Abstract
Aim
Several lines of evidence suggest that NK cell immunotherapy may represent a successful approach in neuroblastoma (NB) patients refractory to conventional therapy. However, homing properties, safety and therapeutic efficacy of NK cell infusions need to be evaluated in a suitable preclinical murine NB model.
Materials and methods
Here, the therapeutic efficacy of NK cell infusions in the presence or absence of NK-activating cytokines have been evaluated in a NB metastatic model set up in NOD/scid mice, that display reduced functional activity of endogenous NK cells.
Results
In NOD/scid mice the injected NB cells rapidly reached all the typical sites of metastatization, including bone marrow. Infusion of polyclonal IL2-activated NK cells was followed by dissemination of these cells into various tissues including those colonized by metastatic NB cells. The early repeated injection of IL2-activated NK cells in NB-bearing NOD/scid mice significantly increased the mean survival time, which was associated with a reduced bone marrow infiltration. The therapeutic effect was further enhanced by low doses of human recombinant IL2 or IL15.
Conclusion
Our results indicate that NK-based adoptive immunotherapy can represent a valuable adjuvant in the treatment of properly selected NB patients presenting with metastatic disease, if performed in a minimal residual disease setting.
Keywords: NK cells, Neuroblastoma, NOD/scid, Metastasis, Immunotherapy
Introduction
Neuroblastoma (NB) is the most common extracranial tumor in infants and the fourth in children. It originates either from primitive sympathetic nervous cells of the adrenal gland or in the spinal roots of extracranial nerves [1, 2]. NB has a broad spectrum of clinical presentation, varying from metastatic disease (stage 4), which occurs in more than 50% of cases at diagnosis, to spontaneous maturation and even regression, which mainly occurs in infants [3]. Indeed, prognosis depends on age and stage, being Myc-N amplification an adverse prognostic factor [4]. In stage 4 patients metastatic spread mainly involves bone and bone marrow (BM) and relapse or progression in this latter site is the major predictor of adverse prognosis. Despite multimodal therapy, followed by autologous hematopoietic stem cell transplant, their overall survival is approximately 20% at 5 years [5].
Not surprisingly, several new therapeutic approaches have been extensively tested in vitro and less frequently in vivo. Among these latter approaches, adjuvant immunotherapy by means of cytokine-engineered cells or anti-GD2 antibody administration has been exploited in NB patients. Complete responses or stabilization of disease have been associated with increase in NK cell number and activity [6, 7], and NK cell mediated-ADCC [8–10].
NK cells have been demonstrated to play an important role in controlling the growth of various tumor cell lines injected in mice [11, 12], including NB [13]. Moreover, NK cell increase in number and activity has been correlated to clinical responses in several gene therapy protocols, including the ones performed in NB patients [6, 7]. On these premises, clinical grade purifications of human NK cells have been reported and phase I trials have been started in patients with various types of cancer, including NB [14–16]. However, no data on homing properties, safety and therapeutic efficacy of NK cell infusions in preclinical murine NB models that closely resemble stage 4 diseases are available at present.
NK cells can kill tumor cells which lack the expression of HLA class I molecules, i.e. the ligands of the inhibitory killer Ig-like receptors (KIRs) [17]. We have recently demonstrated [18] that BM-infiltrating NB cells express little or no HLA class I molecules, while they may express the poliovirus receptor (PVR), which represents a ligand for the activating NK receptor DNAX accessory molecule 1 (DNAM-1) [19]. This latter receptor/ligand interaction appears to play a critical role in the NK-mediated neuroblastoma cell killing by activated NK cells. Thus, the probability of metastatic NB cells to be killed in vitro by activated NK cells depends on the expression or lack thereof of PVR ligand [19].
In the present study, we have set up a NB metastatic model in NOD/scid mice to evaluate the efficacy of NK cells-based immunotherapy. In this model, mice that were not treated with NK cells developed a metastatic disease reminiscent of that observed in stage 4 patients. In our study the therapeutic efficacy of activated NK cell infusions was evaluated in the presence or absence of NK-activating cytokines such as IL2 and IL15. Our results indicate that repeated early NK cell infusions can prolong the survival of neuroblastoma-bearing mice and reduce bone marrow infiltration. Thus, NK-based immunotherapy of NB may represent a valuable adjuvant in a minimal residual disease setting.
Materials and methods
Mice
NOD/scid mice (NOD.CB17-Prkdcscid/J) were purchased from Jackson Laboratories (Bar Harbour, ME, USA) and a colony maintained at the Animal Facility of the Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy. The animals were housed in specific pathogen-free colony and were feed with sterile food and water. The experiments, performed according to the National Regulation on Animal Research Resources, were approved by the Animal care and use Committee of the Istituto Nazionale per la Ricerca sul Cancro. Groups of 4 to 12-week-old mice (both male and female) were injected intravenously (iv) in the tail vein with the indicated amount of tumor cells and of IL2-activated human NK cells in 100 μL of serum free medium. Different schedules of treatment were tested as indicated in the “Results” section. Mice were monitored for disease symptoms and terminated by CO2 asphyxiation. Autopsy was performed on all terminated mice and organs were subjected to histopathological analysis.
NB cell lines
The NB tumor cell lines used were the HTLA-230, GI-LI-N, SH-SY-5Y, cultured as previously described [20]. These cell lines are phenotypically similar to metastatic bone marrow NB cells [19]. Cell viability was checked before injection in mice and always found higher than 95%.
NK cells
NK cells were purified from PBL of healthy donors using the Human NK Cell Enrichment Cocktail-RosetteSep (StemCell Technologies Inc, Vancouver, BC, Canada) and cultured on irradiated feeder cells in the presence of 100 IU/mL human recombinant (hr)IL2 (Proleukin, Chiron Corp., Emeryville, USA) and 1.5 ng/mL Phytohemagglutinin (PHA) (Gibco Ltd, Paisley, UK) in order to obtain polyclonal NK cell populations. Every set of experiments was performed by using the same polyclonal NK cell population. All the polyclonal NK cell population used in the in vivo experiments were derived from the same donor to reduce variability.
Since the NB cells do not express surface HLA class I antigens, the polyclonal NK cell population was not genotyped for KIR expression [17].
Cytotoxicity assay
IL2-activated polyclonal NK cells were tested for cytolytic activity against the indicated cell lines in a 4-h 51Cr-release assay. The effector/target (E/T) ratios are indicated in the text.
Flow cytometry
For one-color cytofluorimetric analysis (FACSCalibur, Becton Dickinson & Co, Mountain View, CA, USA) polyclonal NK cells or HTLA-230 neuroblastoma cell line were washed twice in serum free RPMI-1640 and incubated at a final concentration of 107 cells/ml in 2 μM CFSE (5-(6)-carboxyfluorescein diacetate, succidimylester, Molecular Probes, Paisley, UK) for 7 min at 37°C [21]. Labelled cells (green) were washed twice in RPMI-1640 containing 10% FCS. For cytofluorimetric analysis of surface molecule expression, NK cells were stained with monoclonal antibodies (mAbs) specific for the indicated molecules followed by PE-conjugated goat anti-mouse isotype-specific second reagent. Controls were represented by cells stained with PE-conjugated goat anti-mouse isotype-specific second reagent alone.
Monoclonal antibodies
All the mAbs used: 289 (IgG2a, anti-CD3), BAB281 (IgG1, anti-NKp46), c218 (IgG1, anti-CD56), C127 (IgG1, anti-CD16), MAR93 (IgG1, anti-CD25), c227 (IgG1, anti-CD69), Z231 (IgG1, anti-NKp44) and KRA236 (IgG1, anti-DNAM-1), were produced in A. Moretta’s laboratory.
Cytokines
Purified hrIL2 (Proleukin, Chiron, Emeryville, USA) and hrIL15 (Pepro Tech-London, UK) were used in vivo at the concentration of 100 IU/mouse and 50 ng/mouse, respectively.
Histopathological analysis
All the different organs, as well as the spine, the anterior and posterior leg bones, were systematically removed from terminated mice and fixed for 48 h in formalin. Bones were then decalcified with Decal (DAKO S.P.A, Milano, Italy) for 2.5 h. All the tissues were processed for paraffin embedding, sectioned at 6 μm and stained with hematoxylin-eosin. For homing experiments 4 × 106 CFSE-labelled HTLA-230 cell line or 4 × 106 CFSE-labelled IL2-activated human NK cells, were iv injected in groups of three mice. Mice were then terminated at different time points under laminar flow and blood was immediately withdrawn by cardiac puncture. Bone marrow cells and splenocytes were flushed with sterile tissue culture media from the posterior femurs and tibias and spleen, respectively, and analysed by flow cytometry, together with the blood. All the other organs were immediately snap frozen in liquid nitrogen with OCT compound® (Sakura Europe, Zouterwoude, The Netherlands). Slides of 6 μm thick cryostatic sections were mounted in antifade solution with DAPI (4,6-diamidino-2-phenylindole, Vectashield, Vector Burlingame, CA, USA) and images were captured using a Nikon Eclipse E1000 epifluorescence microscope (Nikon Corp, Tokyo, Japan) equipped with filter sets for DAPI and FITC.
Statistical analysis
Survival curves were constructed by using the Kaplan–Meier method and the generalized Wilcoxon log-rank test (Peto-Prentice) was used to compare the curves. A P value of less than 0.05 was considered statistically significant. Mean survival times were calculated with 95% confidence interval. All tests were two sided. Statistical analyses were performed using the Statsdirect software (Statsdirects Ltd, Cheshire, UK).
Results
Tumorigenicity of NB cell lines in NOD/scid mice
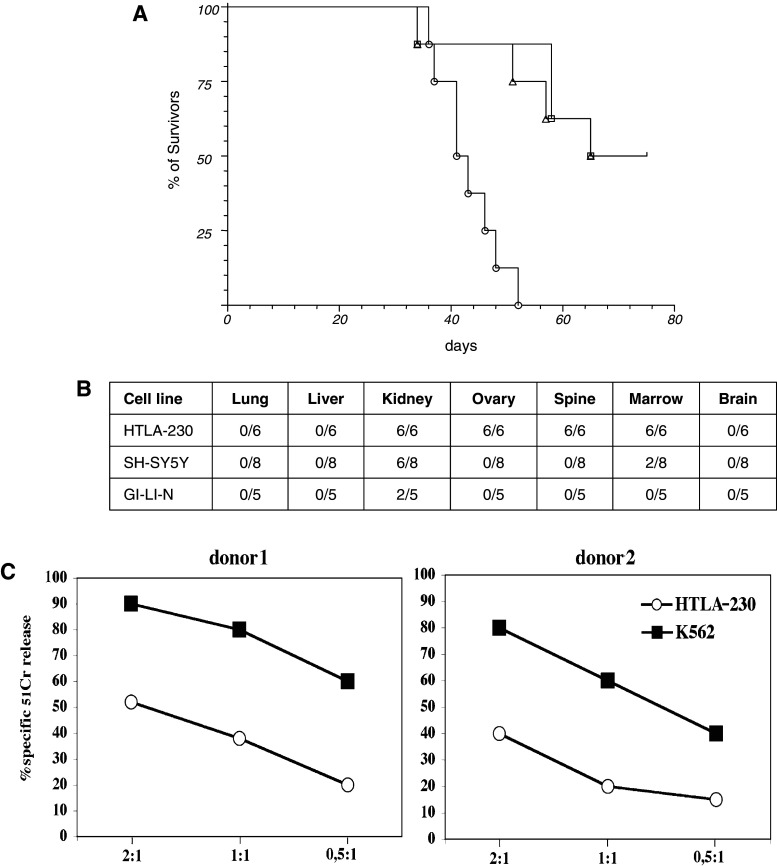
Three NB cell lines HTLA-230, GI-LI-N and SH-SY-5Y, phenotipically similar to metastatic bone marrow-isolated NB cells, were iv injected in groups of 8, 5-week old NOD/scid mice. Animals were terminated either at the appearance of bulky disease or 75 days post injection (pi). In both cases, autopsy was performed and all the organs were subjected to histopathological analysis. As shown in Fig. 1a, the HTLA-230 cell line was fully tumorigenic, leading to the death of all the animals within 60 days pi, while 50% of mice injected with GI-LI-N or SH-SY-5Y survived at day 75.
Fig. 1.
Tumorigenicity of NB cell lines in NOD/scid mice. a HTLA-230 (circle), SH-SY-5Y (square) and GI-LI-N (triangle) cell lines were iv injected in 5-week-old NOD/scid mice (8 mice/group) at the dose of 3 × 106 in 100 μL serum free medium. Mice were monitored and terminated when bulky disease was apparent (varying from 30 to 60 days) or at day 75 post injection. Terminated animals were next evaluated for the presence of metastasis in the various organs. b Presence of metastasis in the different organs evaluated when the mice showed in a were sacrificed. The experiment was performed twice with similar results. c Susceptibility of HTLA-230 cell line to NK-mediated cytolic activity. Effector cells represented by activated NK cell populations derived from two representative healthy donors were tested against the indicated cell lines at different effector to target (E:T) ratios. The results are representative of three independent experiments; the standard deviation of the mean of the triplicates was <5%. All the therapeutic experiments were performed with polyclonal NK cells derived from donor #1
The pattern of metastatization of HTLA-230 cells (Fig. 1b) evaluated at mice sacrifice was characterized in all instances by bone marrow metastasis and by abdominal masses involving the kidney and/or the adrenal glands, thus reproducing a pattern similar to that of advanced human stage 4 disease [3]. For the above reason our experiments were then based on the use of HTLA-230 cells. Quite importantly, as shown in Fig. 1c, this cell line was susceptible to NK-mediated killing [19].
Groups of 5-week-old NOD/scid mice were then iv injected with different amounts of HTLA-230 cells. The minimum tumorigenic dose, leading to the death of 100% of the mice, was approximately 1 × 106 (data not shown). Therapeutic experiments were thus performed using this dose of HTLA-230 cells.
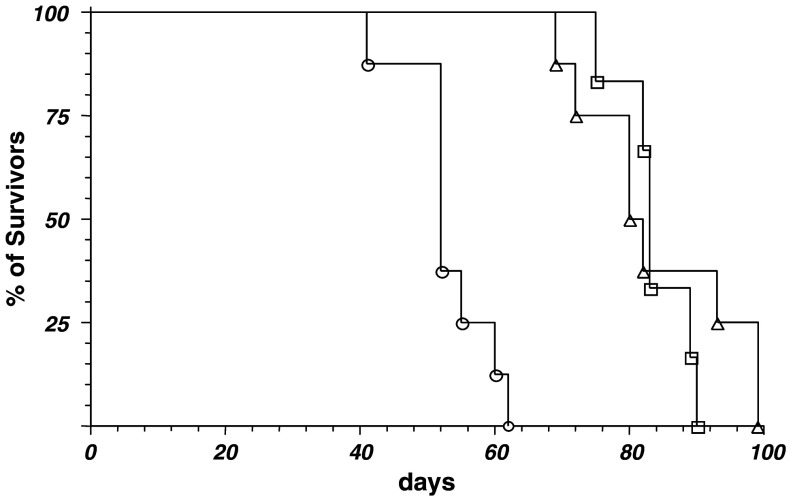
Next, groups of 6 NOD/scid mice of different age were iv injected with 1 × 106 HTLA-230 cells. Survival curves shown in Fig. 2 indicated that tumor growth was faster in young mice than in older ones. Therapeutic experiments were thus performed by using 5-week-old mice.
Fig. 2.
Age-dependent tumorigenicity of HTLA-230 cells. 1 × 106 HTLA-230 cells were iv injected in groups (6 mice/group) of 4 (circle), 8 (triangle) and 12 (square) week-old NOD/scid mice. Animals were monitored and terminated at the appearance of bulky disease. The experiment was performed twice with similar results
Fate of HTLA-230 cells injected in NOD/scid mice
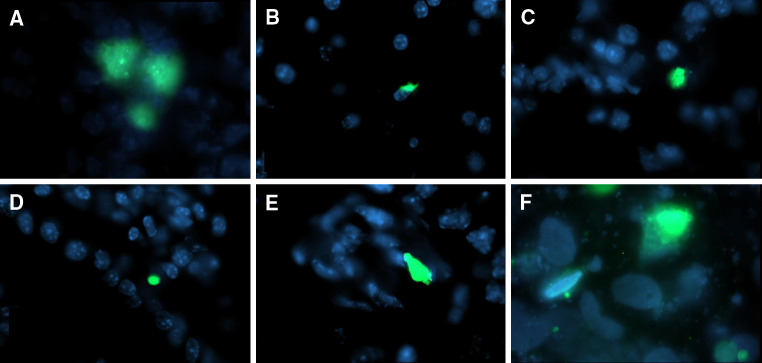
In order to evaluate a suitable time period for injecting NK cells in mice that received HTLA-230 cells we analyzed the distribution of CFSE-labeled tumor cells at various intervals pi. Thus, groups of three mice were terminated at different times pi and tissues and organs were analysed for the presence of fluorescent cells by flow cytometry (blood, spleen and flushed bone marrow) and by fluorescence microscopy (all the other tissues). CFSE-labelled HTLA-230 cells (green) were detected in all the organs analysed, (with the exception of the brain), already after 4 h pi, even in those that were not affected by metastatization (Fig. 3). Conversely, in the blood, HTLA-230 cells were detected only within 2 h from iv injection (data not shown), suggesting that most tumor cells may have reached the various tissues within a short time interval after injection.
Fig. 3.
Fate of injected HTLA-230 cell line; 4 × 106 CFSE-labeled HTLA-230 cells were iv injected in groups of 3 NOD/scid mice. Animals were terminated at different times pi, autopsy performed, organs removed and snap frozen in liquid nitrogen. Cryostat sections (6 μm thick) were then analyzed by fluorescence microscopy. Sections of organs removed 4 h pi are shown; CFSE-labeled HTLA-230 (green), organ cells (blue). a Lung, b liver, c kidney, d gut, e ovary, f bone marrow
Fate of human NK cells injected in NOD/scid mice
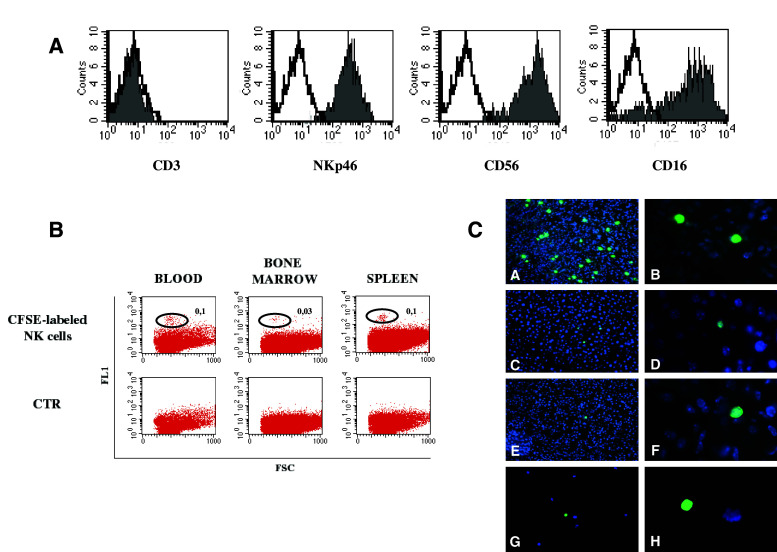
In order to evaluate whether NK cells, similarly to NB cells, could reach different tissues, including possible metastatic sites, highly purified, CD3-/CD56+/NKp46+/CD16+ polyclonal NK cells (Fig. 4a), cultured in vitro in the presence of exogenous hrIL2, were CFSE-labelled and iv injected in groups of three mice. These NK-injected animals were then terminated at different times pi. As shown in Fig. 4b, cytofluorimetric analysis allowed the detection of viable NK cells in blood (0.1%), bone marrow (0.03%) and spleen (0.1%). As expected, the surface phenotype of these NK cells was identical, in terms of activating and inhibitory receptors, to that of the pre-injection NK cells (not shown). Moreover, fluorescence microscopic analysis of tissue sections (Fig. 4c) demonstrated the presence of CFSE-labelled NK cells (green) in all organs analysed (with the exception of the brain). CFSE-labelled NK cells could be detected in the various organs both at 4 and 72 h pi, and in the blood up to 48 h pi. Remarkably, fluorescent NK cells could also be detected in the flushed bone marrow after 18–24 h pi.
Fig. 4.
Fate of injected IL-2 activated NK cells; 4 × 106 CFSE-labeled NK cells were iv injected in groups of 3 NOD/scid mice. Animals were terminated at different times pi. A Cytofluorimetric analysis of in vitro IL-2 activated NK cells demonstrating their purity. B Blood, bone marrow flushed from femurs, and spleen cell suspensions analyzed by flow cytometry. Percentages of CFSE-labeled NK cells (green) with respect to total white blood cell count are indicated. CTR was represented by cells derived from mice injected with unlabelled polyclonal NK cells. C Fluorescence microscopic analysis of cryostat sections of lung (a, b), liver (c, d), kidney (e, f) and bone marrow smears (g, h) at ×20 (a, c, e, g) and ×100 (b, d, f, h) magnification, respectively. Samples were from mice terminated at 24 h pi; CFSE-labeled NK cells (green), organ cells (blue)
Evaluation of the therapeutic efficacy of human NK cells infused in HTLA-230-bearing mice
In a first set of experiments, NOD/scid mice, iv injected with 1 × 106 HTLA-230 cells a week before, were treated with 4 × 106 in vitro IL2-activated human NK cells once a week for 4 weeks. Since no therapeutic effects were observed (data not shown), in a second set of experiments, four groups of 7 NOD/scid mice, iv injected with 1 × 106 HTLA-230 cells at time 0, were treated with medium alone (group 1) or with 4 × 106 in vitro IL2-activated human NK cells, at 24 h pi (group 2), at 4, 24 and 48 h pi (group 3) or at 4, 24, 48 and 96 h pi (group 4). With respect to untreated mice, NK-treated animals had prolonged survivals (Fig. 5a); the mean survival time (52.4, 54.7 and 55.7 vs. 43.4 days of untreated mice, respectively), as well as significance (P = 0.0469 for one treatment, P = 0.041 for three treatments, P = 0.028 for four treatments), increased according to the number of NK cell injections performed.
Fig. 5.
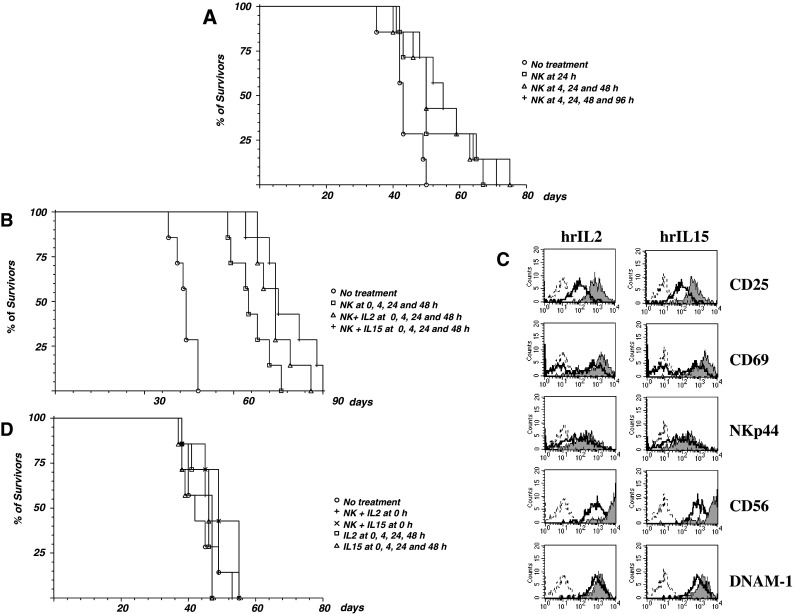
Therapeutic effects of NK cell infusions. Panel a four groups of 7, 5-week-old, NOD/scid mice were iv injected with 1 × 106 HTLA-230 cells, then animals were treated with medium alone (circle) or with 4 × 106 polyclonal NK cells at 24 h pi (square), at 4, 24 and 48 h pi (triangle) or at 4, 24, 48 and 96 h pi (plus sign). Panel b four groups of 7, 5 week-old, NOD/scid mice were iv injected with 1 × 106 HTLA-230 cells, then animals were treated at 0, 4, 24 and 48 h pi with medium alone (circle), with 4 × 106 polyclonal NK cells (square), with 4 × 106 polyclonal NK cells + hrIL2 (triangle) or 4 × 106 polyclonal NK cells + hrIL15 (plus sign). Panel c IL2-activated NK cells from donor #1 were cultured in the absence or in the presence of rhIL2 (100 IU/mL) and rhIL15 (50 ng/mL). After 3 days, cells were stained with mAbs specific for the indicated molecules, followed by PE-conjugated goat anti-mouse isotype-specific second reagent and analyzed by flow cytometry. Sketched profiles cells incubated with second reagent alone, white profiles cells cultured without cytokines, grey profiles cells cultured with the indicated cytokine. Panel d Five groups of 7, 5-week-old, NOD/scid mice were iv injected with 1 × 106 HTLA-230 cells, then animals were treated with medium alone (circle), with 4 × 106 polyclonal NK cells + hr IL2 at 0 h pi (square), with 4 × 106 polyclonal NK cells + hr IL15 at 0 hr pi (triangle), with 100 ng/mouse hrIL2 at 0, 4, 24 and 48 h pi (plus sign) or with 50 ng/mouse hrIL15 at 0, 4, 24 and 48 h pi (multiple sign). All the experiments were performed twice with similar results
In a third set of experiments, we tested whether the earlier treatments with polyclonal NK cells could increase the therapeutic efficacy and whether addition of NK-activating cytokines could further enhance it. As shown in Fig. 5b, mice treated with IL2-activated NK cells at 0, 4, 24 and 48 h had a significant better survival (mean survival time 66 days vs. 43.7 days of untreated mice, P = 0.0005). The difference with respect to the previous schedule of administration was significant (P = 0.042). Addition of hrIL2 (100 IU/mouse) or hrIL15 (50 ng/mouse) further increased survival (Fig. 5b, 74.1 and 77.8 days, mean survival time, respectively, P = 0.0005). The increase in survival obtained with the addition of exogenous cytokines to the polyclonal NK cells was significant (P = 0.040 for IL2 and P = 0.020 for IL15 vs. NK cells without cytokine). In line with these findings, NK cells cultured with these doses of either cytokine displayed substantial increments in the surface expression of several activation markers, including CD25 and CD69 molecules, and up-regulation of CD56 and DNAM-1 molecules (Fig. 5c).
Since anticipation of four treatments with polyclonal NK cells to time 0 significantly increased survival of the HTLA230-bearing NOD/scid mice, we further analyzed whether this effect was solely due to the early killing of circulating HTLA-230 cells. To this end, two groups of mice injected with HTLA-230 cells were infused at time 0 with either NK cells + hrIL2 (100 IU/mouse) or NK cells + hrIL15 (50 ng/mouse). In these groups, however, no further treatments were performed. As shown in Fig. 5d a single treatment at time 0 with NK cells + either cytokine did not affect survival (P = 0.233 for IL2 and P = 0.148 for IL15), ruling out that the therapeutic effect shown in Fig. 5b was merely consequent to the clearance of circulating tumor cells.
Finally, to exclude that the increase in survival time observed with the addition of either hrIL2 or hrIL15 to polyclonal NK cells was due to the activation of endogenous immune effectors or precursors, two groups of HTLA-230-injected mice were treated with hrIL2 (100 IU/mouse) or hrIL15 (50 ng/mouse) at 0, 4, 24 and 48 h pi. Four treatments with either cytokine in the absence of NK cells did not affect survival (Fig. 5d, P = 0.431 for IL2 and P = 0.647 for IL15). No short term toxic effect or long term histopathological changes could be observed in naïve NOD/scid mice injected up to four times with NK cells + either cytokine (data not shown).
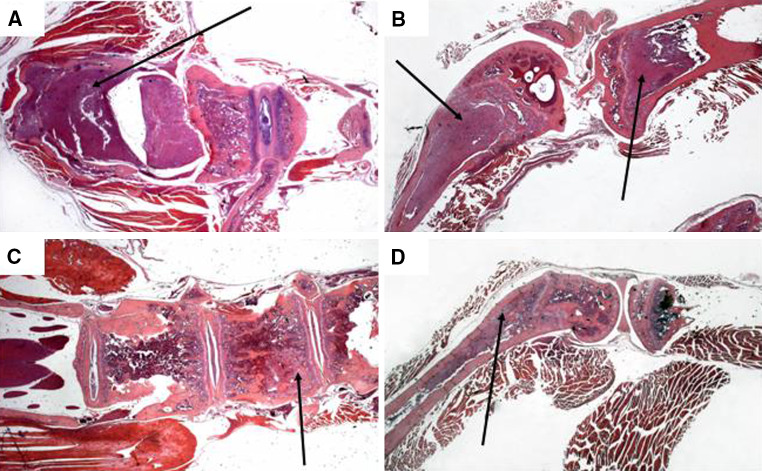
Histological analysis indicated that the pattern of metastatization was not modified by the treatment. However, the extent of bone and bone marrow infiltration was lower in the treated animals. Indeed, 13 out of 28 (46%) untreated mice showed paralysis of posterior legs, due to compression of the spinal cord by NB cells growing inside the vertebrae trabeculae (Fig. 6a), and all animals, but one, had extensive infiltration of femurs’ and tibias’ bone marrow (Fig. 6b). In contrast, the spinal cord of NK + cytokine-treated mice was consistently free of tumor cells (Fig. 6c), with the exception of two mice (7%). In addition, in treated mice, bone marrow infiltration was often limited to femurs, being tibias free of tumor cells in 12 out of the 28 (43%) NK + cytokine-treated mice (Fig. 6d).
Fig. 6.
Histological analysis of untreated and NK + cytokine-treated mice. Hematoxylin-eosin stained sections of spinal cord and vertebrae (left) and femur and tibia (right) from untreated (a, b) and NK cell + cytokine-treated (c, d) tumor-bearing NOD/scid mice. Magnification is ×12.5. Panel a arrow indicates neuroblastoma cells grown outside the vertebral bone towards the spinal cord. Panel b arrows indicate neuroblastoma cells grown in the femur and tibia bones. In the femur NB infiltration destroyed the bone structure while in the tibia NB growth is confined to the bone marrow. Panel c arrow indicates neuroblastoma cells inside the vertebral bone, on the left side the spinal cord appears completely free from metastatic invasion. Panel d arrow indicates neuroblastoma cells grown inside the femur bone marrow without destruction of the bone structure, the tibia bone marrow appears free of neuroblastoma cells
Discussion
In this study, the anti-tumor activity of polyclonal NK cells has been investigated in a model of human metastatic neuroblastoma set up in NOD/scid mice [22] by iv injection of a NB cell line phenotypically similar to metastatic NB [19]. The presence of metastases in the ovaries, which never occur in humans, has been previously reported in another metastatic HTLA-230 model set up in nude mice [23] and in immunocompetent A/J mice injected with murine NB cells [24].
Administration of IL2-cultured NK cells exerted a significant increase in survival time that correlated to the number of injections performed. Furthermore, when human NK cells were infused in combination with low dose hrIL2 or hrIL15, mice showed a further increment in survival as compared to NK-treated mice. Since these cytokines, at the doses used, did not display any direct anti-neuroblastoma effect as shown in Fig. 5d, it is conceivable, as reported in previous studies [25–28], that their role was to sustain the proliferation/survival of the infused NK cells allowing their homing to different organs. Indeed, in line with this concept, NK cells that have been cultured with hrIL2 or hrIL15 displayed incremented expression of various activation markers including CD25 and CD69 molecules. The observation that hrIFN-α, that enhances NK cytotoxicity but not NK proliferation/survival [29] did not increase the therapeutic effect of NK cells (not shown), further support this conclusion.
Following injection in the peritoneum, NK cells have been shown to occasionally reduce NB hepatic metastases [13]. Here, iv administration of NK cells together with tumor cells was able to control subsequent NB cell growth in a very aggressive metastatic model of NB. Within 4 h pi, animals that were not treated with NK cells displayed NB metastatization in various organs including typical sites observed in stage 4 patients. In our model, tumor cells reached all the organs, including sites of metastatization, as soon as 4 h post injection. Since NB cells disappeared from blood 2 h pi, it was unlikely that the observed therapeutic effect was consequent to NK cell-mediated killing occurring within the peripheral blood compartment. A single infusion of NK cells + cytokine, performed simultaneously to NB tumor cell injection, in fact, did not result in any therapeutic effect.
Although we do not show the interaction between NK and NB cells in the same tissue section, the correlation between the number of infusions and the increase in mean survival time strongly suggests that the therapeutic effect of NK cells might take place, at least in part, in tissues infiltrated by tumor cells. Indeed, infused human NK cells were detected in all organs analysed with the exception of brain, starting from 4 h post injection and up to 72 h, confirming previous studies showing that infused NK cells could infiltrate tumor xenografts [30–32] and exert antitumor activity [11–13, 30–32].
In our experimental conditions, the therapeutic effect of human activated NK cells administered with hrIL2 or hrIL15 was significant but incomplete. Conceivably, the therapeutic efficacy of NK cell immunotherapy in this model may have been improved by increasing the number of infusions or the number of NK cells administered each time. However, these modifications would impair the possibility to translate these results in the clinical setting. Several donors need to be envisaged for a single patient when numbers of NK cells and/or infusions are increased. Nonetheless, with doses of NK cells and a schedule of administration that can be scaled up to the patients, we demonstrated that NK cells reached metastatic sites and consistently reduced bone and bone marrow infiltration.
In stage 4 NB patients, bone marrow relapse is a major predictor of poor prognosis [5]. Furthermore, neurological manifestations culminating in spinal cord compression often arise from bone metastasis [33]. In mice treated with activated NK cells and low doses of cytokines, spinal cord compression was rarely observed and tumor infiltration was limited to the bone marrow, leaving the bone structure unaltered. Thus, when extrapolating these results to a clinical setting of minimal residual disease, such as that achieved after high dose chemotherapy, surgery, and autologous hematopoietic stem cell transplant [34], a relevant amelioration of neurological symptoms and a delay in the occurrence of bone marrow relapse may be expected to take place in NB patients following NK cell-based immunotherapy.
Our results therefore support the usefulness of NK-cell-based phase I/II immunotherapeutic protocol if performed in NB patients shortly after high dose chemotherapy and autologous transplant. Following these treatments, in fact, the tumor burden is often reduced to a minimum and metastatic NB cells are few [34], generally below the level of detection by sensitive methods [35]. In addition, depletion of hematopoietic precursors induced by high dose chemotherapy could facilitate homing of infused NK cells to the bone marrow and their activation, as demonstrated for infused T cells [36].
Human NB cells do not express HLA class I or class II antigens and display numerous defects in the expression of antigen processing machinery components [18, 37, 38]. These features make it highly unlikely that NB cells can evoke cytotoxic T lymphocyte responses directed to NB-associated antigens. On the other hand absence of surface HLA class I molecules renders human NB cells suitable targets for NK cell mediated cytotoxicity. However, only a fraction of patients express in their metastatic NB cells the poliovirus receptor (PVR, CD155), a ligand of the DNAM-1 (CD226), NK cytotoxicity activating receptor. Since the expression of PVR correlates with the sensitivity of NB cells to NK cell-mediated killing [19], eligibility of patients to NK-based immunotherapy must take this parameter into proper account.
Nonetheless, infused NK cells may also interact with other effector cells of innate or adoptive immunity likely contributing to enhance anti NB immune response [39]. In particular, NK cells have been shown to induce maturation of monocyte-derived dendritic cells [40, 41], thus promoting their ability to prime naïve T cells [42] by presenting NB-associated antigens. During this NK-DC interaction, cytokines produced by NK cells, including IFN-γ and TNF-α, may also up-regulate the surface expression of HLA class I molecules on NB cells [37, 43], which in turn may allow their recognition and destruction by patient’s CTLs. In essence, according to this model, activated NK cells could directly clear tumor cells left over after high dose chemotherapy and autologous transplant and contribute to the generation of memory CD8+ CTL responses displaying long-lasting anti-tumor immunity.
In conclusion, our data suggest that, after an accurate in vitro evaluation of the patient’s neuroblastoma cell susceptibility to NK-mediated cell lysis and of a minimal residual disease status, NB patients may benefit of NK cell-based immunotherapy.
Acknowledgments
Supported in part by grants from Fondazione Italiana per la Lotta al Neuroblastoma, Genoa, Italy (MVC); Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (AM and LM); Istituto Superiore di Sanità (AM), Ministero della Salute (MVC and LM) and Ministero dell’Università e della Ricerca Scientifica e Tecnologica, Rome, Italy (VP, AM and LM); Fondazione Compagnia di San Paolo, Torino, Italy (VP, AM and LM); and UE grant (European Union FP6, LSHB-CT-2004-503319-AlloStem, AM and LM). AD is the recipient of a FIRB fellowship awarded by Ministero dell’Università e della Ricerca Scientifica e Tecnologica. The excellent secretarial assistance of Ms C. Bernardini and the excellent technical support of Ms B Carlini are deeply acknowledged. Authors are in debt to Dr. E. Bogenman, Los Angeles, USA for the generous gift of the HTLA-230 cell line.
Footnotes
Roberta Castriconi and Alessandra Dondero equally contributed to the work.
References
- 1.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Schwab M, Westermann F, Hero B, Berthold F. Neuroblastoma: biology and molecular and chromosomal pathology. Lancet Oncol. 2003;4:472–480. doi: 10.1016/S1470-2045(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. Philadelphia: Lippincott–Raven; 2001. pp. 895–937. [Google Scholar]
- 4.Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 5.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 6.Brenner MK, Heslop H, Krance R, et al. Phase I study of chemokine and cytokine gene-modified autologous neuroblastoma cells for treatment of relapsed/refractory neuroblastoma using an adenoviral vector. Hum Gene Ther. 2000;11:1477–1488. doi: 10.1089/10430340050057549. [DOI] [PubMed] [Google Scholar]
- 7.Rousseau RF, Haight AE, Hirschmann-Jax C, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 8.Handgretinger R, Baader P, Dopfer R, et al. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 10.Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after stem cell tranplantation: a children’s cancer group study. J Clin Oncol. 2000;18:4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 11.Kuppen PJ, Gorter A, Hagenaars M, et al. Role of NK cells in adoptive immunotherapy of metastatic colorectal cancer in a syngeneic rat model. Immunol Rev. 2001;184:236–243. doi: 10.1034/j.1600-065x.2001.1840121.x. [DOI] [PubMed] [Google Scholar]
- 12.Basse PH, Whiteside TL, Chambers W, Herberman RB. Therapeutic activity of NK cells against tumors. Int Rev Immunol. 2001;20:439–501. doi: 10.3109/08830180109054416. [DOI] [PubMed] [Google Scholar]
- 13.Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci USA. 1994;91:9626–9630. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koehl U, Sorensen J, Esser R, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2004;33:261–266. doi: 10.1016/j.bcmd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7305. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 16.Passweg JR, Sern M, Koehl U, Uharek L, Tichelli A. Use of natural killer cells in hematopoetic stem cell transplantation. Bone Marrow Transplant. 2005;35:637–643. doi: 10.1038/sj.bmt.1704810. [DOI] [PubMed] [Google Scholar]
- 17.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626–633. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castriconi R, Dondero A, Corrias MV, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of dnam-1/pvr interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 20.Airoldi I, Lualdi S, Bruno S, et al. Expression of costimulatory molecules in human neuroblastoma. Evidence that CD40+ neuroblastoma cells undergo apoptosis following interaction with CD40L. Br J Cancer. 2003;88:1527–1536. doi: 10.1038/sj.bjc.6600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 23.Pastorino F, Brignole C, Marimpietri D, Sapra P, Moase EH, Allen TM, Ponzoni M. Doxorubicin-loaded Fab’ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63:86–92. [PubMed] [Google Scholar]
- 24.Croce M, Meazza R, Orengo AM, et al. Sequential immuno-gene therapy with interleukin-12- and interleukin-15-engineered neuroblastoma cells cures metastatic disease in syngeneic mice. Clin Cancer Res. 2005;11:735–742. [PubMed] [Google Scholar]
- 25.London L, Perussia B, Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J Immunol. 1986;137:3845–3854. [PubMed] [Google Scholar]
- 26.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, Croce CM, Baumann H, Caligiuri MA. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunne J, Lynch S, O’Farrelly C, Todryk S, Hegarty JE, Feighery C, Doherty DG. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol. 2001;167:3129–3138. doi: 10.4049/jimmunol.167.6.3129. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 29.Loza MJ, Perussia B. Differential regulation of NK cell proliferation by type I and II IFN. Int Immunol. 2004;16:23–32. doi: 10.1093/intimm/dxh001. [DOI] [PubMed] [Google Scholar]
- 30.Lozupone F, Pende D, Bugio VL, et al. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res. 2004;64:378–385. doi: 10.1158/0008-5472.CAN-03-1501. [DOI] [PubMed] [Google Scholar]
- 31.Hagenaars M, Zwaveling S, Kuppen PJ, et al. Characteristics of tumor infiltration by adoptively transferred and endogenous natural-killer cells in a syngeneic rat model: implications for the mechanism behind anti-tumor responses. Int J Cancer. 1998;78:783–789. doi: 10.1002/(SICI)1097-0215(19981209)78:6<783::AID-IJC17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Basse P, Herberman RB, Nannmark U, Johansson BR, Hokland M, Wasserman K, Goldfarb RH. Accumulation of adoptively transferred adherent, lymphokine-activated killer cells in murine metastases. J Exp Med. 1991;174:479–488. doi: 10.1084/jem.174.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bernardi B, Pianca C, Pistamiglio P, et al. Neuroblastoma with symptomatic spinal cord compression at diagnosis: treatment and results with 76 cases. J Clin Oncol. 2001;19:183–190. doi: 10.1200/JCO.2001.19.1.183. [DOI] [PubMed] [Google Scholar]
- 34.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s cancer group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 35.Seeger RC, Reynolds CP, Gallego R, Stram DO, Gerbing RB, Matthay KK. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a children’s cancer group study. J Clin Oncol. 2000;18:4067–4076. doi: 10.1200/JCO.2000.18.24.4067. [DOI] [PubMed] [Google Scholar]
- 36.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corrias MV, Occhino M, Croce M, et al. Lack of HLA-class I antigens in human neuroblastoma cells: analysis of its relationship to TAP and tapasin expression. Tissue Antigens. 2001;57:110–117. doi: 10.1034/j.1399-0039.2001.057002110.x. [DOI] [PubMed] [Google Scholar]
- 38.Raffaghello L, Prigione I, Bocca P, et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene. 2005;24:4634–4644. doi: 10.1038/sj.onc.1208594. [DOI] [PubMed] [Google Scholar]
- 39.Valteau-Couanet D, Leboulaire C, Maincent K, et al. Dendritic cells for NK/LAK activation: rationale for multicellular immunotherapy in neuroblastoma patients. Blood. 2002;100:2554–2561. doi: 10.1182/blood.V100.7.2554. [DOI] [PubMed] [Google Scholar]
- 40.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 42.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 43.Montaldo PG, Carbone R, Corrias MV, Cornaglia-Ferraris P, Ponzoni M. Synergic differentiation-promoting activity of interferon-γ and tumor necrosis factor: role of receptor regulation on human neuroblasts. J Natl Cancer Inst. 1994;86:1694–1701. doi: 10.1093/jnci/86.22.1694. [DOI] [PubMed] [Google Scholar]