Abstract
Prostate cancer is the most common male cancer and there is an urgent need for adjuvant therapy such as immunotherapy. Recombinant adeno-associated virus type 2 (rAAV) vectors are useful for antigen gene-loading of human dendritic cells (DC) and for the rapid generation of cytotoxic T lymphocytes (CTL). In this study, we report a protocol for AAV-loading of DC with the AAV-loading of self-antigen prostate specific antigen (PSA) resulting in generation of CTL. PSA and cytokine expression, Cell surface marker analysis of DC and CTL cells were done using a FACScalibur flow cytometer. Chromium-51 release assay was used to analyze the killing activity of CTL. It was found that AAV-loading of DC with the PSA gene is superior to PSA protein loading of the same antigen for generating effective CTL. AAV/PSA-loading of DC was found to result in: (1) strong, rapid PSA-specific, MHC Class I-restricted CTL, (2) PSA expression in DC, (3) high CD80, CD83, and CD86 expression on DC, (4) high level of IL-12 and low level of IL-10 in DC, (5) T cell populations with significant interferon γ (IFNγ) expression, but low IL-4 expression, (6) high proliferation of T cell populations, (7) high CD8:CD4 and CD8:CD56 T cell ratios. The reason for generation of robust CTL is partly explained by the characteristics of DC and CTL described. This protocol may be useful for adoptive immunotherapy against self antigens such as PSA for prostate cancer.
Keywords: Dendritic cells, Adeno-associated virus, Prostate cancer, PSA, Integration, Transduction, Immunotherapy
Introduction
Professional antigen presenting dendritic cells (DC) are pivotal in antigen-specific immune responses [34, 4, 31], and are easily obtained from peripheral blood precursors [27, 25]. The genetic manipulation of DC by Adeno-Associated Virus type 2 (AAV) vector-deliveries of self antigen may circumvent and surmount innate immune tolerance. AAV vectors are one of the safest for use in gene therapy. AAV vectors are effective for the delivery of antigens and cytokine genes into human DC, often with 90% efficiency [6, 15, 24, 35, 36]. Transduced DC can stimulate a robust CTL response with one stimulation (1 week co-incubation) against viral antigens. Moreover, these CTL express high levels of IFNγ [6]. Altogether, this is an improvement over currently used techniques [3, 23, 29, 37, 38, 40, 41].
Prostate cancer (PC) is the most common male cancer and the second leading cause of male cancer deaths [8–12, 14, 18, 26, 32, 33]. PC can be treated surgically, radiation and/or by chemotherapy, if detected early. Unfortunately, for many patients with advanced or hormone refractory PC, no effective surgical or medical treatment is available. There is an urgent need to develop alternative treatment approaches such as immunotherapy or gene therapy, as adjunct to standard treatments [9–12, 14, 16–19, 26, 33]. The prostate is not required for normal life. Therefore, the prostate may be a good target for immunotherapy or gene therapy. Effective anti-cancer CTL requires the choosing of an appropriate antigen. Prostate specific antigen (PSA), as a secreted protein, is currently the most widely used test for prostate cancer screening and monitoring [32]. It is expressed in both prostate cancer and normal prostate cells. In this study, we demonstrate that improved anti-PSA CTL can be generated by AAV/PSA transduced DC. In addition, we have characterized both DC and CTL in order to delineate the mechanisms for improved generation of CTL.
Materials and methods
Cells
Prostate cancer cell line, LNCaP–FGC that was obtained from the American Type Culture Collection (ATCC), EBV-transformed B cells (LCL) derived from five healthy donors and negative control cell line K562 (ATCC) were cultured in RPMI 1640 medium (Mediatech) supplemented with 10% fetal bovine serum (FBS) and 2 mM l-glutamine. The peripheral blood mononuclear cells (PBMC) from five healthy donors were separated by routine Ficoll gradient method. All blood donors were given informed consent in writing, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our Human Research Internal Review Board. The HLA haplotype of all donors were compatible with LNCaP-FGC. The PBMC and PBL (non-adherent PBMC) were cultured with 2.5 ml of AIM-V medium (Invitrogen Life Technologies) in six-well culture plate.
Generation of recombinant virus
Human PSA cDNA was amplified by reverse-transcription polymerase chain reaction (RT-PCR). The total mRNA was isolated from LNCaP-FGC. After the first-strand cDNA was generated, PCR amplification for PSA cDNA was carried out using the following primer pair: 5′-ATCCTGTCTCGGATTGTGG-3′ and 5′-TTGATAGGGGTGCTCAGG-3′ that amplify the sequence from nucleotides 252 to 1,506 [30]. PSA cDNA was sequenced and determined to be identical to the published sequence [30]. The AAV/PSA and AAV/PSA/Neo vectors were constructed as previously described [6, 15]. AAV/E6, AAV/BA46, AAV/HM1.24 were also constructed, coding for papilloma virus E6 antigen, breast cancer antigen/milk protein, and multiple myeloma B-cell-restricted antigen, respectively.
The plasmid pSH3 can express AAV type 2 rep and cap genes and adenovirus E2A, VA1 and E4 genes to allow rAAV DNA replication and packaging into particles without contaminating wild type AAV and adenovirus [7]. rAAV vectors were co-lipofected into HK 293 cells (ATCC) with plasmid pSH3. The rAAV were harvested by routine method after 4 days [6, 15]. To generate the purified rAAV the one-step column purification technique described by Auricchio et al. [2]. The rAAV were tittered as described previously by dot blot hybridization [6, 15].
Generation of DC infected by AAV/PSA
After the PBMC (5 × 106) were cultured for two hours, the non-adherent cells were removed. The monocytes (Mo) were infected immediately with 1 × 108 encapsidated genomes (eg)/ml of AAV/PSA virus or the control. After 4 h the medium/virus solution was removed and the cells were finally fed with AIM-V medium containing recombinant human GM-CSF (Immunex, 800 IU/ml). At day 2 and 4, to induce the maturation of Mo into DC, recombinant human IL-4 and TNFα (R & D Systems) at 1,000 IU/ml and 20 ng/ml were added to the medium, respectively. The medium and cytokines were replaced every two days. Finally, at day 6 the DC were mixed with PBL.
Generation of DC pulsed by PSA protein
The method of generation of PSA-lipofected DC described by Santin et al. was employed [28]. In brief, before Mo/DC were treated with TNFα at day 4 PSA protein (Chemicon International, 100μg/ml) and DOTAP liposomal reagent (Roche Diagnostics, 125 μg in 500 μl of AIM-V) were mixed. The complex was added to DC in a total volume of 2.5 ml of AIM-V medium, and the mixture was incubated for 3 h. The cells were washed and resuspended in AIM-V as described above.
Analysis of AAV/PSA Expression and efficient infection
At day six of Mo/DC culture, intracellular staining assay of Pala et al. [22] was employed to analyze the expression and efficient infection of AAV/PSA virus according to the routine method. After the AAV/PSA virus-infected or control suspension cells (DC) were harvested the cells were fixed and permeabilized. The cells were incubated with mouse anti-PSA monoclonal antibody (40 μg/ml; Chemicon International), and were subsequently stained with FITC-conjugated goat anti-mouse monoclonal antibody (1 μg/ml; Chemicon International). A FACSCalibur flow cytometer (Becton–Dickinson) was used for data acquisitions. At least 10,000 events were counted for each sample.
Cell surface marker analysis of DC
For the analysis of DC a panel of FITC- or PE-labeled monoclonal antibodies recognizing the following antigens was used: CD1a (Caltag Laboratories), CD14, CD40 (Chemicon International), HLA-DR, CD80, CD83 and CD86 (BD Pharmingen), Control irrelevant isotype-matched FITC- or PE-conjugated monoclonal antibodies were obtained from BD Pharmingen. After 6 days the non-adherent DC were harvested (>95% viable as assessed by Trypan blue exclusion) the cells counted and distributed. Stained cells were assayed for surface markers as described previously [6].
Analysis of IL-10 and IL-12 expression in the DC
Human IL-12 p70 and IL-10 were measured in the DC supernatants by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Biosource International). The sensitivity of the IL-10 and IL-12 ELISA is <1 and <0.5 pg/ml, respectively. At day 3 of Mo/DC culture (before the addition of TNFα) and day 6 (after the addition of TNFα the supernatants were separated from the culture. IL-10 and IL-12 p70 secretion was measured in duplicate according to the manufacturer’s instructions.
Analysis of T cell proliferation
T cell proliferation, in the presence of variously treated DC, was measured by tritium incorporation.
Generation of target cells
LCL derived from five donors were infected by AAV/PSA/Neo virus, and cultured in the medium plus 50 μg/ml of G418 for more than 15 days. Immunostaining assay was performed to analyze the efficiency of the rAAV infection using mouse anti-PSA monoclonal antibody as described above. K562 cells, the target cells of natural killer cells (NK), and LNCaP–FGC cells were also analyzed for the expression of PSA. In addition, AAV/E6, AAV/BA46 and AAV/HM1.24 virus-infected LCL were generated, respectively, as control targets [6]. A series of non-prostate cell lines were also used as negative control targets.
Generation of cytotoxic T lymphocytes (CTL)
After 6 days of DC culture the mature DC were harvested and mixed with the PBL (ratios 20:1, PBL: DC). The mixtures were cultured in AIM-V containing recombinant human GM-CSF (800 IU/ml), IL-2 (20 IU/ml) and IL-7 (20 ng/ml) at 10–20 × 106 cells/well in 6-well culture plates. The medium and cytokines were replaced every 2 days. After 8 days post-priming the cells were harvested and analyzed further.
Analysis of PSA-specific and MHC Class I-restricted CTL killing activity
Six-hour chromium-51 (51Cr) release assay [23, 28, 42,] was used to analyze the killing activity of CTL elicited by AAV/PSA-infected, PSA-lipofected and control DC against the target cells. The CTL cells and 51Cr-labeled target cells were mixed (20:1) and incubated for 6 h at 37°C with 5% CO2. To determine the structures on the target cells involved in lysis, mouse anti-HLA Class I monoclonal antibodies were used to block cytotoxicity. The 51Cr-labeled targets were pre-incubated with mouse anti-human MHC class I antibody (Serotec) for 1 h. before the 51Cr release assay was performed. The mouse anti-human MHC class II antibody (Serotec) was also used as a control.
CD marker analysis of activated T cells
For the analysis of activated T cells, at day 8 of the experiment the primed T cell populations were analyzed for their surface markers with immunofluorescence staining. A panel of FITC- or PE-labeled monoclonal antibodies recognizing the following antigens was used: CD4, CD8, CD56 (Pharmingen).
Analysis for the level of IFN-γ and IL-4 in the activated T cell populations
At day 8 days post-priming T cells were harvested. The intracellular staining assay was performed to analyze the expression of IFN-γ and IL-4 in the T cells according to the protocol described by Pala et al. [22].
Results
AAV/PSA transduced cells express PSA
One main goal of this study was to determine if AAV-based antigen gene delivery into DC could generate CTL against a self-antigen, PSA, as has been shown previously for viral antigens [6]. The structure of the AAV vectors used in this study includes the PSA cDNA that was ligated into the basic AAV vector dl6-95 to give AAV/PSA. In these vectors the PSA gene was expressed from the AAV p5 promoter, which is known to be active in DC [6]. The titer of AAV/PSA virus stocks was 1 × 1011 encapsidated genomes per ml (eg/ml; data not shown). Virus stock of other AAV vectors was also generated and tittered.
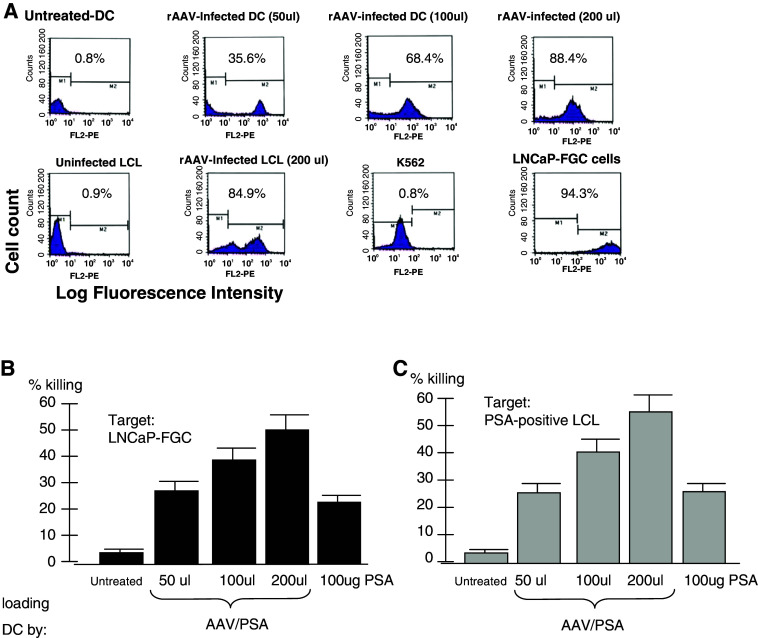
Protocols for the generation of DC by stimulating the differentiation of peripheral blood adherent monocytes (Mo) involve treating adherent Mo with GM-CSF and IL-4. We have modified these protocols in order to promote AAV vector transduction in DC precursor Mo. This altered protocol involves treating adherent Mo with GM-CSF alone for 2 days before the addition of IL-4, TNF-α on day 2 and day 4 [6]. This will allow the Mo to go through a brief period of limited cell division. Cell division is important for promoting higher levels of AAV transduction [23, 38]. The transduction of the Mo/DC population was observed by intracellular staining for PSA. In addition to DC transduction, we also measured the transduction of EB virus transformed B lymphocytes (LCL). The results are shown in Fig. 1a. Both cell types were transduced at levels of 85–90%. We also analyzed the natural level of expression of PSA in LNCaP–FGC cells (a prostate cancer cell line) and K562 cells as these two cell lines will serve as our standard antigen-positive and negative target cells. As expected 94% of LNCaP–FGC cells expressed PSA, while less than 1% of K562 cells did (Fig. 1a).
Fig. 1.
a Transduction efficiency by AAV vectors and analysis of PSA-positive targets. b and c Killing of PSA-positive targets by AAV/PSA-loaded DC derived CTL and PSA protein-loaded DC-derived CTL. CTL were generated as indicated and assayed for their ability to kill either LNCaP-FGC (b) or LCL transduced with AAV/PSA (c)
Analysis of PSA-specific and MHC class I -restricted CTL killing activity
The ability of the AAV/PSA-loaded DC to stimulate effective anti-PSA CTL killing was next analyzed. CTL derived from DC, which was loaded by untreated, PSA protein and AAV/PSA transduction were generated and tested for the ability to kill various targets. Using chromium-release killing assay, Fig. 1b, we compared all primed CTL populations for their ability to kill the PSA-positive prostate cancer cell line LNCaP-FGC. We also tested CTL generated by three different loading doses of the AAV/PSA virus. The data from five different donors, HLA-matched to the LNCaP-FGC cell line, were compiled and the results shown in Fig. 1b. As shown the untreated-derived CTL, at a 20:1 effector:target ratio, were unable to kill LNCaP-FGC cells (3.8%), while the PSA protein-derived CTL killed at 22.9% (P < 0.01). In contrast the CTL derived from increasing doses of AAV/PSA killed at increasingly higher levels of 29.5, 38.7, and 52.5% (P < 0.05). In Fig. 1c, we assayed all three of these primed CTL populations for the ability to kill the PSA-positive autologous LCL. These PSA postive-LCL cell lines were generated by infecting the autologous LCL with AAV/PSA/Neo and transduced cells were selected using G418. We also tested CTL generated by three different loading doses of the AAV/PSA virus. The data from five different donors were compiled and the results shown in Fig. 1c. As shown the untreated-derived CTL, at a 20:1 effector:target ratio, were unable to kill LCL cells (3.3%) while the PSA protein-derived CTL killing was 21.5% (P < 0.01). In contrast, the CTL derived from the three increasing doses of AAV/PSA killed at increasingly higher levels of 22.6, 39.2, and 55.4% (P < 0.01). Thus, the AAV/PSA generated CTL were superior to the PSA protein-derived CTL in killing ability of either the LNCaP-FGC (Fig. 1b) or the autologous PSA positive LCL cells (Fig. 1c). Furthermore, in both experiments, increasing the DC loading dose of AAV/PSA resulted in more effective DC and primed CTL in a loading-dose-dependent fashion.
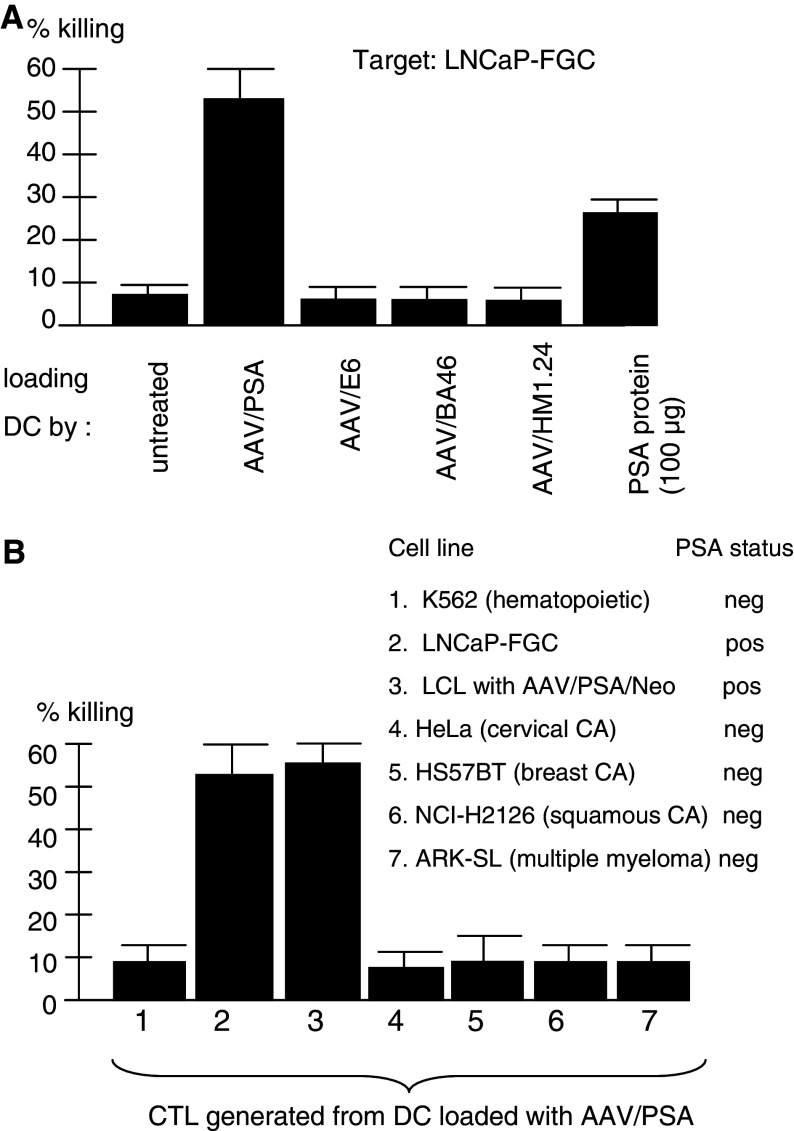
Experiments shown in Fig. 2a were designed to test the antigen specificity of AAV-based DC loading. In this experiment, using five different donors, six different DC loading treatments were carried out, each using one of four different AAV vectors, each containing a different transgene, as well as PSA protein and untreated loading DC. Only one vector, AAV/PSA, contained the target antigen. CTL were generated using the six different DC and then the derived CTL were compared for the ability to kill the PSA-positive LNCaP-FGC. As can be seen, only those T cells incubated with AAV/PSA- and PSA protein-loaded DC were able to kill the LNCaP-FGC at a level of 52 and 23%, respectively (P < 0.001). These data are fully consistent with antigen-loading specificity of the resulting CTL, as expected.
Fig. 2.
a Loading specificity of generated CTL. CTL generated by various AAV vectors were assayed for killing the LNCaP-FGC. Nore that only the CTL generated from DC loaded with AAV/PSA or PSA protein were able to kill these cells. Also note that CTL derived from AAV/PSA-loaded DC could kills at a much higher levevl than those drived from PSA protein-loaded DC. b Target specificity of generated CTL: AAV/PSA-generated CTL response against a variety of targets is shown. Note that only LNCaP-FGC and PSA positive-LCL are killed while all others are not
Experiments shown in Fig. 2b were designed to test the antigen specificity of killing by T cells stimulated by AAV/PSA-loaded DC. Several different HLA-matched antigen-positive and antigen-negative targets were included. As shown, while the LNCaP–FGC and PSA positive-LCL targets were killed, the other five PSA-negative cell lines were not (P < 0.001). These data, and those of the preceeding figures are fully consistent with the resulting CTL being highly PSA-specific.
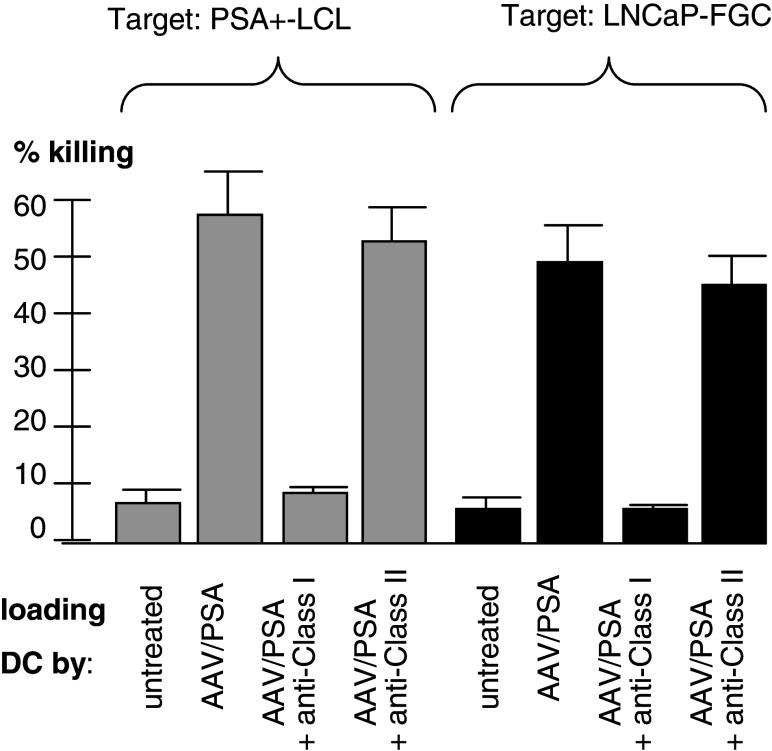
MHC class I-restriction was examined in experiments using both LNCaP–FGC and PSA positive LCL cell lines (Fig. 3). The targets were pre-incubated with anti-MHC class I or anti-MHC class II antibody. CTL was generated by AAV/PSA-loaded DC and incubated with the indicated targets. The CTL killing was blocked by anti-MHC class I antibody, but not by anti-MHC class II antibody, strongly suggesting MHC class I-restriction (P < 0.001).
Fig. 3.
Class I Restriction of CTL killing. CTL generated by various AAV vectors were assayed for killing the LNCaP-FGC. Note that only the CTL generated from DC loaded with AAV/PSA or PSA protein were able to kill these cells. Also, note that CTL derived from AAV/PSA-loaded DC could kill at a much higher level. Note the addition of anti-class I antibody inhibited killing, while anti-class II antibody did not
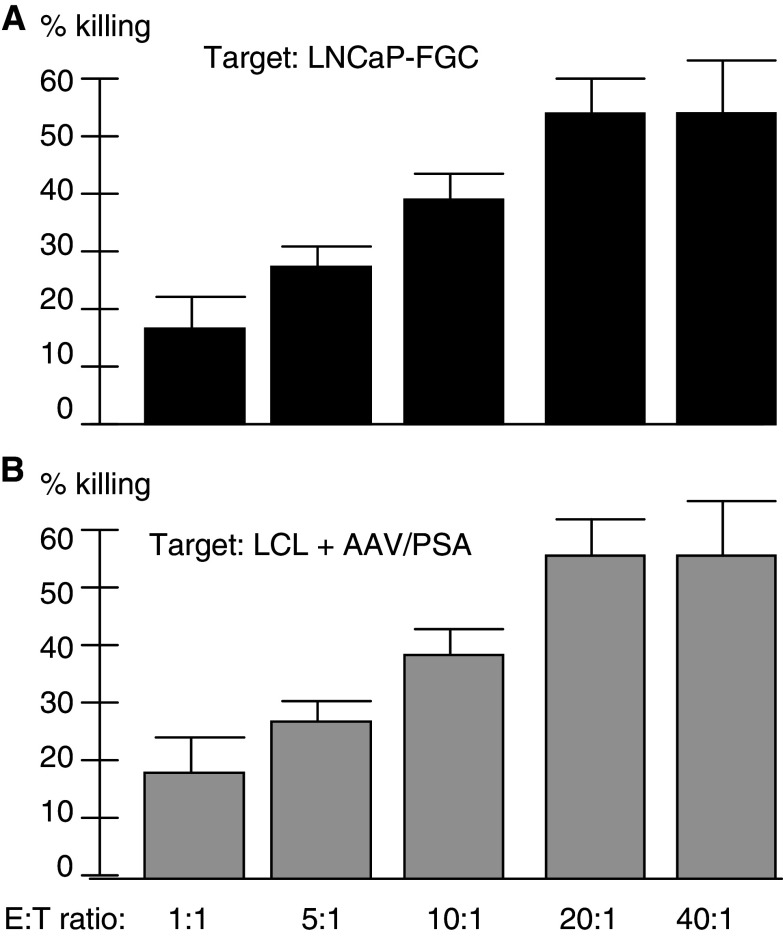
As shown in Fig. 4, we designed an experiment to test the dose-dependent killing ability of the CTL derived from AAV/PSA-loaded DC. Five different effector-to-target ratios were used and the resulting level of killing measured. The results, using the LNCaP-FGC as a target, is shown in Fig. 4a. Note that increased CTL to target ratios resulted in increased killing, with the exception of a plateauing effect at the highest ratio (P < 0.01). A similar analysis using PSA ± LCL cells showed very similar results, including the plateauing effect at the highest ratio (Fig. 4b). These data are fully consistent with target killing being dependent upon the amount of CTL added.
Fig. 4.
CTL killing at various effector to target ratios. Note that increase in CTL to target ratios resulted in increase CTL killing up to 20:1 ratio. CTL were generated as indicated and assayed for their ability to kill either LNCaP-FGC (a) or LCL transduced with AAV/PSA (b)
Characterization of AAV/PSA transduced DC
The DC resulting from the various loading techniques were next characterized to observe if significant differences were induced by the treatments and delineate the mechanisms involved in the robust generation of CTL. We used flow cytometric analysis to determine the surface marker phenotype of untreated and AAV/PSA-loaded DC populations. The results, shown in Fig. 5a, demonstrate that the DC generated from these techniques share common DC markers and low CD14 levels. However, AAV/PSA loaded DC did express significantly higher levels of CD80, CD83, and CD86 (P < 0.05). This is largely in agreement with our other studies, which consistently show CD80 upregulation and sometimes CD40, CD83, and CD86 as well.
Fig. 5.
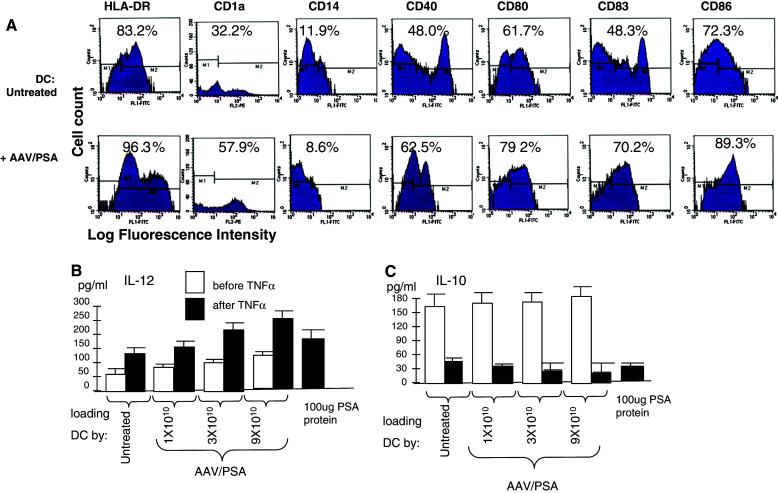
a Characterization of DC under different conditions. Monocytes were treated as indicated, treated with GM-CSF, IL-4, and TNFα, and analyzed by FACS for mean fluorescent intensity (MFI) on day 6. Original histograms are shown with percentage of positivity. b and c Secretion of IL-12 (b) and IL-10 (c) by DC. Shown are the levels of secreted IL-12 and IL-10 by DC under various treatments
The results for IL-12, shown in Fig. 5b, demonstrate that the DC progressively secreted more IL-12 in a dose-dependent manner along with increased AAV/PSA transduction (P < 0.05). Furthermore, the stimulation of DC maturation by TNFα further up-regulated the AAV/PSA dose effect on IL-12 secretion. The results for IL-10, shown in Fig. 5c, demonstrate that DC not treated with TNFα secreted equal amounts of IL-10 independent of AAV/PSA dose. Finally, the stimulation of DC maturation by TNFα was associated with very low IL-10 secretion and this was down-regulated, in a dosage dependent manner by AAV/PSA (P < 0.05).
Characterization of AAV/PSA transduced DC stimulated T cells
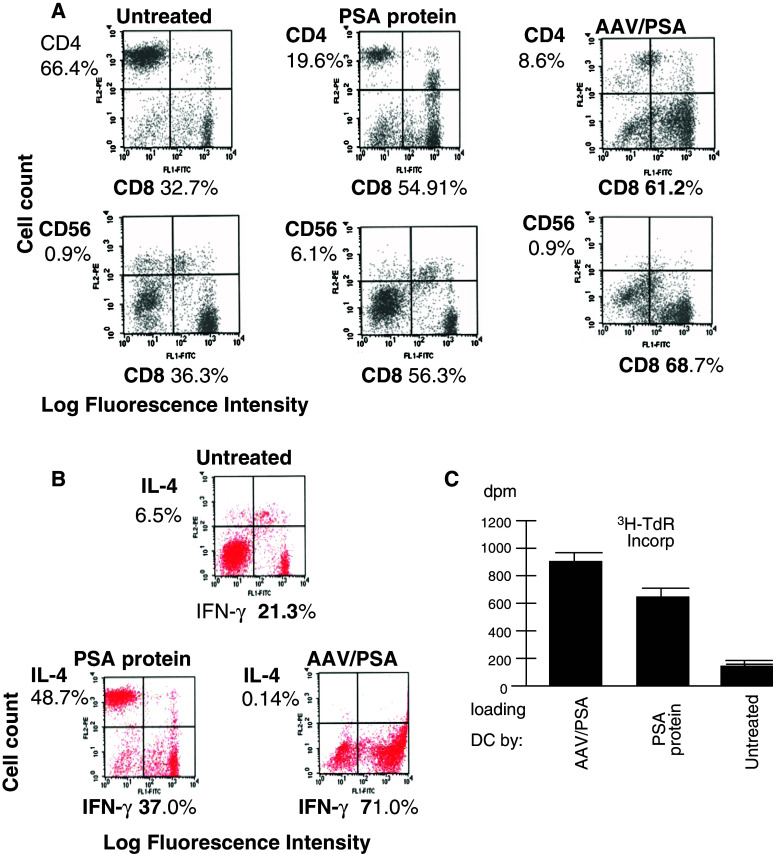
The T cell populations resulting from the variously treated DC were next characterized to observe if significant differences resulted in the stimulated CTL and delineate mechanisms for improved generation of CTL. Immunophenotyping of the AAV/PSA-primed T-cell populations showed that they expressed predominantly CD8 (61%), in contrast to untreated cells (33%; Fig. 6a, P < 0.05). The PSA protein-primed T cells were intermediate at 55% CD8-positive cells. More striking was the increase of the CD8/CD4 ratio in the untreated, protein, and gene-derived T cell populations, rises from 0.49, to 2.8 and then 7.1, respectively (Fig. 6a; P < 0.05). Furthermore, the CD8-to-CD56 ratio was substantially higher in the AAV-loaded situation, increasing from a ratio of 3.1 to 9.2 and finally to 76.3 in the untreated, protein, and gene-derived T cell populations respectively (P < 0.05).
Fig. 6.
a Two-color flow cytometric characterization of surface markers and proliferation of stimulated T cell populations. The top panel shows the CD8 and CD4 prevalence within the activated T cell population resulting from two different pulsing techniques as indicated. The bottom panel shows the CD8 and CD56 prevalence under the same experimental situations as in the top panel. b Two-color flow cytometric characterization of intracellular cytokine expression. Intracellular prevalence of IFNγ and IL-4 within primed T cell populations resulting from untreated-loaded DC (control), PSA protein-loaded, or AAV/PSA-loaded- DC. c T cell proliferation in stimulated T cell populations. The level of proliferation of T cells stimulated by three different treated DC (see Materials and methods section)
To determine the cytokine profile of the T cells generated from co-culture with AAV/PSA-loaded DC, PSA protein-loaded, or untreated DC, we carried out intracellular staining of these T cells for IFNγ and IL-4. Figure 6b demonstrates that most of the stimulated T cells highly expressed IFNγ (71%) and very little IL-4 (0.14%), suggesting that these cells are of strong Th1 phenotype. A much smaller proportion of IFNγ-producing T cells were observed in the T-cell populations primed by PSA protein (37%) or untreated (21.3%). The level of T cell proliferation by variously treated DC was also analyzed by tritium incorporation (Fig. 6c). It was found that the AAV/PSA transduced DC cells stimulated the highest proliferation and untreated DC stimulated the lowest, consistent with the IFNγ expression results (P < 0.05).
Discussion
A number of prostate cancer self antigens have been identified [8–12, 14, 16–19, 26, 32, 33]. We have chosen to target PSA because of its broad expression in normal prostate, prostate carcinomas and minimal expression on other cell types. Previously we have used this technique for the generation of CTL against viral antigens [6]. In this study, we demonstrate that AAV/PSA virus is able to effectively load DC and DC were able to prime and propagate PSA antigen-specific CTL in an efficient manner resulting in robust CTL killing. The protocol described in this study has the ability to generate significant CTL activity against a self-antigen with one stimulation but most DC loading/priming protocols require much longer times. The specific reason for this is not yet known but may be due to as we have shown in this study, (1) high transduction efficiency of AAV/PSA vector, (2) higher CD80, CD 83 and CD86 expression in the DC loaded with AAV/PSA, (3) high IL-12 secretion that is associated with a desirable Th 1 response, and (4) an increase in proliferation of the CD8 + Th1 T cells.
The results in this study are in agreement with the hypothesis that antigen gene loading of DC may be more efficient in CTL killing than protein loading of DC for several reasons. First, due to protein degradation and MHC molecule cycling, protein loading of DC may be an inefficient way to deliver an antigen. In contrast, gene transfer leads to a continuous production of the antigenic protein and may provide the opportunity for repeated rounds of presentation and CTL stimulation. Second, viral entry into cells is usually more efficient than proteins delivered via lipofection [6]. Finally, viral delivery of the full gene encoding the full protein will result in post-translational modifications and multiple epitopes being expressed by the DC. In this study, apart from these theoretical reasons, we have shown that increased CTL killing after AAV-PSA loading compared to PSA protein pulsing may be due to (1) increased proliferation of CTL, (2) high CD8/CD4 ratio and high CD8/CD56 T cell ratios, and (3) T cell population with significant IFNγ expression Therefore, the protocols used in this study resulted in robust CTL killing mainly due to enhanced characteristics of DC and CTL. Further studies are needed to delineate the mechanisms responsible for the rapid and robust CTL killing.
In this study, functional characterization of CD8 + CTL responses included analysis of specificity, using autologous PSA expressing epithelial and lymphoid targets and NK-sensitive targets, (e.g., K562). The generation of significant MHC-class I restricted, antigen specific anti-PSA CTL activity resulting from AAV/antigen gene loading of DC was expected. This is fully consistent with what we know about CTL generation by antigen presenting DC and CTL activity (Th1 immune response). However, some might argue that what we are observing is non-specific killing that the antigen-specific CTL are arising too rapidly. Yet, there was little evidence of CD56 participation as K562 cells were not killed at significant levels and CD56 cells were a minimal subset within the stimulated T cell population. Also, loading specificity results show that only AAV/PSA transduced DC demonstrated high CTL killing whereas AAV/E6 or AAV/BA46 transduced DC did not. Furthermore, the antigen-specificity of the killing was strongly supported by the finding of a lack of cytotoxicity observed against non-PSA-bearing targets. Moreover, as shown in this study CTL killing is dose dependent up to a target ratio of 20:1 and then did not show increase. Therefore, CTL killing is unlikely due to simple cellular toxicity.
We have used androgen sensitive cell line LNCaP as target for CTL killing. We did not use androgen insensitive cell lines such as PC-3 and Du-145 as they have been reported not to express PSA [5, 21]. However, PC-3 and DU-145 PC cell lines can express androgen receptor protein [1] and patients with androgen resistant PC express androgen receptors even at low androgen levels and continue to express PSA [41]. If even low level of PSA is expressed and appropriate epitopes are presented by the DC, CTL killing should be expected in PC-3 and Du-145 cell lines. Further studies are necessary to confirm this.
During the last 10 years there are several reports on different immunological approaches for the treatment of PC [8–12, 14, 16–19, 26, 33]. Both viral and non-viral vectors have been used with limited success. There have been many studies demonstrating the defective nature of T lymphocytes in cancerous hosts and tumor escape mechanisms in PC [13, 20, 39]. As early as 1976 Thomas et al. [37] demonstrated that PC patients had lymphocytes with depressed functions, showing defects is mitogen-induced lymphocyte transformation. In designing adoptive immunotherapy against prostate cancer, it is clear that a more robust in vitro CTL must be generated to allow for tumor cell killing in vivo in spite of surmounting inherent immune tolerance to the cancer. Both clinical and pre-clinical studies indicate that PSA is a good PC antigen for immunotherapy [8–12, 14, 16–19, 26, 33]. While advances are being made in immunotherapy against PC, this is the first report of preclinical demonstration of using AAV vector for gene immunotherapy against a prostate antigen. Rapid generation of CTL by this technique against PSA, after one stimulation is significant. Taken together, the efficient protocol described in this study may be used as an adjuvant immunotherapy for the treatment of PC.
Abbreviations
- AAV
Adeno-associated virus
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cell
Footnotes
Mahendran Mahadevan and Yong Liu contributed equally to this manuscript.
References
- 1.Almirah F, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580:2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio A, Hildinger M, O’Connor E, Gao GP, Wilson JM. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- 3.Bello-Fernandez C, Matyash M, Strobl H, Pickl WF, Majdic O, Lyman SD, Knapp W. Efficient retrovirus-mediated gene transfer of dendritic cells generated from CD34+ cord blood cells under serum-free conditions. Human Gene Ther. 1997;8:1651–1658. doi: 10.1089/hum.1997.8.14-1651. [DOI] [PubMed] [Google Scholar]
- 4.Bender A, Sapp M, Feldman M, Reddy A, Seder R, Schuler G, Steinman RM, Bhardwaj N. Dendritic cells as immunogens for human CTL responses. Adv Exp Med Biol. 1997;417:383–387. doi: 10.1007/978-1-4757-9966-8_62. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson B, Forsberg O, Bengtsson M, Totterman TH, Essand M. Characterization of human prostate and breast cancer cell lines for experimental T cell-based immunotherapy. Prostate. 2007;67:389–395. doi: 10.1002/pros.20498. [DOI] [PubMed] [Google Scholar]
- 6.Chiriva-Internati M, Liu Y, Salati E, Zhou W, Grizzi F, Roman JJ, Lim S, Hermonat PL. Efficient generation of cytotoxic T lymphocytes against cervical cancer cells by adeno-associated virus-human papilloma virus type 16 E7 antigen transduction into dendritic cells. Eur J Immunol. 2002;32:30–38. doi: 10.1002/1521-4141(200201)32:1<30::AID-IMMU30>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Collaco RF, Cao X, Trempe JP. A helper virus-free packaging system for recombinant adeno-associated virus vectors. Gene. 1999;238:397–405. doi: 10.1016/S0378-1119(99)00347-9. [DOI] [PubMed] [Google Scholar]
- 8.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 9.Haakon R, Cavanagh WA, Tjoa BA. Dendritic cell based vaccines: progress in immunotherapy studies for prostate cancer. J Urol. 2004;172:2532–2538. doi: 10.1097/01.ju.0000144211.51111.e4. [DOI] [PubMed] [Google Scholar]
- 10.Harrington KJ, Spitzweg C, Bateman AR, Morris JC, Ville RG. Gene therapy for prostate cancer: current status and future prospects. J Urol. 2001;166:1220–1233. doi: 10.1016/S0022-5347(05)65742-4. [DOI] [PubMed] [Google Scholar]
- 11.Harris DT, Matyas GR, Gomella LG, Talor E, Winship MD, Spitler LE, Mastrangelo MJ. Immunologic approaches to the treatment of prostate cancer. Seminars Oncol. 1999;26:439–447. [PubMed] [Google Scholar]
- 12.Heiser A, Coleman D, Dannul J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI200214364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannides CG, Whiteside TL. T cell recognition of human tumors: implication for molecular immunotherapy of cancer. Clin Immunol Immunopathol. 1993;66:91–106. doi: 10.1006/clin.1993.1012. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman HL, Divgi CR. Optimizing prostate cancer treatment by combining local radiation therapy with systemic vaccination. Clin Cancer Res. 2005;11:6757–6752. doi: 10.1158/1078-0432.CCR-05-0644. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Santin AD, Mane M, Chiriva-Internati M, Parham GP, Ravaggi A, Hermonat PL. Transduction and utility of the granulocyte macrophage-colony stimulating factor gene into dendritic cells by adeno-associated virus. J Interferon Cytokine Res. 2000;20:21–30. doi: 10.1089/107999000312702. [DOI] [PubMed] [Google Scholar]
- 16.MacRae EJ, Giannoudis A, Ryan R, Brown NJ, Hamdy FC, Maitland N, Lewis CE. Gene therapy for prostate cancer: current strategies and new cell-based approaches. Prostate. 2006;66:470–494. doi: 10.1002/pros.20388. [DOI] [PubMed] [Google Scholar]
- 17.Madan RA, Gulley JL, Arlen PM. PSA-based vaccines for the treatment of prostate cancer. Expert Rev Vaccines. 2006;5:199–209. doi: 10.1586/14760584.5.2.199. [DOI] [PubMed] [Google Scholar]
- 18.McNeel DG, Malkovsky M. Immune-based therapies for prostate cancer. Immunol Lett. 2005;96:3–9. doi: 10.1016/j.imlet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Medin JA, Liang S, Hou JW, Kelley LS, Peace DJ, Fowler DH. Efficient transfer of PSA and PSMA cDNAs into DCs generates antibody and T cell anti tumor responses in vivo. Cancer Gene Ther. 2005;12:540–551. doi: 10.1038/sj.cgt.7700810. [DOI] [PubMed] [Google Scholar]
- 20.Miller AM, Pisa P (2005) Tumor escape mechanisms in prostate cancer. Cancer Immunol Immunother. doi:10.1007/100262-005-0110-x [DOI] [PMC free article] [PubMed]
- 21.Mitchell S, Abell P, Ware M, Stamp G, Lalani EN. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 2000;85:932–944. doi: 10.1046/j.1464-410x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 22.Pala P, Verhoef A, Lamb JR, Openshaw PJ. Single cell analysis of cytokine expression kinetics by human CD4+ T-cell clones during activation or tolerance induction. Immunol. 2000;100:209–216. doi: 10.1046/j.1365-2567.2000.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Diez A, Butterfield LH, Li L, Chakraborty NG, Economou JS, Mukherji B. Generation of CD8+ and CD4+ T-cell response to dendritic cells genetically engineered to express the MART-1/Melan-A gene. Cancer Res. 1998;58:5305–5309. [PubMed] [Google Scholar]
- 24.Ponnazhagan S, Mahendra G, Curiel DT, Shaw DR. Adeno-associated virus type 2-mediated transduction of human monocyte-derived dendritic cells: implications for ex vivo immunotherapy. J Virol. 2001;75:9493–9501. doi: 10.1128/JVI.75.19.9493-9501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan CJ, Small EJ. Prostate cancer update: 2005. Curr Opin Oncol. 2006;18:284–288. doi: 10.1097/01.cco.0000219259.83585.f3. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony stimulating factor plus interleukin 4 and down regulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Zhan D, Pecorelli S, Parham GP, Cannon MJ. Generation of an MHC class I restricted cytotoxic T cell response against autologous cervical cancer tumor cells by lipofection of synthetic human papillomavirus types 16 and 18 E7 protein into primary dendritic cells. J Virol. 1999;73:5402–5410. doi: 10.1128/jvi.73.7.5402-5410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroers R, Chen SY. Lentiviral transduction of human dendritic cells. Methods Mol Biol. 2004;246:451–459. doi: 10.1385/1-59259-650-9:451. [DOI] [PubMed] [Google Scholar]
- 30.Schulz P, Stucka R, Feldman H, Combriato G, Klobeck HG, Fittler F. Sequence of a cDNA clone encompassing the complete mature human prostate specific antigen (PSA) and an unspliced leader sequence. Nucleic Acids Res. 1988;16:6226. doi: 10.1093/nar/16.13.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng KC, Pietersz GA, Wright MD, Apostolopoulos V. Dendritic cells: activation and maturation-applications for cancer immunotherapy. Curr Med Chem. 2005;12:1783–1800. doi: 10.2174/0929867054367248. [DOI] [PubMed] [Google Scholar]
- 32.Sokoll LG, Chan DW. Prostate-specific antigen: its discovery and biochemical characteristics. Urol Clin N Am. 1997;24:253–259. doi: 10.1016/S0094-0143(05)70370-0. [DOI] [PubMed] [Google Scholar]
- 33.Steiner MS, Gingrich JR. Gene therapy for prostate cancer: where are we now? J Urol. 2000;164:1121–1136. doi: 10.1016/S0022-5347(05)67127-3. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM. Dendritic cells: from the fabric of immunology. Clin Invest Med. 2004;27:231–236. [PubMed] [Google Scholar]
- 35.Sun JY, Krouse RS, Forman SJ, Senitzer D, Sniecinski I, Chatterjee S, Wong KK., Jr Immunogenicity of a p210 (BCR–ABL) fusion domain candidate DNA vaccine targeted to dendritic cells by a recombinant adeno-associated virus vector in vitro. Cancer Res. 2002;62:3175–3183. [PubMed] [Google Scholar]
- 36.Sun JY, Senitzer D, Forman SJ, Chatterjee S, Wong KK., Jr Identification of new MHC-restriction elements for presentation of the p210 (BCR–ABL) fusion region to human cytotoxic T lymphocytes. Cancer Immunol Immunother. 2003;52:761–770. doi: 10.1007/s00262-003-0415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas JW, Jerkins G, Cox C, Lieberman P. Defective cell-mediated immunity in carcinoma of the prostate. Invest Urol. 1976;14:72–75. [PubMed] [Google Scholar]
- 38.Tillman BW, de Gruijl TD, Luykx-de Bakker SA, Scheper RJH, Pinedo HM, Curiel TJ, Gerritsen WR, Curiel DT. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162:6378–6383. [PubMed] [Google Scholar]
- 39.Timares L, Douglas JT, Tillman BW, Krasnykh V, Curiel DT. Adenovirus-mediated gene delivery to dendritic cells. Methods Mol Biol. 2004;246:139–154. doi: 10.1385/1-59259-650-9:139. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q, Xia D, Carlsen S, Xiang J. Adenovirus-mediated transgene-engineered dendritic cell vaccine of cancer. Curr Gene Ther. 2005;5:237–247. doi: 10.2174/1566523053544272. [DOI] [PubMed] [Google Scholar]
- 41.Yuan X, Li T, Wang H, Zhang T, Barua M, Borgesi RA, Bubley GJ, Lu ML, Balk SP. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Path. 2006;169:682–696. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong L, Granelli-Piperno A, Choi Y, Steinman RM. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]