Abstract
In the present study, either modified IFL regimen (modified irinotecan, fluorouracil and leucovorin, mIFL) alone or in combination with bevacizumab was used to treat patients with metastatic colorectal cancer (CRC). Treatment efficacy was assessed using coupled tomography imaging diagnosis. The toxicity accompany with treatment was evaluated, as well as T cell receptor (TCR) repertoire before and several cycles after therapy was dynamically monitored by analyzing the complementarity-determining region 3 (CDR3) length distribution within CD4+ and CD8+ T cell subsets. The degrees of normalization of the T cell repertoire in CRC patients treated with the two methods were compared. The results showed that mIFL combined with bevacizumab was more effective in treating patients with metastatic CRC, and was accompanied by an increase in side effects such as proteinuria and hematuria. An even more restricted CDR3 profile in patients with metastatic CRC compared with healthy control has been detected. A prominent usage of TCR β chain variable (BV) gene BV12 and BV16 families within the CD4+ T cell subset and BV19 and BV21 families within the CD8+ T cell subset have been found before treatment. Moreover, CD8+ T cells showed more restricted patterns than CD4+ T cells, especially in patients before treatment. For patients with stable disease (SD) or partial remission (PR) after treatment, a less restricted CDR3 profile in post-treatment compared with pre-treatment has been found, but the opposite result was observed for patients with progressive disease (PD). The less restricted CDR3 pattern suggested a trend toward normalization of the TCR repertoire. The normalization of TCR repertoire significantly increased in patients treated with mIFL in combination with bevacizumab, but slightly in patients treated with mIFL alone. The results demonstrate a positive correlation between post-therapy TCR repertoire normalization and remission of metastatic CRC.
Keywords: T cell receptor, Complementarity-determining region 3, Colorectal cancer, Bevacizumab
Introduction
Bevacizumab is a recombinant, humanized, anti-vascular endothelial growth factor (VEGF) monoclonal antibody. It can bind to circulating VEGF with high specificity, inhibiting endothelial cell proliferation and new blood vessel formation in tumor tissues. Thus, bevacizumab can block the nutrient supply to tumor cells and ultimately starve them. Bevacizumab can be used to treat cancers as a single agent or can be administered to improve the delivery of chemotherapeutics by altering tumor vasculature and by decreasing the elevated interstitial pressure in tumors [1]. In treating various cancers, clinical trials that combined bevacizumab with other chemotherapeutics demonstrated increased anti-tumor efficacy and prolonged median survival times compared with trials using a single antibody or chemotherapy [2]. To date, there are only preliminary results on the use of bevacizumab for treating patients with metastatic colorectal cancer (CRC) in China. To assess the effects of bevacizumab, we compared the efficacy and toxicity of two treatments: (1) modified IFL regimen (modified irinotecan, fluorouracil and leucovorin, mIFL) alone and (2) mIFL in combination with bevacizumab.
T lymphocytes are known to play an important role in the anti-tumor immune response. The TCR repertoire can be monitored using complementarity-determining region 3 (CDR3) spectratype analyses, and previous studies have demonstrated T cell proliferation in the peripheral blood of patients with solid tumors [3]. The T cell receptor (TCR) CDR3 spectratype showed different levels of drift; however, it is still unclear how the TCR repertoire or TCR CDR3 spectratype may change during the treatments course of solid tumor. The effectiveness of treatment can be measured by an improvement in response rates, progression-free survival and median survival, which imply that the tumor antigen is gradually being removed in vivo. Questions remain over whether the effectiveness of treatment or the remission of metastatic CRC is accompanied by a trend toward normalization of the TCR repertoire.
To date, dynamic monitoring of the CDR3 spectratypes in patients with metastatic CRC and how this corresponds to different stage characteristic of disease is rarely reported. Furthermore, comparison of the normalization degrees of the TCR repertoire in patients with metastatic CRC after treated with two kinds of methods with different curative effect, and investigation of the association between changes in CDR3 spectratype and the effects of treatment have never been reported.
In the present study, we assessed the efficacy and toxicity accompanying treatment with either mIFL alone or mIFL in combination with bevacizumab in patients with metastatic CRC. The restricted usage of TCR β chain variable (BV) gene families within CD4+ and CD8+ T cell subsets were analyzed. The change of CDR3 spectratypes in patients with metastatic CRC before and several cycles after treatment was dynamically monitored. The normalization degrees of the TCR repertoire in patients with metastatic CRC after treatment with the two methods were compared. The results demonstrated a positive correlation between post-therapy TCR repertoire normalization and remission of metastatic CRC, which suggest that the TCR repertoire normalized toward a polyclonal TCR BV gene usage pattern may originate from removing of tumor antigen through effective treatment.
Patients and methods
Patient eligibility
Between November 2007 and May 2008, a total of 18 patients with metastatic CRC were treated at the Department of Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, PRC. Eighteen patients with advanced, histologically confirmed CRC adenocarcinomas were assessed for eligibility in the study. The eligibility criteria of the study were with ages ≥18 years, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, a life expectancy ≥3 months, adequate hematological, hepatic, and renal function (including urinary excretion of no more than 500 mg protein/d), and a measurable disease according to the response evaluation criteria in solid tumors (RECIST) [4]. Patients who were treated previously with chemotherapy for advanced cancers were not included. Patients who had received adjuvant therapy were eligible provided that >6 months had elapsed between the end of the adjuvant therapy and patient registration in the study. All the patients provided written informed consent for participation in the present study. Patients were considered ineligible for the study if they satisfied any of the following criteria: previous exposure to anti-angiogenesis mAbs, signal transduction inhibitors, or VEGF targeting therapies, thromboembolism that required therapeutic anticoagulation, major surgery 6 weeks prior the study, non-healing wounds, uncontrolled hypertension, bleeding diathesis, brain metastasis, concurrent malignancy, clinically relevant coronary artery disease or a history of a myocardial infarction within the last year, acute or subacute intestinal occlusion or a history of the inflammatory bowel disease, known grade 3 or 4 allergic reaction to any of the treatment components or pregnancy or lactating status. Patients were also considered ineligible if the investigator judged that they had a medical or psychological condition that would prevent them from completing the study or knowingly signing the informed consent.
Six healthy blood donors (four men and two women, the age is 36, 41, 45, 52, 56, and 60, respectively) with no clinical or laboratory evidence of connective tumor diseases or immunological disorders were included in the study, which had approval from the appropriate ethics committee.
Pre-treatment evaluations
The baseline evaluation included patient history, physical examinations (evaluation of vital signs and performance status), recordings of concomitant medications, laboratory tests (hematology and clinical chemistry), and computed tomography (CT) of the thorax and abdomen.
Treatment
Eighteen patients were enrolled in the study. Patients were randomized into arm A (n = 12) and arm B (n = 6). All patients in arm A and arm B were treated as follows: irinotecan 125 mg/m2 intravenous (IV) infusion, leucovorin 20 mg/m2 IV bolus followed by 5-fluorouracil (5-FU) 500 mg/m2 IV infusion (6–8 h), once a week for 4 weeks. Patients in arm A were treated with bevacizumab simultaneously, and the bevacizumab administration always followed the chemotherapy treatment. Patients in arm A received bevacizumab at 5 mg/kg as an IV infusion every 2 weeks. The first infusion was given over 90 min and the second was given over 60 min, and if both were well-tolerated, then subsequent infusions were given over 30 min. Each cycle of therapy was repeated every 42 days. Treatment was administered until the disease progressed, unacceptable toxic effects developed, or the patient refused further treatment.
Dose modifications
Bevacizumab was removed in patients with uncontrolled hypertension or proteinuria of >2 g in 24 h. Bevacizumab was discontinued in patients with grade 3 or 4 hemorrhages, thromboembolic events that required a full dose of anticoagulation, or any type of grade 4 toxicity. Chemotherapy reductions were planned in the event of severe hematological and/or non-hematological toxic effects. In cases of insufficient hematological function (neutrophil count <1500/μl and platelet count <100,000/μl), chemotherapy was delayed for up to 14 days. If no recovery of hematological functions occurred by this point, then the treatment was discontinued. For grade 3 or 4 gastrointestinal toxic effects, thrombocytopenia, and neutropenia, there were 20% dose reductions of irinotecan and 5-FU.
Evaluation during therapy
Routine evaluations of patients were carried out on a weekly basis during therapy. These evaluations included a physical examination, vital signs examination, ECOG PS, laboratory hematological and serum chemistry, and a recording of adverse events.
Efficacy and toxicity assessment
CT was used to evaluate the response of the tumors to therapy. The tumors were evaluated every 6 weeks during treatment and at least every 12 weeks during the follow-up. RECIST was used to assess the type of response [4]. A complete response was defined as the complete disappearance of all target tumor lesions. A partial remission (PR) was defined as at least a 30% decrease in the sum of the longest diameter (LD) of the target lesions, taking as reference the smallest sum LD. Tumor progressive disease (PD) was defined as appearance of one or more new lesions or as at least a 20% increase in the sum of LD of the target lesions, taking as reference the smallest sum LD recorded since the treatment was initiated. Stable disease (SD) was defined as neither a sufficient shrinkage to qualify as a partial response nor a sufficient increase to qualify as PD, taking as reference the smallest sum LD. CT scans were carried out every 6 weeks during treatment. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria Version 3.0 [5].
Isolation of mononuclear cells
Fresh blood samples were obtained from eighteen patients with metastatic CRC and six healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood using Ficoll-Hypaque gradient centrifugation.
Magnetic bead separation of CD4+ and CD8+ T cells
Peripheral blood mononuclear cells were enriched for T cells by passing them through nylon wool columns. The T cells were incubated with anti-CD4 and anti-CD8 monoclonal antibodies coupled with magnetic beads (Miltenyi Biotec, Germany). The cells were then loaded onto MidiMACS columns (Miltenyi Biotec, Germany) and T cells were positively selected according to the manufacturer’s instructions.
The separated cells were centrifuged at 300×g for 5 min and washed twice with RPMI-1640 medium before they were counted. 2 × 105 of cells resuspended in 50 μl of cold FB (2% FCS and 0.1% sodium azide in PBS), incubated with PE-labeled anti-CD8 monoclonal antibodies (mAb) and FITC-labeled anti-CD4 mAb, respectively, cells with PE-labeled rat anti-mouse IgG as an isotype control, the cells were washed twice with cold FB and fixed in PBS containing 2% formaldehyde before they were subjected to FACS analysis. Data were collected on a FACSCalibur flow cytometer (BD Biosciences) and analyzed with CELLQuest software (both from Becton Dickinson). The purity of the separated cells was >95% (data not shown).
RNA extraction and cDNA synthesis
Total RNA was extracted from 2 × 106 CD4+ or CD8+ T cells with a total RNA extraction kit (Omega Biotech Company, USA), according to the manufacturer’s instructions, and quantified by spectrophotometry. One microgram of total RNA was transcribed to first strand cDNA with a cDNA synthesis kit (MBI, Fermentas, USA), according to the manufacturer’s instructions. Total RNA was incubated at 42°C for 1 h with 250 pM oligo-(dT) primer, 200 U Moloney murine leukemia virus (M-MuLV) reverse transcriptase and 250 μM of each dNTP in a total volume of 20 μl.
PCR amplification of cDNA
The primers used for the TCR BV gene family-specific amplification were designed using a previous study [6]. PCR amplifications of BV-specific cDNA were carried out in a volume of 50 μl, containing 2 μl of the 5′ BV primer, 2 μl of the 3′ BC primer, 2.0 mM MgCl2, 10 mM Tris–HCl, 200 mM of each dNTP, and 1 μl of cDNA. Following an initial denaturing step at 94°C for 3 min, the PCR was carried out with 35 cycles of denaturing at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, with a final extension at 72°C for 10 min.
GeneScan analyses of CDR3 spectratypes
An aliquot of 2 μl of the fluorescent PCR products was mixed with 2 μl of formamide and 0.5 μl of loading dye (25 mM EDTA, 50 ng/ml dextran blue). The mixture was denatured at 95°C for 2 min, and 2 μl was loaded on a pre-warmed 6% acrylamide sequencing gel and run for 2 h on a 50 lane Applied Biosystems model 373A DNA sequencer (Applied Biosystems, USA). The data were analyzed by GeneScan software version 672. The relative fluorescence intensity (RI) was measured as: RI (%) = 100 × (clonal peak area)/(total peak area). The following criteria were used to determine whether a given TCR BV gene family was abnormal: a single peak with an RI greater than 35%, twin peaks with each peak having an RI greater than 25%, a skewed distribution where the peak RI of the skewed family was greater than 25%, or no peaks with some families being absent [7]. The rate of abnormal TCR BV gene families measurements before treatment was compared with measurements taken at one to three time points during treatment.
Results
Efficacy
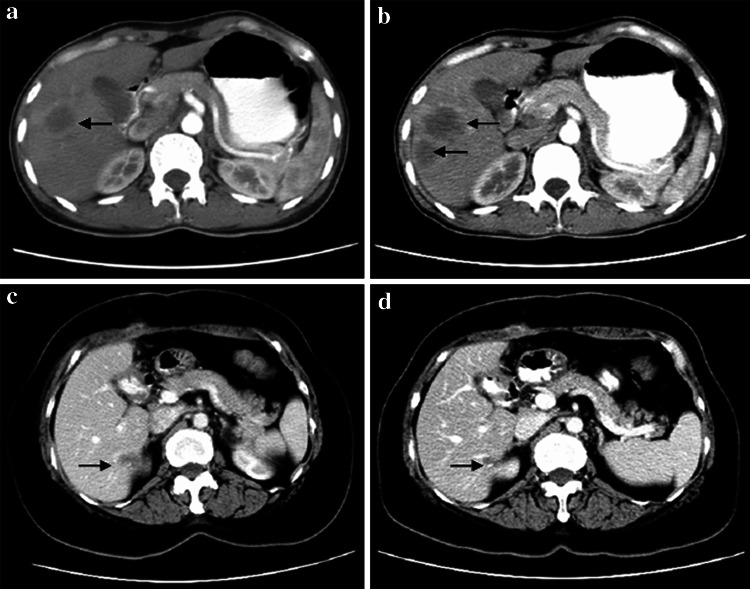
Of the twelve patients in arm A, two patients achieved PR, six patients showed SD, and four patients had PD. The overall response rate (ORR) was 16.7%. Of the six patients in arm B, two patients had PD and four patients had SD, the ORR was 0. The difference in ORR between arm A and arm B was statistically significant (P < 0.05). As can be seen from Fig. 1a and b, which is showing the CT images of tumor in XWJ (patient in arm B), the diameter sum of all tumor lesions is 220 mm pre-treatment (Fig. 1a), and increase to 262 mm and appearance of new tumor lesions after treatment (Fig. 1b). However, in ZQ (patient in arm A), the diameter sum of all tumor lesions is 26 mm pre-treatment (Fig. 1c) and decrease to 13 mm after treatment with mIFL combination of bevacizumab (Fig. 1d). The treatment efficacy of the patients is shown in Table 1.
Fig. 1.
CT images of tumor in two patients. a CT images of tumor in patient XWJ taken at pre-treatment, b CT images of tumor in patient XWJ taken at 2 month after treatment with mIFL, c CT images of tumor in patient ZQ taken at pre-treatment, d CT images of tumor in patient ZQ taken at 2 month after treatment with mIFL combination of bevacizumab. The arrows indicates the site of tumor
Table 1.
Patient characteristics and the rate of abnormal TCR BV gene families
| Case | Sex | Age | Diagnose of cancer | Treatment protocol | ECOG score | Evaluation | Rate of abnormal TCR BV gene families (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of cancer | Sites of metastasis | Baseline | After 1st cycle | After 2nd cycle | After 3rd cycle | After 4th cycle | Imaging | CD4+ T cells | CD8+ T cells | |||||||||||||
| After 1st cycle | After 2nd cycle | After 3rd cycle | After 4th cycle | Baseline | After 1st cycle | After 2nd cycle | After 3rd cycle | Baseline | After 1st cycle | After 2nd cycle | After 3rd cycle | |||||||||||
| JYF | Male | 69 | Rectal | Lung, lymph nodes | mIFL + Avastin | 1 | 1 | 1 | 1 | 1 | SD | SD | SD | SD | 50 | 37.5 | 25 | 66.7 | 62.5 | 54.2 | ||
| LQT | Male | 40 | Colon | Liver, others | mIFL + Avastin | 1 | 1 | 1 | 1 | SD | SD | PD | 62.5 | 58.3 | 58.3 | 54.2 | 70.8 | 66.7 | 62.5 | 66.7 | ||
| ZWT | Male | 46 | Colon | Liver, lymph nodes | mIFL + Avastin | 1 | 1 | 1 | 1 | SD | SD | SD | 41.7 | 37.5 | 29.2 | 37.5 | 33.3 | 20.8 | ||||
| ZQ | Female | 61 | Colon | Liver | mIFL + Avastin | 1 | 1 | PR | 58.3 | 33.3 | 83.3 | 37.5 | ||||||||||
| CJN | Male | 48 | Colon | Lung, liver, others | mIFL + Avastin | 1 | 1 | 1 | 1 | SD | SD | SD | 37.5 | 33.3 | 29.2 | 20.8 | 45.8 | 41.7 | 41.7 | 37.5 | ||
| ZXH | Male | 40 | Rectal | Lung, liver | mIFL + Avastin | 1 | 1 | 1 | SD | PD | 50 | 41.7 | 29.2 | 58.3 | 54.2 | 41.7 | ||||||
| DYL | Male | 51 | Rectal | Lung, others | mIFL + Avastin | 1 | 1 | SD | 33.3 | 29.2 | 45.8 | 41.7 | ||||||||||
| LZX | Male | 62 | Colon | Liver | mIFL + Avastin | 1 | 1 | 1 | 1 | 1 | SD | SD | SD | SD | 58.3 | 54.2 | 50 | 70.8 | 66.7 | 66.7 | ||
| LQY | Male | 55 | Rectal | Lung, liver, others | mIFL + Avastin | 1 | 1 | PD | 50 | 58.3 | 62.5 | 66.7 | ||||||||||
| HYH | Female | 52 | Colon | Liver, others | mIFL + Avastin | 1 | 1 | 1 | 2 | PR | PR | PD | 66.7 | 45.8 | 45.8 | 79.2 | 54.2 | 58.3 | ||||
| DQ | Male | 57 | Rectal | Lung | mIFL + Avastin | 1 | 1 | 1 | 1 | 1 | SD | SD | PR | PR | 50 | 45.8 | 33.3 | 62.5 | 58.3 | 50 | ||
| LSY | Female | 61 | Rectal | Lymph nodes, others | mIFL + Avastin | 1 | 1 | 1 | 1 | 1 | SD | SD | SD | SD | 33.3 | 25 | 20.8 | 62.5 | 58.3 | 45.8 | ||
| XWJ | Female | 40 | Rectal | Lung, liver, others | mIFL | 1 | 2 | PD | 62.5 | 58.3 | 62.5 | 66.7 | ||||||||||
| LQZ | Male | 36 | Colon | Lymph nodes, others | mIFL | 1 | 1 | 1 | 2 | SD | SD | SD | SD | 58.3 | 54.2 | 62.5 | 66.7 | 62.5 | 70.8 | |||
| LST | Female | 49 | Rectal | Liver, others | mIFL | 1 | 1 | 1 | 2 | SD | SD | SD | SD | 54.2 | 50 | 50 | 45.8 | 62.5 | 54.2 | 58.3 | 58.3 | |
| LHS | Male | 45 | Rectal | Lymph nodes, others | mIFL | 1 | 1 | 2 | SD | PD | 37.5 | 45.8 | 45.8 | 58.3 | ||||||||
| LHJ | Female | 38 | Colon | Liver | mIFL | 1 | 1 | SD | 54.2 | 50 | 62.5 | 62.5 | ||||||||||
| HLZ | Female | 47 | Colon | Lymph nodes, others | mIFL | 1 | 1 | 1 | 1 | SD | SD | SD | SD | 45.8 | 50 | 54.2 | 54.2 | 58.3 | 58.3 | |||
Adverse reactions
All patients who received at least one dose of chemotherapy and bevacizumab were assessed for adverse events. Dose modifications or interruptions were required in 13 (72.2%) patients (9 in arm A and 4 in arm B). The incidence of hematological and non-hematological toxicity is summarized in Table 2. The most common grade 3 and 4 hematological toxicities were leukopenia and neutropenia. In one patient, who had colon cancer with liver metastasis, an intestinal obstruction occurred, and the patient was removed from the study. A second patient was removed from the study because of severe nausea and vomiting. The incidence of proteinuria and hematuria was significantly higher among patients in arm A. There were no instances of hypertension, bowel perforation, thromboembolic events, or treatment-related death.
Table 2.
Toxicity
| Site | NCI-CTC grade | |||||||
|---|---|---|---|---|---|---|---|---|
| Bevacizumab treated group (n = 12) | Non-bevacizumab treated group (n = 6) | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Hematological | ||||||||
| Anemia | 3 | 1 | 2 | |||||
| Leukopenia | 1 | 2 | 7 | 1 | 1 | 3 | ||
| Neutropenia | 1 | 3 | 8 | 1 | 1 | 4 | ||
| Thrombocytopenia | 2 | 1 | 2 | 1 | 1 | |||
| Non-hematological | ||||||||
| Nausea/vomiting | 4 | 6 | 2 | 3 | ||||
| Mucositis | 4 | 1 | 1 | 1 | 2 | |||
| Diarrhea | 2 | 1 | 1 | 1 | 1 | |||
| Proteinuria | 2 | 1 | ||||||
| Hematuria | 2 | |||||||
CDR3 spectratypes in patients with metastatic CRC
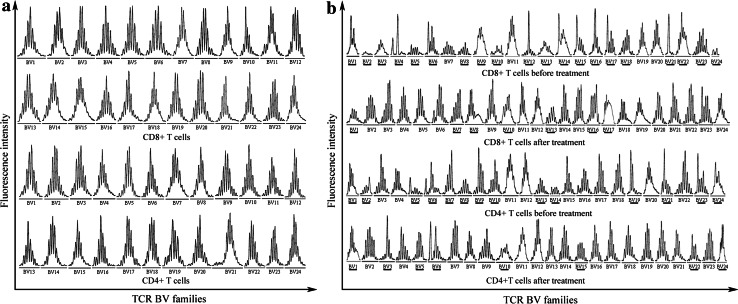
For healthy controls, a normal Gaussian distribution of CDR3 lengths has been observed in each TCR BV gene family, in other words, healthy individuals’ CD4+ and CD8+ T cells CDR3-length distribution showed a polyclonal CDR3 pattern. Figure 2a is a representative chart showing the CD4+ and CD8+ T cells’ CDR3 spectratypes for all TCR BV gene families in normal control-1. For patients with metastatic CRC, however, an abnormal distribution with several peaks fewer than eight or even a single peak and skewed distribution of CDR3 spectratypes has been found in certain TCR BV gene families, as well as several TCR BV gene families being absent or almost no peaks. In other words, an even more restricted CDR3 profile in patients with metastatic CRC has been detected. The CDR3 distribution in the patients showed different degree of restriction. The representative chart showing the CD4+ and CD8+ T cells’ CDR3 length distribution of all TCR BV gene families for patient before and after treatment is shown in Fig. 2b.
Fig. 2.
CDR3 spectratypes of 24 TCR BV gene families within CD4+ and CD8+ T cells. a Representation of a healthy control, b pre- and post-treatment spectratype profiles of representation of patient treated by mIFL combination of bevacizumab. TCR BV gene families showing evidence of abnormal are underlined
Analysis of the pre-treatment CDR3 length distribution in the T cell populations of patients indicated a preferential usage of TCR BV12 and BV16 gene families within the CD4+ T cell subset and BV19 and BV21 families within the CD8+ T cell subset.
CDR3 spectratypes of CD4+ and CD8+ T cells
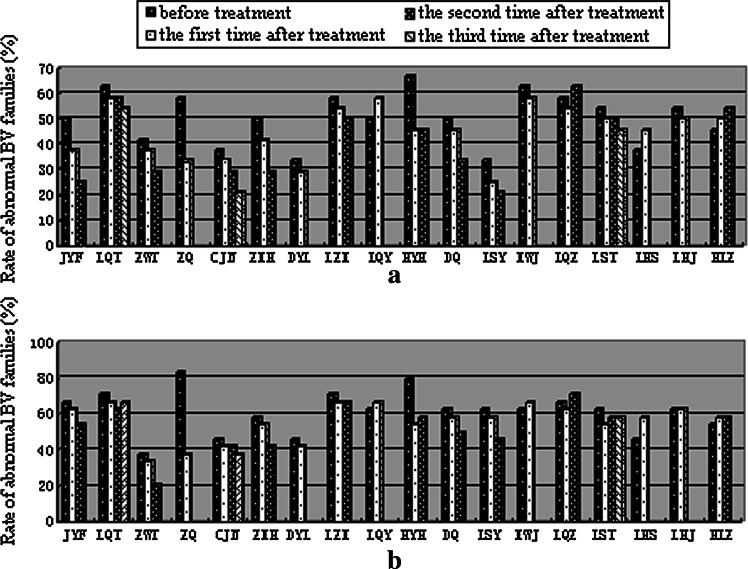
In pre-treatment, CDR3-length distribution showed an even more restricted profile in CD8+ T cells compared with CD4+ T cells for most of patients. However, in post-treatment, the discrepancy of restricted profile in CD4+ T cells and CD8+ T cells was reduced. For example, the rate of abnormal BV families was 58.3% within CD4+ T cells and 83.3% within CD8+ T cells in patient ZQ before treatment and decreased to 33.3% in CD4+ T cells and 37.5% in CD8+ T cells after treatment (Table 1; Fig. 3).
Fig. 3.
The rate of abnormal TCR BV gene families within CD4+ (a) and CD8+ T cells (b) in all of patients before and after treated by mIFL combination of bevacizumab (the first 12 bars on the X-axis) or mIFL alone (the last 6 bars on the X-axis)
Monitoring the CDR3 spectratype over time in patients
The TCR β chain CDR3 spectratypes were detected dynamically for all patients. When compared the CDR3 spectratypes of each BV gene family in patients before treatment with one or several stages after treatment. Some BV gene families showed evidence of oligoclonal expansion before treatment were restored to normal Gaussian distributions or to more skewed distributions post-treatment. Certain BV gene families, such as BV19 and BV21 in the CD4+ T cells of patient ZQ, showed oligoclonal pattern that was changed to polyclonal pattern with Gaussian distribution after treatment. Other BV gene families, such as BV2, BV5, BV8, BV13, and BV14 in the CD4+ T cells of patient ZQ, changed from being absent before treatment to showing polyclonal patterns after treatment. The number of BV gene families with abnormal patterns decreased in both CD4+ and CD8+ T cells in all patients with SD or PR after treatment. However, the number of BV gene families with abnormal patterns increased in both CD4+ and CD8+ T cells in the patients whose CT showed PD. For example, in patient ZQ who showed PR, most of the BV families had either single or several peaks before treatment had about eight peaks with a normal Gaussian distribution after treatment (Fig. 2b). However, the results were opposite for patient XWJ, who showed PD. Most BV gene families in patient XWJ showed polyclonal expansion before treatment changed to oligoclonal expansion post-treatment.
In almost all patients of group A during mIFL combined with bevacizumab therapy, the restricted profile was significantly reduced followed by a gradual increase in the number of peaks within the CDR3 region of different TCR BV gene families. But in half patients of group B during mIFL treatment alone, the CDR3 spectratypes shift toward a more restricted pattern (Table 1; Fig. 3).
Discussion
Bevacizumab has demonstrated encouraging clinical results in the treatment of metastatic colorectal cancer. Combination of bevacizumab with mIFL is an effective first-line of treatment for metastatic colorectal cancer and has been shown to improve overall survival and the ORR [8]. In this pilot study, we evaluated either mIFL alone or mIFL in combination with bevacizumab as a first-line of treatment for patients with metastatic CRC in southern China. The efficacy and relative safety of the two treatment strategies were compared. Our results demonstrate that patients with metastatic CRC treated with a combination of bevacizumab and mIFL had an improved ORR than patients treated with mIFL alone.
The addition of bevacizumab to chemotherapies may induce an increase in side effects. Previous phase 1 and 2 clinical trials suggested that treatment with bevacizumab alone or with chemotherapy resulted in an increased incidence of thrombosis, bleeding, proteinuria, and hypertension [9, 10]. In this pilot study, the incidence of proteinuria and hematuria was higher among patients treated with bevacizumab.
In an attempt to characterize the change of TCR repertoire before treatment and several cycles after treatment, we analyzed the TCR CDR3 spectratypes of T cells in the peripheral blood of patients with metastatic CRC. CDR3 spectratyping analysis is useful for identifying clonal expansion in T cells, since clonal T cell expansion results in decrease in the number of peaks within the CDR3 region, which can be measured as CDR3 skewing. T cell clonal expansion has been observed in tumor tissue and the peripheral blood of patients with solid tumors using CDR3 spectratype analysis [3]. The CDR3 spectratype of tumor-infiltrating lymphocytes (TILs) derived from non-small-cell lung cancers showed a restricted TCR usage, which was associated with an anti-tumor immune response [11]. TCR CDR3 length distribution in CD4 and CD8 T cells have been assessed before and after alemtuzumab (anti-CD52 monoclonal antibody) therapy in five B cell chronic lymphocytic leukemia patients [12]. Still limited information is available which compares the CDR3 spectratypes dynamic change by two treatment methods in patients with solid tumors. T cells clonal expansion in patients with CRC has been reported [13], and a restricted TCR repertoire in the TILs from patients with CRC has been described [14]. Additionally, oligoclonality of CD8+ T lymphocytes has been reported in patients with CRC [15]. Our study expands on this work by examining previously unstudied questions.
Researchers once believed that the TILs from tumor tissues caused clonal expansion, since oligoclonal and monoclonal T cells were not observed in the peripheral blood of patients with solid tumors [3]. Later research demonstrated that clonal T cell expansions could be measured in the blood of patients with solid tumors [16]. The results of our study confirm these results, showing that a number of TCR BV gene families in both CD4+ and CD8+ T cells show a restricted TCR BV gene usage in the peripheral blood of patients with metastatic CRC. The restricted TCR BV profiles may result from a specific response to certain tumor antigen [11]. There are several reasons that can explain the clonal proliferation of antigen-specific T cells in peripheral blood. One possibility is that tumor cells and related antigens infiltrating into blood circulation. These tumor-associated antigen or tumor-specific antigen can stimulate the T cells in peripheral blood constantly, leading to tumor-specific clonal T cell amplification. A second possibility is that under certain conditions, the T cells from the TILs in tumor tissue enter into the peripheral blood circulation and lead to clonal T cell proliferation. The results obtained from analyses T cells of local can reflect local immune situations, but the immune state of the entire body are more likely to be reflect from analysis of T cells in the peripheral blood.
A restricted TCR repertoire in the peripheral blood of a patient with a solid tumor can suggest an anti-tumor immune response. Therefore, analysis of TCR CDR3 spectratypes after therapy may clarify the extent of immune recovery and the degree of tumor elimination. Here, we performed an in-depth analysis of the restricted use of TCR BV gene families and the CDR3 spectratypes in CD4+ and CD8+ T cells before and after treatment in patients with metastatic CRC. Our findings support the notion that an effective treatment is reflected by a return to a more normal TCR repertoire pattern. The CDR3-length distribution and TCR BV CDR3 spectratypes changed dynamically in accordance with patient condition during treatment. Some BV families showed a skewed spectratype before treatment were restored to a normal Gaussian distribution when the patient was in PR. The number of BV gene families that showed evidence of clonal expansion before treatment reduced gradually after treatment in patients where the CT imaging evaluation indicated SD or PR. In contrast, in the patients with PD, according to their CT evaluation, the number of oligoclonal or monoclonal TCR BV gene families increased. This suggests that an abnormal distribution of BV gene families correlates with different stages of disease and the patient prognosis. Therefore, analysis of changes in T cell clonal expansion and in CDR3 spectratypes may be helpful when evaluating treatment efficiency and disease prognosis.
The changes in CDR3 spectratypes and T cell recovery during therapy were different in CD8+ T cells compared with CD4+ T cells. CD8+ T cells showed a more clonal expansion pattern than CD4+ T cells before treatment compared with after treatment. This may because CD8+ T cells play a chief role in the anti-tumor immune response. The existence of a small amount of tumor cells in peripheral blood primarily activate cytotoxic T lymphocytes to exert an anti-tumor response, which results in a more restricted pattern in CD8+ T cells than in CD4+ T cells. As the tumor antigens are gradually removed with treatment, the CD8+ T cell levels return to normal. It has been reported that a comprehensive CD4+ T cell repertoire is required for protective immunity against a wide range of pathogens [17]. It is possible that a more restricted TCR repertoire in CD4+ T cells may result in an increased probability of opportunistic infections during therapy. In the present study, we observed that the CD4+ T cell repertoire normalized during treatment, corresponding with fewer infections after treatment with mIFL alone and mIFL combine bevacizumab.
The results of the present study demonstrate that there is a higher degree of normalization of the TCR repertoire in both the CD4+ and CD8+ T cells of patients with metastatic CRC treated with mIFL in combination with bevacizumab in contrast with patients treated with mIFL alone. The addition of bevacizumab can more effectively remove tumor cell, since very few, if any, tumor-associated antigens remained in vivo to induce T cell proliferation. Therefore, the restricted pattern observed pre-treatment normalized following mIFL and bevacizumab combinatorial treatment. The results demonstrate a positive correlation between post-therapy TCR repertoire normalization and remission of patients with metastatic CRC, which suggest that TCR repertoire characterization based on CDR3 spectratypes analyses maybe have a potential prognostic value in clinical.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (30771952, 30771971), Program for New Century Excellent Talents in University (NCET-07-0410), Natural Science Foundation of Guangdong Province (07117783), and the Major State Basic Research Development Program (973) (2007CB512405).
Abbreviations
- CRC
Colorectal cancer
- 5-FU
5-Fluorouracil
- LV
Leucovorin
- VEGF
Vascular endothelial growth factor
- ECOG
Eastern Cooperative Oncology Group
- PS
Performance status
- RECIST
Response evaluation criteria in solid tumors
- CT
Computed tomography
- LD
Longest diameter
- TCR
T cell receptor
- CDR3
Complementarity-determining region 3
- SD
Stable disease
- PR
Partial remission
- PD
Progressive disease
- IV
Intravenous
- PBMCs
Peripheral blood mononuclear cells
- M-MuLV
Moloney murine leukemia virus
- RI
Fluorescence intensity
- TILs
Tumor-infiltrating lymphocytes
- ORR
Overall response rate
Footnotes
W. Luo and W.-J. Liao have equally contributed to this article.
References
- 1.Giantonio BJ, DE Levy, O’dwyer PJ, Meropol NJ, Catalano PJ, Benson AB, 3rd, Eastern Cooperative Oncology Group A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol. 2006;17:1399–1403. doi: 10.1093/annonc/mdl161. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 3.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute (2003) Common Terminology Criteria for Adverse Events v3.0 (CTCAE) March 31, 2003, (http://ctep.cancer.gov), Publish Date: August 9, 2006
- 6.Soroosh P, Shokri F, Azizi M, Jeddi-Tehrani M. Analysis of T-cell receptor beta chain variable gene segment usage in healthy adult responders and nonresponders to recombinant hepatitis B vaccine. Scand J Immunol. 2003;57:423–431. doi: 10.1046/j.1365-3083.2003.01256.x. [DOI] [PubMed] [Google Scholar]
- 7.Luo W, Ma L, Wen Q, Wang N, Zhou MQ, Wang XN. Analysis of the interindividual conservation of T cell receptor alpha- and beta- chain variable regions gene in the peripheral blood of patients with systemic lupus erythematosus. Clin Exp Immunol. 2008;154:316–324. doi: 10.1111/j.1365-2249.2008.03770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 11.Echchakir H, Asselin-Paturel C, Dorothee G, Vergnon I, Grunenwald D, Chouaib S, Mami-Chouaib F. Analysis of T-cell-receptor beta-chain-gene usage in peripheral-blood and tumor-infiltrating lymphocytes from human non-small-cell lung carcinomas. Int J Cancer. 1999;81:205–213. doi: 10.1002/(SICI)1097-0215(19990412)81:2<205::AID-IJC7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Rezvany MR, Tehrani MJ, Karlsson C, Lundin J, Rabbani H, Osterborg A, Mellstedt H. Reconstitution of the T-cell repertoire following treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with B-cell chronic lymphocytic leukaemia. Br J Haematol. 2006;135:475–485. doi: 10.1111/j.1365-2141.2006.06324.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosolits S, Markovic K, Frodin JE, Virving L, Magnusson CG, Steinitz M, Fagerberg J, Mellstedt H. Vaccination with Ep-CAM protein or anti-idiotypic antibody induces Th1-biased response against MHC class I- and II- restricted Ep-CAM epitopes in colorectal carcinoma patients. Clin Cancer Res. 2004;10:5391–5402. doi: 10.1158/1078-0432.CCR-04-0425. [DOI] [PubMed] [Google Scholar]
- 14.Baier PK, Wimmenauer S, Hirsch T, von Specht BU, von Kleist S, Keller H, Farthmann EH. Analysis of the T cell receptor variability of tumor-infiltrating lymphocytes in colorectal carcinomas. Tumour Biol. 1998;19:205–212. doi: 10.1159/000030008. [DOI] [PubMed] [Google Scholar]
- 15.Akolkar PN, Gulwani-Akolkar B, McKinley M, Fisher SE, Silver J. Comparisons of T cell receptor (TCR) V beta repertoires of lamina propria and peripheral blood lymphocytes with respect to frequency and oligoclonality. Clin Immunol Immunopathol. 1995;76:155–163. doi: 10.1006/clin.1995.1110. [DOI] [PubMed] [Google Scholar]
- 16.Mosolits S, Markovic K, Fagerberg J, Frodin JE, Rezvany MR, Kiaii S, Mellstedt H, Jeddi-Tehrani M. T-cell receptor BV gene usage in colorectal carcinoma patients immunised with recombinant Ep-CAM protein or anti-idiotypic antibody. Cancer Immunol Immunother. 2005;54:557–570. doi: 10.1007/s00262-004-0620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Curr Opin Immunol. 2004;16:775–779. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]