Abstract
The Her2/neu oncogene is overexpressed in various human cancers of epithelial origin and is associated with increased metastatic potential and poor prognosis. Blocking the Her2/neu signalling has been the focus of most therapeutic approaches. In this paper, the Her2/neu extracellular domain expressed in soluble form in yeast Pichia pastoris was used in order to isolate a fully human Fab fragment from a combinatorial Fab phage display library, derived from invaded lymph nodes of a breast cancer patient. The isolated fully human Fab63 binds specifically the native Her2/neu receptor and competes with Herceptin for binding to soluble Her2/neu receptor. In Her2/neu overexpressing cancer cells, Fab63 is rapidly internalized and has significant antiproliferative effects, where ligand-independent mechanisms dominate signal induction. Moreover, in the presence of the ligand heregulin, growth inhibition was also detected by Fab63. The human Fab63 is a non-immunogenic agent with unique properties that can be applied in diagnosis and cancer therapy, with great potential for further manipulation towards the generation of an effective anticancer molecule.
Keywords: Her2/neu, Human Fab, Phage display, Cancer immunotherapy
Introduction
The Her2/neu (ErbB2) gene encodes a 185,000 kDa transmembrane glycoprotein that belongs to the erbB family of epidermal growth factor receptors [36]. Her2/neu is a ligand-less receptor and it is the preferred heterodimerization partner for ligand-bound EGFR family Her1 (EGFR), Her3 and Her4. By functioning as co-receptor, Her2/neu mediates signal transduction, resulting in mitogenesis, apoptosis, angiogenesis and cell differentiation. Any alteration of the tightly regulated erbB receptor signalling pathways result in major cellular abnormalities and tumourigenesis [43].
The Her2/neu gene is amplified and overexpressed in ~20–30% of invasive breast carcinomas and is associated with increased metastatic potential and poor prognosis [33, 35]. Overexpression of Her2/neu receptor is also observed in various human cancers, including ovary, prostate, gastric, lung, bladder and kidney carcinomas [21]. Several reports from experimental models and clinical studies have shown that the Her2/neu is an immunogenic molecule since it generates antibody response and activation of Her2/neu peptide-specific CTLs and T helper (TH) cells [7, 14, 19, 24, 41].
Blocking the Her2/neu signalling and limiting the number of available membrane molecules, has been the focus of many therapeutic approaches. For example, Herceptin, the humanized version of murine 4D5 mAb, is the first antibody which entered the clinic, displaying anti-tumour activity in only up to 30% of patients overexpressing Her2/neu [16]. Herceptin exerts its antiproliferative activity by preventing the cleavage of the receptor’s extracellular domain and receptor downmodulation [6, 39]. Another agent, mAb 2C4, sterically blocks the association of Her2 with other erbB family members and disrupts ligand activation [1]. Pertuzumab, the humanized 2C4 mAb is recently introduced in clinical trials targeting low expressing Her2/neu cancers [2, 17]. Moreover, Trastuzumab and Pertuzumab synergistically inhibit the survival of Her2/neu overexpressing breast cancer cell lines [29].
Advances in antibody engineering allowed the isolation of fully human antibody Fab and single-chain Fv (scFv) fragments from phage display human antibody libraries [10]. The ability to generate fully human antibody fragments is important because it overcame the host anti-mouse antibody (HAMA) response produced by antibody chimerization or humanization. The small size of Fab and scFv fragments is also advantageous in penetrating solid tumours and targeting malignant cells. Internalized Fabs and scFvs have also been isolated as more potent tools for delivering cytotoxic or cytostatic load [31, 40], beyond the membrane barrier into the cytoplasm. Recently, an internalized human scFv displayed on phage (Erbicin) was isolated with cytostatic/cytotoxic effect on Her2/neu positive cell lines [12].
In this paper, we describe the isolation of a fully human Fab fragment (Fab63) against the recombinant extracellular domain of Her2/neu receptor (Her2/neu-ECD), by using a combinatorial Fab phage display library, derived from invaded lymph nodes of a breast cancer patient. The Fab63 competes with Herceptin for binding to soluble Her2/neu receptor and can bind to the native receptor in the surface of Her2/neu overexpressing cells. Unlike Herceptin, Fab63 is strongly internalized at significantly higher rate from that reported for Erbicin. Furthermore, Fab63 has significant antiproliferative effects on Her2/neu overexpressing SKBR3 and MDA-MB-453 cancer cells where ligand-independent mechanisms dominate signal induction. Moreover, in the presence of the ligand heregulin (HRG-β1), growth inhibition was detected in MDA-MB-453 cells, whereas Herceptin could not cause a similar effect. These unique properties of Fab63 make it a very promising candidate as an anti-cancer agent for diagnosis and therapeutics, as well as a valuable tool for the investigation of Her2/neu receptor structural and biochemical properties.
Materials and methods
Soluble expression of ECD-Her2/neu in yeast Pichia pastoris
The human Her2/neu-ECD (aminoacid residues 1–627) was previously enzymatically amplified by PCR and subcloned into the expression vector pPICZαC (Invitrogen, CA) for soluble expression in yeast P. pastoris (D. Papanastasiou, E. Merkouri, A. Mamalaki, manuscript in preparation) The culture supernatant was passed through a 0.22-μm membrane filter, concentrated by using the Minitan Ultrafiltration System (Millipore, Bedford, MA) equipped with 30 kDa cut-off membrane and then dialysed extensively against phosphate buffer (5.8 mM KH2PO4, 40 mM Na2PO4, pH 8). The concentrated protein was then purified using Ni2+–NTA affinity chromatography (QIAgen, Hilden, Germany), under native conditions, according to the manufacturer’s protocol.
Cell lines and culture
Breast cancer cell lines used included the SKBR3 and MDA-MB-453 overexpressing Her2/neu and the low-expressing Her2/neu MDA-MB-435 [22]. These cells as well as HeLa cells were maintained in DMEM or RPMI-1640 supplemented with 10% FBS (Gibco-BRL, Rockville, MD) at 37°C under a 5% CO2–95% air atmosphere.
Construction and biopanning of the combinatorial human Fab-phage library
Library construction and panning was performed as described previously [4, 8] using lymphocytes from a patient with Her2/neu positive breast cancer and isolating total RNA from subtracted B cells. Library panning was performed in microtitre plate wells, coated with the recombinant Her2/neu-ECD (1 μg/well) antigen, expressed in yeast P. pastoris. Non-specific binders were removed by following a mode of 1×, 3×, 6×, 10× increasing stringency wash steps. Soluble Fabs were produced from phagemid clones isolated from the last round of panning and purified as described [4, 5]. The eluates were subjected to 12% SDS-PAGE and the Fab was visualized with Coomassie or silver stain in SDS-PAGE and detected in western blot with the goat anti-human F(ab′)2, followed by an anti-goat HRP conjugated (DAKO, Copenhagen, Denmark). The concentration of protein was determined by Bradford method (Biorad, Hercules, CA).
Fab characterization by ELISA and immunoprecipitation
Microtitre plates coated with antigen (10 μg/ml) were incubated with crude lysates containing the Fab, for 1 h at 37°C [8]. For sandwich ELISA, solubilized Her2/neu receptor extracted from SKBR3 cells as described [23], was captured in microtitre plates with 0.5 μg/ml mouse anti-Her2 antibody (TAB250, Zymed, Laboratories Inc., CA).
For competition ELISA, Her2/neu was preincubated with purified Fab63 or competitors-antibodies, overnight at 4°C and the receptor complexes were captured by 5 μg/ml Fab63 or 1 μg/ml Herceptin (Genentech Inc., South San Francisco, CA) in microtitre plates.
For immunoprecipitation, semi-confluent cells were incubated with 500 nM Fab or 66 nM Herceptin for 30 min. Complexes were immunoprecipitated from membrane extracts with 10 μl gel slurry of goat anti-human F(ab′)2 prebound on protein G Sepharose beads (Sigma, St. Louis, MO) for 2 h at 25°C and analysed by western blot.
In all cases, Fab or Herceptin light chain was detected by goat anti-human F(ab′)2 (Pierce, Rockford, IL) and Her2/neu receptor by C-18 polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Flow cytometry analysis
Live cells were harvested, washed with PBS and saturated in blocking buffer (1% FBS in PBS). Aliquots of 1×105 cells were incubated with Fab63 or control antibodies. The human light chain was detected as above followed by a FITC-conjugated rabbit anti-goat antibody (DAKO). Cells were washed, resuspended in PBS and analysed at a FACScan flow cytometer using CellQuest software (Becton-Dickinson, Oxford, UK).
Confocal laser scanning microscopy
Semi-confluent SKBR3 or MDA-MB-453 cells, grown on poly-l-lysine (Sigma, St. Louis, MO) coated glass lamelles, were washed with PBS and incubated with Fab63 or Herceptin for the indicated time and temperature. Cells were then fixed with 4% paraformaldehyde, while for internalization studies they were permeabilized with ice cold acetone for 30 s [32]. Cells were washed and blocked in Mab’s buffer (10% FBS, 60 mM lysine in PBS) and fluorescein labelling of Fab63 and Herceptin was performed as in flow cytometry. Finally, lamelles were washed, mounted with Citifluor and inverted on slides. Immunofluorescence analysis was performed in a Leica TCS-SP confocal microscope.
Antibody internalization assay
The detection and quantification of internalized Fab63 fragments was performed with the use of radiolabelled Fab63 as previously described [30]. Briefly, Fab63 was labelled with Na125I (PerkinElmer) (1 mCi/mg of protein) using the chloramine T method [26]. The radioiodinated Fabs were purified by gel-filtration on Sephadex G-25. SKBR3 cells were incubated in triplicate with 125I-Fab63 (8×106 cpm/5×104 cells) at 37°C for 30 min and 2 h in the presence of culture media. The cells were washed four times with PBS and two times with ice cold glycine buffer pH 2.5, for the removal of surface bound 125I-Fab63. Finally, the cells were lysed with 2% SDS and cell lysate radioactivity was measured in γ-counter. The total amount of bound 125I-Fab63 to cells was estimated from the sum of the acid washes (surface bound 125I-Fab) and cell lysate (internalized 125I-Fab) radioactivity.
Cell proliferation assay
Single cell suspensions of 105 cells/ml were left to adhere overnight at 96-well culture plates at 100 μl/well, while next day were incubated with medium supplemented with 10% FBS ± Fab63 or Herceptin at a range of concentrations. To test Fab63-Herceptin synergy, cells were cultured in the presence of both agents at 500 and 66 nM, respectively. For ligand-activated studies, MDA-MB-453 cells were treated with HRG-β1 (50 ng/ml) 30 min subsequently to the addition of Fab63 or Herceptin in the presence of 10% FBS. The number of living cells was determined by CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Southampton, UK) at 72 h. Cell survival was expressed as the percent growth of cells cultured without Fab63 or Herceptin. Statistical analysis was performed by the use of Student’s t-test.
Results
Construction of the combinatorial human Fab library: isolation of the specific anti-Her2/neu-ECD Fab63
The phage display Fab library was constructed from B-lymphocytes isolated from invaded lymph nodes of a patient with Her2/neu positive breast cancer. The light chain gene repertoire (κ and λ) was cloned into the pComb3H phagemid, and, subsequently, the Fd heavy chain gene repertoire of the γ1 isotype was inserted into the light chain library. A human combinatorial Fab library with a complexity of 1×107 cfu/μg was created. For selection of specific anti-Her2 Fab fragments the combinatorial library was panned with the recombinant Her2/neu-ECD antigen expressed in yeast P. pastoris in soluble form. The final round of panning yielded 100-fold amplification in the phage titres compared to the minimum eluates from the third round of panning, this indicating enrichment in clones with enhanced affinity towards the specific antigen (data not shown). Phagemid DNA was isolated from the eight round of panning and modified to produce soluble Fabs. Several clones were tested in ELISA with Her2/neu-ECD and clone 63 was selected for large-scale production and further characterization (Fig. 1). The yield of purified Fab was approximately 1 mg/l.
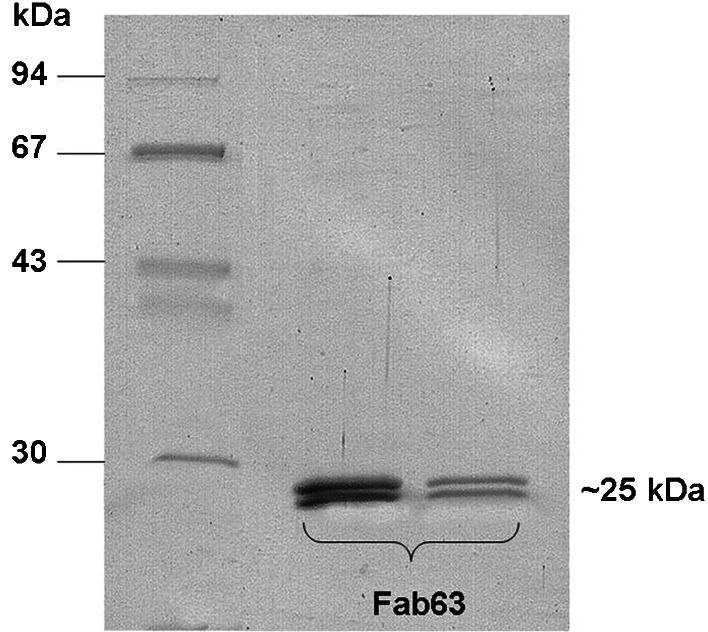
Fig. 1.
SDS-PAGE analysis of purified Fab63. Purified Fab63 from different eluates was subjected to SDS-PAGE electrophoresis and stained with Coomassie. Fab63 is observed as a double band, corresponding to the light and heavy chain fragments, at ~25 kDa according to the molecular weight standard
The specific binding of human Fab63 was first tested in ELISA assay. Fab63 bound specifically to the Her2/neu-ECD and not to antigens such as human serum albumin (HSA), the alpha1-ECD of human AChR [34] (both produced in P. pastoris) or to the bovine serum albumin (BSA) (Fig. 2a).
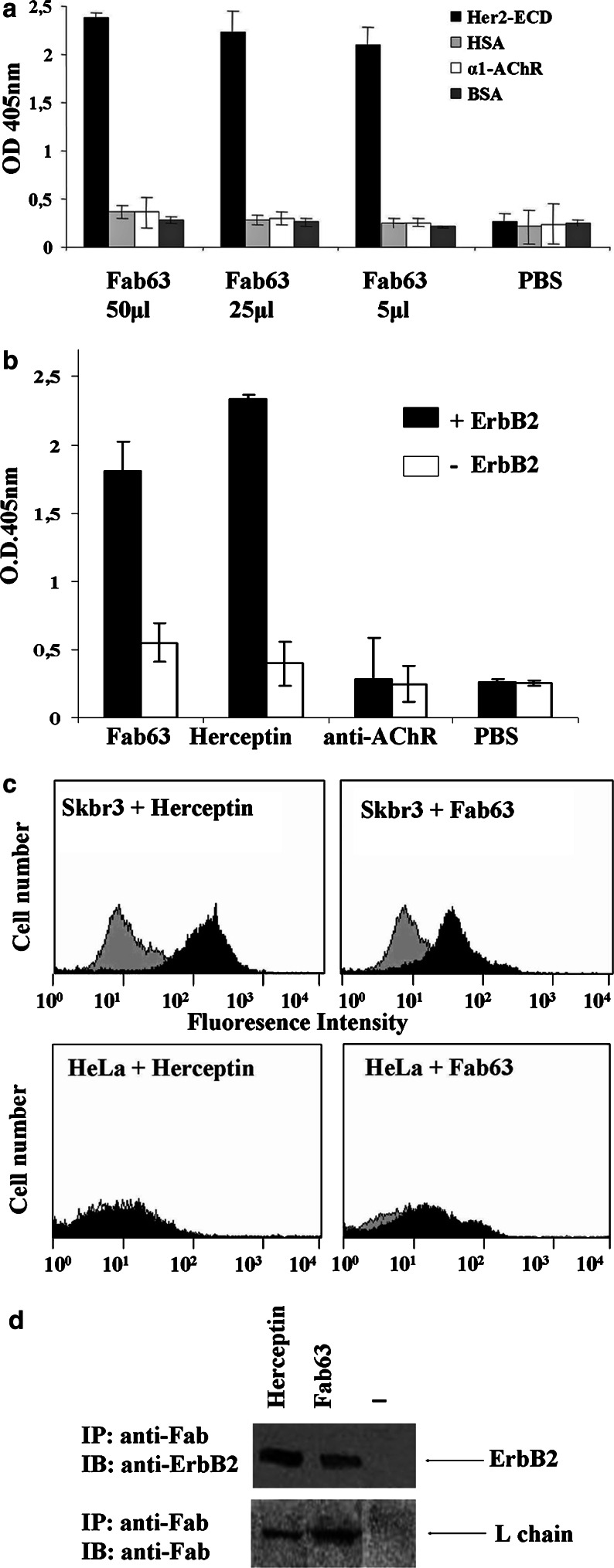
Fig. 2.
Fab63 specificity to native Her2/neu receptor. a Different quantities of bacteria crude lysates containing Fab63 were tested in ELISA for binding to different antigens. Fab63 specifically recognized the Her2/neu-ECD recombinant antigen. Values represent the mean ± SD for four independent experiments. b Sandwich ELISA with soluble Her2/neu receptor captured by the mAb TAB250. Fab63 specifically bound to the receptor in its native conformation. Values represent the mean ± SD for four independent experiments. c FACS analysis of Fab63 binding to positive and negative Her2/neu expressing cells. At the upper panel, Fab63 binds well to Her2/neu expressing SKBR3 cells compared to the positive antibody Herceptin (black histograms) and to cell background (grey histograms). Fab63 and Herceptin showed no significant binding to negative Her2/neu HeLa cell line (lower panel). d Co-immunoprecipitation of Fab63 with Her2/neu receptor in SKBR3 cells. Western blot analysis of immunoprecipitates from cell lysates indicates the presence of the Her2/neu protein (185 kDa) and that of Herceptin or Fab63 light chain (~25 kDa)
Fab63 specificity for native Her2/neu receptor
Fab63 was able to bind to solubilized Her2/neu receptor extracted from the high expressing Her2/neu SKBR3 cells. The soluble receptor was captured in ELISA plates by the anti-Her2/neu TAB250 mouse antibody (Zymed). Fab63 was added at a concentration of 250 nM, whereas Herceptin or an anti-AChR Fab were used as positive or negative controls at 10 or 250 nM, respectively. Detection of Fab63 and Herceptin with an anti-human Fab antibody produced a strong signal only in those wells where Her2/neu receptor was captured. Irrelevant Fab showed background binding in any case (Fig. 2b).
The ability of Fab63 to bind to intact cells was tested by flow cytometry. Fab63 bound to SKBR3 cells and produced only background signal in the Her2/neu negative HeLa cells (Fig. 2c). Sequentially, the specificity of Fab63 against the Her2/neu receptor was further investigated by immunoprecipitation assay after treatment of SKBR3 cells with Fab63. Figure 2d shows that Her2/neu and Fab63 complexes were co-immunoprecipitated with an anti-human F(ab′)2 mAb, which recognize the human light chain and both proteins were detected by western blot analysis with anti-Her2 and anti-Fab antibodies.
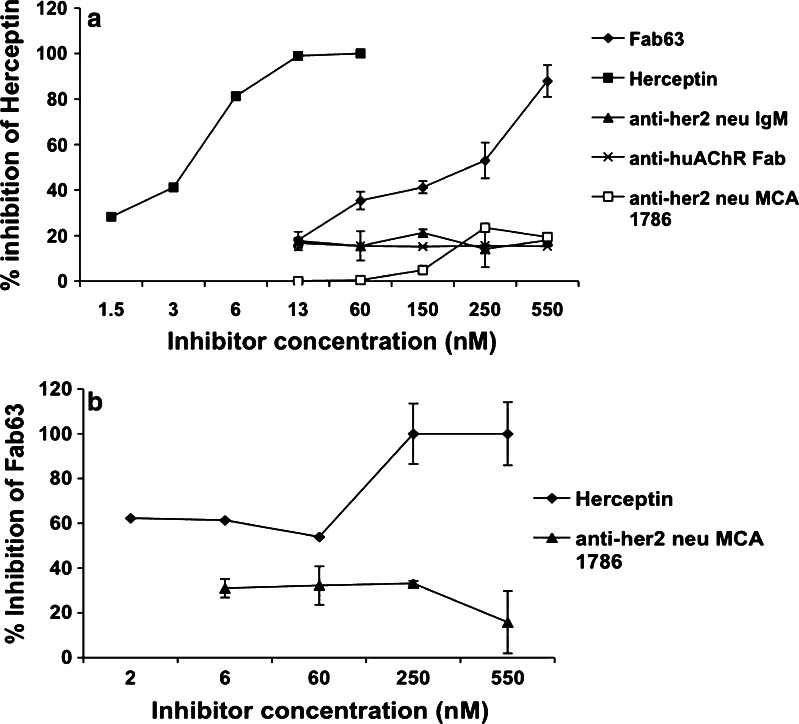
In an attempt to identify the site in Her2/neu that is recognized by Fab63, we used several commercial antibodies in competition assays. Fab63 inhibited Herceptin binding to the receptor when it was preincubated with Her2/neu, showing a 88% inhibition at 550 nM (Fig. 3a). Herceptin as an autocompetitor inhibited self-binding at 100% in a concentration of 66 nM, while the mAb MCA1786 (Serotec, Ltd, NC) that recognizes the extracellular domains of the Her2/neu, an anti-Her2/neu IgM human Ab and an anti-AChR Fab showed no competition. On the other hand, in Fig. 3b it was shown that Herceptin competed Fab63 binding to Her2/neu, at a concentration as low as 2 nM (~60% inhibition) showing maximum inhibition (~100%) at 250 nM, while MCA1786 did not show any significant inhibition. From the above data, we can speculate that Herceptin and Fab63 might be in close proximity since we have to consider the possibility of allosteric effects and the differences between Fab and whole IgG molecules.
Fig. 3.
Competition assays between Fab63 and Herceptin by sandwich ELISA. a Herceptin was used as the captured antibody for the binding of the Her2/neu receptor that had been pre-incubated with various Fabs/Abs (competitors). b Fab63 ability to bind the receptor that had been pre-incubated with competitor antibodies was also studied. A strong competition effect was shown only between Fab63 and Herceptin while no significant competition is observed with the other tested Fab/Abs. Percentage of inhibition was deducted from Her2/neu binding to captured antibodies in the absence of competitors. Bars represent the average ± SD from three individual experiments
Fab63 is internalized in SKBR3 cells
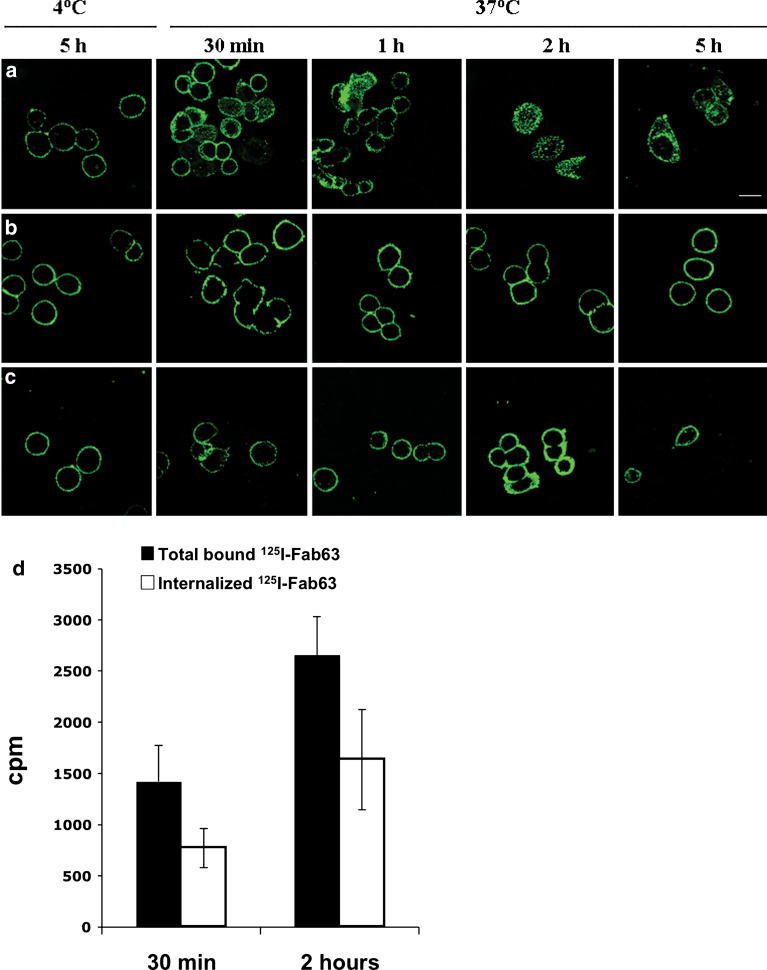
The binding of Fab63 to SKBR3 cells were analysed by using confocal microscopy. SKBR3 cells were incubated with Fab63 for 2 h at 37°C and high intracellular levels of Fab63 was detected using anti-human F(ab′)2 mAb (Fig. 4). Similar results were obtained with MDA-MB-453 cells (data not shown). Time-course analysis of Fab63-SKBR3 interaction was performed at 30 min, 1, 2 and 5 h after addition of Fab63. Figure 4a shows that Fab63 was rapidly internalized within 30 min of incubation with the cells. In contrast, Herceptin was not internalized since it stained the membrane of the cells only, at all time intervals (Fig. 4b). As a negative control, the specificity of this phenomenon was investigated by allowing the Fab63 to interact with the cells at 4°C or by performing the immunodetection under conditions where the cell membrane was not permeabilized (Fig. 4c). In both cases Fab63 was detected only on the cell membrane. The internalization of Fab63 was tested also by using radioiodinated antibody fragments. SKBR3 cells were incubated with 125I-Fab63 at 37°C for 30 min and 2 h as above. Between the two time points tested, there was a ~twofold increase in the amount of the total 125I-Fab63 that bound to cells, as well as in the internalized fraction of 125I-Fab63 (Fig. 4d). The proportion of the internalized Fab63 compared to the total pool of bound radioactivity remained relatively constant and was 54.4 and 62% at 30 min and 2 h of incubation, respectively.
Fig. 4.
Time course internalization of Fab63 in SKBR3 cells. Live SKBR3 cells grown on cover slips were incubated with Fab63 (a, c) or Herceptin (b). For endocytosis detection, cells were fixed and permeabilized, and intracellular antibodies were detected by confocal microscopy using an anti-human F(ab′)2 antibody followed by a FITC-conjugated rabbit anti-goat antibody. a Intracellular staining of Fab63 was observed within 30 min of incubation at 37°C and reached the highest intensity at 2 h of incubation with the cells. Fab63 was also detectable after 5 h of incubation, while no internalization was observed at 4°C. b Herceptin immunodetection showed only membrane staining. c Fab63 was immunodetected only on the cell membrane, on cells that had not been permeabilized subsequently to their incubation with Fab63. Scale bar: 20 μm. d Quantification of Fab63 internalization using 125I-Fab63 radiolabelled fragment. SKBR3 cells were incubated with labelled Fab63 at 37°C for 30 min and 2 h. The amount of total bound Fab63 and its internalization fraction was detected within 30 min and was dramatically increased during the first 2 h of incubation. ±SD represent the average values of three independent experiments
Growth inhibitory effects of human Fab63 on Her2/neu expressing cell lines
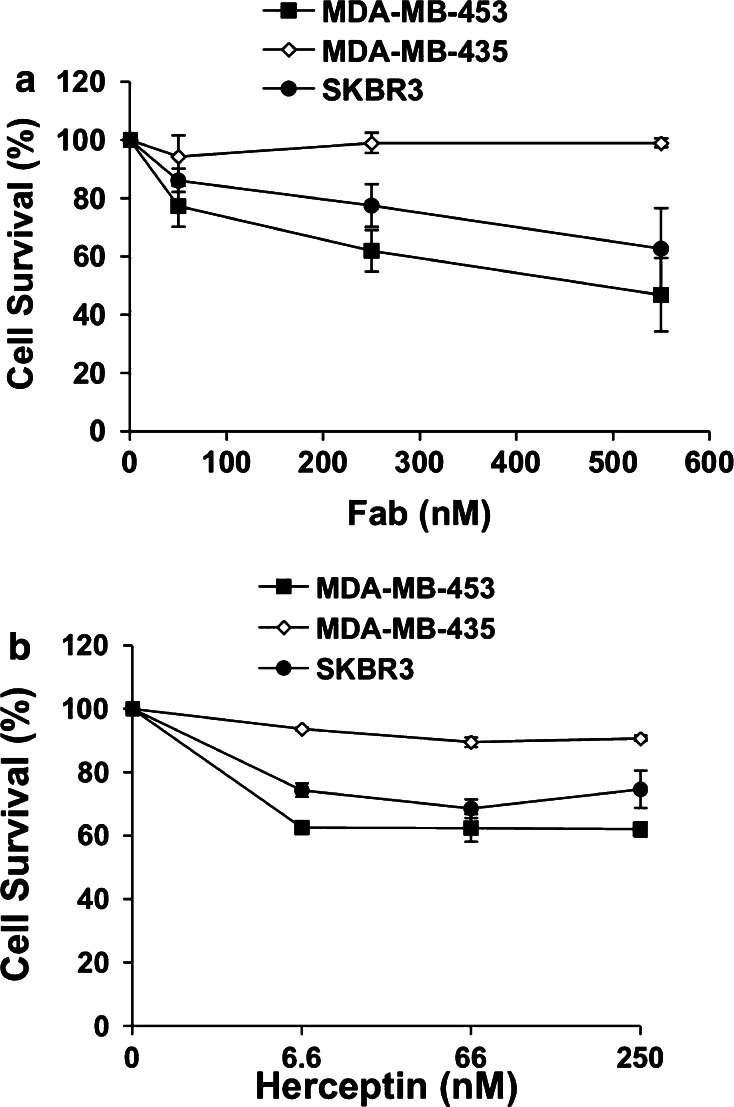
We next exploited the antiproliferative effects of Fab63 on breast cancer cell lines. Fab63 in concentrations of 66, 250 or 550 nM, inhibited cell growth up to ~44 and ~37% in the Her2/neu overexpressing cell lines MDA-MB-453 and SKBR3, respectively (Fig. 5a). This suggests a dose-dependent effect of inhibition by Fab63. In contrast, no cell growth arrest was noted in the MDA-MB-435 cells, which express Her2/neu in low levels (Fig. 5a).
Fig. 5.
Fab63 inhibits proliferation of Her2/neu-positive MDA-MB-453 and SKBR3 cells. Effects of Fab63 (a) or Herceptin (b) in cell growth of Her2/neu-positive (MDA-MB-453, SKBR3) and negative (MDA-MB-435) cell lines. Both agents exhibited antiproliferative effects only in Her2/neu-postive cells. Fab63 showed a dose-dependent growth inhibition. Cell survival is expressed as a percent of the normal growth of control cells, cultured in the absence of Fab/Ab. ±SD is the average value from three independent experiments
Herceptin was used as a positive control antibody, utilizing the whole IgG molecule, since it is known that its Fab fragment does not have any growth inhibitory effect [18]. As it was shown in Fig. 5b, a strong growth inhibition effect of Herceptin was observed even at the low concentration of 6.6 nM (~38% in MDA-MB-453 and ~26% in SKBR3 cells), whereas at concentrations of 66 or 250 nM no additional inhibition of cell growth was observed. These results come with agreement with previously published data on the antiproliferative effect of 4D5 mAb (the original mouse version of Herceptin) in SKBR3 cell line, where its maximum inhibition effect was determined at 10 nM [18].
Simultaneous incubation of SKBR3 with Fab63 and Herceptin, at concentrations of 500 and 66 nM, respectively, did not produce any significant increase in the levels of inhibition, suggesting that there is no synergistic effect between the two compounds (data not shown).
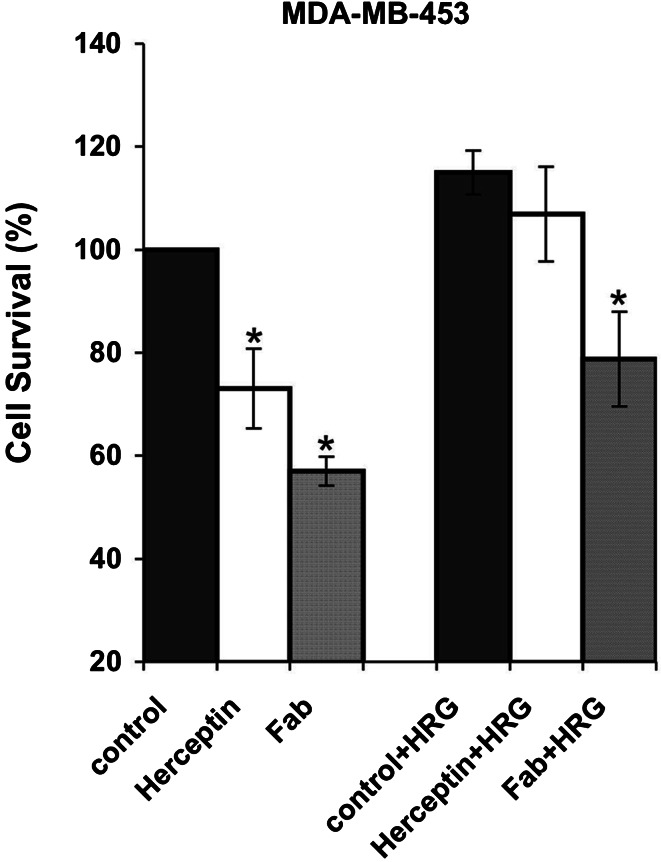
The antiproliferative effect of Fab63 was then tested in the presence of HRG-β1, the ligand of Her3 receptor. Fab63 (550 nM) significantly inhibited cell proliferation in the high Her2/neu expressing MDA-MB-453 cells up to 36.24% (P<0.05), compared to non-Fab treated cells (Fig. 6). Herceptin (66 nM) did not induce any significant inhibition on cell growth (P>0.1) in the presence of HRG-β1.
Fig. 6.
Fab63 inhibits proliferation of MDA-MB-453 cells in the presence of HRG-β1. Fab63 (550 nM) and Herceptin (66 nM) both inhibit cell growth in the high Her2/neu expressing cells MDA-MB-453, while in the presence of HRG-β1 only Fab63 can produce a significant growth inhibition effect P<0.05 (*denote significant inhibition of growth). Results and ±SD values are the average values from three independent experiments
These data show that Fab63 has significant antiproliferative effects in SKBR3 and MDA-MB-453 cancer cells where ligand-independent mechanisms dominate signal induction, while it is also able to negatively affect cell growth in the presence of HRG-β1 growth factor in MDA-MB-453 cells, implicating involvement in ligand-dependent proliferation.
Discussion
A characteristic event in metastatic breast cancers is the elevated levels of Her2/neu ECD in patient sera [28, 42]. The presence of Her2/neu antibodies and their correlation with Her2/neu positive cancer, implies that immunity of Her2/neu develops as a result of exposure of patients to Her2/neu protein expressed by their own cancer [15]. Several groups have produced mouse and rat mAbs against the Her2/neu ECD [20] which represent immunotherapeutic modalities via their antiproliferative effect on tumour cells. However, their non-human origin has limited their use since the repeated treatment produce human anti-mouse antibodies (the HAMA response). The ability to convert rodent antibodies into more human variants has allowed the production of therapeutic agents with reduced immunogenic potential. Herceptin and Pertuzumab, the humanized variants of two mouse mAbs, have been introduced as therapeutic agents for targeting high and/or low expressing Her2/neu cancers, respectively [17, 38]. Recently, by using the phage display technology, fully human scFvs or Fabs are selected from large combinatorial libraries of human antibody fragments [9, 25]. Moreover, the in vivo antibody responses to tumour-associated antigens may be exploited in vitro for the production of tumour-specific recombinant antibodies. Human scFv combinatorial phage display libraries were panned on recombinant ECD expressed in CHO cells, without successful isolation of antibody fragments with antiproliferative properties [37]. Recently, a phage scFv (Erbicin) was selected by panning the phage library on live Her2/neu overexpressing cells, using a subtractive selection strategy based on the use of two combinations of Her2/neu-positive and -negative cell lines [12]. This scFv fragment in order to have a more stable conformation was reconstructed as a compact reduced version of an IgG that retains the antiproliferative properties of the original antibody fragment [13].
In this study, we used the extracellular domain of the Her2/neu antigen expressed in the methylotrophic yeast P. pastoris in near-native conformation, in order to isolate anti-Her2/neu Fab fragments from an immune phage display library. This fragment was previously used as immunogen in experimental animals and anti-Her2/neu mAbs were efficiently produced. A phage antibody recognizing specifically the Her2/neu antigen was efficiently selected from a combinatorial Fab library constructed from infiltrated B lymphocytes of a patient with Her2/neu overexpressing tumour.
Since our selection was based on a purified recombinant fragment of the Her2/neu antigen, it was essential to confirm that Fab63 is able to recognize the native Her2/neu protein and mimic the in vivo conditions. We showed that Fab63 recognized the soluble Her2/neu receptor and bound specifically to the high Her2/neu expressing cancer cells. In a first step, to direct Fab63 binding towards a proximate site along the ~630 residue domain, we used a number of available anti-Her2 antibodies in competition assays with Her2/neu receptor. We found a strong competition effect only between Fab63 and Herceptin, suggesting a putative relationship between the binding sites of the two antibodies. Herceptin Fab-Her2/neu ECD complex has been crystallographically resolved, and revealed antibody binding at the C-end of the ECD IV domain [11]. A considerable credit of the anti-tumour properties of Herceptin was given to this juxtamembrane binding site, which inhibits the cleavage of ECD in breast cancer cells and blocks Her2/neu self-association and constitutive kinase activation [27]. Although this site was proposed as a potent anti-tumour target and it could explain some of the antiproliferative properties of Fab63, it is yet an initial observation, which requires additional data from the Fab63/ECD crystal complex.
What differentiates Fab63 from Herceptin and other antibodies is that it combines its antiproliferative effects with its ability to strongly and rapidly internalize into the target cells. Previous studies have shown that neither monovalent nor bivalent scFv forms of the mAb4D5, the original mouse version of Herceptin, or Herceptin monovalent Fab fragment, have any significant growth altering effects on ErbB2 overexpressing cells [18, 30]. Furthermore, there are no reports for anti-Her2/neu Fab monovalent fragments pairing these two dynamics although internalized Fabs without anti-tumour properties have been described before [32]. The previously described phage scFv or the respective soluble scFv (Erbicin) with anti-tumour properties, have been internalized in SKBR3 cells within 16 h of incubation at 37°C [12]. In contrast, Fab63 was rapidly internalized within 30 min of incubation with the same cells showing maximum intracellular staining within the first 2 h at 37°C. Under the same conditions, Herceptin stained only the cell membrane indicating that it was not internalized in this study. However, a recent publication showed that Trastuzumab (Herceptin) can be internalized after long periods of incubation with SKBR3 cells [3].
Fab63 has the ability to inhibit cell growth of Her2/neu overexpressing SKBR3 and MDA-MB-453 cells. Moreover, the inhibitory effect on cell proliferation was detected in the presence of Her3 ligand, HRG-β1, in MDA-MB-453 cells. One possible explanation for this effect is that Fab63 can block the Her2/neu signalling by limiting the number of membrane receptors after internalization of receptor–Fab complex in either homo- or heterodimerization form. The mechanisms of Fab63 cell growth inhibition are under investigation.
In conclusion, a new fully human Fab fragment was isolated against the extracellular domain of Her2/neu protein expressed in yeast P. pastoris, that has strong antiproliferative effects and internalization properties, which to our knowledge is a novel combination of characteristics among human Fabs isolated so far. In addition Fab63 is originated from a human library and thus is a non-immunogenic agent if administrated in humans. Furthermore, Fab63 internalization into the cytoplasm can potentially limit cytotoxicity effects, compared to antibodies that are constantly exposed on the membrane surface. Its use as a therapeutic tool should be further explored, either as a ‘naked’ antibody or as an ‘armed’ molecule, adopting enhancement manipulation techniques.
Acknowledgements
This work was supported by grants from the GSRT No PENED-01EΔ55 and 97EKBAN-19. We thank Drs. C.F. Barbas and D.R. Burton (The Scripps Research Institute, La Jolla, CA) for the generous gift of the pComb3HSS vector, Dr. A. Lavdas for assistance in confocal microscopy and Dr. S. Avrameas and D. Thanos for critical reading of the manuscript.
Abbreviations
- Fab
Fragment antigen binding
- EGFR
Epidermal growth factor receptor
- mAb
Monoclonal antibody
- PCR
Polymerase chain reaction
- AChR
Acetylcholine receptor
- Ni2+–NTA
Nickel–nitrilotriacetic acid
- FACS
Fluorescence-activated cell sorting
- HRP
Horseradish peroxidase
References
- 1.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/S1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 2.Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, Kelsey SM, Fyfe G. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 3.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbas CF, III, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbas CF, Burton DR (1994) Monoclonal antibodies from combinatorial libraries (1994 Cold Spring Harbor Laboratory Course). Cold Spring Harbor
- 6.Baselga J, Albanell J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol. 2001;12(Suppl 1):S35–S41. doi: 10.1023/A:1011163824080. [DOI] [PubMed] [Google Scholar]
- 7.Baxevanis CN, Sotiropoulou PA, Sotiriadou NN, Papamichail M. Immunobiology of HER-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol Immunother. 2004;53:166–175. doi: 10.1007/s00262-003-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton DR. Human and mouse monoclonal antibodies by repertoire cloning. Trends Biotechnol. 1991;9:169–75. doi: 10.1016/0167-7799(91)90055-M. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR, Barbas CF., III Human antibodies from combinatorial libraries. Adv Immunol. 1994;57:191–280. doi: 10.1016/S0065-2776(08)60674-4. [DOI] [PubMed] [Google Scholar]
- 10.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–1129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 11.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 12.De Lorenzo C, Palmer DB, Piccoli R, Ritter MA, D’Alessio G. A new human antitumor immunoreagent specific for ErbB2. Clin Cancer Res. 2002;8:1710–1719. [PubMed] [Google Scholar]
- 13.De Lorenzo C, Tedesco A, Terrazzano G, Cozzolino R, Laccetti P, Piccoli R, D’Alessio G. A human, compact, fully functional anti-ErbB2 antibody as a novel antitumour agent. Br J Cancer. 2004;91:1200–1204. doi: 10.1038/sj.bjc.6602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16–20. [PubMed] [Google Scholar]
- 15.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 16.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, Sneige N, Smith TL, Hortobagyi GN. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 17.Hynes NE, Lane HA. Erbb receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 18.Kelley RF, O’Connell MP, Carter P, Presta L, Eigenbrot C, Covarrubias M, Snedecor B, Bourell JH, Vetterlein D. Antigen binding thermodynamics and antiproliferative effects of chimeric and humanized anti-p185HER2 antibody Fab fragments. Biochemistry. 1992;31:5434–5441. doi: 10.1021/bi00139a003. [DOI] [PubMed] [Google Scholar]
- 19.Kiessling R, Wei WZ, Herrmann F, Lindencrona JA, Choudhury A, Kono K, Seliger B. Cellular immunity to the Her-2/neu protooncogene. Adv Cancer Res. 2002;85:101–144. doi: 10.1016/s0065-230x(02)85004-7. [DOI] [PubMed] [Google Scholar]
- 20.Kita Y, Tseng J, Horan T, Wen J, Philo J, Chang D, Ratzkin B, Pacifici R, Brankow D, Hu S, Luo Y, Wen D, Arakawa T, Nicolson M. ErbB receptor activation, cell morphology changes, and apoptosis induced by anti-Her2 monoclonal antibodies. Biochem Biophys Res Commun. 1996;226:59–69. doi: 10.1006/bbrc.1996.1311. [DOI] [PubMed] [Google Scholar]
- 21.Koeppen HK, Wright BD, Burt AD, Quirke P, McNicol AM, Dybdal NO, Sliwkowski MX, Hillan KJ. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38:96–104. doi: 10.1046/j.1365-2559.2001.01084.x. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 23.Luther MA, Schoepfer R, Whiting P, Casey B, Blatt Y, Montal MS, Montal M, Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989;9:1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamalaki A, Gritzapis AD, Kretsovali A, Belimezi M, Papamatheakis J, Perez SA, Papamichail M, Baxevanis CN. In vitro and in vivo antitumor activity of a mouse CTL hybridoma expressing chimeric receptors bearing the single chain Fv from HER-2/neu- specific antibody and the gamma-chain from Fc(epsilon) RI. Cancer Immunol Immunother. 2003;52:513–522. doi: 10.1007/s00262-002-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-U. [DOI] [PubMed] [Google Scholar]
- 26.McConahey PJ, Dixon FJ. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70:210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- 27.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 28.Molina MA, Saez R, Ramsey EE, Garcia-Barchino MJ, Rojo F, Evans AJ, Albanell J, Keenan EJ, Lluch A, Garcia-Conde J, Baselga J, Clinton GM. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8:347–353. [PubMed] [Google Scholar]
- 29.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.CAN-03-3856. [DOI] [PubMed] [Google Scholar]
- 30.Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001;12(Suppl 1):S9–S13. doi: 10.1023/A:1011199404516. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 32.Poul MA, Becerril B, Nielsen UB, Morisson P, Marks JD. Selection of tumor-specific internalizing human antibodies from phage libraries. J Mol Biol. 2000;301:1149–1161. doi: 10.1006/jmbi.2000.4026. [DOI] [PubMed] [Google Scholar]
- 33.Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, Danyluk J, Godolphin W, Sliwkowski M, Akita R, et al. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 34.Psaridi-Linardaki L, Mamalaki A, Remoundos M, Tzartos SJ. Expression of soluble ligand- and antibody-binding extracellular domain of human muscle acetylcholine receptor alpha subunit in yeast Pichia pastoris. Role of glycosylation in alpha-bungarotoxin binding. J Biol Chem. 2002;277:26980–26986. doi: 10.1074/jbc.M110731200. [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, Symmans WF, Pusztai L, Bloom KJ. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 36.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(Suppl 1):S3–S8. doi: 10.1023/A:1011195320446. [DOI] [PubMed] [Google Scholar]
- 37.Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindquist E, Schier R, Hemingsen G, Wong C, Gerhart JC, Marks JD. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci USA. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 39.Wang JN, Feng JN, Yu M, Xu M, Shi M, Zhou T, Yu XD, Shen BF, Guo N. Structural analysis of the epitopes on erbB2 interacted with inhibitory or non-inhibitory monoclonal antibodies. Mol Immunol. 2004;40:963–969. doi: 10.1016/j.molimm.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Liu B, Schmidt M, Lu Y, Wels W, Fan Z. Antitumor effect of an HER2-specific antibody-toxin fusion protein on human prostate cancer cells. Prostate. 2001;47:21–28. doi: 10.1002/pros.1043. [DOI] [PubMed] [Google Scholar]
- 41.Ward RL, Hawkins NJ, Coomber D, Disis ML. Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Hum Immunol. 1999;60:510–515. doi: 10.1016/S0198-8859(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 42.Wu JT. C-erbB2 oncoprotein and its soluble ectodomain: a new potential tumor marker for prognosis early detection and monitoring patients undergoing Herceptin treatment. Clin Chim Acta. 2002;322:11–19. doi: 10.1016/S0009-8981(02)00134-1. [DOI] [PubMed] [Google Scholar]
- 43.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]