Abstract
Choosing a reliable source of tumor-specific T lymphocytes and an efficient method to isolate these cells still remains a critical issue in adoptive cellular therapy (ACT). In this study, we assessed the capacity of MHC/peptide based immunomagnetic sorting followed by polyclonal T cell expansion to derive pure polyclonal and tumor-reactive Melan-A specific T cell populations from melanoma patient’s PBMC and TIL. We first demonstrated that this approach was extremely efficient and reproducible. We then used this procedure to compare PBMC and TIL-derived cells from three melanoma patients in terms of avidity for Melan-A A27L analog, Melan-A26–35 and Melan-A27–35, tumor reactivity (lysis and cytokine production) and repertoire. Regardless of their origin, i.e., fresh PBMC, peptide stimulated PBMC or TIL, all sorted populations (from the three patients) were cytotoxic against HLA-A2+ melanoma cell lines expressing Melan-A. Although some variability in peptide avidity, lytic activity and cytokine production was observed between populations of different origins in a given patient, it differed from one patient to another and thus no correlation could be drawn between T cell source and reactivity. Analysis of Vβ usage within the sorted populations showed the recurrence of Vβ3 and Vβ14 subfamilies in the three patients but differences in the rest of the Melan-A repertoire. In addition, in two patients, we observed major repertoire differences between populations sorted from the three sources. We especially documented that in vitro peptide stimulation of PBMC, used to facilitate the sort by enriching in specific T lymphocytes, could significantly alter their repertoire and reactivity towards tumor cells. We conclude that PBMC which are easily obtained from all melanoma patients, can be as good a source as TIL to derive high amounts of tumor-reactive Melan-A specific T cells, with this selection/amplification procedure. However, the conditions of peptide stimulation should be improved to prevent a possible loss of reactive clonotypes.
Keywords: Melan-A, MHC/peptide, Immunomagnetic sorting, Melanoma, Adoptive immunotherapy
Introduction
Adoptive cell therapy (ACT) of cancer patients consists in the isolation, in vitro expansion and reinjection of tumor-reactive lymphocytes. While initial ACT trials in melanoma were performed with tumor-infiltrating lymphocytes (TIL) of undefined specificities [14, 24] the extensive characterization of numerous melanoma antigens has allowed the development of new ACT protocols targeting defined epitopes. Among melanoma-associated antigens recognized by tumor specific CTL, Melan-A is the most commonly expressed antigen by melanoma tumors and Melan-A epitopes presented in the HLA-A0201 context are the most frequently recognized by tumor infiltrating lymphocytes from melanoma patients [2, 17]. Therefore most of the recent ACT protocols have targeted these epitopes and documented encouraging immunological and clinical responses [8, 27, 28]. A remaining challenge to further improve these protocols is to design and validate a fast and simple method to obtain pure reactive Melan-A cytotoxic T cells from PBMC or TIL from all melanoma patients.
In some trials, Melan-A specific effector cells were peripheral blood T lymphocyte populations enriched by in vitro peptide stimulation [18, 20]. However, since none of these populations were pure, this strategy has the major drawback of injecting activated uncharacterized T cells together with the specific effectors. Purity was achieved in other studies by injecting T cell clones derived from these populations [27, 28] but this strategy presents the risk of picking a clone that, despite its selection in vitro, may turn out to be poorly active in vivo against the tumor. Furthermore, the cloning step and the subsequent expansion of a clone is a lengthy process requiring multiple in vitro stimulations that may drive the clone towards senescence and impair its reactivity in vivo [9, 10, 13, 21]. Because of these concerns, we chose immunomagnetic sorting with HLA/peptide coated beads [3] to isolate pure but yet polyclonal Melan-A specific T cells. The first issue of our study was to evaluate the efficiency of this method to achieve the expansion of polyclonal and tumor reactive T cells in a reproducible manner.
The second issue is the choice of a reliable source of tumor specific T lymphocytes and in this respect, PBMC and TIL (whether from tumor-invaded lymph nodes or from metastatic lesions) from melanoma patients have been used to obtain tumor specific T cells [8, 18, 20, 27, 28]. Although TIL from patients are frequently enriched in melanoma specific T cells [2, 17] and have been used with some success in ACT protocols [7, 16], they are very difficult to isolate except from invaded lymph nodes. The most convenient source of these cells would therefore be PBMC that can be obtained from all patients. Yet, differences in Melan-A/A2 repertoires from PBMC or TIL from the same patient have been reported [5, 19, 25] that could result in significantly different functional properties of Melan-A specific T cells from the two sources. In addition, in vitro stimulation of PBMC with peptide which has been used in these studies to increase the fraction of Melan-A reactive lymphocytes may further alter repertoire diversity. To formally evaluate whether Melan-A specific lymphocytes sorted from PBMC can be as good effectors as those sorted from TIL, we compared the function and repertoire of Melan-A specific T cell lines selected by MHC-multimer sorting from tumor-invaded lymph nodes (TILN) and from PBMC previously enriched or not in specific T cells by peptide stimulation.
Materials and methods
Cell lines
Melanoma cell lines “M” were established in our laboratory from metastatic tumor fragments as previously described [11]. The human mutant cell line CEMx721 T2 (T2) used as presenting cell was a generous gift from T. Boon (Ludwig Institute for Cancer Research, Brussels, Belgium).
Production of melanoma TIL
Short-term cultured TIL (gift from the unit of cellular and gene therapy of Pr. B. Dreno, Nantes) were isolated by culturing cryopreserved fragments of stage III metastatic lymph nodes into 12-well tissue culture plates with X-Vivo 15 serum-free medium (Bio*Whittaker, Walkersville, MD, USA) containing 150 U/ml rIL2 (Eurocetus, Rueil-Malmaison, France) and glutamine (1 nM) (Bio*Whittaker) for 10–14 days and then expanded on feeder cells as previously described [14].
Construction of HLA-A*0201/peptide tetramers
HLA-A0201/peptide α3-mutated monomers were generated as previously described [3]. Recombinant proteins were produced as inclusion bodies in E. coli XA90F’LacQ1, dissolved in 8 M urea, and refolded with 50 μg/ml of Melan-A A27L peptide (ELAGIGILTV), purchased from Eurogentech (Brussels, Belgium). Tetramerisation was performed as previously described [3]. Briefly, HLA monomers were biotinylated for 4 h at 30°C with 6 μg/ml BirA (Immunotech), purified on a monoQ column (Pharmacia, St Quentin en Yvelines, France) and tetramerised with PE-labeled streptavidin (Sigma, St Louis, MO, USA) at a molar ratio 4/0.8.
Tetramer staining
To minimize non-specific staining, HLA-A2-Melan-A tetramer was tittered and used at the lowest concentration that allowed the detection of 100% of a Melan-A specific T cell clone diluted among PBMC. PBMC or TIL were incubated for 1 h at 4°C in the dark with Melan-A tetramer (10 μg/ml). After washing in PBS-0.1% BSA, cells were resuspended in PBS and analyzed on a FACScan.
Immunomagnetic cell sorting and expansion of T cell sorted populations
HLA-A*0201/Melan-A monomers (20 μg/ml) were incubated for 1 h at room temperature with 6.7 × 106 streptavidin-coated beads (Dynabeads M-280 streptavidin, DYNAL, Compiegne, France) and washed in PBS/0.1% BSA. 5 × 106 PBMC were rotated for 4 h at 4°C with monomer-coated beads [3]. After ten washes, bead coated cells were expanded using a polyclonal T cell stimulation protocol [14].
Positive magnetic cell sorting of Vβ8 and Vβ14 specific cells was performed with Dynabeads (Dynal Biotec, Compiegne, France). Briefly, 5 × 106 lymphocytes were incubated with 15 μg/ml mouse anti-human Vβ8 or Vβ14 antibodies (Immunotech, Beckman-Coulter, Marseille, France) on ice for 45 min, washed twice with PBS/0.1% BSA and resuspended in culture medium. Subsequently cells were incubated with sheep anti-mouse IgG coated Dynabeads (Dynal Biotec, Compiegne, France) at a 1:1 ratio for 4 h at 4°C with gentle rotation. Rosetted Vβ + T cells were trapped by a magnet (MPC 6, Dynal Biotec) and washed ten times in PBS/0.1% BSA. The cell/bead suspension was incubated in culture medium in 6-well plates overnight at 37°C to allow beads to detach. After overnight incubation, beads were extracted by the magnet, and sorted lymphocytes were transferred on feeder cells, as previously described [14].
Briefly, 2000 bead-coated T cells/well were distributed in 96-well plates mixed with irradiated feeder cells [LAZ EBV-B cells (2 × 104/well) and allogeneic PBMC (105/well)], in 150 μl of culture medium supplemented with IL-2 (150 U/ml) and PHA (15 μg/ml).
Synthetic peptides
The Melan-A peptides 27–35 AAGIGILTV and 26–35 EAAGIGILTV and the decamer analogue A27L ELAGIGILTV were purchased from Eurogentech (Brussels, Belgium). Purity (>70% for biologic assays and >80% for HLA-A2 folding) was controlled by reversed-phase high-performance liquid chromatography. Peptides were lyophilized, dissolved in DMSO at 10 mg/ml and stored at −80°C.
PBMC stimulation
PBMC from HLA-A2 stage III melanoma patients were stimulated with irradiated allogeneic HLA-A2 melanoma line pulsed with the Melan-A A27L analogue, in presence of 5 ng/ml IL-6 (Sigma, St louis, USA) and 5 ng/ml IL-12 (Sigma), according to a method previously described [12]. Irradiated and peptide-loaded stimulator cells were added again twice, at day 7 and 14, in presence of 10 U/ml IL-2 and 5 ng/ml IL-7.
51Chromium cytotoxicity assay
Cytotoxic activity was measured in a standard 4-h assay against 51Cr-labeled cells. Briefly, target cells (peptide pulsed-T2 or melanoma cells) were incubated with 100 μCi Na512CrO4 (Oris Industrie, Gif-sur-Yvette, France) at 37°C for 1 h. Effector cells were then added at an E/T ratio of 10/1, in a final volume of 100 μl. For peptide recognition assays, T2 cells were preincubated with a range of Melan-A peptide concentrations for 1 h at 37°C. After the 4 h-co-culture, 25 μl of supernatant were mixed with 100 μl of scintillation cocktail (Optiphase Supermix, Wallak, UK) for measurement of radioactive content.
Cytokine production assay
About 1 × 105 lymphocytes were stimulated by 3 × 105 stimulator cells (melanoma cells and peptide-pulsed T2 cells) in 200 μl of RPMI 1640-10% FCS in the presence of brefeldin A, 10 μg/ml (Sigma, St Louis MO, USA) in round-bottom 96-well plates. The cultures were incubated for 6 h at 37°C in 5% CO2 humidified atmosphere. For intracytoplasmic cytokine staining, cells were then fixed 10 min at room temperature in a solution of PBS 4% paraformaldehyde (Sigma), washed and stored at 4°C until labeling. Fixed stimulated lymphocytes were stained for cytokine production using the method described by Jung et al. [15]. Anti-IL2, anti-TNF and anti-IFN-γ specific antibodies were purchased from BD Biosciences, France. After staining, cells were resuspended in PBS and 1.5 × 104 events were analyzed on a FACScan flow cytometer using Cell Quest software (Beckton Dickinson, Grenoble, France).
TCR β-chain V region expression analysis
A panel of 24 anti-Vβ Abs was used in this study (Immunotech, Beckman-Coulter, Marseille, France). Anti-Vβ1, −2, −3, −4, −5.1, −5.2, −5.3, −6, −7, −8, −9, −11, −12, −13.1, −13.2,−13.6, −14, −16, −17, −18, −20, −21.3, −22 and −23 mAbs were used as purified mAbs. Staining and washing were performed in PBS, 0.1% BSA. For indirect fluorescence labeling, cells were incubated (1) with purified anti-Vβ mAbs for 30 min at 4°C and washed, (2) with goat anti-mouse FITC-labeled Ab for 30 min at 4°C and washed. Cells were then analyzed by flow cytometry.
Results
Evaluation of MHC/peptide multimer sorting and expansion to amplify Melan-A specific polyclonal populations from TIL or PBMC
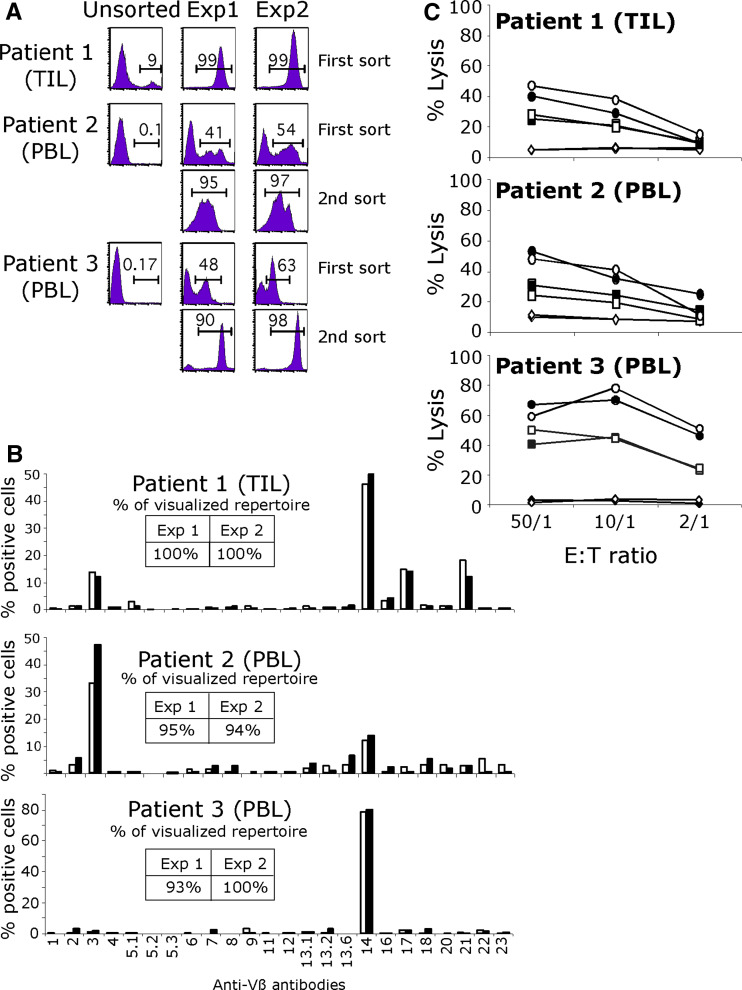
We tested the efficiency and reproductibility of MHC/multimer immunomagnetic sorting on either TIL or PBMC from HLA-A2 melanoma patients. Experiments were performed in duplicate for each tested sample. The fraction of tetramer labeled cells in the starting TIL population was high (9%) but hardly detectable in the two PBMC samples. We obtained almost pure tetramer-labeled populations from each of these samples following one or two sort/amplification cycles (Fig. 1a). The enrichment factor in specific T cells was very similar in duplicated experiments, thus documenting the reproducibility of this sort/amplification method. Moreover, the enrichment factor could be as high as 1940 when starting from PBMC which underlines the efficiency of this strategy (Table 1). In order to evaluate a possible effect of sort/amplification cycles on repertoire diversity, the pattern of TCR Vβ usage of sorted populations was determined. First of all, as shown on Fig. 1b, the Melan-A specific repertoire was very similar in duplicate experiments in all three patients strongly documenting the reproducibility of the procedure. Furthermore, the high degree of polyclonality of the Melan-A specific population obtained from patient 2 after two rounds of sort/amplification suggested that this procedure did not majorly alter repertoire diversity. Finally, we checked the tumor reactivity of each sorted population on HLA-A2 melanoma cell lines expressing the target antigen. All the sorted populations were cytotoxic against these cell lines, and in accordance with the results obtained on repertoire diversity, tumor reactivity was very similar in duplicate experiments (Fig. 1C). Finally, these highly enriched populations remained extremely stable upon multiple restimulations in terms of purity and tumor reactivity (data not shown).
Fig. 1.
a Sorting of Melan-A specific lymphocytes from TIL and PBMC. HLA-A*0201/Melan-A-A27L monomers coated on magnetic beads were used to isolate in duplicate Melan-A-specific populations from melanoma patients’ PBMC or TIL. PE-conjugated tetramers were used to assess the purity of the sorted populations after a 14-day amplification on feeder cells. Values indicate the percentage of cells labeled with tetramers after one or two rounds of sorting/amplification. b Repertoire diversity of sorted populations was assessed by labeling with 24 anti-Vβ antibodies. Inserts indicate the percentage of the specific population characterized with this panel in each experiment: white bars experiment 1, black bars experiment 2. c Lysis of melanoma cell lines by Melan-A sorted populations: white and black symbols correspond to duplicate experiments. Circles and squares represent two HLA-A2 melanoma cell lines expressing the Melan-A antigen and lozenge a HLA-A2 negative melanoma cell line
Table 1.
Enrichment and amplification yields in Melan-A specific T cells after MHC/peptide multimer sorting
| Patient 1 (TIL) | Patient 2 (PBL) | Patient 3 (PBL) | ||||
|---|---|---|---|---|---|---|
| Starting population | ||||||
| Total cell number | 4 × 106 | 5 × 106 | 5 × 106 | |||
| CD8 specific cells (%)a | 9 | 0.1 | 0.17 | |||
| CD8 specific cells nbb | 3.6 × 105 | 5 × 104 | 8.5 × 104 | |||
| First sort | Exp 1 | Exp 2 | Exp 1 | Exp 2 | Exp 1 | Exp 2 |
| D14 after sort/amplification | ||||||
| Amplified cell number | 1.2 × 108 | 1.3 × 108 | 2.2 × 107 | 1.8 × 107 | 2 × 107 | 2 × 107 |
| CD8 specific cells (%)a | 99 | 99 | 41 | 54 | 48 | 63 |
| CD8 specific cell nbb | 1.19 × 108 | 1.29 × 108 | 9 × 106 | 9.7 × 106 | 9.6 × 106 | 1.2 × 107 |
| Enrichmentc | ×330 | ×358 | ×1,800 | ×1,940 | ×1,129 | ×1,482 |
| Second sort | ||||||
| Total cell number | 5 × 106 | 5 × 106 | 5 × 106 | 5 × 106 | ||
| Nb of specific cellsb | 2 × 106 | 2.7 × 106 | 2.4 × 106 | 3.1 × 106 | ||
| D14 after sort/amplification | ||||||
| Amplified cell number | 3.2 × 108 | 2 × 108 | 2.5 × 108 | 2.5 × 108 | ||
| CD8 specific cells (%)a | 95 | 97 | 90 | 95 | ||
| CD8 specific cells nbb | 3.1 × 108 | 1.6 × 108 | 2.3 × 108 | 2.4 × 108 | ||
| Enrichmentc | ×155 | ×61 | ×96 | ×76 | ||
a% of Melan-A specific T cells is determined by CD8/tetramer double labeling
bNumber of specific cells (Percentage of tetramer labeled cells multiplied by the total number of cells)
cEnrichment factor is the ratio between the number of specific cells after and before each sort/amplification cycle
Together these results validated the choice of such a procedure to obtain Melan-A specific populations from blood or TIL. In particular, the demonstration of reproducibility was a prerequisite to be able to compare PBMC and TIL as alternate sources of Melan-A specific T cells, for use in immunotherapy.
Comparison of TIL and PBMC from three melanoma patients as a source of Melan-A specific T cells
We used PBMC, Melan-AA27L-stimulated PBMC and TIL from 3 HLA-A2 melanoma patients, as different sources of Melan-A specific lymphocytes. PBMC stimulation was performed by three rounds of co-incubation with an irradiated HLA-A2 positive Melan-A negative melanoma cell line loaded with the Melan-AA27L analog [12].
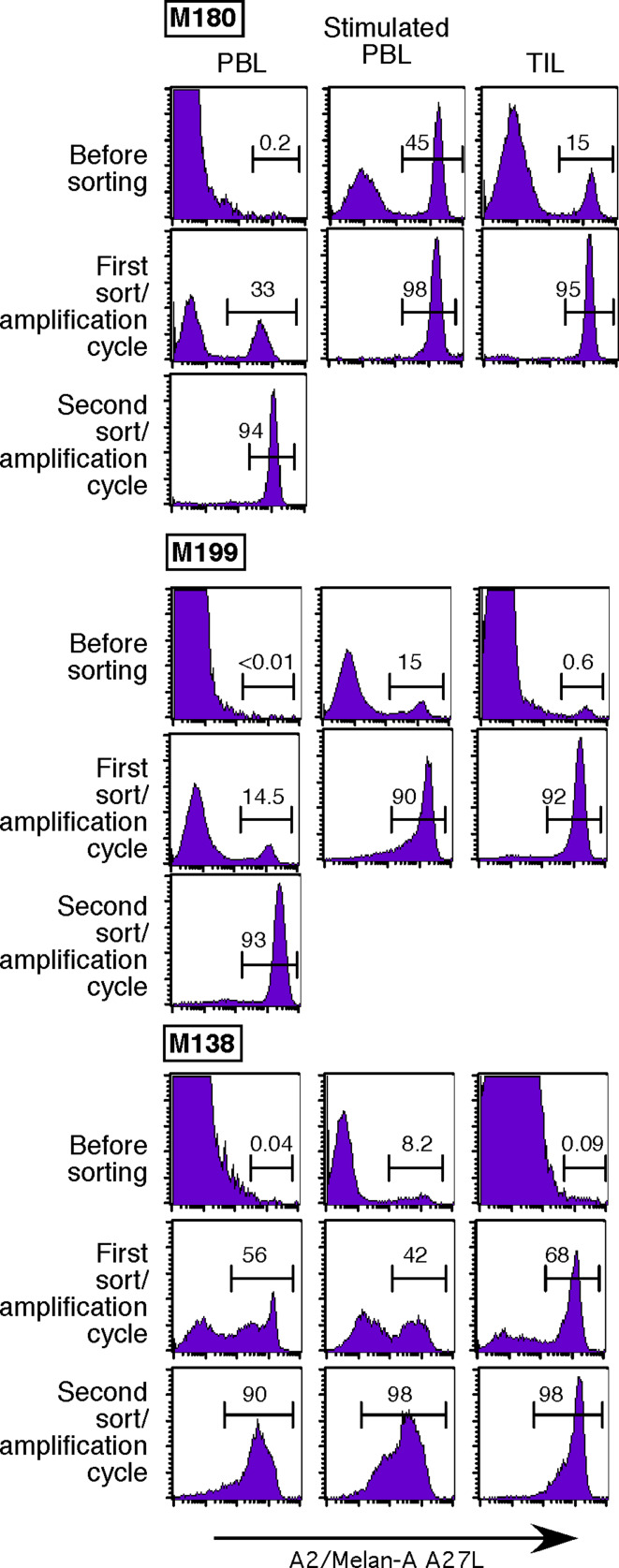
Before sorting, tetramer positive cells were hardly detectable in unstimulated PBMC, but easily detected among TIL (0.09–15%) and peptide-stimulated PBMC (8.2–45%). In 4/9 populations derived from PBMC or TIL, a single sort with monomer-coated beads was sufficient to obtain highly enriched (over 90%) tetramer-labeled cells (Fig. 2). Following polyclonal amplification, a second sort was performed on the remaining five partially enriched populations and resulted in a purity above 90% in every case (Fig. 2). Thus we were able to obtain almost pure tetramer-positive cell-lines from all patients with at most two multimer sorts, regardless of the starting populations (unstimulated or stimulated PBMC or TIL).
Fig. 2.
Sorting of Melan-A specific lymphocytes from different sources. HLA-A*0201/Melan-A-A27L monomers coated on magnetic beads were used to isolate Melan-A-specific populations from unstimulated PBMC, peptide stimulated PBMC and TIL in three melanoma patients. PE-conjugated tetramers were used to assess the purity of the sorted populations. Values indicate the percentage of cells labeled with tetramers after one or two rounds of sorting/amplification steps
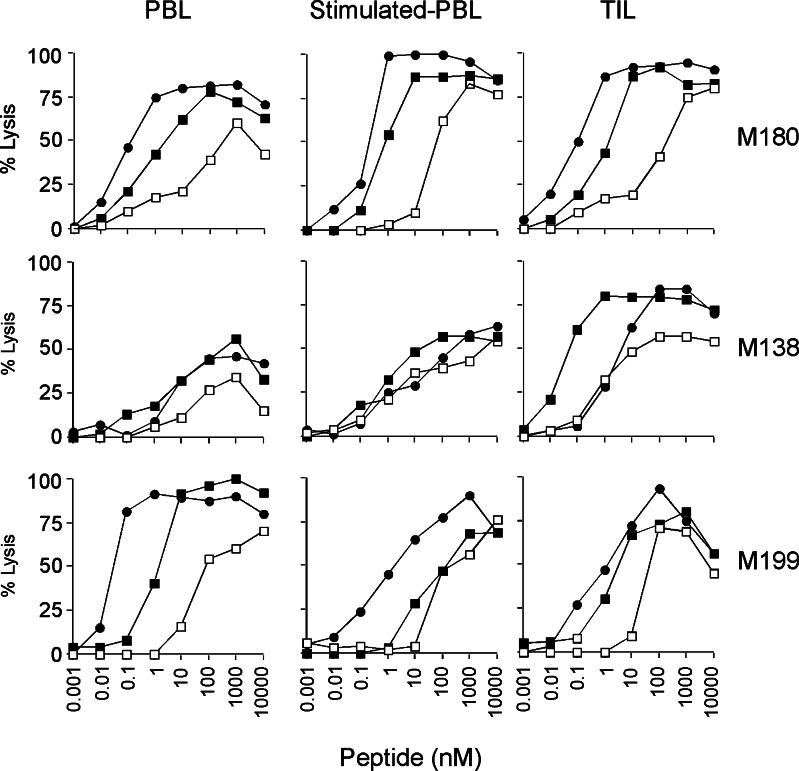
Each sorted T cell line was then tested for peptide specificity and tumor reactivity. To this end, the relative avidity of sorted populations was first evaluated by determining their EC50 against peptide-loaded T2 cells, using a standard 51Cr release assay. In all patients, sorted populations displayed higher avidities towards both decapeptides, i.e. the analog decapeptide Melan-A A27L (EC50 ranging from 0.05 to 10 nM) and the natural Melan-A26–35 decapeptide (EC50 from 0.04 to 20 nM), as compared to their avidity towards the natural Melan-A27–35 nonapeptide (EC50 from 1 to 500 nM) (Fig. 3). Although this may have resulted in part from the fact that the peptide used to sort and/or stimulate Melan-A specific lymphocytes was a decamer, we have previously shown that this decamer was the preferential epitope recognized by clones derived from TIL [23].
Fig. 3.
Avidities of Melan-A-specific sorted CTL lines for Melan-A peptides. The TAP-deficient cell line T2 expressing HLA-A*0201 was loaded with various concentrations of either the natural peptides Melan-A26–35 (filled square) or Melan-A 27–35, (open square) or the modified decapeptide A27L (filled circle). Lytic activity was evaluated at an E:T ratio of 10:1 by the classical 4-h 51Cr release assay
We did not observe any clear correlation between the avidity of sorted populations and their origin (TILN or PBMC). Indeed, in patient M180 (upper panel), the relative avidity of the three sorted populations towards the different Melan-A peptides were rather similar. In patient M138 (middle panel), lymphocytes sorted from TIL exhibited a higher avidity towards the three Melan-A peptides, whereas in patient M199 (lower panel), antigen-specific lymphocytes sorted from unstimulated PBMC displayed a higher avidity than those sorted from TIL or stimulated PBMC. In conclusion, we did not evidence any preferential source to isolate Melan-A specific lymphocytes of high avidity.
Since our final goal was to generate tumor-reactive T cells, the populations under study were also compared for their cytotoxicity and cytokine production in response to HLA-A2 melanoma cell lines that spontaneously express the Melan-A antigen.
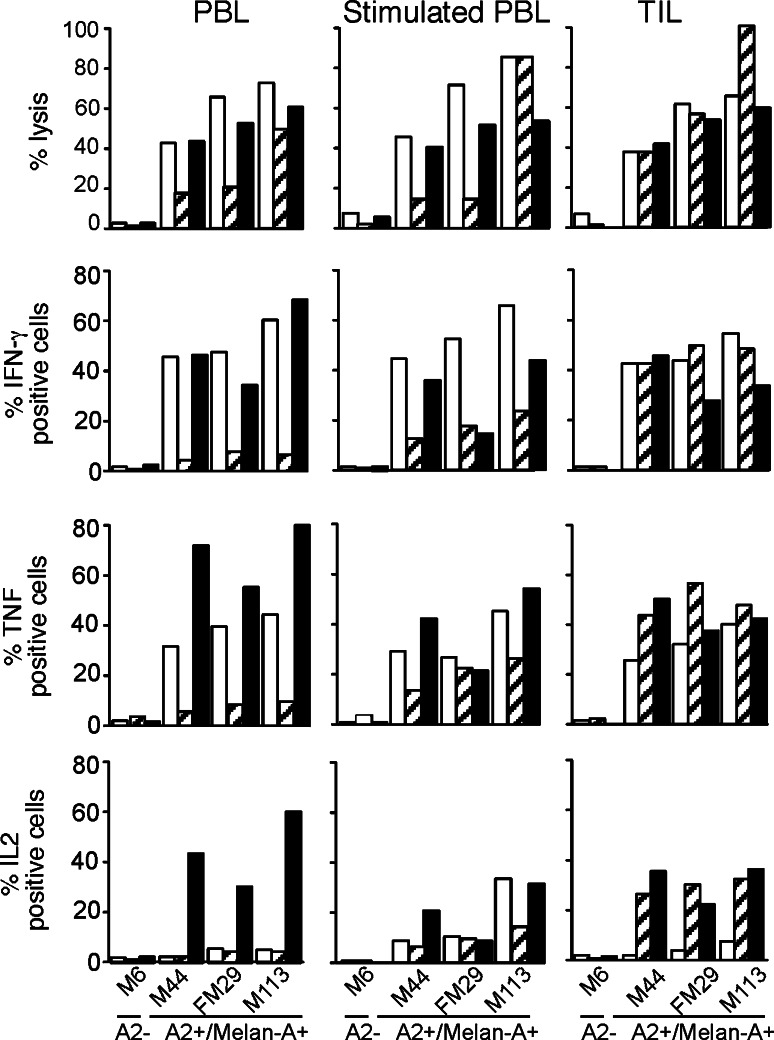
All sorted T cell lines were lytic against A2+/Melan-A+ melanoma cell lines and produced IFN-γ and TNF upon stimulation by these cells (Fig. 4). For each patient, the tumor reactivity of the T cell lines was correlated with their relative avidities towards Melan-A peptides. For example, in patient M138 (hatched bars), tetramer-specific lymphocytes sorted from TIL were the most reactive against melanoma cells whereas, in patient M199 (black bars), cells sorted from unstimulated PBMC were the most reactive (especially in terms of cytokine production). Therefore, tumor-reactivity of sorted populations could not be predicted from their origin. Nonetheless, it is interesting to note that a significant fraction of IL-2 producing cells, a characteristic of highly reactive anti-tumor lymphocytes [12, 27], was observed in all three populations sorted from stimulated PBMC, whereas IL-2 producing cells were present in only one out of three and two out of three populations sorted from unstimulated PBMC and TIL, respectively.
Fig. 4.
Reactivity of Melan-A specific sorted populations towards melanoma cell lines: open square patient M180, (hatched bars) patient M138, filled square patient M199. For lysis experiment, effector and target cells were incubated at a 10:1 ratio. For cytokine production, effector and target cells were incubated at a 1:2 ratio in the presence of Brefeldin A, stained with anti-cytokine antibody and analyzed by flow cytometry (5 × 103 cells)
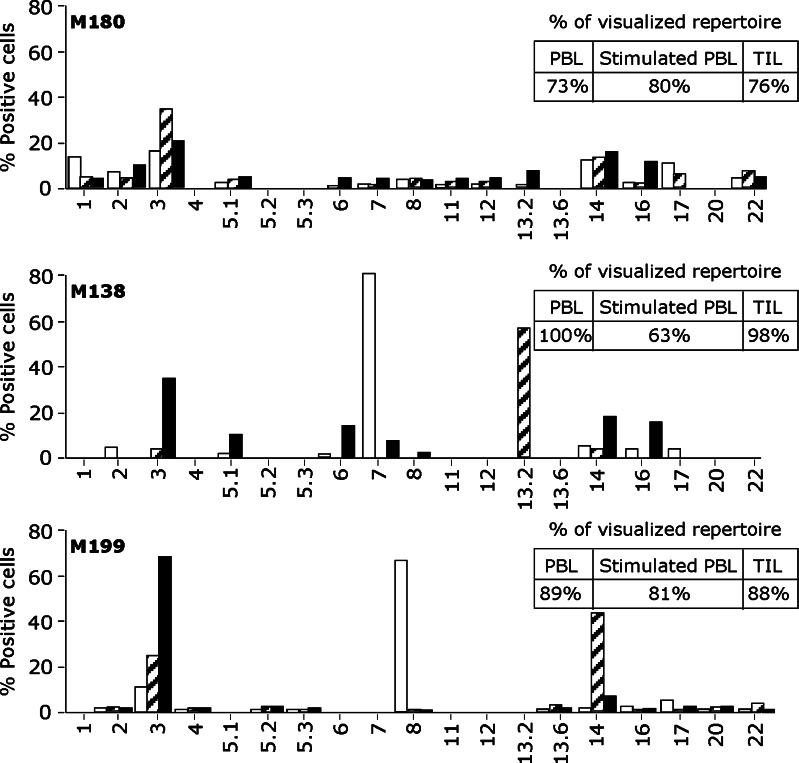
The pattern of TCR Vβ usage of sorted populations was then assessed to determine if repertoire differences could explain their different avidities and tumor reactivities. To this aim, we used a panel of 24 anti-Vβ antibodies representing the most frequently expressed Vβ chains within a normal repertoire. Using this panel of antibodies, the mean fraction of accessible repertoire in our patients was 83 ± 12% (inserts in Fig. 5). Sorted populations were rather polyclonal and a similar degree of diversity in Vβ usage was observed in unstimulated or stimulated PBMC or TIL in two patients (M180 and M199). In contrast, patient M138 displayed a more restricted repertoire, particularly among peptide-stimulated PBMC (hatched bars). However, in this last sample, Melan-A/A2 Vβ TCR diversity was probably underestimated since only 63% of the MelanA repertoire was detected by our panel of antibodies.
Fig. 5.
Analysis of the Vβ repertoire of sorted Melan-A specific cells from unstimulated PBMC (open square), stimulated PBMC (hatched bars) and TIL (filled square) patients. A panel of 24 anti-Vβ Abs was used. Insets indicate the percentage of the specific population characterized with this panel in each patient
A number of recurring Vβ subfamilies were observed among the three patients: Vβ2,Vβ3, Vβ14, Vβ16 and Vβ17 (Fig. 5). Concerning patient M180, the results were in accordance with the similar avidity and reactivity of the three sorted populations, i.e., there were no marked difference between Vβ subfamilies of the sorted populations according to their origins (Fig. 5, upper panel). Sorted populations from the two other patients were more heterogeneous in term of Vβ subfamilies. Concerning patient M138 (middle panel), Vβ7 subfamily was dominant among unstimulated PBMC (white bars), but very low or absent among TIL and stimulated PBMC, respectively. Concerning patient M199, Vβ8 T cells were dominant in Melan-A specific T cells sorted from unstimulated PBMC (white bars), but absent among those derived from TIL and from stimulated PBMC. In contrast, the Vβ14 subfamily was dominant among cell populations derived from peptide-stimulated PBMC (hatched bars), but hardy detectable in nonstimulated PBMC.
In order to check if the different avidities diplayed by PBMC and stimulated PBMC from this last patient (Figs. 3, 4) could be due to the variable proportion of distinct Vβ subfamilies (Fig. 5), the dominant subpopulations Vβ8 and Vβ14 were sorted and then analyzed for their avidity for peptide-loaded T2 cells and their reactivity towards melanoma cells.
Relative avidity of Vβ-sorted populations from M199 patient
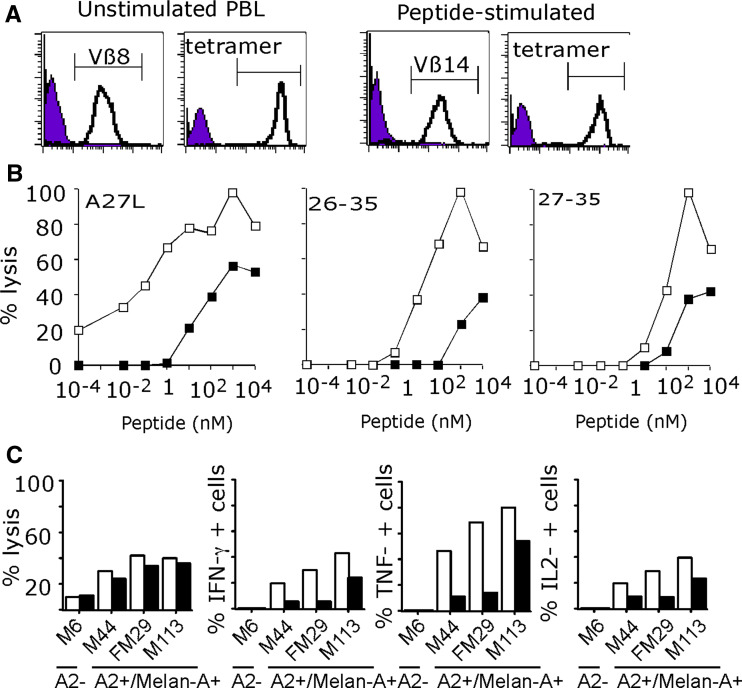
Figure 6a illustrates the purity of these sorted populations in terms of Vβ expression and tetramer labeling. As shown on Fig. 6b, Vβ8 lymphocytes derived from unstimulated PBMC were of higher avidity towards Melan-A peptides than Vβ14 cells derived from peptide-stimulated PBMC. Indeed, EC50 of the Vß8 population were respectively, of 0.1, 30 and 100 nM on Melan-A A27L, Melan-A26–35 and 27–35 whereas EC50 of the Vβ14 sorted population were of 30 nM, 1 μM and 500 nM on these same peptides. Finally, and in accordance with results obtained on Melan-A peptides, the Vβ8 sorted population was more reactive on Melan-A/A2 melanoma lines than the Vβ14 population, as illustrated by cytokine production in response to melanoma cells (Fig. 6c).
Fig. 6.
Analysis of MelanA specific Vβ8 and Vβ14 T lymphocytes from patient M199. a Vβ and tetramer staining of Vβ sorted populations. b Avidities of Vß8 (open square) and Vß14 (filled square) sorted T cells for MelanA peptides. The TAP-deficient cell line T2 was loaded with various concentrations of either the modified decapeptide A27L or the natural peptides Melan-A26–35 and Melan-A 27–35. Lytic activity was measured at an E:T ratio of 10:1 by 4-h 51Cr release assay. c Lysis and cytokine production by Vβ8 (open square) and Vβ14 (filled square) sorted populations in response to HLA-A2 Melan-A positive or negative melanoma cells. For lysis experiment, effector and target cells were incubated at a 10:1 ratio. For cytokine production, effector and target cells were incubated at a 1:2 ratio in the presence of Brefeldin A, stained with anti-cytokine antibody and analysed by flow cytometry (5 × 103 cells)
Discussion
The aim of this study was to compare unstimulated PBMC, peptide-stimulated PBMC and TIL from lymph nodes as alternate sources of functional Melan-A specific lymphocytes. To this aim, we first validated a procedure based on immunomagnetic sorting of specific T cells with MHC/peptide coated beads. The peptide used to generate MHC/peptide complexes and to stimulate PBMC in vitro was the well-characterized Melan-A A27L analog peptide, previously shown to cross-stimulate a large fraction of the T cell repertoire directed against the natural Melan-A epitopes [26].
We demonstrated that this method was efficient to isolate and amplify Melan-A-specific populations hardly detectable by standard tetramer staining. These populations were polyclonal, as assessed by Vβ staining, and tumor-reactive (Fig. 1). Moreover, we documented the high reproducibility of this method by comparing duplicate experiments. Thus, this sorting procedure can be used to analyse the repertoire and function of the whole Melan-A specific population in contrast to the previously reported analyses of clones [5, 19] which can be biased by the variable ability of these clones to proliferate in vitro.
Using this procedure, we were able to obtain over 90% pure Melan-A specific populations from PBMC with two rounds of sorting/expansion whereas a single round was most of the time sufficient to achieve the same purity when starting with peptide-stimulated PBMC or TIL (Figs. 1a, 2). A second round of sorting/expansion did not seem to alter the diversity of the Melan-A repertoire since, as could be documented in patients M180 and M199, a similar number of Vβ subfamilies were obtained from PBMC (two sorts) or from stimulated PBMC and TIL (one sort). This Vβ diversity of the Melan-A repertoire, the general dissimilarity of the repertoire between patients and the recurrence of Vβ3 and Vβ14 subfamilies are in accordance with previous reports [5, 6, 19, 25].
In each of the three patients, a global analysis revealed a similar pattern of Vβ usage in cells sorted from PBMC, stimulated PBMC or TIL although in variable proportions. Nonetheless, we sometimes observed the major expansion of a particular Vβ family in only one of the three sources from the same patient. For example, in patients M138 and M199, Vβ7 and Vβ8 subfamilies were predominant in PBMC while Vβ13.2 and Vβ14 subfamilies were over-represented in stimulated PBMC, respectively. Likewise, Vβ3 subfamily was preferentially expressed in cells sorted from TIL in patient M138 (Fig. 5). Considering that those expanded subfamilies represented a high proportion of MelanA/A2 sorted cells, we wondered whether their preferential expansion in a single sample could result in functional differences between sources in the same patient. As a matter of fact, we did observe differences in reactivity of specific T cells from each source when challenged with Melan-A peptides and melanoma cell-lines, especially in terms of cytokine production. For example, in patient M138, initial peptide stimulation of PBMC dramatically increased the recovery of cytokine producing specific T cells while TIL remained the most reactive towards peptide and melanoma cells (Fig. 4, hatched bars). In contrast, in patient M199, T cells derived from unstimulated PBMC were more reactive than those derived from peptide-stimulated PBMC or TIL (Figs. 3, 4, black bars). In this last patient, peptide stimulation resulted in the complete disappearance of the dominant Vβ8 subfamily and dramatically favored the expansion of the Vβ14 subfamily (Fig. 5). After sorting of these Vβ8 and Vβ14 subfamilies, we observed a significant difference between these two populations in terms of avidity and melanoma reactivity (Fig. 6b, c). This result underlines the fact that in vitro peptide stimulation can majorly affect the Melan-A repertoire leading to either a decrease (patient M199) or an increase (patient M138) in global reactivity towards melanoma cells, especially when the initial repertoire in PBMC is very oligoclonal, as seen in those two patients. In contrast, in patient M180 whose initial Melan-A repertoire in PBMC was the most diverse, peptide stimulation had little effect on repertoire and reactivity. We assume that adjusting the concentration of stimulating peptide and the number of stimulations should allow the recovery of a broader reactive repertoire. Indeed, it has been reported that the amount of peptide used to pulse antigen-presenting cells for in vitro stimulation influences the avidity of peptide specific CTLs [1, 29].
An alternative explanation for the loss of highly reactive T cells would be that multimer sorting itself induced apoptosis of these cells due to a prolonged stimulation of their TCR, as previously reported following incubation of mouse T cells with soluble MHC tetramers at 37°C [4]. In our procedure, the sort is performed at 4°C, thus limiting T cell activation, and then, sorted cells are immediately restimulated with PHA, IL2 and feeder cells. Thus, although apoptosis of some cells certainly occurred, it is impossible for us to delineate precisely which stimuli triggered apoptosis and especially the contribution of persistent multimer stimulation in this process. Nonetheless, we documented that Melan-A specific T cells with high tumor reactivity can be efficiently sorted and expanded from all samples using this procedure, despite a potential loss of some reactive T cell clones. In support of that, a recent study compared the sorting of Melan-A specific T cells using either reversible multimers or classical tetramers and concluded that “reversible multimer-based isolation was not better than the tetramer-based T cell isolation method regarding the generation of CTL clones with superior peptide avidity and/or tumor recognition efficiency” [22].
Finally, multiple cycles of stimulations have been shown to drive specific T lymphocytes towards replicative senescence [9, 10, 13, 21] which may alter their in vivo efficiency after transfer. Thus, as future developments, we consider using a single round of PBMC stimulation with low concentrations of peptide followed by MHC-peptide multimer sorting to obtain enriched Melan-A specific populations at an early stage of differentiation.
In conclusion, we demonstrated that PBMC from melanoma patients can provide a reliable source of Melan-A reactive T lymphocytes that were as good effectors as those derived from TILN and that HLA/peptide multimer sorting is a very efficient method to isolate pure population from this source. We reckon that this strategy could be extended to other melanoma epitopes in other HLA contextes and could thus represent a promising approach for the development of future ACT protocols.
Acknowledgments
This work was supported by grants from the « Ligue Nationale contre le Cancer »: labellisation 2003–2007, by a grant from INCA “Thérapie adoptive cellulaire du cancer” and by a grant from ENACT network number 503306. We thank Pr B. Dreno and the Unit of skin Cancer for providing us with blood and tumor samples from melanoma patients.
Footnotes
Nathalie Labarrière and Nadine Gervois have equally contributed to this work.
Contributor Information
Nathalie Labarrière, Phone: +33-240-084720, FAX: +33-240-356697, Email: nlabar@nantes.inserm.fr.
Francine Jotereau, Phone: +33-240-084720, FAX: +33-240-356697, Email: jotereau@nantes.inserm.fr.
References
- 1.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benlalam H, Labarriere N, Linard B, Derre L, Diez E, Pandolfino MC, Bonneville M, Jotereau F. Comprehensive analysis of the frequency of recognition of melanoma-associated antigen (MAA) by CD8 melanoma infiltrating lymphocytes (TIL): implications for immunotherapy. Eur J Immunol. 2001;31:2007–2115. doi: 10.1002/1521-4141(200107)31:7<2007::AID-IMMU2007>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Bodinier M, Peyrat MA, Tournay C, Davodeau F, Romagne F, Bonneville M, Lang F. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat Med. 2000;6:707–710. doi: 10.1038/76292. [DOI] [PubMed] [Google Scholar]
- 4.Cebecauer M, Guillaume P, Hozak P, Mark S, Everett H, Schneider P, Luescher IF. Soluble MHC-peptide complexes induce rapid death of CD8+ CTL. J Immunol. 2005;174:6809–6819. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 5.Cole DJ, Wilson MC, Rivoltini L, Custer M, Nishimura MI. T-cell receptor repertoire in matched MART-1 peptide-stimulated peripheral blood lymphocytes and tumor-infiltrating lymphocytes. Cancer Res. 1997;57:5320–5327. [PubMed] [Google Scholar]
- 6.Dietrich PY, Walker PR, Quiquerez AL, Perrin G, Dutoit V, Lienard D, Guillaume P, Cerottini JC, Romero P, Valmori D. Melanoma patients respond to a cytotoxic T lymphocyte-defined self-peptide with diverse and nonoverlapping T-cell receptor repertoires. Cancer Res. 2001;61:2047–2054. [PubMed] [Google Scholar]
- 7.Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N, Jotereau F. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–454. doi: 10.1016/S0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gervois N, Heuze F, Diez E, Jotereau F. Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- 12.Gervois N, Labarriere N, Le Guiner S, Pandolfino MC, Fonteneau JF, Guilloux Y, Diez E, Dreno B, Jotereau F. High avidity melanoma-reactive cytotoxic T lymphocytes are efficiently induced from peripheral blood lymphocytes on stimulation by peptide-pulsed melanoma cells. Clin Cancer Res. 2000;6:1459–1467. [PubMed] [Google Scholar]
- 13.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jotereau F, Pandolfino MC, Boudart D, Diez E, Dreno B, Douillard JY, Muller JY, LeMevel B. High-fold expansion of human cytotoxic T-lymphocytes specific for autologous melanoma cells for use in immunotherapy. J Immunother. 1991;10:405–411. doi: 10.1097/00002371-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B, Jotereau F. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother. 2002;51:532–538. doi: 10.1007/s00262-002-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labarriere N, Pandolfino MC, Raingeard D, Le Guiner S, Diez E, Le Drean E, Dreno B, Jotereau F. Frequency and relative fraction of tumor antigen-specific T cells among lymphocytes from melanoma-invaded lymph nodes. Int J Cancer. 1998;78:209–215. doi: 10.1002/(SICI)1097-0215(19981005)78:2<209::AID-IJC15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 19.Mandruzzato S, Rossi E, Bernardi F, Tosello V, Macino B, Basso G, Chiarion-Sileni V, Rossi CR, Montesco C, Zanovello P. Large and dissimilar repertoire of Melan-A/MART-1-specific CTL in metastatic lesions and blood of a melanoma patient. J Immunol. 2002;169:4017–4024. doi: 10.4049/jimmunol.169.7.4017. [DOI] [PubMed] [Google Scholar]
- 20.Meidenbauer N, Marienhagen J, Laumer M, Vogl S, Heymann J, Andreesen R, Mackensen A. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161–2169. doi: 10.4049/jimmunol.170.4.2161. [DOI] [PubMed] [Google Scholar]
- 21.Menzel O, Migliaccio M, Goldstein DR, Dahoun S, Delorenzi M, Rufer N. Mechanisms regulating the proliferative potential of human CD8+ T lymphocytes overexpressing telomerase. J Immunol. 2006;177:3657–3668. doi: 10.4049/jimmunol.177.6.3657. [DOI] [PubMed] [Google Scholar]
- 22.Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, Schmidt T, Germeroth L, Wagner H, Peschel C, Busch DH, Bernhard H. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320:119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Romero P, Gervois N, Schneider J, Escobar P, Valmori D, Pannetier C, Steinle A, Wolfel T, Lienard D, Brichard V, van Pel A, Jotereau F, Cerottini JC. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201-restricted Melan-A/MART-1 antigenic peptide in melanoma. J Immunol. 1997;159:2366–2374. [PubMed] [Google Scholar]
- 24.Topalian SL, Muul LM, Solomon D, Rosenberg SA. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;102:127–141. doi: 10.1016/S0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 25.Valmori D, Dutoit V, Lienard D, Lejeune F, Speiser D, Rimoldi D, Cerundolo V, Dietrich PY, Cerottini JC, Romero P. Tetramer-guided analysis of TCR beta-chain usage reveals a large repertoire of melan-A-specific CD8+ T cells in melanoma patients. J Immunol. 2000;165:533–538. doi: 10.4049/jimmunol.165.1.533. [DOI] [PubMed] [Google Scholar]
- 26.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 27.Vignard V, Lemercier B, Lim A, Pandolfino MC, Guilloux Y, Khammari A, Rabu C, Echasserieau K, Lang F, Gougeon ML, Dreno B, Jotereau F, Labarriere N. Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J Immunol. 2005;175:4797–4805. doi: 10.4049/jimmunol.175.7.4797. [DOI] [PubMed] [Google Scholar]
- 28.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]