Abstract
Cancer cells cannot develop into invasive cancers without interactions with cells and soluble mediators present in the tumor microenvironment. Accumulating evidence indicates that the immune system is a critical determinant of malignant outgrowth; however, the tumor-modulating effects of spontaneous immune responses towards nascent malignancies are rather paradoxical. Both cancer-protective and cancer-promoting features of the immune system have been described. This review will discuss the role of the dynamic inflammatory tumor microenvironment during cancer development and progression, and will focus on the intriguing question: “Do malignancies develop in spite of—or because of—spontaneous immune responses?” Special emphasis will be put on recent progress in our understanding of the immune system’s double-edged sword function during de novo carcinogenesis.
Keywords: Inflammation, Cancer, Innate immune system, Adaptive immune system, Immunosurveillance
Introduction
Conventional therapies against cancer have tremendously increased patient survival; however, still many cancer patients succumb to recurrent and metastatic disease. One of the major impediments to effective cancer therapy is acquisition of unresponsiveness to cytotoxic effects of chemotherapy regimens. There is a growing body of clinical and experimental observations supporting the concept that not only cancer cell autonomous processes determine malignant outcome, but that cancer cell-extrinsic processes occurring in the tumor microenvironment are as important as well. To increase survival of cancer patients, it will be critical to develop combinatorial approaches that not only target cancer cells—which are genetically instable and thus prone to become drug-resistant-, but also target the genetically stable tumor-supportive microenvironment and activate anti-tumor immunity. To successfully reach this goal, it is critical to understand how reciprocal interactions between cancer cells and stromal cells positively or negatively regulate tumor progression.
One of the key stromal players involved in cancer development is the immune system. Nascent tumors do not develop unnoticed by the immune system, and tumor development is almost always associated with recruitment and activation of adaptive and innate immune cells. Such spontaneous immune responses are not just bystander effects of cancer development, but instead modulate malignant outcome [13]. The importance of both the adaptive and innate immune system during cancer development is, however, controversial and a matter of intense debate. Already in the late nineteenth and early twentieth centuries, opposing roles of the immune system during cancer progression were described. In 1863, the German pathologist Virchow was the first to postulate that inflammation is one of the predisposing factors of tumorigenesis. He based this hypothesis on the observation that cancerous tissue frequently contained cells and factors that are hallmark features of inflammatory responses. His hypothesis has been ignored for more than 100 years, but recently experienced a renaissance [2]. As will be discussed in this review, a growing number of recent clinical and experimental data now support his hypothesis by revealing causal connections between inflammation and cancer.
On the other hand, Ehrlich in 1909, and later Thomas and Burnet, proposed the hypothesis that the immune system has the ability to spontaneously identify and eliminate cancer cells, and thus protects against tumor development; a process referred to as cancer immunosurveillance [6]. The first serious attempt to actively utilize the immune system to eradicate tumors was already applied in the 1890s by Coley, who treated cancer patients with bacterial preparations, now referred to as “Coley’s toxin”. Acute activation of the patient’s immune system with Coley’s toxin was reported to result in tumor regression in some cases [37].
More than a century after these contradicting hypotheses regarding the link between immune system and tumorigenesis were postulated, the role of spontaneous immune responses during cancer development is still a poorly understood and controversial topic. Whereas some recent experimental studies have provided convincing data supporting the tumor immunosurveillance theory, other compelling studies reveal that the immune system can promote tumorigenesis [13]. The fact that tumors do appear and develop despite an active and sometimes “efficient” immune response, indicates that at least in these cases the immune system has failed in protecting against cancer, and—as will be discussed here—might actually have contributed to progression of these tumors.
Immune system and cancer: protection, inertia, or promotion?
For a long time, it was assumed that the immune system has a protective role against nascent malignancies, like it is protecting us from infections with foreign pathogens. Indeed, various clinical observations support the concept that the immune system can prevent or inhibit certain cancers. For example, presence of infiltrating T lymphocytes in human colon cancers has been reported to correlate with improved prognosis [24], and patients with a suppressed adaptive immune system, e.g., AIDS or organ transplantation patients, have increased incidence of viral-associated malignancies [4]. However, over the years it has become clear that the role of the immune system during cancer development is more complex and can be opposing. For example, people with an intact immune system do develop malignancies, indicating that the immune system is not powerful enough to protect us completely from cancer. In addition, although cancer cells often express tumor-specific antigens and induce spontaneous anti-tumor T cell responses, these antigenic tumors frequently grow undisturbed. Also activation of anti-tumor T cell responses by various vaccination strategies only sporadically results in tumor eradication. Moreover, whereas patients with suppressed adaptive immune system do have a drastic increased incidence of viral-associated malignancies, incidence of non-viral epithelial malignancies such as breast and prostate cancer is not increased, and sometimes even decreased [13].
Over the last decade, a paradigm shift has occurred regarding the interplay between the immune system and cancer; there is a growing awareness that the immune system can also have a tumor promoting effect on nascent malignancies. This “dark side” of the immune system is supported by multiple clinical observations. For example, tumors are frequently characterized by massive influx of chronically activated innate immune cells. Presence of these cells often correlates with poor prognosis. In addition, cancers frequently arise at sites of chronic inflammation [1]. For example, patients with inflammatory bowel disease have a strong predisposition to developing intestinal malignancies. Moreover, long-term usage of anti-inflammatory drugs, like aspirin or selective COX–2 inhibitors, is associated with a reduced risk of various epithelial malignancies [29].
What have experimental studies taught us about the paradoxical tumor-promoting and tumor-protective effects of the immune system during cancer development and progression? Initial studies on the role of the immune system on cancer growth were performed with old-fashioned tumor transplantation models in which murine or human cancer cells were injected subcutaneously into immune-proficient and immune-deficient recipient mice. These pioneering studies mainly focused on the adaptive immune system, and frequently supported the concept of immunosurveillance and suggested that vaccination against cancer would be a powerful and effective anti-cancer approach. Although these early studies have increased our understanding of tumor antigens and tumor-specific T cells, care should be taken in the evaluation of these initial optimistic studies, as many of the promising, initial findings observed in tumor transplantation settings could not be recapitulated in more sophisticated de novo tumor models that more accurately resemble human cancer formation. For instance, Ochsenbein et al. [35] showed that injection of single cell suspensions of antigenic sarcoma cells resulted in efficient induction of anti-tumor cytotoxic T cell (CTL) responses followed by tumor rejection. In contrast, transplantation of solid tumor pieces containing the same antigenic sarcoma cells failed to induce CTL responses and resulted in tumor outgrowth. Likewise, Garbe et al. utilized a mouse model for de novo adenocarcinoma of the pancreas and found that—although these mice develop spontaneous immune cell responses with specificity for the tumor cells—these mice developed progressively growing spontaneous tumors. On the other hand, cell lines generated from these same tumors were found to be highly immunogenic and did not result in tumor formation upon injection in immune proficient mice [25]. These two examples and many other studies indicate that injection of a large bolus of cancer cells results in a mechanistically different interplay between immune system and cancer cells than is the case during “spontaneous” tumor formation. Several aspects might explain this discrepancy between tumor transplantation experiments and “spontaneous” tumor formation. For example, inoculation of suspensions of cancer cells results in massive tumor cell necrosis and early release of tumor antigens which could trigger acute adaptive immune responses, whereas spontaneously arising tumors frequently trigger more chronic innate immune responses [49]. Injected and sporadic tumors also have different growth kinetics, transplanted tumor cells ‘develop’ into palpable tumors without going through a premalignant phase, and the stromal microenvironment of injected tumors does not reflect the microenvironment of sporadic tumors. The artificial nature of lesions produced by inoculation of cancer cells has frequently resulted in skewed results not representative for the clinical situation, and might have misled us regarding the active interplay between immune system and cancers.
The recent availability of de novo mouse tumor models has allowed investigators to more carefully dissect the role of the immune system during spontaneous tumor development. Which lessons have we learnt from these more sophisticated and more clinically relevant spontaneous mouse tumor models? Thus far, many studies have revealed that sporadic tumors do induce specific adaptive immune responses; however, the degree of tumor control by these “natural” responses varies greatly per tumor model. Overall, the malignant outcome of the dynamic interplay between adaptive immune system and nascent malignancies can be divided into three scenarios.
Scenario 1: Protection
In this scenario, spontaneous immune responses elicited by nascent tumors recognize and eliminate cancer cells; however, after a phase of equilibrium, immunosurveillance leads to inadvertent selection of tumor escape variants which ultimately develop into clinically apparent neoplasms with reduced immunogenicity. Support for this so-called cancer immunoediting process, initially postulated by the group of Schreiber [17], has been provided by various experimental studies. The central model that has been utilized to document the existence of immunosurveillance and immunoediting is the chemical 3-methylcholanthrene (MCA)-induced carcinogenesis model. For example, IFNγ-insensitive mice displayed an increased sensitivity to MCA-induced sarcoma formation as compared to wild type mice [41]. In addition, RAG-2 deficient mice, which lack all mature T and B lymphocytes, were reported to have increased susceptibility to chemically induced tumor development [41]. Mice with other immune-deficiencies, e.g., perforin deficient mice, TCRβ deficient mice, STAT1 deficient mice and IL12p40 deficient mice, were also reported to have increased susceptibility to carcinogen-induced tumor formation (reviewed in [18]). Interestingly, few studies also reported that certain spontaneously arising tumors occur with increased frequency in aged RAG-2−/− and IFNγ−/− mice [18]. In contrast, however, another group has reported that RAG-1- deficient and control mice developed MCA-induced tumors at similar tumor frequencies [39]. In addition, SCID mice, nude mice and CD8 deficient mice do not show increased tumor incidence or reduced latency [3, 20, 44, 46]. Thus, whereas some studies support the concept that immunosurveillance is involved in controlling MCA-induced tumor formation, other studies do not support this concept. It remains to be established whether these discrepancies are caused by differences in administration route or dose of MCA, by differences in pathogenic status or genetic background of immune-deficient and immune-proficient mouse colonies, or by other variables.
In a recent study, it was demonstrated that the adaptive immune system is also critical for maintaining MCA-induced cancer cells in a “dormant” phase, i.e., in stable tumor masses; antibody-mediated elimination of adaptive immune cells at this tumor phase resulted in outgrowth of the tumor masses, thus providing support for the existence of an immune-dependent equilibrium phase [31]. Whether similar mechanisms play a role during formation of other types of cancers that are not initiated by exposure to carcinogens, but rather by loss of tumor suppressor genes or expression of oncogenes remains to be established.
Based on some studies that did find that MCA-induced tumors that developed in the presence of a functional immune system do grow out after transplantation into immune-proficient mice, whereas MCA-induced tumors from immune-deficient mice are rejected upon transplantation into immune proficient mice, it was hypothesized that the immune system not only has a cancer protective effect, but also a “tumor sculpting” effect [19]. This tumor-sculpting effect is an implicit consequence of immune-mediated elimination of immunogenic tumor cells, followed by inadvertent outgrowth of tumor escape variants with reduced immunogenicity. For further details on tumor escape mechanisms, the reader is referred to some excellent reviews [10, 17, 30]. It will be important to address whether this same mechanism also plays a role during formation of sporadic tumors that are not initiated by exposure to chemicals. As will be discussed below, there are clear indications that such tumors might be less prone to the process of cancer immunoediting.
In summary, these mechanistic studies indicate that certain tumor types, and in particular MCA-induced cancers, might be suppressed and edited by various components of the adaptive immune system. However, as discussed below, multiple spontaneous tumor model systems do not provide evidence for an “elimination” or “escape” phase by the endogenously activated adaptive immune system, but rather indicate alternative interactions between the adaptive immune system and spontaneously arising cancers.
Scenario 2: Inertia
In this scenario, de novo tumor formation is not suppressed by spontaneous adaptive immune responses, and selection of less immunogenic tumor escape variants does not occur. Various studies, including ours, did not find a protective and/or editing effect of the adaptive immune system during sporadic tumor formation. For example, Willimsky and Blankenstein utilized a mouse tumor model based on rare spontaneous activation of a dormant oncogene and found that tumors grew progressively, despite presence of spontaneous humoral and cellular immune responses with specificity for the tumor. In addition, these sporadic tumors did not lose their intrinsic immunogenicity, as they were rejected after transplantation in immune-competent mice. Mechanistic studies revealed that the sporadic tumors induced T cell tolerance, and thus were not affected by the adaptive immune system [47]. Likewise, in another tumor model, spontaneous pancreatic adenocarcinomas grew progressively in the presence of spontaneous tumor-specific adaptive immune responses, whereas cell lines generated from these same spontaneous tumors were found to be highly immunogenic and were rejected upon injection in immune-proficient mice [25]. These studies indicate that the spontaneous tumors were not sculpted by the adaptive immune system, as they did not represent escape-variants that had lost their immunogenicity; instead, it appears that these tumors rather avoided immune destruction by sculpting the immune system.
Whereas some studies have shown that absence of adaptive immune cells increases susceptibility to MCA-induced carcinogenesis, genetic elimination of adaptive immune cells in transgenic mouse models for de novo tumorigenesis does not affect malignant outcome. For example, in a transgenic mouse model for pancreatic islet cell carcinogenesis, e.g., RIP1–Tag2 mice, it was shown that genetic elimination of the T and B cell compartment did not affect tumor progression [7], suggesting absence of elimination of cancer cells by the adaptive immune system. Whereas the MCA chemical carcinogenesis model has been the central model to document the existence of cancer immunosurveillance and immunoediting, another chemical carcinogenesis model, e.g., 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol 13-acetate (TPA)-induced carcinogenesis, appeared not to be inhibited by immunosurveillance mechanisms, as tumor incidence was unaltered in perforin deficient animals [46], and even reduced in TNFα−/− mice [34], and in αβ T cell deficient mice [26].
Why does the adaptive immune system not suppress tumor growth and/or affect tumor immunogenicity in these studies? Several underlying mechanisms have been described, including, but not limited to, failure of T cell homing to the tumor [5, 25], induction of T cell tolerance [47], presence of local immunosuppressive networks [50], and collaboration with pro-tumor inflammatory responses [14]. Thus, besides the concept of immunoediting in which the adaptive immune system sculpts developing tumors, these studies suggest an alternative concept in which a spontaneously developing tumor sculpts or avoids anti-tumor adaptive immune responses. Moreover, as will be discussed in more detail below, there is a third scenario by which the adaptive immune system can influence tumor progression; the adaptive immune system can also promote tumor formation through modulation of the inflammatory tumor microenvironment.
Scenario 3: Promotion
Thus far, tumor–host interactions have been largely ignored in studies exploring the significance of the adaptive immune system during de novo tumor formation. Recently, however, people have begun to realize that tumors do not develop without extensive reciprocal interactions with stromal cells, including immune cells, endothelial cells, fibroblasts, adipocytes, and their soluble mediators. Importantly, these tumor-microenvironmental processes also influence systemic and local anti-tumor adaptive immune responses. Many recent studies have underscored the significance of chronically activated innate immune cells in determining malignant outcome [1]. For example, mast cells are essential for full tumor development in experimental mouse models for de novo skin and pancreatic islet carcinogenesis [8, 43]. Likewise, macrophages play critical roles in late stage mammary tumor and metastasis formation in the PyMT mouse mammary tumor model [33]. Also other studies have revealed that genetic alterations alone are not sufficient for tumor promotion, but that signals from the inflammatory tumor microenvironment are critical for tumorigenesis [23, 27, 28, 38]. As chronic inflammation is a complex and dynamic process with different cells and soluble mediators involved, it is no surprise that multiple mechanisms have been identified via which inflammatory states can promote cancer development [1, 9, 33, 38, 48]. Inflammation directly contributes to cancer development by production of reactive oxygen species that can promote malignant mutations [36], or by paracrine regulation of signal transduction pathways inside cancer cells [12]. In addition, inflammatory cell-derived soluble mediators can modulate proliferation, migration and survival of mutated epithelial cells. Indirect mechanisms via which inflammation promotes malignant outcome are activation of angiogenesis and stimulation of tissue remodeling [12]. Importantly, chronic inflammation also has a suppressive effect on anti-tumor adaptive immune responses. A subset of innate immune cells, e.g., myeloid derived suppressor cells, frequently accumulates in tumors and lymphoid organs, and indirectly enhances tumorigenesis through active suppression of anti-tumor immunity via induction of T lymphocyte dysfunction by direct cell-cell contact and by production of immunosuppressive factors [32, 40]. Likewise, malignant tissues attract regulatory T cells that are known to suppress anti-tumor immunity [11]. Thus, studies aimed at dissecting the importance of the immune system during cancer development or at developing immunotherapeutical approaches, should not focus on the adaptive immune system alone, but should encompass the entire context in which a tumor is developing, including the inflammatory tumor microenvironment. This is underscored by our studies in which we revealed that the adaptive immune system can actively contribute to a pro-tumor inflammatory tumor microenvironment in a transgenic mouse model for skin carcinogenesis, e.g., HPV16 mice. One of the earliest characteristics of skin cancer development in HPV16 mice is massive influx of innate immune cells, e.g., mast cells and neutrophils, and deposition of immunoglobulins in the dermis. To assess the functional significance of the adaptive immune system, we took a genetic approach and intercrossed HPV16 mice with RAG-1−/− mice. Surprisingly, absence of T and B lymphocytes did not accelerate tumor progression, but instead resulted in an almost complete absence of chronic inflammation in (pre-) malignant skin. As a consequence, levels of VEGF-A and gelatinolytic matrix metalloproteinases remained at steady-state levels, activation of an angiogenic vasculature was attenuated, oncoprotein-positive keratinocytes failed to attain a hyperproliferative phenotype, and overall carcinoma incidence was significantly reduced. Transfer of B lymphocytes or serum derived from HPV16 mice into T and B cell deficient-HPV16 mice was sufficient to restore chronic inflammation and other hallmarks of premalignancy [14]. This study indicates that the adaptive immune system, through crosstalk with the innate immune system, can modulate the tumor microenvironment in favor of tumor development and progression. It will be critical to investigate the underlying mechanisms, and whether similar inflammatory pathways are critical during formation of other tumor types. Consistent with our data, a link between humoral immunity and cancer progression was described by a study of Siegel et al., in which active immunization of cancer-prone immune-proficient mice resulted in induction of tumor-specific humoral immune responses and subsequent increased chemical-promoted tumorigenesis [42]. Likewise, interstitial antibody deposition does occur in cancer patients [13], and early presence of autoantibodies in serum of cancer patients is associated with poor prognosis [45]. My own studies are currently focusing on addressing the interplay between adaptive and innate immune systems in (conditional) mouse models for mammary carcinogenesis [16], where, similar to the HPV16 mouse model, immunoglobulins can be found in tumor stroma (Fig. 1). In order to develop strategies aimed at interfering with inflammation-driven tumorigenesis, it will be critical to elucidate the mechanistic link between humoral immune responses and pro-tumor inflammatory responses. Immunoglobulins and/or cytokines might be the critical soluble mediators linking B lymphocytes and innate immune cells. Since both immunoglobulins and complement components are deposited in stroma of (pre-) malignant skin lesions in HPV16 mice, we hypothesized that the complement system might link the humoral immune response with chronic inflammation; however, mechanistic studies showed that the complement system is not required for initiation or maintenance of inflammation in pre-malignant skin of HPV16 mice [15]. Alternatively, B lymphocytes might activate resident innate immune cells through crosslinking of Fc receptors expressed on resident immune cells by immunoglobulins, or via production of pro-inflammatory cytokines.
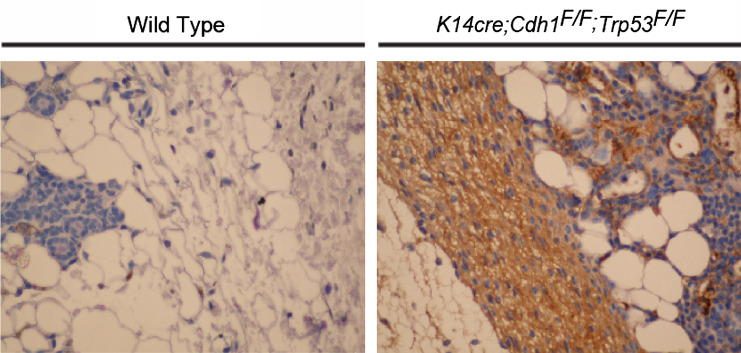
Fig. 1.
Immunoglobulin deposition is a hallmark of mammary tumorigenesis. Similar to (pre-) malignant skin of HPV16 mice [14] and human breast and prostate cancers [13], robust humoral immune responses are found during mammary tumor development in a conditional mouse model for invasive lobular carcinoma, e.g., K14cre; Cdh1F/F; Trp53F/F mice [16]. The immunohistochemical images show immunoglobulin deposition (IgG; brown staining) in interstitial stroma of malignant mammary glands from K14cre; Cdh1F/F; Trp53F/F mice. IgG depositions are absent in negative littermate controls, with exception of serum present in blood vessels. ×40 magnifications
In conclusion, the tumor microenvironment is frequently characterized by an immunological balance in favor of chronic inflammatory responses that foster cancer promotion and prevent or counteract anti-tumor adaptive immune responses.
Tumor intrinsic and extrinsic parameters influencing the nature of the crosstalk with the immune system
What determines which process—immune-mediated tumor protection or immune-mediated tumor promotion—is dominant in a particular tumor setting? Very likely, these different immune functions are not mutually exclusive, but rather co-exist in a dynamic balance that—in a spatiotemporal manner—can tip over in favor of tumor progression or tumor inhibition. This immune balance likely depends on many different cancer cell-intrinsic and cancer cell-extrinsic parameters. For example, the cancer-initiating trigger might be critical in skewing the immune system towards an anti-tumor or pro-tumor direction. Patients with a suppressed adaptive immune system, e.g., AIDS or organ transplantation patients, suffer from a drastic increased incidence of viral-associated malignancies, indicating that the immune system in healthy individuals frequently prevents these viral-associated malignancies from developing. Likewise, chemical-induced cancer might similarly be more easily prevented by adaptive immune responses than tumors initiated by genetic alterations [18, 47]. However, even different chemical-carcinogenesis models, e.g., the MCA versus the DMBA/TPA model, are shaped in a different degree by the interaction with the immune system [26, 46]. Similarly, the tissue of origin and tumor type might play a role in determining the nature of the interplay between cancer cell and immune system. Possibly, epithelial tumors trigger different natural immune responses as compared to non-epithelial tumors. Also cancer growth kinetics might determine whether the balance of the inflammatory tumor microenvironment is tipped over in favor of chronic pro-tumor inflammatory responses or acute anti-tumor immune cell responses. Recent observations suggest that expression of particular oncogenes directly instructs an inflammatory phenotype. For example, acute activation of a switchable form of Myc in a β-cell tumor model induced immediate expression of multiple chemokines, resulting in recruitment of mast cells which subsequently promoted further expansion of Myc-induced tumors [43]. Oncogene-driven expression of a certain array of inflammatory mediators might thus polarize the inflammatory microenvironment in favor of tumor progression, suggesting that the genetic make-up of a particular tumor might be critical in determining which leukocytes will be recruited to the genetic lesion, and how these leukocytes will behave. The microbiological status of the host can also influence the interplay between immune system and cancer, as exemplified by a study showing that increased tumor susceptibility of cytokine-deficient animals could be attributed to increased sensitivity to opportunistic infections, as antimicrobial therapy could prevent solid tumor formation in these animals [22]. Likewise, inflammation-associated colon cancer development in TGFβ1-deficient mice is blocked in a germ-free environment, and reintroduction of Helicobacter hepaticus reinstates inflammation and cancer development, indicating that enteric flora can be a driving force in establishing chronic inflammation and subsequent colon cancer development [21].
A deeper understanding of tumor intrinsic and extrinsic characteristics that influence the nature of the crosstalk with the immune system might open opportunities to suppress tumor-promoting immune responses and tip the balance over in favor of anti-tumor immune responses.
Conclusions
Do malignancies develop in spite of—or because of—natural immune responses? At least one thing is evident: the immune system exerts a tremendous effect on tumor development and progression. However, the nature and malignant outcome of the interplay between immune system and evolving cancer is dynamic and complex, involving extensive reciprocal interactions between genetically altered cells, adaptive and innate immune cells, their soluble mediators and other stromal cells present in the neoplastic microenvironment. The context in which a malignancy is developing largely determines whether the balance between immune system and malignancy will be tipped over in favor of undesirable tumor-promotion or desirable tumor-suppression. The fact that many clinical and experimental tumors do appear and develop despite an active immune response indicates that in these cases the immune system has failed in protecting against cancer. Recent experimental studies now clearly indicate that these spontaneous tumors are not devoid of immune cells, but instead are characterized by massive influx of innate immune cells, and that interference with the chronic inflammatory tumor microenvironment actually can prevent or inhibit tumor progression. Thus, these “successful” tumors largely progress because of their ability to avoid or redirect anti-tumor adaptive immune responses and to directly or indirectly exploit signals derived from the stromal immune cells.
Tremendous efforts have been and are being put into development and validation of successful immunotherapeutical approaches against cancer. Some of these efforts do seem to have potential in preventing development of malignancies; however, therapy of full-blown tumors remains relatively unsuccessful. One of the likely underlying mechanisms for this failure is presence of an immunosuppressive tumor microenvironment counteracting the activation status of adaptive immune cells or preventing penetration of anti-tumor T cells. For efficient eradication of well-established tumors, it may therefore be critical to combine standard anti-cancer approaches that target cancer cells directly with strategies aimed at perturbation of the cancer-supportive tumor stroma and with cancer immunotherapeutical approaches (Fig. 2).
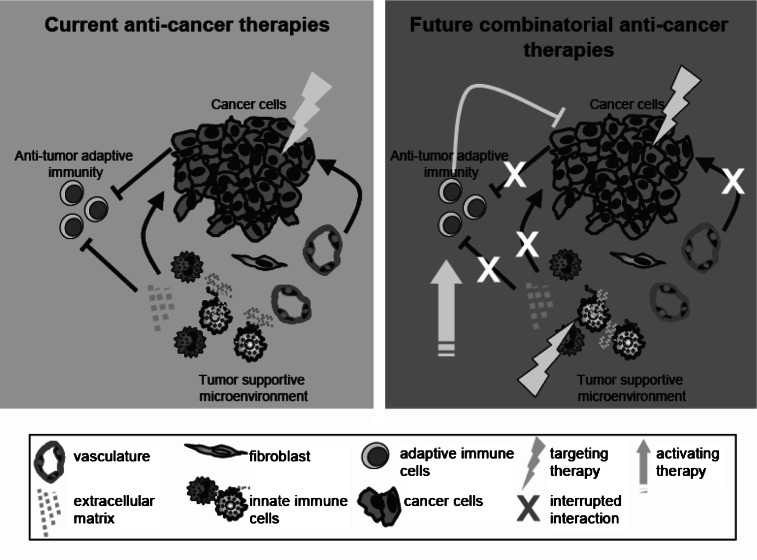
Fig. 2.
Schematic model of anti-cancer combinatorial approaches. Conventional anti-cancer therapies, such as chemotherapy, radiotherapy, and targeted therapies, target cancer cells directly (left panel). These standard therapies have tremendously increased patient survival; however, many tumors eventually become unresponsive towards their cytotoxic effects. It is now generally accepted that not only cancer cell autonomous processes determine malignant outcome, but that cancer cell-extrinsic processes occurring in the tumor microenvironment are as important as well. For efficient eradication of well-established tumors, it will be critical to develop combinatorial approaches that not only target cancer cells—which are genetically instable and thus prone to become drug-resistant-, but also target the genetically stable tumor-supportive microenvironment and activate anti-tumor adaptive immunity (right panel). To successfully reach this goal, it is critical to understand how reciprocal interactions between cancer cells and stromal cells positively or negatively regulate tumor progression
Acknowledgments
I thank Dr. Michiel de Bruin and Dr. Jos Jonkers for critically reading this manuscript. KEdV is supported by a grant from the Dutch Cancer Society (NKI2006-3715).
Footnotes
This article is a symposium paper from the conference “The European Society for Medical Oncology (ESMO) and the European Society for Cancer Immunology and Immunotherapy (ESCII) International Symposium on Immunology”, held in Athens, Greece, on 15–17 November 2007.
References
- 1.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 3.Blankenstein T. Do autochthonous tumors interfere with effector T cell responses? Semin Cancer Biol. 2007;17:267–274. doi: 10.1016/j.semcancer.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2:373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 5.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 6.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 7.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/S0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croci DO, Zacarias Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56:1687–1700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 12.de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–137. doi: 10.1159/000092969. [DOI] [PubMed] [Google Scholar]
- 13.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 14.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.de Visser KE, Korets LV, Coussens LM. Early neoplastic progression is complement independent. Neoplasia. 2004;6:768–776. doi: 10.1593/neo.04250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 18.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 19.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Engel AM, Svane IM, Rygaard J, Werdelin O. MCA sarcomas induced in scid mice are more immunogenic than MCA sarcomas induced in congenic, immunocompetent mice. Scand J Immunol. 1997;45:463–470. doi: 10.1046/j.1365-3083.1997.d01-419.x. [DOI] [PubMed] [Google Scholar]
- 21.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- 22.Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, Mihm M, Dranoff G. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–1219. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 25.Garbe AI, Vermeer B, Gamrekelashvili J, von Wasielewski R, Greten FR, Westendorf AM, Buer J, Schmid RM, Manns MP, Korangy F, Greten TF. Genetically induced pancreatic adenocarcinoma is highly immunogenic and causes spontaneous tumor-specific immune responses. Cancer Res. 2006;66:508–516. doi: 10.1158/0008-5472.CAN-05-2383. [DOI] [PubMed] [Google Scholar]
- 26.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 27.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 30.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 32.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 35.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohshima H. Genetic and epigenetic damage induced by reactive nitrogen species implications in carcinogenesis. Toxicol Lett. 2003;140–141:99–104. doi: 10.1016/S0378-4274(02)00506-4. [DOI] [PubMed] [Google Scholar]
- 37.Parish CR. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol. 2003;81:106–113. doi: 10.1046/j.0818-9641.2003.01151.x. [DOI] [PubMed] [Google Scholar]
- 38.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 39.Qin Z, Blankenstein T. A cancer immunosurveillance controversy. Nat Immunol. 2004;5:3–4. doi: 10.1038/ni0104-3. [DOI] [PubMed] [Google Scholar]
- 40.Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 42.Siegel CT, Schreiber K, Meredith SC, Beck-Engeser GB, Lancki DW, Lazarski CA, Fu YX, Rowley DA, Schreiber H. Enhanced growth of primary tumors in cancer-prone mice after immunization against the mutant region of an inherited oncoprotein. J Exp Med. 2000;191:1945–1956. doi: 10.1084/jem.191.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 44.Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974;183:534–536. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- 45.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 46.van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 48.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 49.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]