Abstract
The repertoire of oligosaccharide components of cellular glycoproteins significantly contributes to cell adhesion and communication. In tumor cells, alteration in cellular glycosylation may play a key role in giving rise to invasive and metastatic potential. Over 100 melanoma cell lines deposited in the ESTDAB Melanoma Cell Bank (Tubingen, Germany) were studied for the characteristic glycan composition related to tumor progression. Analysis of: (1) cell adhesion to extracellular matrix proteins—fibronectin, laminin, and collagen; (2) the expression of selected glycosyltransferases—α2,3(Galβ1,3)- and α2,3(Galβ1,4)-sialyltransferases, α1,2- and α1,3-fucosyltransferases, and N-acetylglucosaminyltransferase V; (3) characterization of N-glycans was carried out on uveal (4), primary cutaneous (6), and metastatic (96) melanoma cell lines. Results showed that uveal cells did not adhere to any of the substrates and, in general, possessed less glycans containing α-2,6- and α-2,3-linked sialic acid. The average number of polypeptides bearing β-1,6-branched tri- and tetra antennary glycans(characteristic of the metastatic phenotype)were similar in uveal, primary cutaneous, and metastatic melanoma cell lines. Characterization of N-glycans may open a new perspective in the search for specific glycoproteins that could become targets for the therapeutic modulation of melanoma.
Keywords: Melanoma, N-glycans, Glycosyltransferases, Adhesion, Extracellular matrix proteins, Metastasis
Introduction
Most secreted and cell surface proteins are glycosylated. Cell surface glycoproteins contribute to a variety of interactions between the cell and its extracellular matrix [1, 22, 23]. The attached sugar chains have many biological functions, for example, cell-cell communication, signal transduction, and protein folding. Therefore, it is not surprising that aberrant glycosylation accompanies various diseases, including cancer [8, 11, 15, 19, 28]. The structural variability of glycans is dictated by tissue-specific regulation of glycosyltransferase genes, the availability of sugar nucleotides, and competition between enzymes for acceptor intermediates during glycan elongation [20]. Aberrant glycosylation occurs in essentially all types of experimental and human cancers, and many glycosyl epitopes constitute tumor-associated antigens. Many recent studies indicate that aberrant glycosylation is the result of initial oncogenic transformation, as well as a key event in the induction of invasion and metastasis [16]. The most widely occurring glycosylation change including malignancy is enhanced β1-6 GlcNAc side chain branching of N-linked structures caused by enhanced activity of N-acetylglucosaminyltransferase V (GnT-V) and counteracting β1-4 GlcNAc (bisecting GlcNAc) synthesized by N-acetylglucosaminyltransferase III (GnT-III) [9, 11, 12, 32]. The level of both epitopes is determined by the balance between GnT-V and GnT-III. The results of recent studies indicated that important adhesion proteins, such as integrins and cadherins, are heavily glycosylated and that alterations in their glycosylation accompany tumor progression [3–5, 14, 33]. Human cancers of the breast, colon, bladder, and melanomas show increased levels of β1-6GlcNAc branched N-glycans formed due to the increased activity of GnT-V [11, 33]. Artificial and spontaneous melanoma hybrids of high metastatic potential showed enhanced expression of GnT-V mRNA and increased enzymatic activity, accompanied by increased β1-6GlcNAc branching and enhanced cell surface expression of GnT-V protein substrates: LAMP-1 and β1 integrin [6]. β1-integrins with altered glycans are essential for cell adhesion to ECM proteins [3, 14] and altered glycans are probably involved in metastasis of melanoma cells [10, 18]. β1-6GlcNAc branched structures in integrins, like α5β1 and α3β1 result in reduced adhesion to fibronectin and enhanced motility of epithelial and melanoma cells [9, 33]. It has been recently shown in melanoma cell lines that adhesion molecules such as N-cadherin [4, 5] and integrins [24, 33] undergo altered glycosylation. Comparison of N-cadherin glycans from primary (WM35) and metastatic (WM9, WM239, A375) melanoma cell lines, employing MALDI mass spectrometry, showed that N-cadherin from primary melanoma cells possesses high-mannose and biantennary complex type glycans, while N-cadherin from metastatic cells possesses mostly tri- and tetraantennary type glycans [5]. These data are in good agreement with our prior lectin analysis [24] in which we compared the glycosylation profile of proteins from these cell lines. We have found that the metastatic cell line A375 possessed the greatest number of proteins reacting with PHA-L, a lectin specific for branched N-linked oligosaccharides, as compared to the primary WM35 cell line. Recently, employing tandem mass spectrometry we have identified these proteins in this A375 line [27]. Among the identified proteins were N-cadherin and integrin subunits (α2, α3, α5, αv, β1), as well as lysosome-associated membrane proteins (LAMPs). Interestingly, in addition, L1, Mac-2 binding protein CD44, and melanotransferrin were also shown to possess branched N-glycans. Some of these proteins are associated mainly with nervous tissue or the immune system and play a crucial role in cell adhesion processes. In a separate analysis, we have shown by MALDI-MS that in A375 cells only, both subunits of integrin α3β1 carried the β1-6 branched oligosaccharide with sialic acid. The involvement of these proteins in adhesion and migration processes, and the importance of their glycans have also been clearly shown in our studies [5, 33]. Blocking of N-cadherin-mediated interactions by specific antibodies significantly inhibited melanoma cell migration. Inhibition of synthesis of complex type N-glycans by swainsonine (mannosidese II inhibitor) led to a 50% decrease in cell migration in in vitro chemotactic cell migration [5] and wound healing assays (unpublished data). On the other hand, enhanced GnT-III gene expression inhibits β1,6 GlcNAc branching leads to suppression of metastasis in B16 melanoma cells. One of the targets appears to be E-cadherin, in which enhanced β1,4 GlcNAc reduces β1,6 GlcNAc branching, leads to enhanced cadherin-dependent cell-cell adhesion and suppression of metastasis [35]. Thus, GnT-V displays a prometastatic effect, whereas GnT-III is antimetastatic. In addition, apart from adhesion processes, cancer cells use their carbohydrate moieties to escape recognition by the immune system as they migrate and colonize target tissues [13, 25, 26]. Melanoma has become one of the fastest-rising malignancies in the last several decades and there are no successful tools for its early diagnosis or therapeutic procedures for its treatment, since it usually goes unnoticed and rapidly progresses to a disseminated, metastatic stage [17, 22, 23]. Many biological events and molecules are responsible for the metastatic process [2]. As almost all of these molecules are glycoproteins, the alteration of enzyme activity which catalyzes the formation of sugar chains may change the structure and composition of N-glycans. This may also affect the physiological function of the proteins responsible for metastasis. Therefore, glycosylation which promotes or inhibits tumor cell invasion and metastasis is of crucial importance in current cancer research [16]. Presented here are the results of the first large scale analysis of the adhesion properties, glycosyltransferase expression, and N-glycan characterization of glycoproteins of uveal, primary cutaneous, and metastatic melanoma cell lines deposited in the large European Melanoma Bank (ESTDAB), see http://www.medizin.uni-tuebingen.de/estdab/ and http://www.ebi.ac.uk/ipd/estdab/index.html
Materials and methods
Cell culture
Cells—uveal (n=4), primary cutaneous (n=6), and metastatic (n=96) were cultured in RPMI-1640 medium supplemented with 10% Fetal Calf Serum (Gibco). Frozen cell pellets (0.5–3×106 cells/pellet) received from the ESTDAB Melanoma Cell Bank (Tübingen, Germany) were thawed and suspended in a small volume (1 ml) of complete medium, split into two or three equal portions, and grown to confluence in 58 cm2 culture dishes (Corning). Afterwards, cells were harvested either with a rubber policeman (for glycan characterization) or using the standard trypsin/EDTA method (adhesion study).
Glycan characterization
Equal amounts of total protein from cell extracts (15 micrograms, as determined by the Peterson method [30]) were separated by 8% SDS-PAGE under reducing conditions according to Laemmli [21] and then electrotransferred overnight at 4°C to a PVDF membrane at a constant current of 100 mA. Glycan chain analysis of melanoma cell glycoproteins was performed according to the modified manufacturer’s procedure (Glycan Differentiation Kit, Roche). PVDF sheets were treated for 1 h with a solution of 0.05 M Tris-HCl and 0.15 M NaCl, pH 7.5 (TBS) containing a blocking agent. The blots were washed twice with TBS and once with TBS containing 1 mM MgCl2, 1 mM MnCl2, and 1 mM CaCl2. Then the blots were incubated for 1 h with the digoxigenin-labeled lectins: Datura stramonium agglutinin (DSA) specific for Gal (β1-4) GlcNAc (1:2000), Galanthus nivalis agglutinin (GNA) specific for terminal Man (α1-2, 1-3, or 1-6) Man units (1:2000), Maackia amurensis agglutinin (MAA) specific for NeuNAc (α2-3) Gal (1:800) or Sambucus nigra agglutinin (SNA) specific for NeuNAc (α2-6) Gal (1:2000). After washing with TBS, the blots were incubated for 1 h with polyclonal sheep anti-digoxigenin Fab fragments (1:4000) conjugated with alkaline phosphatase. Alternatively the blots were incubated for 1 h with the biotin-labeled lectins: Phaseolus vulgaris agglutinin (PHA-L) specific for (β1-6) branched N-glycans (1:1000) or Aleuria aurantia agglutinin (AAA) specific for fucose residues (1:4000). After washing with TBS; 0.1% Tween, the blots were incubated for 1 h with ExtrAvidin (Sigma) (1:4000) conjugated with alkaline phosphatase. After further washings, the conjugated alkaline phosphatase introduced either with the anti-digoxigenin antibodies or ExtrAvidin was detected by reduction of the 4-nitroblue tetrazolium salt in the presence of 5-bromo-4-chloro-3-indol phosphate in 0.1 M Tris-HCl, 0.05 M MgCl2, and 0.1 M NaCl, pH 9.5.
Expression of glycosyltransferases
RNA isolation and cDNA synthesis
RNA was extracted using RNeasy Mini Kit (QIAGEN). The concentration and quality of RNA samples were measured with a Spectrophotometer UV/VIS (Beckman). Then 1 μg of total cellular RNA was reverse transcribed by reverse transcriptase Omniscript (QIAGEN) with oligo dT23. The reaction mixture contained 2 μl 10× RT-PCR buffer, 2 μl 5 mM dNTPs, 1 μl reverse transcriptase Omniscript, 2 μl oligo dT23 primer (concentration 10 μM) and water in a final volume of 20 μl. RNA was incubated at 65°C for 5 min and with the reaction mixture at 37°C for 1 h, followed by inactivation of the enzyme at 95°C for 5 min.
RT-PCR for glycosyltransferases
PCR amplification of the sample was performed with both specific primer pairs for each of the studied glycosyltransferases: α1,2-fucosyltransferase—FUT-1, α1,3-fucosyltransferase—FUT-4, β1,6-N-acetylglucosaminyltransferase V—MGAT-5, α2,3(Galβ1,3)-sialyltransferase—SIAT-3, α2,3(Galβ1,4)-sialyltransferase—SIAT-4C, and human glyceraldehydes-3-phosphate dehydrogenase (GAPDH) genes.
The PCR reaction comprised 30 cycles and consisted of denaturing at 94°C (1 min), annealing at 60.5°C (1 min), and extension at 72°C (2 min). The PCR mixture contained: 2.5 μl 10× PCR buffer, 5 μl Q-solution, 1 μl MgCl2, 2 μl sample cDNA, 1.6 μl 10 mM dNTPs, 0.25 μl Ampli Taq polymerase (Perkin, Elmer), 1 μl of each glycosyltransferase specific primer (concentration 10 μM), and water in a final volume of 22 μl. The negative control reaction was performed simultaneously and was otherwise identical but without cDNA. Reaction products obtained after 30 cycles were electrophorezed on 3% agarose containing ethidium bromide. Glycosyltransferase mRNA expression of each sample was determined in at least two independent experiments (separate RNA isolation).
Sequences of forward (F) and reverse (R) oligonucleotide primers for glycosyltransferase genes and lengths of the amplification products were as follows [31]:
FUT-1 (173 bp) F: 5′-TATTCCGCATCACCCTGC-3′ R: 5′-CTGTTCCCGGAGATGGTG-3′
FUT-4 (319 bp) F: 5′-GGTGCCCGAAATTGGGCTCCTGCACAC-3′ R: 5′-CCAGAAGGAGGTGATGTGGACAGCGTA-3′
MGAT-5 (856 bp) F: 5′-GTGGATAGCTTCTGGAAGAA-3′ R: 5′-CAGTCTTTGCAGAGAGCC-3′
SIAT-3 (307 bp) F: 5′-AACAAGTCTCTGGGGTCACG-3′ R: 5′-TGAGGATTCGAATCTCAGGG-3′
SIAT-4C (320 bp) F: 5′-CTTCTTCATGGAGATTGCAGC-3′ R: 5′-CTACAGCTCTTGCCCAGGTC-3′
Adhesion to extracellular matrix proteins
Cell adhesion assays were performed using a 96-well plate, coated with mouse collagen IV, human fibronectin, or mouse laminin (BD Biosciences). The plates were washed with the PBS buffer and non-specific binding sites were blocked with 1% BSA for 1 h at 37°C.
The wells were pretreated by washing them twice with the PBS buffer and once with serum-free medium. In the meantime, cells were starved in serum-free medium for 30 min at 37°C in a humidified atmosphere containing 5% CO2. Afterwards, cells were harvested and washed with serum-free medium, added (5×104 cells in 100 μl volumes) to the pretreated wells, and left for 1 h at 37°C (CO2 incubator).
Wells were washed three times with PBS and adherent cells were fixed with 200 μl of 96% ethanol for 10 min at room temperature. Afterwards, they were washed three times with PBS and stained with 100 μl of 0.1% crystal violet water solution.
Finally, cells were washed with water and treated with 50 μl 0.5% Triton X-100 overnight (18 h). Absorbance, which is proportional to the number of adherent cells, was measured at 600 nm. The arbitrary scale (− : OD=0.00–0.05; +: OD=0.06–0.25; ++: OD=0.26–0.50; +++: OD=0.51–0.75; ++++: OD=0.76−) was used to evaluate and compare adherence of various cell lines.
Results and discussion
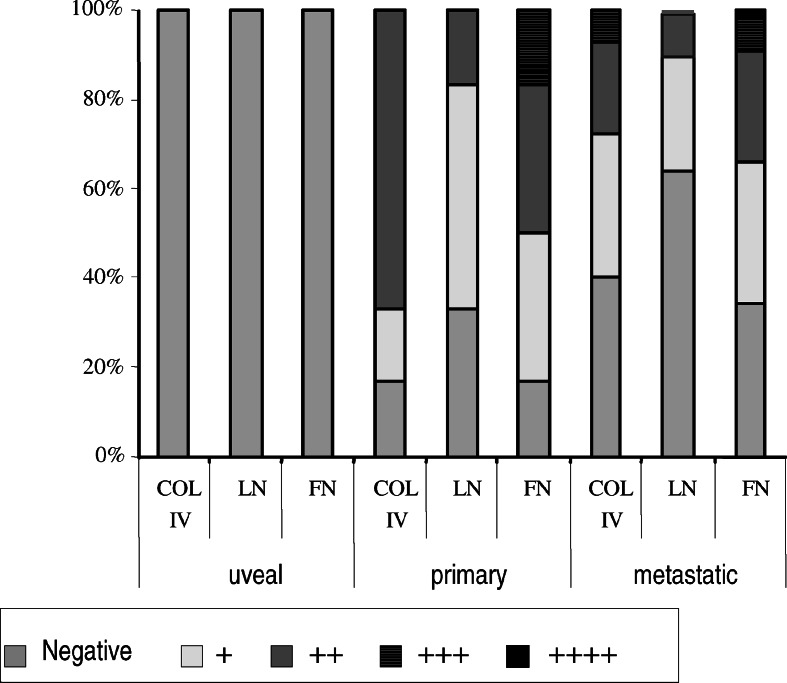
The analysis of adhesion performed on 106 melanoma cell lines (4 uveal, 6 primary cutaneous, and 96 metastatic) showed that they differ significantly in their ability to bind to the major extracellular matrix proteins: fibronectin, laminin, and collagen IV (Fig. 1).
Fig. 1.
Adhesion of melanoma cells; uveal (n=4), primary cutaneous (n=6), metastatic (n=96) to extracellular matrix proteins: FN fibronectin; LN laminin; COL IV collagen. See Materials and methods for details. Y-axis: % of cell lines adhering to a given ECM protein
Uveal cells did not adhere to any of these proteins, while among cutaneous melanomas 17–33% of primary cell lines and 34–64% of metastatic cell lines did not adhere to a given ECM protein. Even when cutaneous melanoma cells adhere to any of the examined ECM components, most of them did so weakly (+, ++). Nevertheless, a few primary melanoma cell lines showed strong binding (+++, ++++), but only to fibronectin, whereas some metastatic melanoma cell lines strongly adhered to all ECM proteins. The smallest percentage of cell lines adhered to laminin. Our results may suggest that cutaneous metastatic melanoma cells reveal more diverse adhesion properties (from not adherent to highly adherent) to ECM proteins in comparison to primary melanoma cells. It could indicate that these cells differ in their cell adhesion receptor repertoire, or that the difference in cell behavior might be due to changes in the glycosylation profile of these cell surface proteins.
Analysis of total RNA isolated from 106 melanoma cell lines was performed with the aim of determining the expression of glycosyltransferases responsible for synthesis of cancer-related glycans (data not shown). The RT-PCR method indicated high expression of sialyltransferase mRNAs (both SIAT 4C and SIAT 3) in all cell lines. Fucosyltransferase mRNAs (FUT 1 and FUT 4)were not detected in 25 and six of the cell lines, respectively. The lack of expression, of FUT 1 in almost 25% of all studied cell lines may indicate that its expression as well as the synthesis of fucosylated glycans may be important in cancer progression and metastasis, as was suggested recently in prostate and colon cancers [7, 13, 25]. The majority of cell lines expressed mRNA for N-acetylglucosaminyltransferase V (MGAT 5). In general, the expression of MGAT 5 transcript was low. However, in 10% of cell lines the expression of MGAT 5 was high while it was absent in only one cell line.
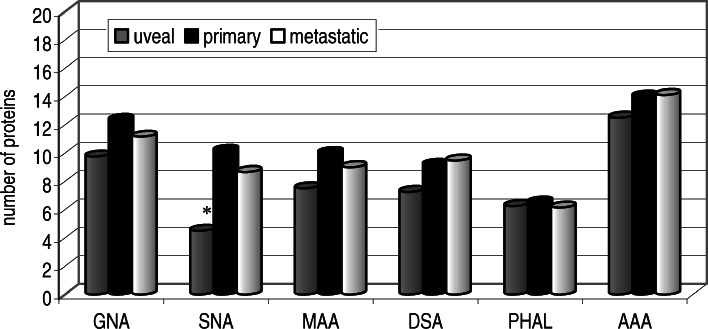
In the present study six lectins of well known and narrow specificity were employed to gain information on N-glycosylation of these 106 melanoma cell lines. In all studied cell lines the following numbers of protein bands reacting with lectins were detected: ca. 5–19 for GNA (a lectin specific for high-mannose type glycans), ca. 5–18 for DSA (a lectin specific for Gal(β1-4)GlcNAc in hybrid or complex type glycans), ca. 9–15 reacting with MAA (a lectin specific for α2,3-linked sialic acid), ca. 1–17 reacting with SNA (a lectin specific for α2,6-linked sialic acid), ca. 0–15 for PHA-L (a lectin specific for β1-6 branched complex type glycans), ca. 1–24 fore AAA (a lectin specific for fucose). In general, each subgroup of melanoma, uveal, primary skin, and metastatic, showed the presence of glycans reacting with each of the lectins used (Fig. 2). The only significant difference was observed with respect to glycans bearing α2,6-linked sialic acid, as uveal cell glycoproteins carried much less of such linked sialic acid than either primary cutaneous and metastatic melanoma (Fig. 2).
Fig. 2.
The average number of glycoproteins of uveal (n=4), primary cutaneous (n=6) and metastatic (n=96) melanoma cells reacting with digoxigenin-labeled lectins (GNA, SNA, MAA, DSA) and biolin-labeled lectins (PHAL, AAA). See Materials and methods for details. Y-axis: average number of proteins reacting with a given lectin. Asterisk: Statistically significant differences (p<0.5)
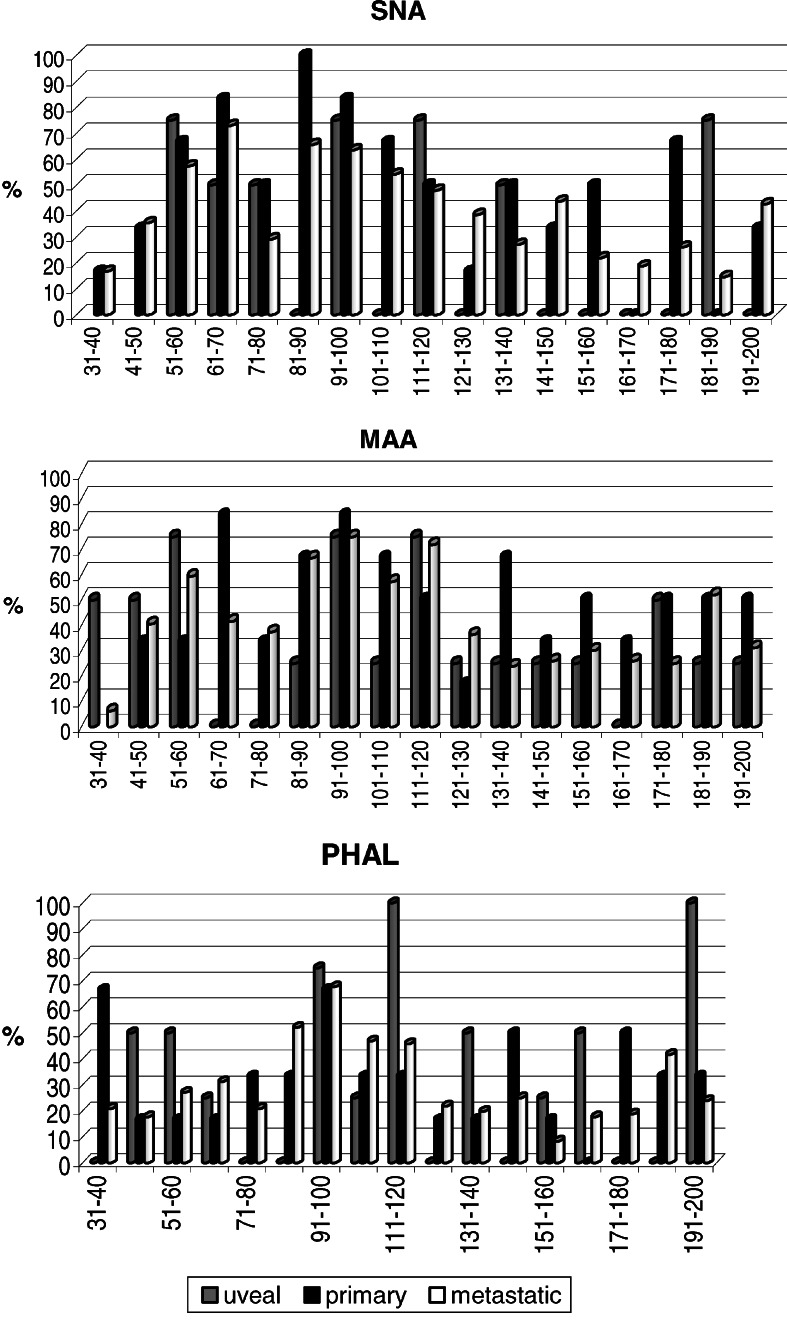
It is very difficult to conclude at present, based on the general type of comparative analysis, and keeping in mind that number of uveal cells is low, the importance of such structures. However, it seems that it is worthwhile to continue the search for a characteristic link between uveal melanoma and α2,6-linked sialic acid bearing glycans. More detailed insight into N-glycan content of various glycoproteins, as presented in Fig. 3, allows for a cautious search of protein candidates that might be selectively linked to cancer progression either due to their altered expression and/or glycosylation. Among these proteins are cell adhesion molecules belonging to the integrin, cadherin, as well as immunoglobulin superfamilies, which have been implicated in tumor progression in cutaneous melanoma [17, 34]. As indicated in Fig. 3, the significant difference between metastatic and primary cutaneous, as well as between metastatic and uveal cell lines, involves proteins of MW140–180 kDa reacting with SNA, which indicates the importance of the presence of α2-6 linked sialic acid residues. In particular, elevated α2-6 sialylation is correlated with changes in cell motility and invasiveness [3]. Numerous results strongly suggest that integrin glycosylation is necessary for cell adhesion and spreading on integrin ligands. Integrins are major carries of N-glycans. Changes in the N-glycan structures of integrins, especially α3β1 and α5β1, the major receptors for laminin and fibronectin, can affect cell-cell and cell-ECM interactions thereby affecting tumor malignancy [14, 33]. A recent study of Ozaki et al. [29] pointed to another glycoprotein, Mac-2 binding protein (M-2BP), which could be a novel target molecule in the immunotherapy of lung cancer. M-2BP has been reported to be a 90–70 kDa secreted glycoprotein that is a ligand of galectin 3 with multiple N-glycosylation sites. M-2BP is thought to play a key role in cellular immune responses and is also involved in cell aggregation by cross-linking with galectins. From our analysis we know that M-2BP is also present in melanoma cells, and that its glycosylation changes with melanoma progression (data not shown).
Fig. 3.
The percentage of glycoproteins of uveal (n=4), primary cutaneous (n=6) and metastatic (n=96) melanoma cells as present within consecutive MW ranges (31->200 kDa, 10 kDa step) due to possession of α2,6-linked sialic acid (SNA), α2,3-linked sialic acid (MAA), and β1-6-branched glycans (PHA-L). See Materials and methods for details. X-axis: ranges of MW, Y-axis: percent of cell lines possessing a glycoprotein(s) reacting with a given lectin within a particular MW range
Data collected during the characterization of N-glycans of studied melanoma cells opens a new perspective in the search for a specific glycoprotein candidate that could become a target for immunotherapy of melanoma. We are currently continuing with a more detailed analysis of the obtained results.
Acknowledgements
The studies were carried out and fully financed within the FP5 EU project “European Searchable Tumor Cell Lines Data Bank” – ESTDAB-NAS: QLRI-CT-2001-01325. The authors would like to thank Prof. Graham Pawelec for comments and fruitful discussion.
Abbreviations
- AAA
Aleuria aurantia agglutinin
- BSA
Bovine serum albumin
- ECM
Extracellular matrix
- DSA
Datura stramonium agglutinin
- GAPDH
Glyceraldehydes-3-phosphate dehydrogenase
- GlcNAc
N-acetylglucosamine
- GnT-V
N-acetylglucosaminyltransferase V
Footnotes
This article is a symposium paper from the conference “Progress in Vaccination against Cancer 2004 (PIVAC 4)”, held in Freudenstadt-Lauterbad, Black Forest, Germany, on 22–25 September 2004
References
- 1.Aplin A, Howe A, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Option Cell Biol. 1999;11:737–744. doi: 10.1016/S0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 2.Bani MR, Giavazzi R. Invasion and metastasis. In: Bronchud MH, Foote MA, Peters WP, Robinson MO, editors. Principles of molecular oncology. Totawa: Humana Press; 2000. pp. 298–320. [Google Scholar]
- 3.Bellis SL. Variant glycosylation an underappreciated regulatory mechanism for β1 integrins. Biochim Biophys Acta. 2004;1663:52–60. doi: 10.1016/j.bbamem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Ciołczyk-Wierzbicka D, Gil D, Hoja-Łukowicz D, Lityńska A, Laidler P. Carbohydrate moieties of N-cadherin from human melanoma cell lines. Acta Biochim Pol. 2002;4:991–998. [PubMed] [Google Scholar]
- 5.Ciołczyk-Wierzbicka D, Amoresano A, Casbarra A, Hoja-Łukowicz D, Lityńska A, Laidler P. The structure of the oligosaccharides of N-cadherin from human melanoma cell lines. Glycoconjugate J. 2004;20:483–492. doi: 10.1023/B:GLYC.0000038294.72088.b0. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty AK, Pawelek J, Ikeda Y, Miyoshi E, Kolesnikova N, Funasaka Y, Ichihashi M, Taniguchi N. Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyltransferase V, β 1-6 branching and metastasis. Cell Growth Differ. 2001;12:623–630. [PubMed] [Google Scholar]
- 7.Chandrasekaran EV, Chawda R, Locke RD, Piskorz CF, Matta KL. Biosynthesis of the carbohydrate antigenic determinants, globo H, blood group H and Lewis b: a role for prostate cancer cell alpha 1,2-L-fucosyltransferase. Glycobiology. 2002;12:153–162. doi: 10.1093/glycob/12.3.153. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Olio F. Protein glycosylation in cancer biology: an overview. J Clin Pathol. 1996;49:126M–135M. doi: 10.1136/mp.49.3.M126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetriou M, Nabi I, Coppolino M, Dedhar S, Dennis JW. Reduced contract-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-Transferase V. J Cell Biol. 1995;130:383–392. doi: 10.1083/jcb.130.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis JW. N-linked oligosaccharides processing and tumor cell biology. Cancer Biol. 1991;2:411–420. [PubMed] [Google Scholar]
- 11.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 12.Granovsky M, Fata J, Pawling J, Muller W, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat-5 deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 13.Goupille C, Marionneau S, Bureau V, Hallouin F, Meichenin M, Rocher J, Le Pendu J. α1,2 Fucosyltransferase increase resistance to apoptosis of rat colon carcinoma cells. Glycobiology. 2000;10:375–382. doi: 10.1093/glycob/10.4.375. [DOI] [PubMed] [Google Scholar]
- 14.Gu J, Taniguchi N. Regulation of integrin function by N-glycans. Glycoconjugate J. 2004;21:9–15. doi: 10.1023/B:GLYC.0000043741.47559.30. [DOI] [PubMed] [Google Scholar]
- 15.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 16.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herlyn M, Berking C, Li G, Satyamoorthy K. Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. 2000;10:303–312. doi: 10.1097/00008390-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Humphries MJ, Matsumoto K, White SL, Olden K. Inhibition of experimental metastasis by castanospermine in mice: blockage of two distinct stages of tumor colonization by oligosacharide processing inhibitors. Cancer Res. 1986;46:5215–5222. [PubMed] [Google Scholar]
- 19.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoprotein in cancer. Glycoconjugate J. 1997;14:569–576. doi: 10.1023/A:1018580324971. [DOI] [PubMed] [Google Scholar]
- 20.Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur J Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- 23.Li G, Satyamoorthy K, Herlyn M. Dynamic of cell interactions and communications during melanoma development. Crit Rev Oral Biol Med. 2002;13:62–70. doi: 10.1177/154411130201300107. [DOI] [PubMed] [Google Scholar]
- 24.Lityńska A, Przybyło M, Pocheć E, Hoja-Łukowicz D, Ciołczyk D, Laidler P. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Res. 2001;11:1–8. doi: 10.1097/00008390-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Marionneau S, Le Moullac-Vaidye B, Le Pendu J. Expression of histo-blood group A antigen increase resistance to apoptosis and facilitates escape from immune control of rat colon carcinoma cells. Glycobiology. 2002;12:851–856. doi: 10.1093/glycob/cwf103. [DOI] [PubMed] [Google Scholar]
- 26.Nangia-Makker P, Conklin J, Hogan V, Raz A. Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol Med. 2002;8:187–192. doi: 10.1016/S1471-4914(02)02295-5. [DOI] [PubMed] [Google Scholar]
- 27.Ochwat D, Hoja-Łukowicz D, Lityńska A. N-glycoproteins bearing β1-6 branched oligosaccharides from the A375 human melanoma cell line analysed by tandem mass spectrometry. Melanoma Res. 2004;14:479–485. doi: 10.1097/00008390-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Orntoft TF, Vestergaard EM. Clinical aspect of altered glycosylation of glycoproteins in cancer. Electrophoresis. 1999;20:362–371. doi: 10.1002/(SICI)1522-2683(19990201)20:2<362::AID-ELPS362>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki Y, Kontani K, Teramoto K, Fujita T, Tezuka N, Sawai S, Watanabe H, Fujino S, Asai T, Ohkubo I. Identification of antigenic epitopes recognized by Mac-2 binding protein-specific cytotoxic T lymphocytes for use in cancer immunotherapy. Biochim Biophys Res Commun. 2004;317:1089–1095. doi: 10.1016/j.bbrc.2004.03.155. [DOI] [PubMed] [Google Scholar]
- 30.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 31.Petretti T, Kemmner W, Schulze B, Schlag PM. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–366. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce M, Buckhaults P, Chen L, Fregien N. Regulation of N-acetylglucosaminyltransferase V and Asn-linked oligosaccharide β(1,6)-branching by a growth factor signalling pathway and effects on cell adhesion and metastatic potential. Glycoconjugate J. 1997;14:623–630. doi: 10.1023/A:1018592627696. [DOI] [PubMed] [Google Scholar]
- 33.Pocheć E, Lityńska A, Amoresano A, Casbarra A. Glycosylation profile of integrin α3β1 changes with melanoma progression. Biochim Biophys Acta. 2003;1644:113–123. doi: 10.1016/j.bbamcr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Sanders DS, Blessing K, Hassan GA, Bruton R, Marsden JR, Jankowski J. Alterations in cadherin and catenin expression during the biological progression of melanocytic tumours. Mol Pathol. 1999;52:151–157. doi: 10.1136/mp.52.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura M, Ihara Y, Matsuzawa Y, Taniguchi N. Aberrent glycosylation of E-cadherin enhanced cell-cell binding to suppress metastasis. J Biol Chem. 1996;271:13811–13815. doi: 10.1074/jbc.271.34.20265. [DOI] [PubMed] [Google Scholar]