Abstract
Non-specific immunopotentiators, such as polysaccharide K (PSK), also known as OK-432, induce anti-tumor effects via immunological responses. The efficacy of combination immunochemotherapy using these immunopotentiators has been examined by multiple previous studies. The survival benefits of immunochemotherapy for patients with curative resections of gastric cancers are not widely accepted. To clarify this issue, we performed a meta-analysis to evaluate the effect of immunochemotherapy on survival in patients with curative resections of gastric cancer. For this study, we compared the results of chemotherapy and immunotherapy using the biological response modifier PSK as an immunopotentiator. The meta-analysis included 8,009 patients from eight randomized controlled trials after central randomization. The overall hazard ratio for eligible patients was 0.88 (95% confidence interval, 0.79–0.98; P = 0.018) with no significant heterogeneity [χ 2(8) for heterogeneity = 11.7; P = 0.16]. The results of this meta-analysis suggest that adjuvant immunochemotherapy with PSK improves the survival of patients after curative gastric cancer resection.
Keywords: Adjuvant immunochemotherapy, Gastric cancer, Meta-analysis, Polysaccharide K
Introduction
Gastric cancer remains one of the leading causes of cancer-related deaths worldwide; the mortality rate for gastric cancer is now second only to that for lung cancer [1]. While surgery remains the mainstay of curative treatment, the relapse rate is high; survival, even after surgical resection with curative intent, remains low. Several types of postoperative adjuvant chemotherapy have been administered in the hopes of preventing relapse and increasing cure rates. These protocols include systemic adjuvant chemotherapy, peritoneal adjuvant chemotherapy, and adjuvant immunochemotherapy [2].
Adjuvant immunochemotherapy is expected to exert a synergistic effect with surgical resection. Polysaccharide K (PSK; Kureha Corporation, Tokyo, Japan), with a putative mean molecular weight of 100 kD following extraction from mycelia of Coriolus versicolor strain CM-101, is one of the most commonly used non-specific immunopotentiators [3]. PSK has a life-prolonging effect when administered with chemotherapy for leukemia [4] and colorectal cancer [5]. In the few prospective randomized controlled studies, a number of studies have reported definite benefits from PSK for gastric cancer [6, 7]. The efficacy of PSK, however, remains controversial due to a lack of robust evidence in clinical practice [8]. In the treatment of gastric cancer, PSK, widely prescribed for oral ingestion, is typically used as postoperative adjuvant immunochemotherapy [9]. This study sought to evaluate the effect of adjuvant immunochemotherapy with PSK to that of chemotherapy or surgery alone by means of a meta-analysis of randomized trials for curative resections of gastric cancer.
Materials and methods
Search strategy
We performed both computerized and manual searches of the MEDLINE electronic database to identify all randomized controlled trials (RCT) of PSK (or Krestin) use for patients with curative resections of gastric cancer. The search strategy utilized a combination of medical subject headings and text words related to the use of adjuvant immunochemotherapy with PSK for gastric cancer patients. Review papers were also examined for published results. We avoided duplications of data by examining the body of each publication and the names of all authors. When such duplications were identified, the latest version was included into our study. To ensure that all relevant studies were included, researchers with area expertise were also queried about the possible existence of unpublished trials.
We used the following eligibility criteria for the inclusion of trials into our analysis: (a) The aim of the study was the evaluation of the effect of the adjuvant chemotherapy regimen with or without PSK administration on patient survival. (b) The study was a central RCT, including trials using envelope methods. (c) Adjuvant therapy was administered after curative tumor resection. (d) A control arm received the same adjuvant chemotherapeutic regimen as the therapeutic arm. (e) The trial was concluded before the end of 2005.
Quality assessment of trials
We further assessed the quality of eligible studies using an assessment form designed for this review [10] comprised of the following criteria: (1) Was the allocation truly random? (2) Was the treatment allocation properly concealed? (3) Were the important prognostic factors of each group similar at baseline? (4) Were the numbers of withdrawals, dropouts, and losses to follow-up described accurately and in detail for each group? Was the dropout rate documented? (5) Was the analysis based on intention-to-treat? (6) Were the types and schedules of follow-up similar for the control groups? The answers to these questions were categorized as yes, no, or unclear.
The envelope randomization methods proved to be problematic in several respects. Participating physicians sometimes interfered with the randomization process, especially in studies initiated in the early 1980s. For this reason, we were skeptical of authentic random allocation in clinical studies using the envelope method. Using these criteria, clinical studies examining PSK activity were broadly subdivided into four quality categories. A+++: All criteria met; A++: Criteria for questions 1, 2, and 3 was met, but one or more of questions 4–6 were not met; A+: Criteria for question 1 was met, but one or more of questions 2–6 were not met; A: Unclear. This classification was then used as the basis of a sensitivity analysis.
Statistical methods
We used the hazard ratio (HR) to assess the survival benefit of PSK. If HR or variances of log HR were not included in the original studies, we calculated them according to the method proposed by Parmar et al. [11]. The DerSimonian–Laird method was used to estimate the pooled HR [12]. After consideration of the inter-study variations with the random effect, we examined the trials for heterogeneity by means of the Q statistic. We also performed a sensitivity analysis according to study quality including only those studies with a quality score of A+++ or A++. For multiple-treatment studies, we treated each comparison of treatment with PSK to that without PSK in the same trial as an independent study.
An HR of less than 1.0 indicates a beneficial effect of immunochemotherapy, while a ratio equal to or greater than 1.0 demonstrates a harmful effect. We defined a statistical test result with a P-value less than 0.05 as significant. SAS for Windows, release 8.02 (SAS Institute Inc., Cary, NC, USA), was used for all analyses.
Results
Evaluation of trials
After screening the references resulting from the MEDLINE search and handsearching for additional review papers based on those abstracts, we identified 44 papers that met our eligibility criteria [13–56]. Since 1981, the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMTC) has been conducting research in Japan into adjuvant immunochemotherapy. We identified three additional eligible references (JFMTC-1, JFMTC-5, and JFMTC-11) among previous JFMTC studies, although only the unpublished study reports were available. After assessing 47 potentially suitable papers in greater detail, we selected eight trials for this meta-analysis.
The characteristics of the eight references are shown in Table 1. Of the 39 references excluded from this meta-analysis, 11 references were not comparisons of treatment with and without PSK [18–28], 10 references were duplications of eligible trial data [29–38], 5 were not randomized studies [39–43], 5 contained target populations that differed significantly from ours [44–48], 5 were not clinical trials [49–53], and 3 evaluated endpoints different from overall survival [54–56]. The total number of patients in the group receiving PSK in combination with any chemotherapy (PSK group) was 4,037, with 3,972 patients in the group receiving the same chemotherapy alone (control group). In JFMTC-1, we divided the two comparisons in this trial into JFMTC-1a (regimen A vs. regimen B) and JFMTC-1b (regimen C vs. regimen D).
Table 1.
Characteristics of included trials
| No. | Study | Interventions | Participants no. | Excluded population (%) | Analyzed cases | Male (%) | Stage III over (%) | Five-year survival rate (%) | Deatha | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Niimoto et al. [13] | A: Mitomycin C (MMC) 20 mg i.v. day 1, futraful (FT) 600 mg/day orally for 1 year | 256 | 22.0 | 199 | 58.5 | 83 | 0.004 | ||
| (SACG-Kyushu) | B: MMC 20 mg i.v. day 1, PSK 3 g/day and FT 600 mg/day orally for 1 year | 264 | 28.0 | 191 | 71.7 | 54 | – | |||
| 2 | Kondo et al. [14] | A: FT 600—800 mg/day orally for 3 months with 2 months rest | 208 | 23.6 | 159 | 63.6 | 58 | 0.135 | ||
| (SACG-Chubu) | B: FT 600—800 mg/day orally for 3 months and PSK 3 g/day during 2 months rest | 198 | 26.8 | 145 | 71.3 | 42 | – | |||
| 3 | Kondo et al. [15] | A: Carbazilquinone (CQ) 2 mg/m2 i.v. on day 0, 8, 15 in each course | 75 | 34.7 | 49 | 59.2 | 40.7 | 47.1 | 27 | 0.690 |
| (TGOG) | B: CQ 2 mg/m2 i.v. on day 0, 8, 15 and PSK 2 mg/m2 orally from day 15 for 4 weeks | 65 | 27.7 | 47 | 63.2 | 65.3 | 45.3 | 27 | – | |
| 4 | Ogawa et al. [16] | A: MMC 0.4 mg/kg i.v. day 0 and 0.2 mg/kg day 1, HCFU 400 mg/day orally for 1 year | 69 | 20.2 | 55 | 21.0 | 81.1 | 9 | 0.810 | |
| (KGSG) | B: MMC 0.4 mg/kg i.v. day 0 and 0.2 mg/kg day 1, HCFU 400 mg/day and PSK 3 g/day orally for 1 year | 70 | 20.0 | 56 | 41.0 | 80.2 | 11 | – | ||
| 5 | Nakazato et al. [6] | A: MMC 6 mg/m2 i.v. day 0 and 1, fluorouracil (FU) 150 mg/day orally for 4 weeks with 4 weeks rest | 129 | 0.0 | 129 | 66.7 | 65.1 | 60.0 | 52 | 0.044 |
| (SIP) | B: MMC 6 mg/m2 i.v. day 0 and 1, FU 150 mg/day orally for 4 weeks and PSK 3 g/day orally for 4 weeks | 124 | 0.0 | 124 | 66.9 | 64.5 | 73.0 | 33 | – | |
| 6 | JFMTC-1 | A: MMC 20 mg bolus day 0 and 1, tegafur 600 mg/day orally for 8 months | 1,897 | 20.1 | 1,509 | 63.9 | 65.8 | 60.2 | 620 | 0.500 |
| B: MMC 20 mg bolus day 0 and 1, tegafur 600 mg/day orally and PSK 3 g/day for 8 months | 1,942 | 16.7 | 1,601 | 61.8 | 66.8 | 61.2 | 638 | – | ||
| C: MMC 20 mg bolus day 0 and 1, tegafur 600 mg/day orally, and OK-432 totally 100KE for 8 months | 1,907 | 18.5 | 1,543 | 63.8 | 69.1 | 58.7 | 660 | – | ||
| D: MMC 20 mg bolus day 0 and 1, tegafur 600 mg/day orally, OK-432 totally 100KE, and PSK 3 g/day for 8 months | 1,891 | 17.8 | 1,544 | 65.7 | 68.5 | 60.2 | 633 | – | ||
| 7 | JFMTC-5 | A: Tegafur 600 mg/day orally | 222 | 2.3 | 217 | 65.9 | 53.0 | 47.0 | 115 | 0.350 |
| B: Tegafur 600 mg/day orally and PSK 3 g/day | 220 | 2.5 | 215 | 60.7 | 51.2 | 52.8 | 101 | |||
| 8 | JFMTC-11 | A: Surgery alone | 114 | 1.8 | 112 | 65.8 | 8.7 | 83.3 | 20 | 0.769 |
| B: PSK 3 g/day orally for 6 months | 114 | 0.0 | 114 | 67.0 | 5.4 | 84.8 | 19 | – |
SACG The Co-operative Study Group of Surgical Adjuvant Chemotherapy for Gastric Cancer, TGOG Tokai Gastrointestinal Oncology Group, KGSG Kumamoto Gastrointestinal Immunochemotherapy Study Group, SIP The Study of Immunochemotherapy with PSK for Gastric Cancer, 95% CI 95% confidence interval
aThe number of death after 5 years
Based on our quality assessment, SIP [17] and the JFMTC-11 studies attained quality scores of A+++. JFMTC-5 was given a score of A++, while the remaining studies (SACG-Kyushu [13], SACG-Chubu [14], TGOG [15], KGSG [16], and JFMTC-1) received a score of A (Table 2).
Table 2.
Detailed quality assessment of included trials
| No. | Study | Quality assessment | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 |
|---|---|---|---|---|---|---|---|---|
| 1 | SACG-Kyushu | A | Yes | No | Yes | Yes | No | Yes |
| 2 | SACG-Chubu | A | Yes | No | Yes | Yes | No | Yes |
| 3 | TGOG | A | Yes | No | Yes | Yes | No | Yes |
| 4 | KGSG | A | Yes | No | Yes | No | No | Yes |
| 5 | SIP | A+++ | Yes | Yes | Yes | Yes | Yes | Yes |
| 6 | JFMTC-1 | A | Yes | No | No | Yes | No | Yes |
| 7 | JFMTC-5 | A++ | Yes | Yes | Yes | Yes | No | Yes |
| 8 | JFMTC-11 | A+++ | Yes | Yes | Yes | Yes | Yes | Yes |
Result of meta-analysis
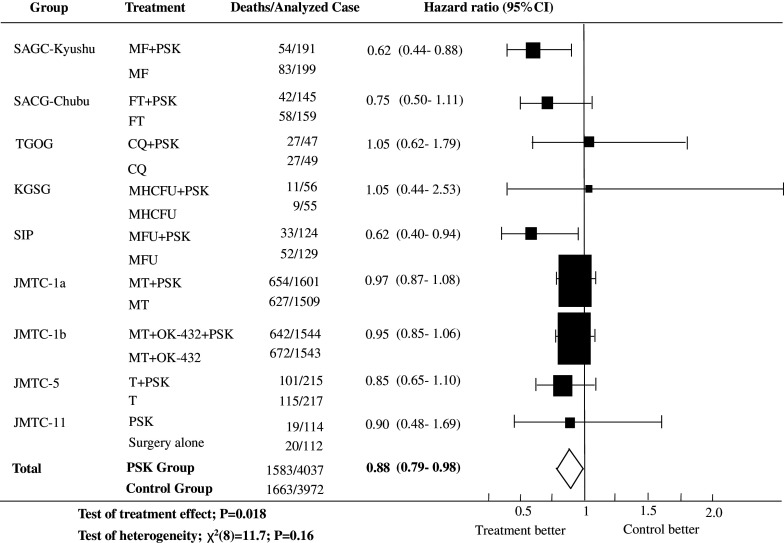
The HR and corresponding 95% confidence interval (CI) for each trial as well as the overall HR and 95% CI are presented in Fig. 1. The estimated overall HR was 0.88 (95% CI, 0.79–0.98; P = 0.018) with no significant heterogeneity between the treatment effects observed in different studies [χ 2(8) for heterogeneity = 11.7; P = 0.16].
Fig. 1.
Survival HRs and 95% CIs based on the eight selected trials. MF mitomycin C plus futraful, FT futraful, CQ carbazilquinone, MHCFU mitomycin C plus HCFU, MFU mitomycin C plus 5-fluorouracil, MT mitomycin C plus tegaful, T tegaful
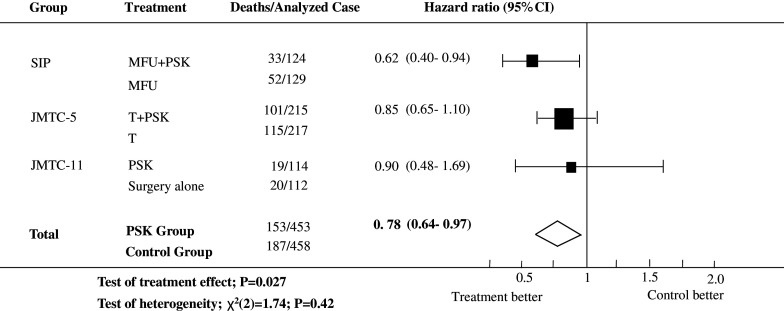
A sensitivity analysis using the SIP, JFMTC-5, and JFMTC-11 studies, those studies with high quality scores, was also performed (Fig. 2). The estimated overall HR for these trials was 0.78 (95% CI, 0.64–0.97; P = 0.027) with no significant heterogeneity between the treatment effects in different studies [χ 2(2) for heterogeneity = 1.74; P = 0.42].
Fig. 2.
Survival HRs and 95% CIs based on the three trials with quality scores of A+++ and A++. MF mitomycin C plus futraful, FT futraful, CQ carbazilquinone, MHCFU mitomycin C plus HCFU, MFU mitomycin C plus 5-fluorouracil, MT mitomycin C plus tegaful, T tegaful
Discussion
This study sought to analyze data from published and unpublished randomized clinical trials to evaluate the effect of adjuvant immunochemotherapy using PSK in comparison to therapeutic modalities without a PSK regimen. Although a considerable number of randomized trials have been performed to determine the effect of PSK as adjuvant chemotherapy, not all have been published. To broaden our research, we also searched for relevant meeting presentations, doctors performing such trials, and pharmaceutical industry endeavors. As a result, we detected a number of additional unpublished trials that met our criteria.
Combining data from eight selected studies yielded an HR of 0.88; these results from our meta-analysis indicated a significant improvement in survival resulting from PSK immunochemotherapy (95% CI, 0.79–0.98; P = 0.018). This improvement may well be both statistically and clinically significant. We further conducted a sensitivity analysis using the three trials with the best quality to verify the robustness of our results. The beneficial effect of PSK therapy was confirmed in our sensitivity analysis (HR was 0.78; 95% CI, 0.64–0.97; P = 0.027), which did not yield significantly different results from the larger analysis. We therefore conclude that PSK is effective as adjuvant immunochemotherapy for patients with gastric cancer.
Immunochemotherapy with OK-432, which has similar immunomodulatory effects as PSK, has also proved to be effective as adjuvant chemotherapy for gastric cancer in recent years [57]. The mechanisms by which PSK and other biological response modifiers are beneficial for patients after curative resection of gastric cancer remain unclear. Recent advances in molecular biology, however, suggest that immunomodulation by oral PSK may sensitize peripheral blood lymphocytes (PBL) to PSK, leading to the subsequent activation and proliferation of cytotoxic effector cells [58]. Unlike PBL, regional node lymphocyte suppressor cells were suppressed, and T helper cells increased in proportion. NK cells play an important role in immunological surveillance against tumors, potently inhibiting tumor metastasis [59, 60]. Thus, long-term administration of PSK after surgery may be beneficial to inhibit recurrences due to tumor metastasis. Reports detailing the efficacy of PSK as an adjuvant immunotherapy agent after surgery support this model [61].
Oral fluorinated pyrimidine therapy is one of the standard adjuvant chemotherapies in Japan. Addition of immunopotentiator PSK results in an even higher efficacy of therapy [15, 17]. In 2005, the Hokuriku-Kinki Immunochemotherapy Study Group started a Phase III RCT of Postoperative Adjuvant Therapy with S-1 Alone versus S-1 plus PSK for patients with Stage II/IIIA Gastric Cancer [62]. S-1 is an additional candidate for postoperative adjuvant chemotherapy in Japan. The initial success of this trial has drawn intense attention with the expectation that it can be established as the standard immunochemotherapy regimen in the future.
In conclusion, the results of this meta-analysis suggest that the addition of PSK to standard chemotherapy offers significant advantages in survival over chemotherapy alone for patients with curative resections of gastric cancers. We hope our results will result in a wider acceptance of immunochemotherapy as effective treatment of gastric cancer.
Acknowledgments
The sponsor of this study had no role in data collection, data analysis, data interpretation, or writing this report. There were no funding sources. The corresponding author had full access to all data in this report and assumes final responsibility for the decision to submit this publication.
References
- 1.Ferlay J, Bray F, Pisani P et al (2004) GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide. IARC CancerBase No. 5, version 2.0. IARC, Lyon
- 2.Sastre J, García-Saenz JA, Díaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol. 2006;12:204–213. doi: 10.3748/wjg.v12.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi H, Matsunaga K, Oguchi Y. Antimetastatic effects of PSK (Krestin), a protein-bound polysaccharide obtained from basidiomycetes: an overview. Cancer Epidemiol Biomarkers Prev. 1995;4:275–281. [PubMed] [Google Scholar]
- 4.Ohno R, Yamada K, Masaoka T, et al. A randomized trial of chemoimmunotherapy of acute nonlymphocytic leukemia in adults using a protein-bound polysaccharide preparation. Cancer Immunol Immunother. 1984;18:149–154. doi: 10.1007/BF00205503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto J, Morita S, Oba K, et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunol Immunother. 2006;55:404–411. doi: 10.1007/s00262-005-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima T, Inokuchi K, Hattori T, et al. Multi-institutional cooperative study of adjuvant immunochemotherapy in gastric cancer—five-year survival rate (in Japanese) Gan To Kagaku Ryoho. 1989;16:799–806. [PubMed] [Google Scholar]
- 8.Kim R, Yoshida K, Toge T. Current status and future perspectives of post-operative adjuvant therapy for gastric carcinoma. Anticancer Res. 2002;22:283–289. [PubMed] [Google Scholar]
- 9.Ikuzawa M, Matsunaga K, Nishiyama S, et al. Fate and distribution of an antitumor protein-bound polysaccharide PSK (Krestin) Int J Immunopharmacol. 1988;10:415–423. doi: 10.1016/0192-0561(88)90128-2. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S (eds) (2005) Assessment study quality. Cochrane handbook for systematic reviews of interventions 4.2.5 [updated May 2005]; Section 6. The Cochrane Library, Issue 3. Wiley, Chichester, UK
- 11.Parmar MB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Niimoto M, Hattori T, Tamada R, Sugimachi K, Inokuchi K, Ogawa N. Postoperative adjuvant immunochemotherapy with mitomycin C, futraful and PSK for gastric cancer. An analysis of data on 579 patients followed for five years. Jpn J Surg. 1988;18:681–686. doi: 10.1007/BF02471530. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Ichihashi H, Nakazato H, Ogawa N. Result of adjuvant immunochemotherapy on 8 year survival using Krestin and Futraful for gastric cancer patients who underwent radical gastrectomy—a randomized controlled trial by cooperative study group (in Japanese) Biotherapy. 1989;3:655–664. [Google Scholar]
- 15.Kondo T, Sakamoto J, Nakazato H. Alternating immunochemotherapy of advanced gastric carcinoma: a randomized comparison of carbazilquinone and PSK to carbazilquinone in patients with curative gastric resection. Biotherapy. 1991;3:287–295. doi: 10.1007/BF02221321. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Kako H, Kitano T, et al. Cooperative study of adjuvant immunochemotherapy using HUFU (Mifurol®) for gastric cancer patients who underwent curative resection (in Japanese) Rinsyo To Kenkyu. 1994;71:201–206. [Google Scholar]
- 17.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 18.Ochiai T, Sato H, Sato H, et al. Randomly controlled study of chemotherapy versus chemoimmunotherapy in postoperative gastric cancer patients. Cancer Res. 1983;43:3001–3007. [PubMed] [Google Scholar]
- 19.Fujimoto S, Furue H, Kimura T, et al. Clinical evaluation of schizophyllan adjuvant immunochemotherapy for patients with resectable gastric cancer—a randomized controlled trial. Jpn J Surg. 1984;14:286–292. doi: 10.1007/BF02469643. [DOI] [PubMed] [Google Scholar]
- 20.Kanabe S, Tamakuma S, Mimura K, et al. Comparison of immunochemotherapy and chemotherapy of stage IV gastric carcinoma (in Japanese) Gan No Rinsho. 1985;31:1805–1809. [PubMed] [Google Scholar]
- 21.Sato S. Study of validation of post-operative adjuvant chemotherapy following curative resection of stage II, III gastric cancer (in Japanese) Tokyo Ika Daigaku Zasshi. 1989;47:297–308. [Google Scholar]
- 22.Nio Y, Tobe T. Immunity of gut-associated lymphoid tissue and the role of the oral immunotherapy in multi-disciplinary treatment of the digestive organ cancer (in Japanese) Nippon Geka Gakkai Zasshi. 1989;90:1436–1438. [PubMed] [Google Scholar]
- 23.Imaizumi M, Kondo T, Kamei H, Ichihashi H. Cooperative study on surgical adjuvant immunochemotherapy for prevention of postoperative recurrence of gastric cancer (II). Cooperative study group on surgical adjuvant immunochemotherapy for prevention of postoperative recurrence of gastric cancer supported by the Ministry of Health and Welfare (Kondo’s group) (in Japanese) Gan to Kagaku Ryoho. 1990;17:2397–2403. [PubMed] [Google Scholar]
- 24.Fujimoto S, Furue H, Kimura T, et al. Clinical outcome of postoperative adjuvant immunochemotherapy with sizofiran for patients with resectable gastric cancer: a randomised controlled study. Eur J Cancer. 1991;27:1114–1118. doi: 10.1016/0277-5379(91)90306-X. [DOI] [PubMed] [Google Scholar]
- 25.Maehara Y, Sugimachi K, Akagi M, Kakegawa T, Shimazu H, Tomita M. Early postoperative chemotherapy following noncurative resection for patients with advanced gastric cancer. Br J Cancer. 1992;65:413–416. doi: 10.1038/bjc.1992.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imaizumi M, Kondo T, Kamei H. Cooperative study of postoperative long-term adjuvant immunochemotherapy for prevention of postoperative recurrence of gastric cancer (second trial) (in Japanese) Gan No Rinsho. 1994;40:1243–1250. [Google Scholar]
- 27.Tanaka N, Aichi A, Nagata H, et al. Preoperative intra tumor biological response modifier injection therapy for patients with stomach neoplasm (in Japanese) Nihon Rinsho Meneki Gakkai Kaishi. 1995;18:626. [PubMed] [Google Scholar]
- 28.Osawa S, Shiroto H, Kondo Y, et al. Randomized controlled study on adjuvant immunochemotherapy with carmofur (HCFU) for noncuratively resected and unresected gastric cancer (in Japanese) Gan To Kagaku Ryoho. 1996;23:327–331. [PubMed] [Google Scholar]
- 29.Imaizumi M, Kondo T, Kamei H, Ichihashi H. Cooperative studies on surgical adjuvant immunochemotherapy for prevention of postoperative recurrence of gastric cancer (in Japanese) Gan to Kagaku Ryoho. 1984;11:60–68. [PubMed] [Google Scholar]
- 30.Nakajima T, Inokuchi K, Hattori T, et al. A multi-institutional study on postoperative adjuvant immunochemotherapy of gastric cancer (in Japanese) Gan To Kagaku Ryoho. 1985;12:1850–1863. [PubMed] [Google Scholar]
- 31.Tamada R, Inokuchi K, Hattori T, et al. A multi-institutional study on postoperative adjuvant immunochemotherapy of gastric cancer (II) (in Japanese) Gan to Kagaku Ryoho. 1987;14:716–722. [PubMed] [Google Scholar]
- 32.Niimoto M, Hattori T, Tamada R, Sugimachi K, Inokuchi K, Ogawa N (1987) Postoperative adjuvant immunochemotherapy with mitomycin C, futraful and PSK for curatively resected cases of gastric cancer. In: Salmon SE (ed) Adjuvant therapy of cancer V. Grune & Stratton, Orlando, pp 515–524
- 33.Ichihashi H, Kondo T, Nakazato H. Clinical results of a randomized controlled trial on the effect of adjuvant immunochemotherapy using Esquinon and Krestin in patients with curatively resected gastric cancer—7-year survival—Cooperative Study Group for Cancer Immunochemotherapy, Tokai Gastrointestinal Oncology Group (in Japanese) Gan to Kagaku Ryoho. 1987;14:2758–2766. [PubMed] [Google Scholar]
- 34.Nakazato H, Koike A, Ichihashi H, Saji S, Danno M, Ogawa N. An effect of adjuvant immunochemotherapy using krestin and 5-FU on gastric cancer patients with radical surgery (first report)—a randomized controlled trial by the cooperative study group. Study Group of Immuno-chemotherapy with PSK for Gastric Cancer (in Japanese) Gan to Kagaku Ryoho. 1989;16:2563–2576. [PubMed] [Google Scholar]
- 35.Nakazato H, Koike A, Ichihashi H. Results of a randomized controlled trial using adjuvant immunochemotherapy in patients surgically treated for gastric cancer (in Japanese) Biotherapy. 1989;3:1356–1360. [Google Scholar]
- 36.Sakaguchi Y, Moriguchi S, Maehara Y, et al. Effect of postoperative long-term cancer chemotherapy (PLCC) on 15 year survival rate of gastric cancer patients (in Japanese) Biotherapy. 1989;3:1353–1355. [Google Scholar]
- 37.Hattori T, Nakajima T, Nakazato H, et al. Postoperative adjuvant immunochemotherapy with mitomycin C, tegafur, PSK and/or OK-432 for gastric cancer, with special reference to the change in stimulation index after gastrectomy. Jpn J Surg. 1990;20:127–136. doi: 10.1007/BF02470759. [DOI] [PubMed] [Google Scholar]
- 38.Kondo M. Immunotherapy as adjuvant treatment after curative resection of gastric cancer [comment] Lancet. 1994;344:274. doi: 10.1016/S0140-6736(94)93042-2. [DOI] [PubMed] [Google Scholar]
- 39.Mitomi T, Ogoshi K. Clinical study of PSK as an adjuvant immunochemotherapeutic agent against gastric cancer (in Japanese) Gan To Kagaku Ryoho. 1986;13:2532–2537. [PubMed] [Google Scholar]
- 40.Saji S, Takao H, Kida H, et al. Effects of postoperative adjuvant immunochemotherapy using PSK for patients of gastric cancer, and its preventive effects on the recurrence (in Japanese) Prog Med. 1987;7:1703–1709. [Google Scholar]
- 41.Maehara Y, Moriguchi S, Sakaguchi Y, et al. Adjuvant chemotherapy enhances long-term survival of patients with advanced gastric cancer following curative resection. J Surg Oncol. 1990;45:169–172. doi: 10.1002/jso.2930450307. [DOI] [PubMed] [Google Scholar]
- 42.Maehara Y, Emi Y, Sakaguchi Y, et al. Postoperative long-term cancer chemotherapy (PLCC) is effective for patients with gastric cancer following curative resection (in Japanese) Nippon Geka Gakkai Zasshi. 1990;9:1368–1370. [Google Scholar]
- 43.Maehara Y, Inutsuka S, Takeuchi H, Baba H, Kusumoto H, Sugimachi K. Postoperative PSK and OK-432 immunochemotherapy for patients with gastric cancer. Cancer Chemother Pharmacol. 1993;33:171–175. doi: 10.1007/BF00685337. [DOI] [PubMed] [Google Scholar]
- 44.Nakao I, Yokoyama T, Urushizaki I, et al. Clinical outcome of PSK for advanced gastric cancer (in Japanese) Oncologia. 1985;14:163–169. [Google Scholar]
- 45.Nakazato H, Ichihashi H, Kondo T. Clinical results of a randomized controlled trial on the effect of adjuvant immunochemotherapy using Esquinon and Krestin in patients with curatively resected gastric cancer. Cooperative Study Group of Cancer Immunochemotherapy, Tokai Gastrointestinal Oncology Group (in Japanese) Gan To Kagaku Ryoho. 1986;13:308–318. [PubMed] [Google Scholar]
- 46.Nakajima T, Inokuchi K, Hattori T, et al. Multi-institutional cooperative study of adjuvant immunochemotherapy in gastric cancer—five-year survival rate (in Japanese) Gan to Kagaku Ryoho. 1989;16:799–806. [PubMed] [Google Scholar]
- 47.Nio Y, Tsubono M, Tseng CC, et al. Immunomodulation by orally administered protein-bound polysaccharide PSK in patients with gastrointestinal cancer. Immunomodulation by orally administered protein-bound polysaccharide PSK in patients with gastrointestinal cancer (in Japanese) Biotherapy. 1992;4:117–128. doi: 10.1007/BF02171756. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi Y, Mai M, Nakazato H. Preoperative CEA and PPD values as prognostic factors for immunochemotherapy using PSK and 5-FU. Anticancer Res. 2005;25:1377–1384. [PubMed] [Google Scholar]
- 49.Saji S, Kida T, Kunieda K, et al. Five-year survival of gastric cancer patients treated with postoperative long term adjuvant immunochemotherapy. Prog Med. 1985;5:485–492. [Google Scholar]
- 50.Akimoto M, Nishihira T, Kasai M. Modulation of the anti-tumor effect of BRM under various nutritional or endocrine conditions (in Japanese) Gan to Kagaku Ryoho. 1986;13:1270–1276. [PubMed] [Google Scholar]
- 51.Sugimachi K, Maehara Y, Ogawa M, Kakegawa T, Tomita M. Dose intensity of uracil and tegafur in postoperative chemotherapy for patients with poorly differentiated gastric cancer. Cancer Chemother Pharmacol. 1997;40:233–238. doi: 10.1007/s002800050652. [DOI] [PubMed] [Google Scholar]
- 52.Hanazaki K, Sodeyama H, Yokoyama S, et al. Postoperative chemotherapy may improve prognosis in unresectable gastric cancer. J Clin Gastroenterol. 1998;26:269–273. doi: 10.1097/00004836-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Kuroda Y, Horikawa N, Tsuji M, et al. Usefulness of polysaccharide K (PSK) as postoperative adjuvant immunotherapy in patients with stage IV gastric cancer. Int J Clin Oncol. 1998;3:311–316. [Google Scholar]
- 54.Ogoshi K, Miyaji M, Iwata K, Kondo Y, Tajima T, Mitomi T. Effect of postoperative adjuvant immunochemotherapy on panperitonitis carcimatous stomach cancer (in Japanese) Biotherapy. 1990;4:676–679. [Google Scholar]
- 55.Sakamoto J, Koike A, Saji S, Teramukai S, Ohashi Y, Nakazato H. Preoperative serum immunosuppressive acidic protein (IAP) test for the prognosis of gastric cancer: a statistical study of the threshold level and evaluation of the effect of the biological response modifier PSK. Jpn J Surg. 1992;22:530–536. doi: 10.1007/BF00308899. [DOI] [PubMed] [Google Scholar]
- 56.Toge T, Yamaguchi Y. Protein-bound polysaccharide increases survival in resected gastric cancer cases stratified with a preoperative granulocyte and lymphocyte count. Oncol Rep. 2000;7:1157–1161. doi: 10.3892/or.7.5.1157. [DOI] [PubMed] [Google Scholar]
- 57.Sakamoto J, Teramukai S, Nakazato H, et al. Efficacy of adjuvant immunochemotherapy with OK-432 for patients with curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials. J Immunother. 2002;25:405–412. doi: 10.1097/00002371-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Algarra I, Collado A, Garrido F. Protein bound polysaccharide PSK abrogates more efficiently experimental metastases derived from H-2 negative than from H-2 positive fibrosarcoma tumor clones. J Exp Clin Cancer Res. 1999;16:373–380. [PubMed] [Google Scholar]
- 59.Nio Y, Tsubono M, Tseng CC, et al. Immunomodulation by orally administered protein-bound polysaccharide PSK in patients with gastrointestinal cancer. Biotherapy. 1992;4:117–128. doi: 10.1007/BF02171756. [DOI] [PubMed] [Google Scholar]
- 60.Pedrinaci S, Algarra I, Garrido F. Protein-bound polysaccharide (PSK) induces cytotoxic activity in the NKL human natural killer cell line. Int J Clin Lab Res. 1999;29:135–140. doi: 10.1007/s005990050079. [DOI] [PubMed] [Google Scholar]
- 61.Shibata M, Nezu T, Fujisaki S, Andou K, Tomita R, Fukuzawa M. Clinical potential of biological response modifiers combined with chemotherapy for gastric cancer. Dig Surg. 2002;19:255–260. doi: 10.1159/000064577. [DOI] [PubMed] [Google Scholar]
- 62.Ueda Y, Fujimura T, Kinami S et al (2006) A randomized phase III trial of postoperative adjuvant therapy with S-1 alone versus S-1 plus PSK for stage II/IIIA gastric cancer: Hokuriku-Kinki Immunochemo-Therapy Study Group-Gastric Cancer (HKIT-GC). Jpn J Clin Oncol [Epub ahead of print] [DOI] [PubMed]