period by the TLR9 ligand CpG in respect to their ability to elicit tumor-specific CTL responses in vivo. DC were differentiated in the presence of Flt3L in order to generate both mDC and pDC subsets [2, 3, 37]. We show that activation of DC by CpG for as little as 4 h irreversibly programs DC to produce Th1-associated cytokines and is highly effective in inducing an antitumoral T-cell response. We also demonstrate that short-term activated DC acquire migratory function towards a CCR7 ligand and overcome the immune suppression mediated by Treg cells. Thus, short-term activation of DC by a TLR9 ligand induces fully functional DC for DC-based tumor immunotherapy.
Materials and methods
Mice
Female Balb/c and C57BL/6 mice were purchased from Harlan–Winkelmann (Borchen, Germany). TCR transgenic OT-I and OT-II mice were kindly provided by Prof. Th. Brocker (Institute of Immunology, Munich, Germany). Mice were 6–10 weeks of age at the onset of experiments. All animal studies were approved by the local regulatory agency (Regierung von Oberbayern, Munich, Germany).
Generation and activation of Flt3L bone marrow-derived DC
Immature murine fms-like tyrosine kinase 3 ligand DC (FL-DC) were generated from bone marrow as described [2, 3], with minor modifications. Briefly, bone marrow was extracted and red blood cells were lysed with ammonium chloride buffer (BD Biosciences). Cells were cultured at 2–3 × 106 cells/ml in RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 μg/ml streptomycin, 100 IU/ml penicillin, 1 mM sodium pyruvate, 1% non-essential amino acids (MEM-NEAA) and 3.75 × 10−4% 2-mercaptoethanol (DC medium) containing 20 ng/ml recombinant Flt3L (Tebu-Bio, Offenbach, Germany) for 7–8 days at 37°C. CD11c+ DC generally represented >80% of the non-adherent cells with 40% pDC (B220+) and 60% mDC (B220−, CD11b+). DC were activated with the oligodeoxynucleotide CpG 1826 (6 μg/ml) (5′-TCCATGACGTTCCTGACGTT-3′, Coley Pharmaceutical Group, Langenfeld, Germany), the TLR7/8 ligand R848 (0.5 μg/ml, Alexis Biochemicals, Lausen, Switzerland), or the TLR7 ligand CL087 (1 μg/ml, Invivogen, San Diego, USA). To examine plasmacytoid (B220+ CD11c+ cells) and myeloid (B220− CD11c+ cells) subsets separately, pDC were isolated using magnetic CD45R (B220) MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s instructions on day 8 of FL-DC culture. pDC-depleted FL-DC were used as mDC. The purity of both subsets was greater than 95% of CD11c+ cells in all experiments.
DC phenotyping and cytokine production
Concentration of IL-12p70, IL-6 and IL-10 in culture supernatants was determined in triplicate by ELISA according to the manufacturer’s instructions (BD Biosciences). IFN-α was measured according to the following protocol: rat monoclonal antibody to mouse IFN-α (clone RMMA-1) was used as the capture antibody, rabbit polyclonal antibody to mouse IFN-α for detection (both from PBL Biomedical Laboratories, Piscataway, NJ, USA) together with HRP-conjugated donkey antibody to rabbit IgG as the secondary reagent (Jackson ImmunoLaboratories, Bar Harbor, ME, USA). Recombinant mouse IFN-α (PBL Biomedical Laboratories) was used as standard (IFN-α concentration in IU/ml). For analysis of activation markers and CCR7 expression, cells were stained with fluorochrome-conjugated monoclonal antibodies (B220, CD11b, CD11c, CD40, CD80, CD86, MHC I and MHC II antibodies from BD Pharmingen, CCR7 antibody from BioLegend) and analyzed by flow cytometry. Data were acquired on a FACSCalibur (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR).
T-cell proliferation assays
FL-DC were pulsed with different concentrations of ovalbumin (OVA) (Sigma-Aldrich, St Louis, MO, USA) or recombinant survivin protein (expressed in HEK293 cells; kindly provided by J. Wei, Munich) as negative control for 18 h before activation with CpG for 0, 4 or 20 h. For peptide loading, unpulsed DC were cultured for the last hour of CpG stimulation with OVA257–264 peptide (SIINFEKL) (Metabion, Martinsried, Germany) or the control peptide T1Db (SAINNYAQKL) derived from the SV40 large T antigen (Metabion). To analyze the induction of CD8 T-cell responses, 2 × 105 splenocytes from OT-I mice were co-cultured with the indicated number of FL-DC for 48 h. To analyze CD4 responses, splenocytes from OT-II mice were magnetically sorted using CD4 MicroBeads according to the manufacturer’s instructions (Miltenyi Biotech) and 105 CD4 T cells were co-cultured with the same number of FL-DC. 5′-bromo-2′-deoxy-uridine (BrdU; 7.5 μM) was added for the last 6–12 h of culture to measure cell proliferation. BrdU incorporation was assessed by ELISA according to the manufacturer’s instructions (Roche, Mannheim, Germany) and chemiluminescence was measured in relative light units (rlu) with a multilabel plate reader (Mithras, Berthold Technologies, Bad Wildberg, Germany). IFN-γ secretion by T cells was determined by ELISA according to the manufacturer’s instructions (BD Biosciences). For Treg cell suppression assays, CD4+ CD25− effector cells and CD4+ CD25+ Treg cells were isolated from spleens of wild-type C57BL/6 mice using the CD4+ CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotech) according to the manufacturer’s instructions. The purity of Treg cells was greater than 97%. CD4+ CD25− effector T cells (7.5 × 104 cells per well) were cultured in DC medium in the presence of soluble anti-CD3 antibody (clone 500A2, 0.1 μg/ml, BD Biosciences) with 3.5 × 103 FL-DC and 6 × 104 CD4+ CD25+ Treg cells for 48 h. BrdU incorporation in the last 12 h was measured in triplicate by chemiluminescence as described above.
Immunization with FL-DC
FL-DC were harvested and stimulated with 6 μg/ml for 4 h or 20 h. Cells were either pulsed with OVA (100 μg/ml) for 24 h before CpG stimulation or were loaded with OVA257–264 peptide (100 ng/ml) for the last hour of CpG stimulation. After removal of unbound peptide by washing with PBS, 4 × 105 DC were injected s.c. together with 100 μg CpG twice at a 7-day interval. OVA-specific CTL responses were determined 1 week after the second immunization. For the detection of OVA-specific CD8 T cells, peripheral blood lymphocytes were stained with OVA257–264-H-2kb-PE pentamers (Proimmune, Oxford, UK) and anti-CD8 (BD Pharmingen) after red blood cell lysis. The percentage of OVA-specific CD8 T cells was determined by flow cytometry.
In vivo cytotoxicity assays
Targets were prepared from C57BL/6 splenocytes. The suspension was divided into two populations, unpulsed or pulsed with OVA257–264 peptide (10 μg/ml) for 1 h at 37°C, washed extensively and labeled with a low concentration (1.5 μM) or a high concentration (15 μM) of carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR, USA), respectively. Peptide-pulsed CFSEhigh cells and unpulsed CFSElow cells were mixed 1:1 and a total of 107 target cells were injected i.v. into immunized mice. Three days later, CFSE-labeled target cells from peripheral blood were analyzed by flow cytometry. Specific lysis was calculated as follows: specific lysis (%) = 100 × [1 − (CFSEhigh cells/CFSElow cells)/(CFSEhigh cells in naïve mice/CFSElow cells in naïve mice)].
DC migration
2 × 104 FL-DC (unstimulated, 4 h-DC or 20 h-DC) in 5 μm transwell inserts (Costar, Corning, NY, USA) were placed into 24-well plates containing 600 μl DC medium with or without 100 ng/ml CCL21 and incubated for 2 h at 37°C. The medium in the lower chambers was concentrated to 50 μl and cells were counted with a hemocytometer. All conditions were performed as triplicates.
Tumor experiments
For tumor induction, 2.5 × 105 murine colon carcinoma C26 cells on a Balb/c background (Cell Lines Service, Heidelberg, Germany) were injected subcutaneously into the right flank of Balb/c mice. Tumor growth was monitored for 120 days after tumor challenge and was expressed as the product of the perpendicular diameters of individual tumors. Animals were sacrificed when tumor size exceeded 225 mm2. For DC therapy, mice received 2 × 105 FL-DC s.c. together with 100 μg CpG in the non tumor-bearing (contralateral) flank. Prior to injection, immature DC were loaded with UV-irradiated tumor cells (0.7 J/cm2) in a ratio of 5:1 24 h before CpG stimulation. In some experiments, mice were additionally injected with 100 μg CpG peritumorally as indicated. Treatment was initiated at day 8 after tumor injection when tumor size was 10–20 mm2. The interval between the first and second vaccination was 5 days, and two more vaccinations were performed at 7-day intervals. Mean tumor size curves of therapy groups were plotted until three mice of that group died or were killed.
Statistics
Statistical analyses were performed by unpaired, one-way analysis of variance (ANOVA) with the Newman–Keuls multiple comparison test. Significance was set at P < 0.05. Comparisons in tumor size among groups were made using the Mann–Whitney test for various time points. Comparisons among groups regarding survival time were made using the log-rank test. Fisher’s exact test was used for comparing tumor incidence in re-challenged mice. Statistical analyses were performed using SPSS software (SPSS, Chicago, IL, USA).
Results
Short-term activation of FL-DC with CpG rapidly induces Th1 cytokine production
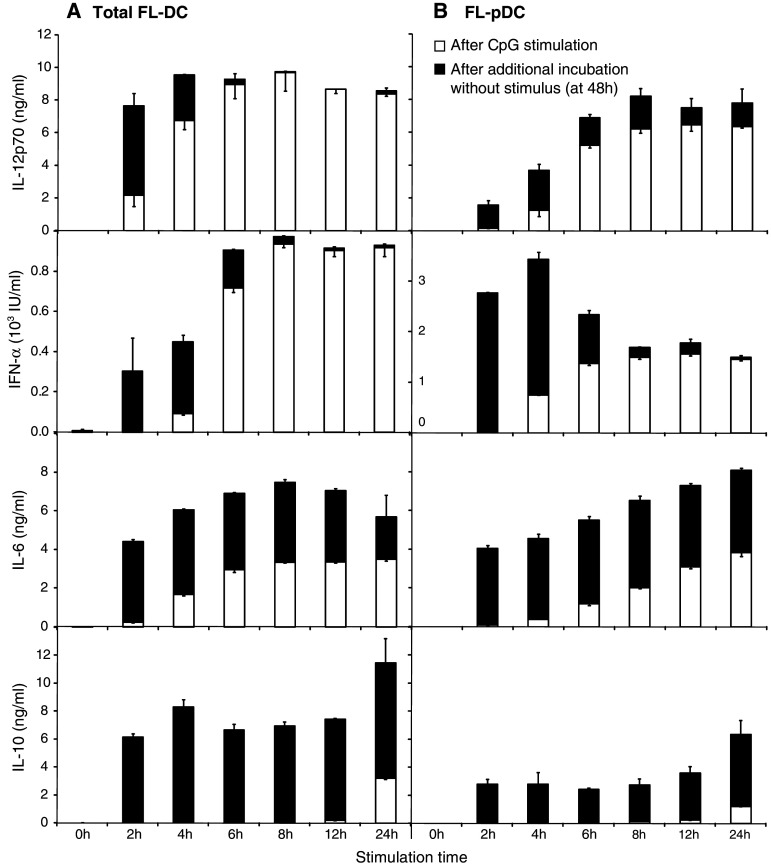
We investigated the kinetics of cytokine production by FL-DC after stimulation for increasing times with the TLR9 ligand CpG 1826 (CpG). DC were stimulated with CpG for 2 h (short-term) up to 24 h (long-term). After the stimulation period, cytokine levels were measured in the supernatant (Fig. 1, white bars). Cells were washed to remove free CpG and culture was continued for a total of 48 h. Cytokine secretion after this additional incubation without stimulus indicates the therapeutically relevant fraction of cytokines that can be produced in vivo following DC transfer (Fig. 1, black bars). In total FL-DC (Fig. 1a), the bioactive heterodimer of IL-12, IL-12p70, was detected as early as 2 h after stimulation with CpG. Secretion persisted after removal of the stimulus, so that a 2 h activation period was sufficient to induce subsequent IL-12 production, but production ceased after 8 h. IFN-α was also produced early, with most of the secretion taking place in the first 8 h after activation. The time course of IL-6 secretion showed a sustained production for over 24 h even with short activation times. In contrast, IL-10 secretion was initiated late between 12 and 24 h after the onset of stimulation, and levels were highest after a stimulation of 24 h. As IL-10 suppresses the differentiation of Th1 cells, a short stimulation may help to support a Th1-inducing cytokine milieu. We further analyzed differential cytokine production by the pDC and mDC subpopulations isolated from FL-DC. IL-12p70 production by pDC was rapid and occurred mainly in the first 8 h after stimulation (Fig. 1b). pDC are the main producers of IFN-α upon TLR stimulation and levels were accordingly high upon activation. For this cytokine, production also ceased nearly entirely after 8 h. Secretion of IL-6 was sustained over 48 h, whereas the onset of IL-10 production was late. Cytokine secretion by mDC showed a very similar pattern to that of total FL-DC, except for IFN-α, which is not produced by mDC upon CpG stimulation (data not shown). Thus, activation of DC for as little as 4 h induces efficient production of all cytokines examined. Importantly, the Th1-type cytokines IL-12p70 and IFN-α are produced nearly exclusively during the first 8 h after initiation of activation, independently of the duration of stimulation.
Fig. 1.
Short-term activation of FL-DC and FL-pDC with CpG rapidly induces high levels of cytokines. Murine FL-DC a and pDC sorted from FL-DC cultures b were stimulated with 6 μg/ml CpG for 2–24 h, extensively washed to remove excess CpG and cultured without further stimulation for a total of 48 h. Cytokine concentration in the supernatant was measured directly after the 2 to 24 h stimulation period (white bars) and at 48 h, after additional incubation without stimulus (black bars). Results show mean and SEM of one representative experiment of four
Short-term activated FL-DC upregulate activation markers
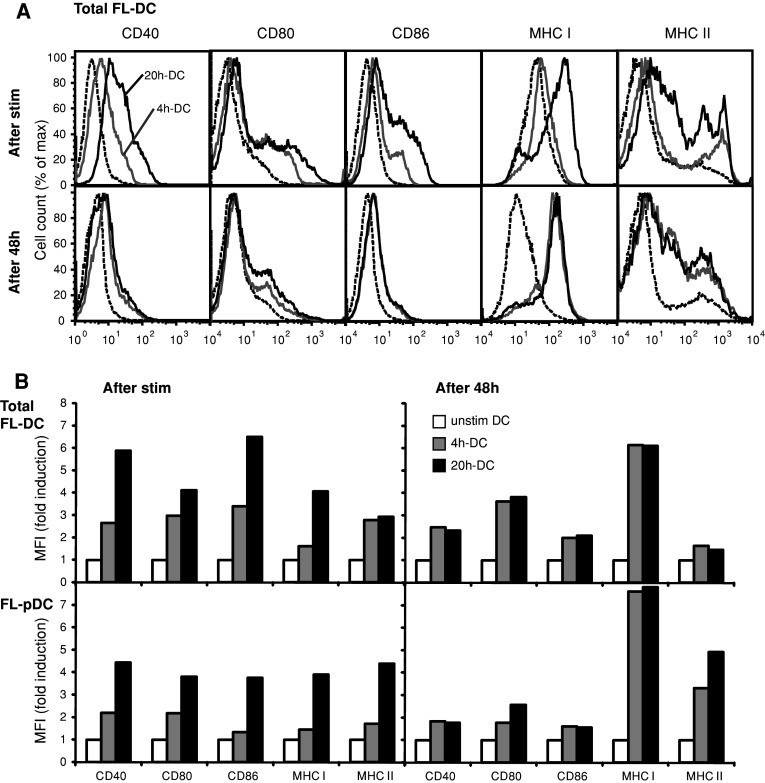
To further characterize the potential of short-term CpG-activated FL-DC to initiate T-cell responses, we examined the expression of co-stimulatory and MHC molecules on total FL-DC that were stimulated either transiently for 4 h (4 h-DC) or for 20 h (20 h-DC). 4 h-DC showed a 2.5-fold to threefold increase in expression of the co-stimulatory molecules CD40, CD80 and CD86 relative to unstimulated DC (Fig. 2a, b). Expression of MHC class I and MHC class II molecules was also increased. In 20 h-DC, co-stimulatory and MHC molecules were further upregulated. In contrast, 48 h after the initiation of stimulation, both 4 h-DC and 20 h-DC expressed similar levels of co-stimulatory and MHC molecules. Expression at 48 h was increased compared to unstimulated DC, although to a lesser extent than at earlier time points (Fig. 2a, b). We further assessed the distribution and activation phenotype of the pDC and mDC subpopulations within FL-DC. Whereas the pDC proportion in unstimulated and 4 h-DC represented 35–42% of FL-DC after 48 h, the pDC percentage was slightly reduced in 20 h-DC (28%). The kinetics of activation marker expression on pDC and mDC were similar to those of total FL-DC, whereby mDC showed an overall higher expression of co-stimulatory molecules (Fig. 2b and data not shown). Thus, short-term stimulation is as efficient as a long-term stimulation to induce an activated phenotype of both pDC and mDC at 48 h. Furthermore, expression of co-stimulatory molecules decreases after an initial peak, suggesting that the immunostimulatory potential of DC may be higher at early time points after activation.
Fig. 2.
Short-term activation of FL-DC induces upregulation of costimulatory and MHC molecules. FL-DC were stimulated with CpG for 4 h (4 h-DC) or 20 h (20 h-DC), washed to remove excess CpG and cultured without further stimulus for a total of 48 h. Surface expression of the indicated markers was measured by flow cytometry directly after stimulation and at 48 h. Histograms a illustrate the expression of depicted markers on total FL-DC for unstimulated cells (dashed line), 4 h-DC (grey line) and 20 h-DC (black line). Graphs b show mean fluorescence intensity (MFI) depicted as fold increase relative to unstimulated DC for total FL-DC and for gated pDC within FL-DC (FL-pDC). One representative experiment of four is shown
Short-term DC efficiently cross-present antigen and induce Th1 responses
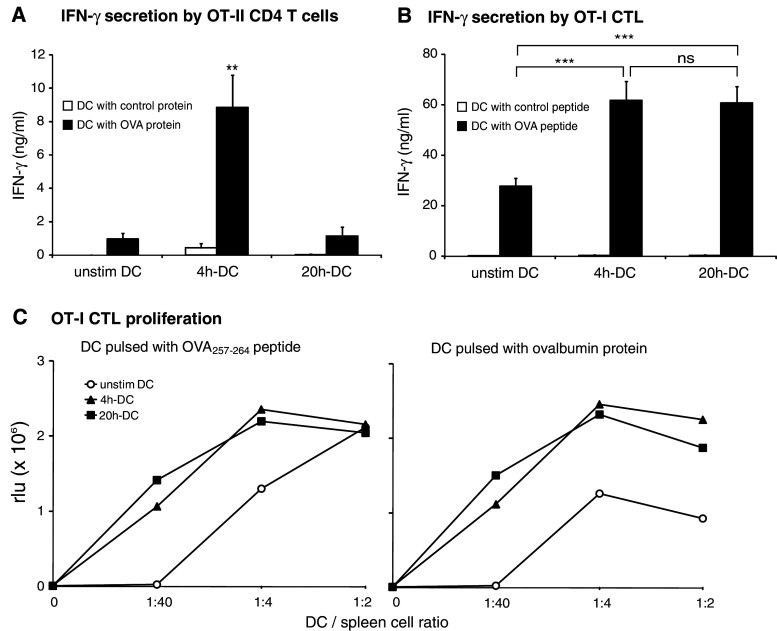
The early production by FL-DC of the Th1-inducing cytokines IL-12p70 and IFN-α suggested that short-term activated DC could be more efficient than long-term activated DC in the induction of Th1 responses. To examine the DC capacity for generating Th1 responses, OVA protein-pulsed DC were co-cultured for 48 h with OVA-specific CD4 T cells from OT-II mice. The hallmark cytokines for Th1- and Th2-type responses, IFN-γ and IL-4, were measured in the supernatants. As predicted, 4 h-DC induced high levels of IFN-γ, whereas T cells co-cultured with 20 h-DC produced no more IFN-γ than T cells cultured with unstimulated DC (Fig. 3a). No IFN-γ was produced when DC were pulsed with a control protein. FL-DC did not promote Th2 responses, as IL-4 was not detected in any supernatants (data not shown).
Fig. 3.
Short-term activated FL-DC induce Th1 differentiation of CD4 cells and proliferation of CTL. a DC were pulsed with OVA (black bars) or survivin protein as control antigen (white bars), stimulated for 4 h or 20 h with CpG and co-cultured with sorted OT-II CD4 T cells. IFN-γ production was measured in culture supernatants after 48 h. b OT-I splenocytes were co-cultured with peptide-pulsed DC to induce IFN-γ secretion by CTL. DC were loaded with OVA257–264 peptide (black bars) or the irrelevant T1Db peptide as control antigen (white bars). Results show mean + SEM of quadruplicates of two independent experiments (**P < 0.01, ***P < 0.001). c DC were pulsed with OVA257–264 peptide or OVA protein and co-cultured with splenocytes from OT-I mice. DC-induced T-cell proliferation was assessed after 48 h by measuring BrdU incorporation. One representative experiment of three is shown
One of the main goals of DC vaccination is the induction of an efficient CTL response against tumor-associated antigens. To assess the efficiency of short-term activated DC to present antigen to CTL, 4 h-DC and 20 h-DC were pulsed with the MHC I-restricted peptide OVA257–264 and co-cultured with splenocytes from OT-I mice. Both 4 h-DC and 20 h-DC promoted IFN-γ secretion by OT-I CTL more efficiently than unstimulated DC (Fig. 3b). When DC were pulsed with a control peptide, no IFN-γ was induced. Furthermore, OT-I CTL proliferation was induced by peptide-pulsed short- and long-term CpG-stimulated DC to a similar extent (Fig. 3c). To initiate a protective CTL response in vivo, extracellular antigens must be taken up, then processed and presented in the context of MHC class I molecules, a mechanism termed cross-priming [12]. To determine the ability of 4 h-DC and 20 h-DC to cross-prime antigen-specific T cells, FL-DC were pulsed with OVA protein before activation with CpG. Antigen-pulsed, activated DC were then co-cultured with OT-I splenocytes and proliferation was measured after 48 h (Fig. 3c). While unstimulated DC induced little CTL proliferation, activation of DC with CpG increased their efficiency to induce CTL proliferation independently of the duration of CpG stimulation. Thus, both 4 h-DC and 20 h-DC efficiently cross-prime CTL, but 4 h-DC induce more potent Th1 responses in CD4 cells.
Short-term activation of FL-DC by CpG is sufficient to overcome immune suppression mediated by regulatory T cells
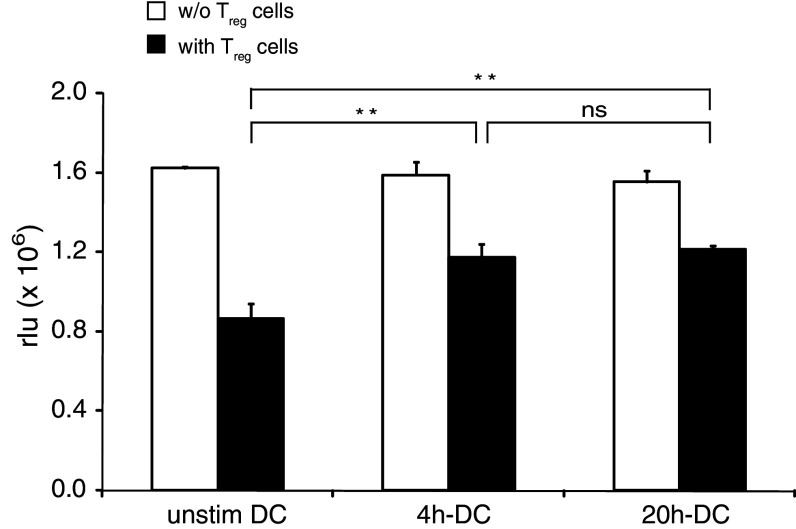
Activation of DC by TLR ligands can overcome the suppression of effector CD4 T-cell proliferation mediated by Treg cells [39]. Since inhibition of Treg cell function is an essential step for the success of DC vaccination, we investigated whether short-term activated DC can suppress Treg cell function. FL-DC were activated with CpG for 4 h or 20 h, washed extensively to remove remaining CpG and added to co-cultures of CD4 T cells and Treg cells in the presence of anti-CD3 mAb. While Treg cells suppressed CD4 T-cell proliferation down to 53% of initial levels when co-cultured with unstimulated DC, 4 h-DC and 20 h-DC restored CD4 T-cell proliferation to 74 and 78%, respectively (Fig. 4).
Fig. 4.
Short-term activation of FL-DC overcomes immune suppression by regulatory T cells. 4 h-DC or 20 h-DC were added to CD4 T cells stimulated by anti-CD3 mAb in the absence (white bars) or presence (black bars) of T reg cells. CD4 T-cell proliferation was assessed by measuring BrdU incorporation and is shown in relative light units. Results show means and SEM of one representative experiment of three (**P < 0.01)
Short-term activated FL-DC upregulate CCR7 and acquire migratory function towards CCL21
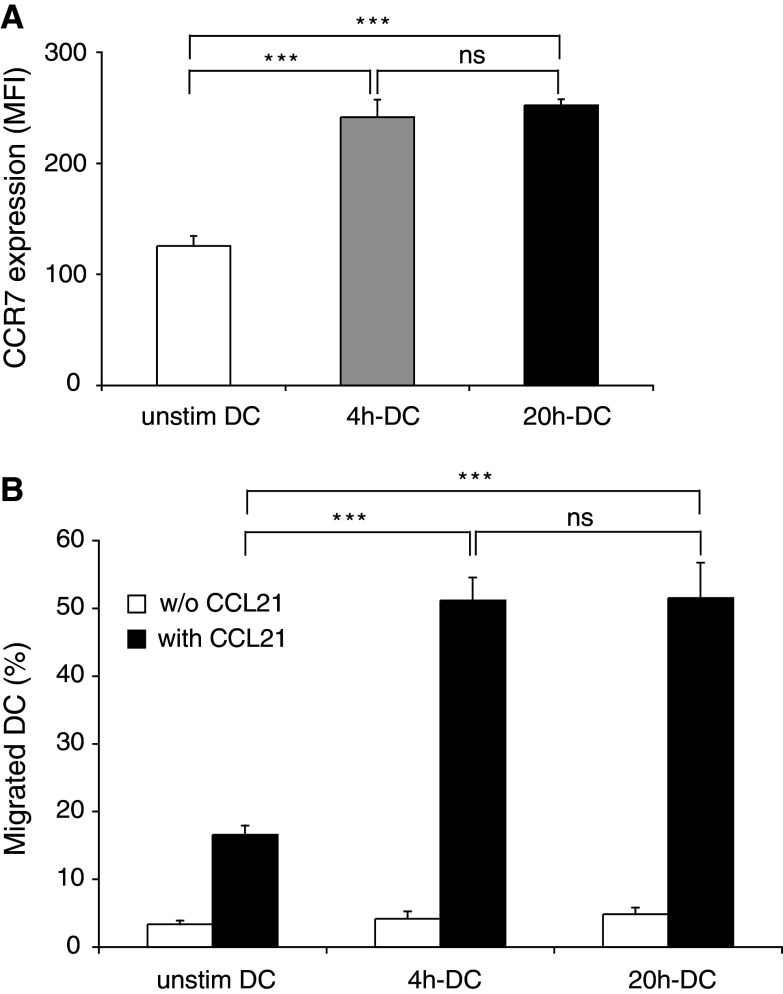
To assess the migratory potential of FL-DC, we examined the expression of the chemokine receptor CCR7 on DC after activation with CpG. CCR7 is upregulated during DC maturation and guides DC to lymph nodes [10, 41]. CCR7 expression was rapidly upregulated by CpG activation after 4 h of stimulation and did not further increase when the stimulation was extended to 20 h (Fig. 5a). In a standard transwell chemotaxis assay we found that 4 h-DC migrated towards a gradient of the CCR7 ligand CCL21 (6Ckine) as efficiently as 20 h-DC (Fig. 5b).
Fig. 5.
Short-term activated FL-DC acquire migratory function toward the CCR7 ligand CCL21. DC were activated for 4 h or 20 h with CpG. a Surface expression of CCR7 was measured by FACS directly after stimulation. CCR7 expression is shown as mean and SEM of MFI. One representative experiment of three is shown. b Migration of FL-DC toward CCL21 was assessed in a standard transwell chemotaxis assay. Mean and SEM from two experiments are shown (***P < 0.001)
Short-term activated DC generate an efficient antigen-specific immune response in vivo
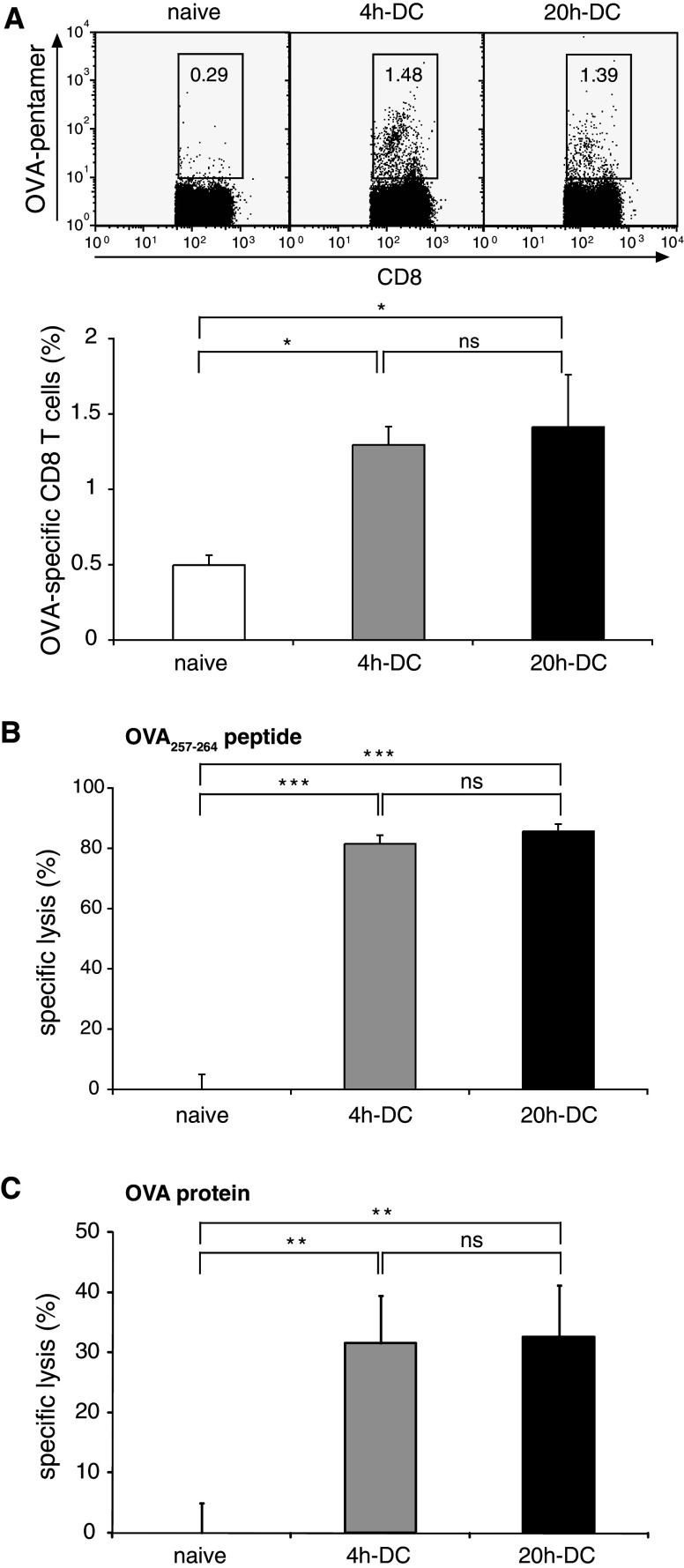
We demonstrated that short-term activated FL-DC induce antigen-specific CD8 T-cell proliferation and overcome Treg cell-mediated suppression in vitro. To examine the induction of antigen-specific CTL responses in vivo, naïve C57BL/6 mice were immunized s.c. with peptide-pulsed FL-DC stimulated with CpG for 4 h or 20 h. Specific CTL induction was analyzed 1 week after the second immunization. Immunization with short-term activated FL-DC was sufficient to induce a strong OVA-specific CTL response determined by MHC class I pentamer staining (Fig. 6a). Furthermore, specific target cell lysis in vivo demonstrated cytotoxic function of the induced CTL (Fig. 6b). Longer activation of DC for 20 h did not result in an increase in the frequency or cytotoxic activity of specific CTL. Similarly, no increase of cytotoxic activity was seen in mice immunized with 20 h-DC compared to 4 h-DC when DC were pulsed with whole OVA protein (Fig. 6c). Hence, 4 h-activated FL-DC induced an efficient CTL response in vivo that was not increased by longer CpG activation.
Fig. 6.
Short-term activated FL-DC generate an antigen-specific immune response in vivo. C57BL/6 mice were immunized twice s.c. with 2 × 105 FL-DC stimulated for 4 or 20 h with CpG and pulsed with OVA257–264 peptide. a Antigen-specific CD8 T cells in peripheral blood were measured by flow cytometry with H2-Kb OVA257–264 pentamers 1 week after the last immunization. Dot plots show representative data gated on CD8 T cells. Numbers indicate the percentage of CTL that are OVA-pentamer-positive. Graph shows mean and SEM of eight mice from two independent experiments. b, c To assess function of CTL, an in vivo cytotoxicity assay was performed by transferring CFSE-labeled unpulsed (CFSElow) and peptide-pulsed (CFSEhigh) target cells. Labeled cells were detected by flow cytometry in peripheral blood on day 3. Mice were immunized with peptide-pulsed DC b or protein-pulsed DC c. Mean specific lysis and SEM of five mice per group is shown. Results are representative of two independent experiments (*P < 0.05, ***P < 0.001)
Short-term activated DC induce a potent antitumor immune response
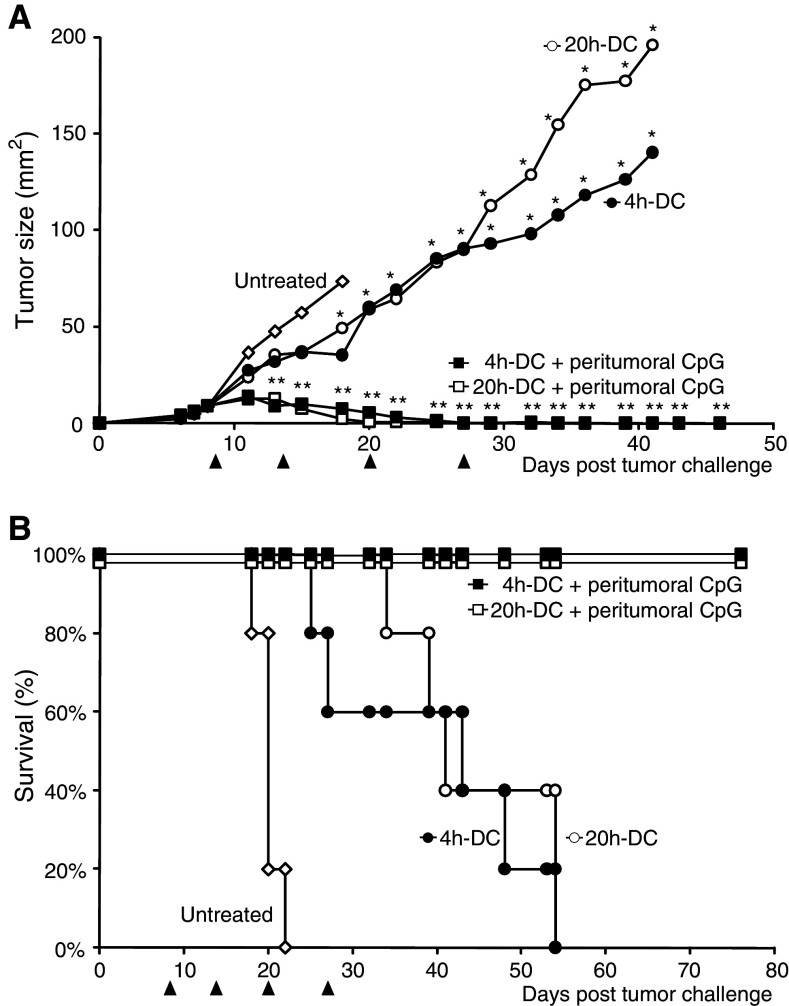
To examine whether the CTL response generated by short-term activated FL-DC controls tumor growth, mice bearing established C26 tumors were treated with an immunotherapy protocol combining CpG-activated DC with peritumoral CpG injections [14]. Immature DC pulsed with UV-irradiated tumor cells were activated in vitro with CpG. Antigen-pulsed, activated FL-DC injected together with 100 μg CpG on the flank opposite the tumor reduced tumor growth and led to a significant increase in survival compared to untreated mice (Fig. 7a, b). This therapeutic effect was independent of the duration of the in vitro activation of the DC. When the therapy was supported with an additional injection of CpG in the peritumoral area, all mice rejected their tumors and remained tumor-free for 4 months after the last DC vaccination. We have previously shown that using this protocol, peritumoral CpG injection alone does not result in a significant reduction of tumor growth [14]. In addition, immunization with irradiated tumor cells and CpG in the absence of DC did not protect against a subsequent tumor challenge and could not cure established C26 tumors [15]. To evaluate long-term protection, the cured mice were re-challenged s.c. with C26 tumor cells 4 months after the last treatment. None of the cured mice developed a tumor. In contrast, all naïve control mice developed tumors (P = 0.008, data not shown). We did not detect a difference between the mice treated with 4 h-DC or 20 h-DC, indicating the development of an efficient memory response for both stimulation regimens.
Fig. 7.
Short-term activated FL-DC are efficient for tumor therapy. Balb/c mice bearing palpable C26 tumors were injected s.c. with 100 μg CpG and CpG-activated FL-DC (4 h-DC: 4 h in vitro activation, 20 h-DC: 20 h in vitro activation) pulsed with irradiated tumor cells in the flank opposite the tumor. Two groups received an additional 100 μg CpG peritumorally. The treatment was administered four times at 5- to 7-day intervals (arrows). a 4 h-DC and 20 h-DC significantly reduced tumor growth compared to untreated mice (*P < 0.05 from day 18 onwards). The addition of peritumoral CpG led to complete tumor regression in all treated mice (**P < 0.01 compared to untreated mice at all time points from day 13). Mean tumor size of treatment groups (n = 5) is plotted until two mice per group remain. b Treatment with either 4 h-DC or 20 h-DC significantly increased survival (P < 0.01). Similar results were obtained in four independent experiments
TLR7/8 ligands trigger rapid activation of FL-DC
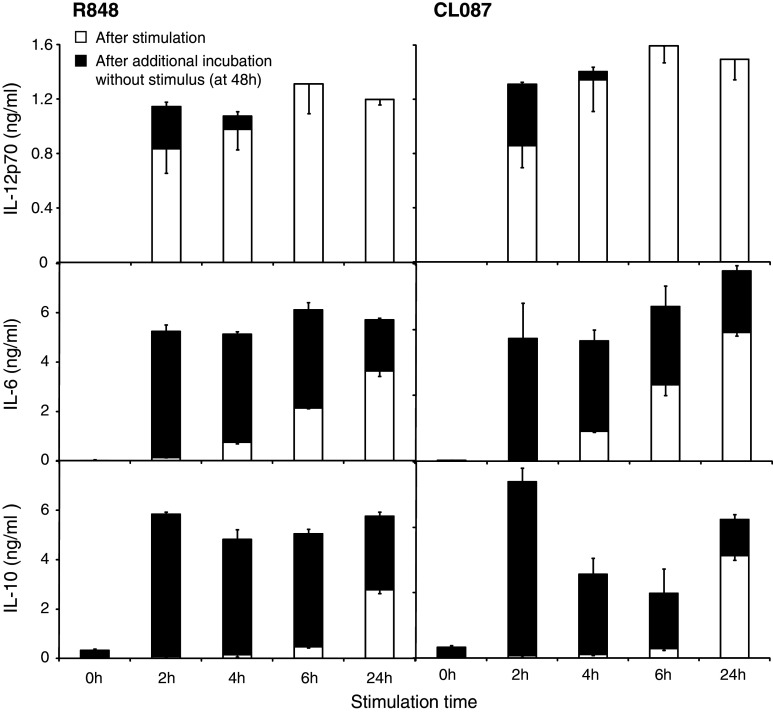
CpG as a TLR9 ligand enhances immune responses in many murine tumor models [26]. However, the distribution of TLR9 differs substantially between murine and human DC populations. In mice TLR9 is expressed by pDC and mDC, whereas in humans TLR9 expression is mainly restricted to pDC [27]. We therefore investigated the kinetics of FL-DC activation by ligands for TLR7 and 8, which are expressed on both human and murine mDC and pDC [19]. DC were stimulated with R848 as ligand for murine TLR7 (and TLR8 in humans) or CL087 as TLR7 ligand for 2 h up to 24 h (Fig. 8). After the stimulation period, cytokine levels were measured in the supernatant as in Fig. 1 (white bars). Cells were washed and culture was continued for a total of 48 h (black bars). For both ligands, IL-12p70 secretion was induced even more rapidly than with CpG and persisted after removal of the stimulus (Fig. 8). As with CpG stimulation, production ceased after 6 h. IL-6 showed a sustained secretion for over 24 h. IL-10 was again secreted late, and levels were highest after long stimulation times. Thus, as for TLR9, TLR7/8 activation induces rapid and efficient production of Th1 cytokines by DC that is restricted to the first 6–8 h after initiation of stimulation.
Fig. 8.
TLR7 activation triggers rapid activation of FL-DC. FL-DC were stimulated with the TLR7/8 ligand R848 or CL087 as TLR7 ligand for 2 to 24 h, extensively washed to remove excess ligands and cultured without further stimulation for a total of 48 h. Cytokine concentration in the supernatant was measured directly after the 2–24 h stimulation period (white bars) and at 48 h, after additional incubation without stimulus (black bars). Results show mean and SEM of one representative experiment of three
Discussion
For the optimization of DC-based cancer vaccines, key questions that remain to be resolved include the usage of the appropriate DC subtype and the most effective activation of DC. Maturation of DC is critical for the induction of antigen-specific CTL, but the most efficient strategies for DC maturation remain to be determined [7, 22]. In the majority of DC vaccination trials to date, DC have been extensively activated for 24–48 h [7, 22, 52]. However, such prolonged activation can result in extinction of cytokine production, in particular for the Th1-polarizing cytokine IL-12p70. This phenomenon, termed DC exhaustion, occurs independently of the stimulus used for maturation [17, 24, 28] and is associated with the absence of DC response to further stimulation both in vitro and in vivo [24, 28, 43]. In this study, we show that short activation of DC for 4 h by a TLR9 ligand triggers a complete maturation program including the rapid production of Th1-type cytokines, upregulation of co-stimulatory molecules, migration towards a CCR7 ligand and efficient antigen presentation to CTL. Importantly, the Th1-polarizing cytokines IL-12p70 and IFN-α were produced only in the early phase following DC stimulation, leading to a more efficient induction of IFN-γ-producing Th1 cells by short-term activated DC. Taken together, our results suggest that vaccination with short-term activated DC may ensure the benefit of fully mature migratory DC that still produce high levels of Th1 cytokines and thus promote the differentiation of Th1 cells.
Dendritic cell activation with the TLR9 ligand CpG leads to a Th1-type cytokine profile and to the efficient generation of antigen-specific cytotoxic T cells in vivo [26]. In most clinical studies to date DC maturation was however induced by a cytokine cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2 [23]. Although DC activated in this manner develop a mature phenotype and can drive T-cell proliferation, these DC are poor producers of IL-12p70 and thus defective in their ability to induce the Th1 cells essential for a productive antitumor CTL response [24, 29, 48]. Moreover, DC matured with cytokines such as TNF-α are tolerogenic and induce suppressive Treg cells [32, 36]. Activation of DC with TLR ligands may therefore provide more effective antitumoral immunity. Indeed, maturation of human DC with a combination of the TLR3 ligand poly I:C and inflammatory cytokines induced more potent CTL responses in vitro than the classical cytokine cocktail [34].
Migration of DC towards gradients of the lymph node-directing chemokine CCL21 is key for enabling antigen-loaded DC to efficiently interact with T cells in draining lymph nodes [10]. Exposure of DC to PGE2 induces a migratory phenotype, but inhibits IL-12p70 production, thus impairing Th1-directed immunity [25, 31, 44, 45]. In this study, we show that short-term activation of DC by CpG enhances the migratory potential of DC to lymph-node directing chemokines. In contrast, extensive activation of DC for 24 h through CD40 stimulation inhibits DC migration [51]. Thus, short-term activation of DC with a TLR9 ligand has the advantage of inducing multifunctional DC capable of both lymph node migration and IL-12p70 production.
While many DC vaccination studies have utilized conventional or myeloid DC [40], we have differentiated DC in the presence of Flt3L in order to generate both mDC and pDC [2, 3, 37]. pDC can, upon TLR stimulation, produce high levels of type-I interferon that enhance the generation of Th1 responses by mDC [4, 11, 20, 30]. We demonstrate that a short activation is sufficient to induce strong IFN-α production by pDC. Indeed, because of the early extinction of IFN-α production, a short-term activation is essential in order to obtain the benefit of IFN-α secretion in vivo after DC transfer. We further show that the combination of short-term activated mDC and pDC is effective for the immunotherapy of established tumors. In addition, as has been previously described for long-term activated myeloid DC [14], we show a potent enhancement of the antitumoral effect of short-term activated Flt3L-derived DC when the vaccine is combined with a peritumoral application of CpG. Combination of myeloid and plasmacytoid DC is a potential strategy in clinical studies, as both these subsets can be generated for immunotherapy in tumor patients by the in vivo administration of Flt3L [6].
To date, the TLR9 ligand CpG has been identified as the most potent immune enhancer in mouse tumor models [26]. However, TLR9 expression in humans is mainly restricted to pDC [27]. Nevertheless, CpG has to date shown substantial evidence of antitumor activity in human clinical trials [26]. DC can also be stimulated via TLR7/8 and ligands of TLR7/8 have already been successfully used as vaccine adjuvants in clinical trials [47]. We have shown that stimulation with ligands that activate both TLR7 and TLR8 in humans trigger a rapid and efficient production of Th1-type cytokines similarly to CpG. In the future, other ligands for TLR7 such as specific RNA oligonucleotides may prove suitable for tumor therapy, as immunostimulatory RNA oligonucleotides induce potent T-cell responses in mice and activate both myeloid and plasmacytoid human DC through TLR7 [1, 16, 18]. Furthermore, the combination of ligands for different TLR can lead to potent synergy for the induction of IL-12 and Th1-polarization by DC in both mice and men [38, 50].
In summary, we show that short-term activation of FL-DC with a TLR ligand induces a potent antitumor CTL response. Thus, the kinetics of DC activation may play an important role for the induction of objective clinical responses by DC vaccines. We propose that shorter DC maturation protocols in human clinical trials may result in strong antitumoral immunity with the benefit of multifunctional migratory DC that produce high levels of Th1 cytokines.
Acknowledgments
This study was supported by grants from the Else-Kröner Fresenius Foundation (Carole Bourquin and Stefan Endres), the Deutsche Krebshilfe (10-2214-En3 to Stefan Endres, Max-Eder research fellowship to Max Schnurr), the German Research Foundation (DFG En 169/7-2 to Stefan Endres and Carole Bourquin, Graduiertenkolleg 1202 to Carole Bourquin, Stefan Endres, and Max Schnurr, SFB-TR 36 to Stefan Endres) and from LMUexcellent (cluster CiPSM 114 and research professorship to Stefan Endres). We thank Nadja Sandholzer for her expert technical assistance and Jiwu Wei and Raffaela Tyroller for providing survivin protein.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- FL-DC
Flt3L-derived DC
- Flt3L
Fms-like tyrosine kinase 3 ligand
- mDC
Myeloid DC
- OVA
Ovalbumin
- pDC
Plasmacytoid DC
- TLR
Toll-like receptor
- Treg cell
Regulatory T cell
References
- 1.Bourquin C, Schmidt L, Hornung V, Wurzenberger C, Anz D, Sandholzer N, Schreiber S, Voelkl A, Hartmann G, Endres S. Immunostimulatory RNA oligonucleotides trigger an antigen-specific cytotoxic T-cell and IgG2a response. Blood. 2007;109:2953–2960. doi: 10.1182/blood-2006-07-033258. [DOI] [PubMed] [Google Scholar]
- 2.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- 3.Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169:6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178:1655–1663. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Dehm S, Bonham K, Kamencic H, Juurlink B, Zhang X, Gordon JR, Xiang J. DNA array and biological characterization of the impact of the maturation status of mouse dendritic cells on their phenotype and antitumor vaccination efficacy. Cell Immunol. 2001;214:60–71. doi: 10.1006/cimm.2001.1883. [DOI] [PubMed] [Google Scholar]
- 6.Davis ID, Chen Q, Morris L, Quirk J, Stanley M, Tavarnesi ML, Parente P, Cavicchiolo T, Hopkins W, Jackson H, Dimopoulos N, Tai TY, MacGregor D, Browning J, Svobodova S, Caron D, Maraskovsky E, Old LJ, Chen W, Cebon J. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 7.de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 8.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 10.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/S0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher R. Tagging T cells: TH1 or TH2? Science. 1997;275:1615. doi: 10.1126/science.275.5306.1615. [DOI] [PubMed] [Google Scholar]
- 12.Groothuis TA, Neefjes J. The many roads to cross-presentation. J Exp Med. 2005;202:1313–1318. doi: 10.1084/jem.20051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 14.Heckelsmiller K, Beck S, Rall K, Sipos B, Schlamp A, Tuma E, Rothenfusser S, Endres S, Hartmann G. Combined dendritic cell- and CpG oligonucleotide-based immune therapy cures large murine tumors that resist chemotherapy. Eur J Immunol. 2002;32:3235–3245. doi: 10.1002/1521-4141(200211)32:11<3235::AID-IMMU3235>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdorfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Hokey DA, Larregina AT, Erdos G, Watkins SC, Falo LD., Jr Tumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunity. Cancer Res. 2005;65:10059–10067. doi: 10.1158/0008-5472.CAN-05-1692. [DOI] [PubMed] [Google Scholar]
- 18.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 19.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Amakawa R, Inaba M, Hori T, Ota M, Nakamura K, Takebayashi M, Miyaji M, Yoshimura T, Inaba K, Fukuhara S. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol. 2004;172:4253–4259. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 22.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 23.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 24.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 25.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.V97.11.3466. [DOI] [PubMed] [Google Scholar]
- 26.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::AID-IMMU3026>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 28.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 29.Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/S0264-410X(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 30.Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178:1534–1541. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 31.Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA, Maliszewski C, Shortman K, Cebon J, Maraskovsky E. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 32.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/S1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 33.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 35.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 36.Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, Erb K, Schuler G, Lutz MB. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8-dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 38.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4 + CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 40.Proudfoot O, Pouniotis D, Sheng KC, Loveland BE, Pietersz GA. Dendritic cell vaccination. Expert Rev Vaccines. 2007;6:617–633. doi: 10.1586/14760584.6.4.617. [DOI] [PubMed] [Google Scholar]
- 41.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 42.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Reis e Sousa C, Yap G, Schulz O, Rogers N, Schito M, Aliberti J, Hieny S, Sher A. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–647. doi: 10.1016/S1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 44.Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 45.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 46.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 47.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, Chen Q, Parente P, Jefford M, Masterman KA, Caron D, Chen W, Maraskovsky E, Cebon J. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immunol. 2004;4:9. [PubMed] [Google Scholar]
- 48.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 49.Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, Thielemans K, Bonehill A. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1513–1537. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–550. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe S, Kagamu H, Yoshizawa H, Fujita N, Tanaka H, Tanaka J, Gejyo F. The duration of signaling through CD40 directs biological ability of dendritic cells to induce antitumor immunity. J Immunol. 2003;171:5828–5836. doi: 10.4049/jimmunol.171.11.5828. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, Tanaka R. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 53.Zhong H, Shurin MR, Han B. Optimizing dendritic cell-based immunotherapy for cancer. Expert Rev Vaccines. 2007;6:333–345. doi: 10.1586/14760584.6.3.333. [DOI] [PubMed] [Google Scholar]