Abstract
Continuous efforts are dedicated to develop immunotherapeutic approaches to neuroblastoma (NB), a tumor that relapses at high rates following high-dose conventional cytotoxic therapy and autologous bone marrow cell (BMC) reconstitution. This study presents a series of transplant experiments aiming to evaluate the efficacy of allogeneic BMC transplantation. Neuro-2a cells were found to express low levels of class I major histocompatibility complex (MHC) antigens. While radiation and syngeneic bone marrow transplantation (BMT) reduced tumor growth (P < 0.001), allogeneic BMT further impaired subcutaneous development of Neuro-2a cells (P < 0.001). Allogeneic donor-derived T cells displayed direct cytotoxic activity against Neuro-2a in vitro, a mechanism of immune-mediated suppression of tumor growth. The proliferation of lymphocytes from congenic mice bearing subcutaneous tumors was inhibited by tumor lysate, suggesting that a soluble factor suppresses cytotoxic activity of syngeneic lymphocytes. However, the growth of Neuro-2a cells was impaired when implanted into chimeric mice at various times after syngeneic and allogeneic BMT. F1 (donor-host) splenocytes were infused attempting to foster immune reconstitution, however they engrafted transiently and had no effect on tumor growth. Taken together, these data indicate: (1) Neuro-2a cells express MHC antigens and immunogenic tumor associated antigens. (2) Allogeneic BMT is a significantly better platform to develop graft versus tumor (GVT) immunotherapy to NB as compared to syngeneic (autologous) immuno-hematopoietic reconstitution. (3) An effective GVT reaction in tumor bearing mice is primed by MHC disparity and targets tumor associated antigens.
Keywords: Neuroblastoma, Bone marrow transplantation, Syngeneic, Allogeneic, MHC disparity
Introduction
Transplantation of autologous bone marrow cells (BMC) is currently used for lympho-hematopoietic reconstitution of neuroblastoma (NB) patients after aggressive radio and chemotherapy [1]. However, most therapies are largely inefficient in eradication of the disease and many of these children will relapse [2]. It is unclear whether disease relapse is affected by possible contamination of the autologous BMC graft with NB micrometastases, which may be below the threshold of flow cytometric and even PCR detection [3]. Furthermore, attempts to enhance anti-tumor immunity after high-dose chemotherapy and autologous BMC transplantation have been concluded with modest clinical benefit [2, 4–6]. A major disadvantage of autologous transplants is the already proven lack of effective reactivity against the tumor [7]. Allogeneic transplants may benefit NB patients, as a more efficient way to generate an effective graft versus tumor (GVT) reaction [8]. Recent reports suggest effective induction of GVT reactions and superior outcomes of recipients of BMC allografts suggesting a need for further experimentation of reduced-intensity allogeneic BMC and umbilical cord blood transplants in neuroblastoma patients [9–12].
In this study we attempted to define immune reactivity after allogeneic bone marrow transplantation in a murine model of subcutaneous Neuro-2a implants. There are two possible scenarios. The first implies that Neuro-2a might be excluded from the state of mutual donor–host tolerance after allogeneic BMC transplantation due to absence of MHC antigens, rendering the tumor susceptible to GVT activity against tumor-associated antigens. The second scenario implies that alloreactivity against Neuro-2a cells is mediated by MHC antigens that may be present at a frequency lower than their detection by flow cytometry [13].
In formulation of the experimental design, we adopted several assumptions. First, neuroblastoma appears to be a particular case of a tumor characterized by low-immunogenicity antigens [14, 15]. It is unclear whether this tumor is submitted to immune surveillance, either because innate immunity is tolerant to fetal tumors, and/or neuroblastoma shares multiple antigens with normal mature tissues of ectodermal origin. Second, this malignancy is characterized by expression of classes I and II major histocompatibility (MHC) antigens at low levels [16–18], rendering NB cells relatively resistant to immune attack. Several approaches have been conceived to circumvent this limitation, including the use of natural killer (NK) cells to initiate an immune reaction [14, 19]. Interestingly, MHC antigen expression is induced in neuroblastoma cells under inflammatory conditions, induced primarily by interferon-γ (INF-γ) and interkeukin-2 (IL-2) [20–23]. Consequently, therapeutic approaches have focused on the use of these cytokines to foster anti-tumor immunity [24–26]. An interesting approach used allogeneic neuroblastoma cells to foster immune reactivity in the presence of lymphotactin and IL-2 [27].
Materials and methods
Animal model
Mice used in this study were A/J (H2Ka, CD45.2), C57BL/6 [H2 Kb, CD45.2], B6.SJL-Ptprca Pepcb/BoyJ (H2 Kb, CD45.1), C57BL/6-TgN(ACTbEGFP)1Osb [GFP, H2 kb], inbred A/C57BL/6 chimeras (H2Ka/b, CD45.2), and NOD.SCID mice purchased from Jackson Laboratories. Mice were housed in a barrier facility in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Tumor cells
Neuro-2a wild type cells, a mouse neuroblastoma of strain A origin, were obtained from the American Type Culture Collection (ATCC). Cells were cultured to maximum 12 passages in Minimal Essential Medium (GIBCO), supplemented with 10% fetal bovine serum (FCS, HyClone), MEM non-essential amino acids, 100 units/ml penicillin, and 100 mg/ml streptomycin (Life Technologies). Tumors were induced by subcutaneous (sc) implantation into the shaved right flank of 106 Neuro-2a cells in 100 μl of phosphate-buffered saline (PBS). Tumor growth was measured with a caliper and the volume (mm3) was calculated according to (width2 × length × 0.4).
Bone marrow cell preparation
Whole BMC (wBMC) were harvested from femurs and tibia of donors, and low-density cells were collected as previously described [28]. Immunomagnetic T cell depletion was performed by incubated for 45 min at 4°C with saturating amounts of biotinylated anti-mouse monoclonal antibodies (mAb) specific to CD4, CD5, and CD8. All antibodies were obtained from hybridoma cell cultures (ATCC). mAb-coated cells were washed twice with PBS containing 2% FCS and were incubated with sheep-anti-rat IgG conjugated to M-450 magnetic beads at a ratio of 4 beads per cell (Dynal Inc.). Conjugated cells were precipitated by exposure to a magnetic field. The efficiency of T cell depletion was reassessed by flow cytometry using a cocktail of primary-labeled mAb against the T cell markers listed above.
Splenocyte preparation
Spleens were harvested from mice, minced, passed through 40-μm mesh, and dispersed into a single-cell suspension in PBS. Red blood cells were lysed with medium containing 0.83% ammonium chloride, 0.1% potassium bicarbonate, 0.03% disodium EDTA. After 4 min, the reaction was arrested with excess of ice-cold PBS. T cells were enriched by elution through a cotton wool column (preferential retention of B lymphocytes and myeloid cells by differential charge than the eluted T cells), or immunomagnetic depletion using hybridoma-derived antibodies against GR-1, Mac-1, and B220.
Flow cytometry
Measurements were performed with a Vantage SE flow cytometer (Becton Dickinson). Positive staining was determined on a log scale, normalized with control cells stained with isotype control mAb. Donor chimerism was determined from the percentage of donor and host peripheral blood lymphocytes (PBL) and splenocytes. Blood was collected in heparinized serum vials in 200 μl M199, centrifuged over 1.5-ml lymphocyte separation media (Cedarlane), and red blood cells were lysed. Nucleated cells were incubated for 45 min at 4°C with phycoerythrin (PE)-anti-H2 Kb (Caltag) and fluorescein isothiocyanate (FITC)-anti-H2Kk mAb (eBioscience). Minor antigen disparity was assessed using CD45.1-PE and CD45.2-FITC antibodies (eBioscience). T cells were quantified using CD4-allophycocyanin and CD8-FITC antibodies (BD Pharmingen).
Conditioning and transplantation
Recipients were conditioned with total body irradiation (TBI) at 700 rad using an x-ray irradiator (Rad Source 2000; Rad Source Technologies Inc., Alpharetta, GA) at a rate of 106 rad/min. After 6 h, the cells were injected into the lateral tail vein in 200 μl PBS. GVHD was assessed using a semiquantitative clinical scale including weight loss, posture (hyperkeratosis of the foot pads impairs movement), activity, diffuse erythema (particularly of the ear) or dermatitis, and diarrhea. GVHD was validated by histology in H&E sections of the ear and liver according to 0-no infiltration, 1-scarce infiltrates, 2-patchy infiltration, and 3-diffuse infiltration with deterioration of tissue structure.
Proliferation assays
Splenocytes were labeled with 2.5 μM 5-(and-6-)-carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) and 5 × 107 cells were plated on petri dishes for 45 min to enrich for lymphocytes. After 45 min, the non-adherent cells were collected, washed, and incubated in DMEM supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 13.6 μM folic acid, 270 μM l-asparagine, 548 μM L-arginine HCL, 10 mM HEPES, 50 μM 2β-Mercaptoethanol (2-ME), 100 mg/ml streptomycin, 100 U/ml penicillin, 5% heat-inactivated FBS, and 1% heat-inactivated mouse serum (all the ingredients purchased from Beit Haemek and Sigma). Triplicate cultures were harvested after 3–5 days and the dilution of CFSE was analyzed by flow cytometry by gating on the live lymphocytes. Data were analyzed using the ModFit software (Verity Software House).
Cytotoxic assays
Effector splenocytes harvested from naïve mice and chimeras were passed through wool mesh to enrich for T lymphocytes (~70%). These cells were incubated with 5 × 105 Neuro-2a target cells for 7 h at 37˚C in 150 μl at 1:10 to 1:100 target:effector ratios. Cytolysis was quantified by lactate dehydrogenase (LDH) release and normalized for background values [29].
Statistical analysis
Data are presented as mean ± standard deviations for each experimental protocol. Results in each experimental group were evaluated for reproducibility by linear regression of duplicate measurements. Differences between the experimental protocols were estimated with a post hoc Scheffe t-test and significance was considered at P < 0.05.
Results
Tumor growth is affected by the immune system
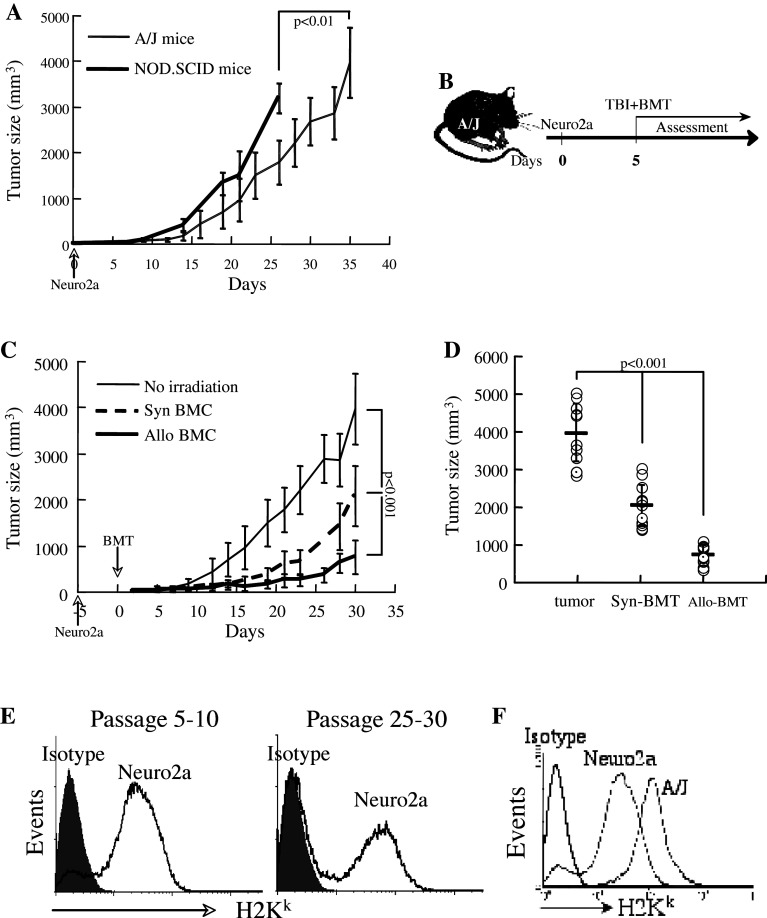
In preliminary studies, the appropriate number of Neuro-2a cells which generate detectable subcutaneous tumors that grow slow enough to allow a therapeutic intervention was determined to be in the range of 0.8–1 × 106. Transplantation of 0.8–1 × 106 Neuro-2a cells allowed a follow up of several weeks before reaching limiting sizes, while lower inoculum failed to induce tumors in some mice. Notably, we aimed at a critical mass for established tumors to perform continuous measurements, a model that hinders possible eradication of small tumors under various experimental conditions. In first stage, we examined the possible involvement of immunogenic mechanisms in NB tumor growth. Neuro-2a cells transplanted into congenic (H2Ka) mice formed subcutaneous tumors at a slower tempo as compared to immunocompromized NOD.SCID mice (Fig. 1a), suggesting that a lymphocyte-mediated mechanism slowed the development of these tumors. Notably, NOD.SCID mice have competent NK cells.
Fig. 1.
Neuro-2a growth is affected by T cell immunity. a 106 Neuro-2a cells were injected subcutaneously into congenic H2Ka (n = 15) and immunocompromized NOD.SCID mice (n = 8). Tumor volume was evaluated according to: length × width2 × 0.4, until the tumors reached maximal allowed sizes. Data represent means ± standard deviations (SD). b The time sequence for transplant experiments: total body irradiation at 700 rad and intravenous infusion of bone marrow cells were performed 5 days after subcutaneous implantation of 106 Neuro-2a cells. c Transplantation of 5 × 106 syngeneic (n = 11) and allogeneic (n = 19) whole bone marrow cells (BMC) resulted in reduced growth rate of the tumors. Data represent means ± SD. d Tumor size at 30 days after syngeneic (H2Ka → H2Ka) and allogeneic (H2 Kb → H2Ka) bone marrow transplantation as compared to growth in naïve congenic (H2Ka) mice. e Expression of H2Ka antigen in Neuro-2a cells using a cross-reactive anti-H2Kk antibody: left panel passage 6, right panel passage 25. f Low level expression of H2Kk in Neuro-2a cells as compared to splenocytes of A/J mice
The impact of conditioning and bone marrow transplantation on tumor growth was determined according to the experimental setting detailed in Fig. 1b. To simulate conditions of a pre-existing tumor, Neuro-2a cells were implanted subcutaneously 5 days before total body irradiation (TBI) at 700 rad and bone marrow transplantation. Irradiation and transplantation of 5 × 106 syngeneic BMC (n = 11) reduced tumor size as compared to naïve subcutaneous tumors (P < 0.001; Fig. 1c), consistent with growth inhibition of cultured cells following irradiation (not shown). This does not exclude the participation of immunogenic mechanisms in suppression of tumor growth after syngeneic transplantation. Under the same experimental conditions, allogeneic (H2Kb → H2Ka) bone marrow transplantation (n = 19) further reduced tumor growth (P < 0.001). The significant differences observed on day 30 post-transplantation (Fig. 1d) imply that Neuro-2a cells serve as targets of MHC-disparate immune cells of donor origin. Measurements of MHC antigen expression using anti-H2Kk antibody, which is cross-reactive to H2Ka, revealed variable expression of the antigen in Neuro-2a cells (Fig. 1e). Downregulation of MHC antigens might explain the slower growth rates on Neuro-2a cells from high passages (>25) as compared to low passages (5–10). The levels of expression in cultured Neuro-2a cells are lower than those of splenocytes of congenic A/J mice (Fig. 1f).
Allografted mice develop cytotoxic GVT reactivity
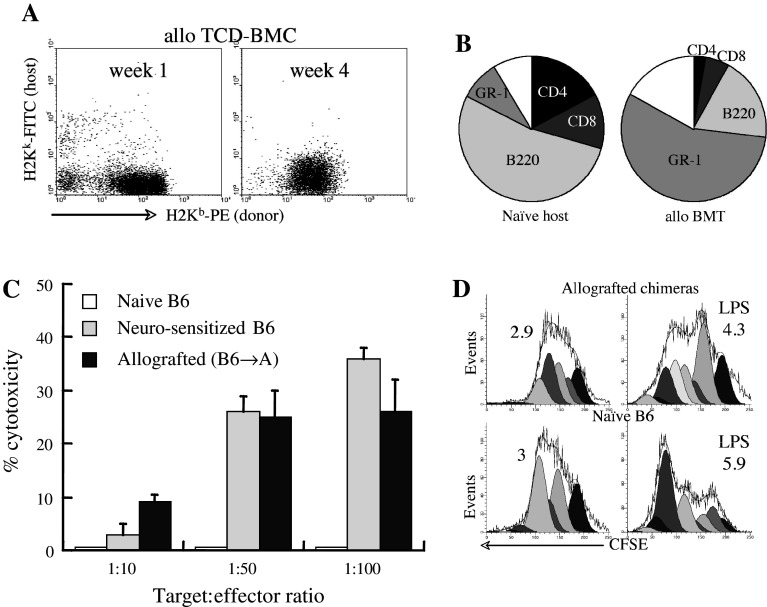
Radiated (700 rad) recipients of 5 × 106 allogeneic TCD-BMC (B6 → A) showed the levels of donor chimerism in peripheral blood of 73 ± 6 and 92 ± 4% after 2 and 4 weeks from transplantation, respectively. During the first 4 weeks post-transplantation, the spleen was predominantly donor-type (Fig. 2a) demonstrating that the conditioning regimen was largely lymphoablative. At this point of time, the spleens contained ~60% of the cellularity of naïve H2Ka mice, with a marked reduction in fractions of CD4+ and CD8+ T cells (Fig. 2b), consistent with the predominant myeloid and B lymphocyte reconstitution of the spleen at early times after transplantation.
Fig. 2.
Immune responses after allogeneic bone marrow transplantation. a A/J mice radiated at 700 rad and allografted (H2 Kb → H2Ka) with 5 × 106 T cell depleted (TCD) BMC display full donor chimerism in the spleen. b At 4 weeks post allogeneic transplantation the chimeric spleens include small numbers of CD4+ and CD8+ T cells (n = 13) as compared to naïve A/J hosts (H2Ka, n = 7). c Cytotoxicity Neuro-2a cells (target) by splenocytes (effector) harvested at 4 weeks after allogeneic bone marrow transplantation (n = 6), as determined from LDH release. Cytolysis of lymphocytes from naïve B6 donors and B6 mice immunized with two intravenous injections of 3 × 106 Neuro-2a cells (at 3 day intervals) are presented as controls (n = 5). d Proliferation of splenocytes from allografted mice (H2 Kb → H2Ka) in response to LPS in vitro, as determined from CFSE dilution (n = 4). Demonstrative for assays were performed in triplicates
For positive identification of an effective GVT reaction as the basis of tumor growth inhibition, splenocytes of the chimeric mice were evaluated for cytotolysis of target tumor cells. Mice bearing subcutaneous tumors were grafted with allogeneic BMC (as detailed in Fig. 1b). Splenocytes harvested after 4 weeks were co-incubated with Neuro-2a cells for 7 h. LDH release from tumor cells increased proportionally to the splenocyte:Neuro-2a ratio, positively identifying effective GVT reactivity (Fig. 2c). To ascertain cytolysis, B6 mice were immunized twice with Neuro-2a cells at 3-day interval, and after 3 days the splenocytes were assayed for cytolytic activity. In variance from naïve B6 splenocytes, which lacked cytotoxic activity upon short-term in vitro stimulation, the splenocytes of pre-sensitized B6 mice elicited effective cytotoxicity. Considering the low numbers of T cells in spleens of chimeric mice-bearing tumors (Fig. 1b), proliferation was determined from CFSE dilution under an LPS challenge for 5 days. The proliferation index increased from 2.9 ± 1.2 to 4.9 ± 1.2 under the influence of LPS, used as a non-specific stimulus (Fig. 2d). In comparison, the splenocytes of naïve B6 donor mice (H2Kb) displayed marginally higher proliferation rates upon LPS stimulation. Thus, immune cells of the reconstituted chimeras proliferated in response to non-specific stimuli and displayed tumor-specific cytotoxicity.
Dissociation between GVT and GVH reactions
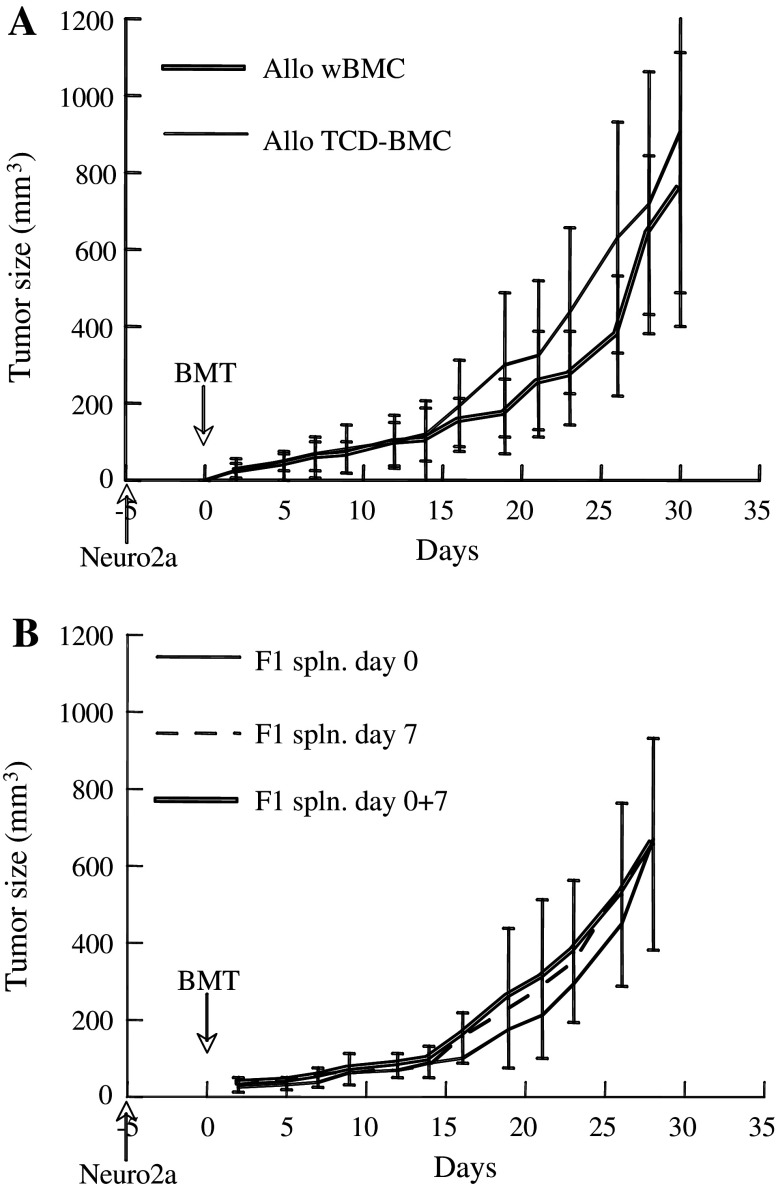
Recipients of allogeneic whole BMC developed GVHD grade 1–2 at variable levels. To determine whether effective GVT activity may be achieved in the absence of grafted donor T lymphocytes, the tumor growth was monitored after transplantation of T cell depleted BMC (TCD-BMC). Depletion of CD4+, CD5+, and CD8+ T cells from donor BMC inoculum had a minor effect on tumor growth as compared to recipients of whole BMC (Fig. 3a), suggesting that the infused donor lymphocytes had little or no contribution to the GVT reaction. Thus, effective GVT may be achieved without causing GVHD by transplantation of TCD-BMC.
Fig. 3.
Involvement of T cells in tumor growth inhibition. a Mice bearing subcutaneous tumors were infused with 5 × 106 allogeneic whole bone marrow cells (wBMC, n = 19) or T cell depleted (TCD) BMC (n = 26) according to the experimental sequence detailed in Fig. 1b. Data represent means ± SD. b Recipients allografted with 5 × 106 TCD-BMC (H2 Kb) were infused intravenously with 2 × 107 splenocytes (F1 spln.) from F1 mice (H2Ka/b) on days 0 or 7 and on both days (0 + 7)
Infusion of F1 lymphocytes does not foster GVT reactivity
The superior outcome after allogeneic versus syngeneic transplants and the finding that Neuro-2a cells express MHC class I antigens implies a requirement of MHC disparity in induction of GVT reactivity. However, the chimeric mice accepted both host type (H2Ka) and donor-type (H2 Kb) skin grafts at 4 weeks post-transplantation, demonstrating induction of transplant tolerance through hematopoietic chimerism (n = 4). If GVT reactivity could be achieved under a state of tolerance to MHC antigens, infusion of F1 splenocytes should rather foster the reaction. In an attempt to foster reactivity, 2 × 107 splenocytes from F1 mice (H2Ka/b) were added to 5 × 106 H2Kb TCD-BMC. Infusion of F1 splenocytes on days 0 or 7, or on both days (0 + 7) did not affect, significantly, the tumor growth rates (Fig. 3b). There are two possible explanations: (a) ineffective engraftment of F1 splenocytes in the chimeric mice, (b) F1 splenocytes did not acquire GVT reactivity in the absence of MHC mismatch to the tumor.
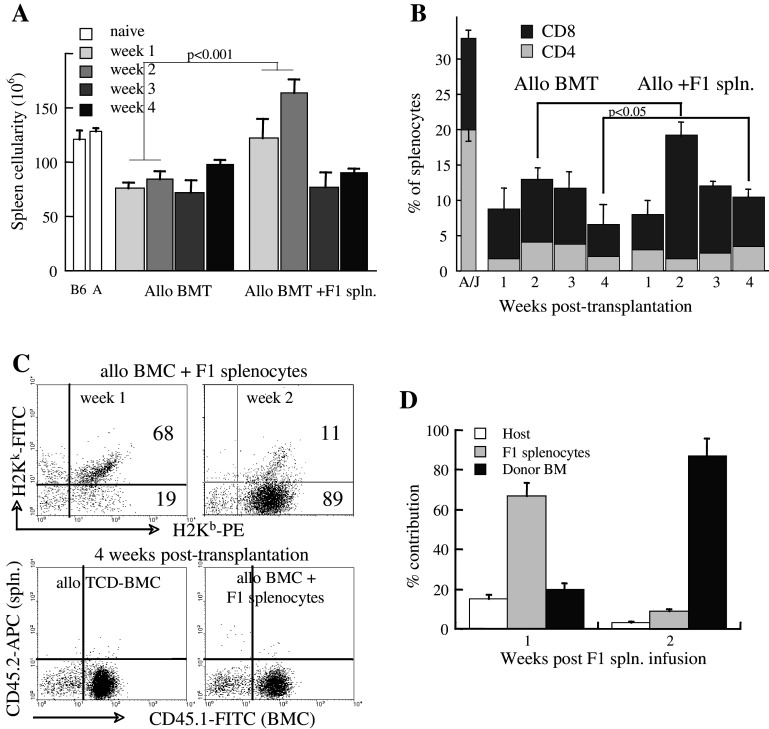
The pattern and tempo of lymphoid reconstitution were monitored in the grafted mice with and without F1 splenocyte infusion on day 0. Spleen cellularity increased gradually after transplantation, with an expected early quantitative advantage (at 2 weeks) in recipients of F1 splenocytes (Fig. 4a). However, an unexpected decrease occurred afterwards, indicating that the infused F1 splenocytes were short lived. Similar data were observed after infusion of F1 splenocytes on day +7 post-transplantation (not shown), supporting their transient engraftment. A significant difference was a higher fraction of 11 ± 1.2% CD4+ and CD8+ T cells in the spleens of recipients of F1 splenocytes at 4 weeks post-transplantation (P < 0.05 vs 6.5 ± 3% of spleens of TCD-BMC recipients) (Fig. 4b).
Fig. 4.
Immune reconstitution after allogeneic BMT and F1 splenocyte infusion. a Spleen cellularity measured at weekly intervals in mice grafted with 5 × 106 allogeneic TCD-BMC (Allo BMT) and with 2 × 107 F1 (H2Ka/b) splenocytes on day 0 (Allo + F1 spln.), according to the experimental setting detailed in Fig. 1b. Spleen cellularity in naïve donors (B6) and recipients (A/J) is given as control. b Fractional distribution of CD4+ and CD8+ T cells in the spleen measured at weekly intervals after transplantation of TCD-BMC (Allo BMT) and co-transplantation of F1 splenocytes (Allo + F1 spln.). The distribution of these subsets in naïve H2Ka (A/J) recipients is shown as control. c Dissociation between splenocytes derived from the bone marrow (H2 Kb) and F1 splenocytes (H2Ka/b) at 1 and 2 weeks post-transplantation (upper panels). At 4 weeks post-transplantation (lower panels), the spleen was reconstituted from the bone marrow cells (CD45.1), without evidence of residual F1 splenocytes (CD45.2). d Switch in splenic contribution from adoptively transferred F1 splenocytes to bone marrow-derived cells during the first two weeks after F1 splenocyte in fusion
To determine the origin of splenic T cells, minor antigen disparity was used to dissociate between bone marrow-derived lymphocytes (H2 Kb, CD45.1) and F1 splenocytes (H2Ka/b, CD45.2). Although F1 splenocytes (H2Ka/b) were observed at 1–2 weeks post-transplantation, virtually all cells originated from the bone marrow (CD45.1) at 4 weeks post-transplantation (Fig. 4c). The quantitative switch occurred during the second week after F1 splenocyte infusion, when bone marrow-derived immune cells became dominant (Fig. 4d). Consistent results were observed when F1 splenocytes were infused on day +7 post-transplantation (not shown). Thus, F1 splenocytes engrafted transiently and enhanced the immune recovery from the bone marrow. It is possible that early homeostatic expansion of the F1 splenocytes, although transient, contributed to lymphoid reconstitution by secretion of cytokines in the hypocellular lymphoid organs, fostering homeostatic expansion of bone marrow-derived cells. These cells did not cause GVHD and did not affect significantly tumor growth rates.
Target antigens of the GVT reaction
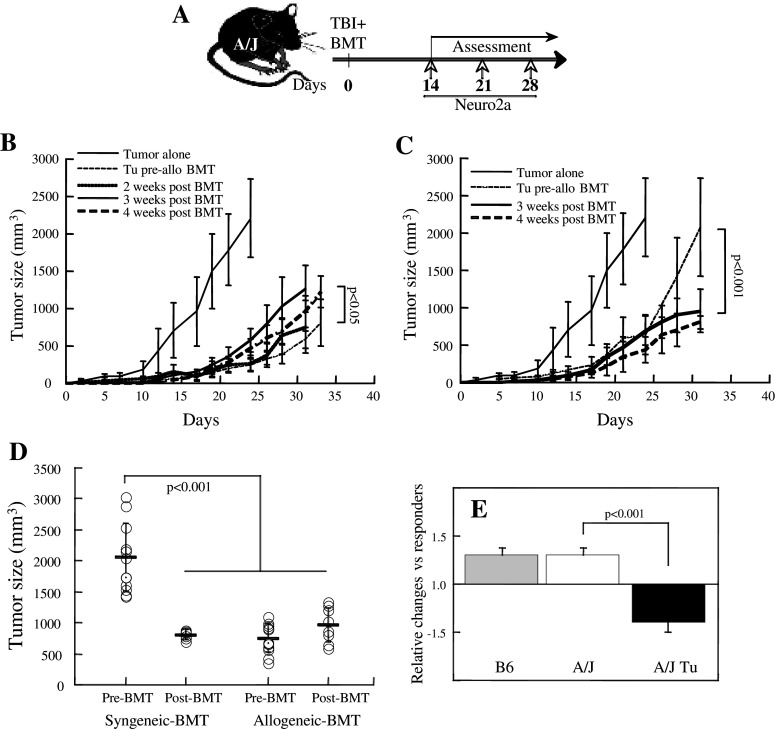
The GVT reaction presented apparently controversial information in reference to MHC antigen specificity. On the one hand, the significant tumor growth inhibition attained by allotransplant as compared to syngeneic reconstitution suggests that alloreactivity to MHC antigens is essential to the induction of an effective GVT reaction. On the other hand, tumor-specific reactivity was displayed by splenocytes reconstituted primarily from the donor bone marrow, which are tolerant to the tumor (and host) MHC antigens. Therefore, a challenge experiment was performed with allogeneic bone marrow transplantation preceding the implantation of subcutaneous tumors (Fig. 5a). Notably, in this setting the Neuro-2a cell implants were not exposed to radiation. Subcutaneous grafting of NB cells at 2, 3, and 4 weeks after allogeneic BMT showed remarkably slower rates of tumor growth (P < 0.001) as compared to naïve mice (Fig. 4b). Furthermore, early implantation of tumors at 2 weeks post-transplantation resulted in slowest growth rates, which were similar to tumors implanted prior to allogeneic BMT (Fig. 5b). Taken together these data indicated that Neuro-2a cells were submitted to immune surveillance and the presence of tumor cells during the phase of immune reconstitution fostered anti-tumor immunity.
Fig. 5.
Adaptive immunity inhibits tumor growth. a The time sequence for the transplant experiments where transplantation of 5 × 106 TCD-BMC preceded a tumor challenge (subcutaneous implantation of 106 Neuro-2a cells). b Comparative growth rates in of Neuro-2a cells in mice allografted (H2 Kb → H2Ka) after tumor implantation (Tu pre-allo BMT), and implantation of tumors at 2 weeks (n = 5), 3 weeks (n = 9) and 4 weeks (n = 11) after allogeneic transplantation (post-BMT). c Comparative growth rates in of Neuro-2a cells in mice grafted with syngeneic TCD-BMC (H2Ka → H2Ka) after tumor implantation (Tu pre-syn BMT), and implantation of tumors at 3 and 4 weeks (n = 6) after syngeneic transplantation (post-BMT). d Tumor size at 30 days after subcutaneous implantation of 106 Neuro-2a cells in mice with syngeneic and allogeneic BMT. Tumors were implanted 5 days before transplantation (pre-BMT) or 4 weeks after transplantation (post-BMT). e Splenic lymphocytes from naïve B6 (n = 12) and A/J mice (n = 5) and A/J mice bearing subcutaneous tumors (n = 6) were stimulated with ConA for 5 days in culture and proliferation was determined from CFSE dilution. The data represent the ratio between control responders and incubation with the soluble fraction of Neuro-2a tumor lysate
The limited efficacy of syngeneic transplants in reducing tumor growth rates as compared to allogeneic transplants in tumor-bearing mice (Fig. 1c) questioned the importance of transition to adaptive immunity by transplantation. Therefore, tumors were subcutaneously implanted at 3 and 4 weeks after syngeneic BMT (Fig. 5a), showing a significant reduction of tumor growth rates (P < 0.01; Fig. 5c). In fact, the growth rates of tumors implanted at 4 weeks post-transplantation were similar after syngeneic and allogeneic reconstitution, similar to allografted mice bearing tumors and significantly lower than mice reconstituted with syngeneic BMC (P < 0.001; Fig. 5d). Thus, transition to adaptive immunity after transplantation is favorable for the induction of GVT reactivity whereas the presence of tumors prior to transplantation results in faster growth rates. These data questioned the relative low efficacy of syngeneic transplants to induce GVT reactivity in tumor-bearing mice. To evaluate this phenomenon, the proliferation of splenocytes was determined in the presence of the soluble fraction of tumor lysates. While tumor lysates equally stimulated splenocytes of naïve B6 and A/J mice, proliferation was inhibited in splenocytes from A/J mice bearing subcutaneous tumors (Fig. 5e). These data imply that the tumors release inhibitory factors, with apparent specificity to inactivation of syngeneic splenic lymphocytes remote from the subcutaneous tumor.
Discussion
Difficulties in eradication of neuroblastoma in significant numbers of pediatric patients have prompted the discussion surrounding possible immunotherapeutic approaches. In this study, we demonstrate that effective anti-tumor immune reactivity is elicited by allogeneic bone marrow transplantation in tumor-bearing mice through development of tumor-specific cytotolytic responses.
Neuroblastoma is submitted to immune surveillance, as evidenced by the faster growth rates in immunocompromized as compared to congenic mice, growth inhibition by allogeneic transplantation in tumor bearing mice, and tumor suppression in mice that underwent BMT before the tumor challenge. Attempting to simulate the pathological conditions where bone marrow transplantation is performed in children with pre-existing neuroblastoma, mice bearing subcutaneous tumors were grafted with bone marrow. The differences in growth rates following allogeneic and syngeneic immuno-hematopoietic reconstitution revealed several features of this experimental model. First, direct cytolysis of Neuro-2a cells by lymphocytes of the chimeric mice demonstrates specific sensitization to tumor antigens in vivo. Importantly, effective GVT reaction was attained under conditions of partial T cell reconstitution, which demonstrates their functional competence early after transplantation. Second, an inhibitory mechanism appears to be involved in suppression of GVT reactivity in mice bearing tumors at the time of syngeneic transplantation. Soluble factors within tumor lysates inhibited the proliferation of T cells from congenic mice bearing subcutaneous tumors. Third, the implantation of tumors after BMT is equally efficient in suppressing tumor growth by syngeneic and allogeneic immune cells. Recovery of the immune system after transplantation is associated with a period of immune activation, which apparently abolished the inhibition of syngeneic cell activity against the tumor. These features of the GVT reaction reveal complex patterns of cellular responses to the tumor that involve several mechanisms of activation while the tumor attempts to evade immune surveillance.
Within this complex pattern of regulation of the GVT reaction, it is questioned what is the role of MHC disparity. On the one hand, suppression of tumor growth following allogeneic BMT and the cytotoxic activity of mismatched lymphocytes (from immunized and chimeric mice) suggest that MHC disparity is essential to generate a potent GVT reaction. Several data support a requirement for MHC disparity: (a) Using a cross-reactive antibody, Neuro-2a cells were found to express low levels of MHC class I molecules. Although neuroblastoma is traditionally considered to express low levels of MHC class I and II antigens [16–18], as few as one MHC class I molecule per cell is sufficient (in principle) for T cell activation and lysis of tumor cells [13, 30, 31]. In fact, infiltration of cytolytic T cells into the tumor increases the expression of MHC antigens through secretion of INF-γ and IL-2, contributing to priming of additional T cells against tumor associated antigens [20–23]. (b) Naïve B6 mice (H2 Kb) reject the tumor cells and immunization with Neuro-2a cells elicits tumor-specific cytotoxic activity. This type of rejection is a consequence of MHC incompatibility between host immunity and non-self tissue. (c) Infusion of F1 splenocytes (H2Ka/b), which are unresponsive to H2Ka and engraft transiently, failed to further suppress tumor growth. These data suggest a requirement for MHC mismatch to elicit an effective GVT reaction and imply that MHC antigens serve as targets of cytotoxic lymphocytes.
On the other hand, MHC antigen disparity per se is insufficient to explain the inhibition of tumor growth for several reasons: (a) The differences in tumor growth rates were marginally affected by exclusion of T cells from the donor inoculum, thus GVT cannot be considered to be a GVH-like reaction mediated by mature donor lymphocytes within the graft. (b) Cytolytic activity was attributed to lymphocytes derived from the bone marrow after allogeneic transplantation, which are tolerant to host MHC antigens. The state of tolerance to host MHC antigens was demonstrated by acceptance of both donor (H2 Kb) and host-type (H2Ka) skin grafts by the chimeric mice. (c) Tumor growth was equally suppressed in mice undergoing immune reconstitution after syngeneic and allogeneic BMT preceding the tumor challenge. Thus, the faster growth rates following syngeneic (as compared to allogeneic) reconstitution in tumor bearing mice are attributed to inhibitory mechanisms, rather than ineffective immunization. The apparent mechanism of GVT reactivity involves a facilitating effect of MHC disparity without specific targeting of the MHC antigens.
The evolving scenario suggests that MHC disparity is essential to initiate a GVT response and overcome inhibitory mechanisms, however, the MHC antigens themselves are not targeted in the tumor. The MHC-independent nature of tumor cytolysis is evident from the effective GVT reactivity of donor lymphocytes tolerant to tumor (and host) MHC in allografted tumor bearing mice, and tumor growth suppression following syngeneic immune reconstitution. By elimination, the cytotoxic cells target tumor-associated antigens, which are considered as essential targets of T cell-mediated immunotherapy [32]. None of the tumor-associated antigens detected in NB are specific and the majority is shared by normal tissue, including disialogangliosides GD2 and GD3 [33], survivin [34], transferrin receptor [35], the adhesion molecule L1-CAM [36], T cell acute lymphoblastic leukemia-associated antigen 1 (TALLA-1) [37], and the B7 costimulatory family member 4Ig-B7-H3 [38]. However, if tumor antigen uptake is performed by the lymphocytes themselves, this process should be MHC-unrestricted as well, to explain the similarity in growth suppression after syngeneic and allogeneic BMC transplantation. This scenario does not explain the role of MHC antigens in priming the GVT reaction in tumor-bearing mice. Therefore, antigen presenting cells may be involved in the process of GVT reactivity, providing the context of MHC-dependent antigen presentation along with MHC-unrestricted cytolysis. It is therefore postulated that antigen presenting cells are involved in the physiological process of sensitization against the tumor [39–41], a mechanism that includes the requirement for priming by MHC antigen disparity in the process of induction of anti-tumor reactivity. Inhibition of tumor progression upon challenging early after allogeneic transplantation (under limiting numbers of T cells) is suggestive of superior antigen uptake capacity, which is characteristic of immature dendritic cells. In addition, the GVT reaction might include a MHC-independent process mediated by NK cells, which are among first subsets that develop after bone marrow transplantation. Tumor cell lysis by MHC class I-independent NK activity may be involved in all situations analyzed in this study [42–44], however it cannot explain the variations in outcomes with reference to MHC disparity, despite the close interaction between NK and dendritic cells [40, 41]. The data presented here suggest that lymphocytes are the primary effectors of cytotoxic activity within the GVT reaction.
As a tumor that is often present during the pre-natal phase, it is questioned what is the involvement of innate immunity and the shared antigens with the immune-privileged ectodermal tissue in failure of the immune system to suppress neuroblastoma [18]. Although in our models the tumors were implanted in mature mice, it is evident that the developing immune system after syngeneic and allogeneic transplantation restores immune surveillance of this tumor. Furthermore, tumor lysate was found to inhibit the proliferation of lymphocytes of mice-bearing subcutaneous tumors, which explains in part the lower efficacy of tumor suppression by syngeneic transplants against pre-implanted tumors. Neuroblastoma was also shown to inhibit dendritic cell development and function [45–48], in addition to other mechanisms of evasion from immune surveillance by direct killing of cytotoxic cells [49]. It is therefore a complex pattern of communication between the tumor and cytotoxic cells, mediated by antigen presenting cells and affected by soluble inhibitory factors that operate also at remote sites such as the spleen.
The results of these studies challenge some of the prevalent notions concerning this tumor. First, some studies described low or absent expression of MHC class I and class II antigens in primary human neuroblastoma, suggesting that this tumor is not a potential target of alloimmune responses [50]. These data raised the possibility that the tumor might be excluded from the mutual donor–host tolerance induced by allogeneic bone marrow transplantation, rendering it susceptible to attack by MHC-independent mechanisms. However, we found that Neuro-2a cells, a prevalent experimental model, display H2Ka antigens at low levels. In contradiction to our initial assumption, alloreactivity was an essential ingredient in generation of an effective GVT reaction against established tumors, as seen in tumor growth suppression after allogeneic transplantation in tumor bearing mice. Second, the prior studies suggested that immune reactivity against neuroblastoma is impaired by the low immunogenicity of antigens specific to this tumor [14, 15]. This concept may be true only in relative terms [32]. Our data show that MHC antigens are not targeted, rather participate in priming of the GVT reaction against tumor associated antigens. Third, although initially considered that robust lympho-hematopoietic reconstitution is a prerequisite to achieve anti-tumor activity, partial immune reconstitution was sufficient and effective. The fact that bone marrow-derived T cells initiate the GVT reaction which provides a significant pragmatic advantage in reference to possible dissociation between GVT and GVH reactions by elimination of donor T cells in the graft. Furthermore, this observation is important in the context of haploidentical transplants, which elicit more vigorous GVH responses and require stringent T cell depletion [8, 10]. Possible induction of GVT reactions by non-myeloablative transplants should be explored, considering the superior capacity of cells to track micrometastases, reach intra-marrow spread of neuroblastoma, and restore the mechanisms of immune surveillance [51, 52]. Finally, donor-derived lymphocytes appear to be the primary effectors of tumor cytolysis, dendritic cells are the mediators, and NK cells may participate as an additional mechanism. The evidence for this proposition includes faster growth rates in lymphocyte-deficient NOD.SCID mice, the requirement for MHC mismatch to overcome inhibition and initiate GVT, and the absence of significant differences when T cells are depleted from the donor inoculum.
In summary, although lympho-hematopoietic reconstitution of hosts with syngeneic bone marrow is superior in terms of safety and reduced intensity of conditioning, our results suggest that this approach is rather ineffective except extreme cases where administration of cytotoxic agents causes irreversible bone marrow aplasia. Allotransplants are evidently efficient in inducing a potent GVT reaction against neuroblastoma, where MHC disparity at the level of antigen presenting cells is important in induction of cytolytic responses against tumor associated antigens.
Acknowledgments
This work was supported by grants from the Israel Cancer Research Fund, Israel Ministry of Health, Israel Cancer Research Association, and the Frankel Trust for Experimental Bone Marrow Transplantation. The excellent technical assistance of Mrs. Natalia Binkovsy, Mrs. Ela Zuzovsky, and Mrs. Ana Zemlianski is gratefully acknowledged.
References
- 1.Ladenstein R, Pötschger U, Hartman O, EBMT Paediatric Working Party et al. 28 years of high-dose therapy and SCT for neuroblastoma in Europe: lessons from more than 4000 procedures. Bone Marrow Transplant. 2008;41(Suppl 2):S118–S127. doi: 10.1038/bmt.2008.69. [DOI] [PubMed] [Google Scholar]
- 2.Kanold J, Yakouben K, Tchirkov A, et al. Long-term results of CD34(+) cell transplantation in children with neuroblastoma. Med Pediatr Oncol. 2000;35:1–7. doi: 10.1002/1096-911X(200007)35:1<1::AID-MPO1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Burchill SA, Kinsey SE, Picton S, et al. Minimal residual disease at the time of peripheral blood stem cell harvest in patients with advanced neuroblastoma. Med Pediatr Oncol. 2001;36:213–219. doi: 10.1002/1096-911X(20010101)36:1<213::AID-MPO1052>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Bowman LC, Grossmann M, Rill D, et al. Interleukin-2 gene-modified allogeneic tumor cells for treatment of relapsed neuroblastoma. Hum Gene Ther. 1998;9:1303–1311. doi: 10.1089/hum.1998.9.9-1303. [DOI] [PubMed] [Google Scholar]
- 5.Lode HN, Xiang R, Dreier T, et al. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 6.Haight AE, Bowman LC, Ng CY, et al. Humoral response to vaccination with interleukin-2-expressing allogeneic neuroblastoma cells after primary therapy. Med Pediatr Oncol. 2000;35:712–715. doi: 10.1002/1096-911X(20001201)35:6<712::AID-MPO50>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Slavin S, Morecki S, Weiss L, Or R. Donor lymphocyte infusion: the use of alloreactive and tumor-reactive lymphocytes for immunotherapy of malignant and nonmalignant diseases in conjunction with allogeneic stem cell transplantation. J Hematother Stem Cell Res. 2002;11:265–276. doi: 10.1089/152581602753658457. [DOI] [PubMed] [Google Scholar]
- 8.Kanold J, Paillard C, Tchirkov A, et al. Allogeneic or haploidentical HSCT for refractory or relapsed solid tumors in children: toward a neuroblastoma model. Bone Marrow Transplant. 2008;42(Suppl 2):S25–S30. doi: 10.1038/bmt.2008.279. [DOI] [PubMed] [Google Scholar]
- 9.Teshima T, Mach N, Hill GR, et al. Tumor cell vaccine elicits potent antitumor immunity after allogeneic T-cell-depleted bone marrow transplantation. Cancer Res. 2001;61:162–171. [PubMed] [Google Scholar]
- 10.Inoue M, Nakano T, Yoneda A, et al. Graft-versus-tumor effect in a patient with advanced neuroblastoma who received HLA haplo-identical bone marrow transplantation. Bone Marrow Transplant. 2003;32:103–106. doi: 10.1038/sj.bmt.1704070. [DOI] [PubMed] [Google Scholar]
- 11.Li JM, Waller EK. Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10:540–551. doi: 10.1016/j.bbmt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama M, Azuma E, Araki M, et al. Evidence of graft-versus-tumor effect in refractory metastatic neuroblastoma. Transplantation. 2006;82:142–144. doi: 10.1097/01.tp.0000225780.90991.49. [DOI] [PubMed] [Google Scholar]
- 13.Sykulev Y, Joo M, Vturina I, et al. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 14.Main EK, Lampson LA, Hart MK, et al. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985;135:242–246. [PubMed] [Google Scholar]
- 15.Coze C, Aalto-Setala K, Brenner M, Chiang Y. Characteristics and immunomodulatory properties of human neuroblastoma cells after retrovirus-mediated gene transfer of the cytokine genes IL-2 and IFN-gamma. Transgenics. 1995;1:585–595. [Google Scholar]
- 16.Ponzoni M, Guarnaccia F, Corrias MV, Cornaglia-Ferraris P. Uncoordinate induction and differential regulation of HLA class-I and class-II expression by gamma-interferon in differentiating human neuroblastoma cells. Int J Cancer. 1993;55:817–823. doi: 10.1002/ijc.2910550521. [DOI] [PubMed] [Google Scholar]
- 17.Murphy C, Nikodem D, Howcroft K, et al. Active repression of major histocompatibility complex class I genes in a human neuroblastoma cell line. J Biol Chem. 1996;271:30992–30999. doi: 10.1074/jbc.271.48.30992. [DOI] [PubMed] [Google Scholar]
- 18.Wolfl M, Jungbluth AA, Garrido F, et al. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–406. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans A, Main E, Zier K, et al. The effects of gamma interferon on the natural killer and tumor cells of children with neuroblastoma: a preliminary report. Cancer. 1989;64:1383–1387. doi: 10.1002/1097-0142(19891001)64:7<1383::AID-CNCR2820640702>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Lampson LA, Whelan JP, Fisher CA. HLA-A, B, C and beta 2-microglobulin are expressed weakly by human cells of neuronal origin, but can be induced in neuroblastoma cell lines by interferon. Prog Clin Biol Res. 1985;175:379–388. [PubMed] [Google Scholar]
- 21.Sugimoto T, Horii Y, Hino T, et al. Differential susceptibility of HLA class II antigens induced by gammainterferon in human neuroblastoma cell lines. Cancer Res. 1989;49:1824–1828. [PubMed] [Google Scholar]
- 22.Hock H, Dorsch M, Kunzendorf U, et al. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci USA. 1993;90:2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadanaga N, Nagoshi M, Lederer JA, et al. Local secretion of IFN-gamma induces an antitumor response: comparison between T cells plus IL-2 and IFN-gamma transfected tumor cells. J Immunother. 1999;22:315–323. doi: 10.1097/00002371-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Katsanis E, Orchard PJ, Bausero MA, et al. Interleukin-2 gene transfer into murine neuroblastoma decreases tumorigenicity and enhances systemic immunity causing regression of preestablished retroperitoneal tumors. J Immunother. 1994;15:81–90. doi: 10.1097/00002371-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Bauer M, Reaman GH, Hank JA, et al. A phase II trial of human recombinant interleukin-2 administered as a 4-day continuous infusion for children with refractory neuroblastoma, non-Hodgkin’s lymphoma, sarcoma, renal cell carcinoma, and malignant melanoma. A Childrens Cancer Group study. Cancer. 1995;75:2959–2965. doi: 10.1002/1097-0142(19950615)75:12<2959::AID-CNCR2820751225>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Bowman L, Grossmann M, Rill D, et al. IL-2 adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood. 1998;92:1941–1949. [PubMed] [Google Scholar]
- 27.Rousseau RF, Haight AE, Hirschmann-Jax C, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 28.Pearl-Yafe M, Yolcu ES, Stein J, et al. Expression of Fas and Fas-ligand in donor hematopoietic stem and progenitor cells is dissociated from the sensitivity to apoptosis. Exp Hematol. 2007;35:1601–1612. doi: 10.1016/j.exphem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Barker SE, Grosse SM, Siapati EK, et al. Immunotherapy for neuroblastoma using syngeneic fibroblasts transfected with IL-2 and IL-12. Br J Cancer. 2007;97:210–217. doi: 10.1038/sj.bjc.6603857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christinck ER, Luscher MA, Barber BH, Williams DB. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama S, Tsomides TJ, Sykulev Y, Eisen HN. Variations in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J Immunol. 1995;154:567–576. [PubMed] [Google Scholar]
- 32.Foss FM. Immunologic mechanisms of antitumor activity. Semin Oncol. 2002;29:5–11. doi: 10.1053/sonc.2002.33076. [DOI] [PubMed] [Google Scholar]
- 33.Saito M, Yu RK, Cheung NK. Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochem Biophys Res Commun. 1985;127:1–7. doi: 10.1016/S0006-291X(85)80117-0. [DOI] [PubMed] [Google Scholar]
- 34.Croce M, Meazza R, Orengo AM, et al. Immunotherapy of neuroblastoma by an Interleukin-21-secreting cell vaccine involves survivin as antigen. Cancer Immunol Immunother. 2008;57:1625–1634. doi: 10.1007/s00262-008-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugimoto T, Tatsumi E, Kemshead JT, et al. Determination of cell surface membrane antigens common to both human neuroblastoma and leukemia-lymphoma cell lines by a panel of 38 monoclonal antibodies. J Natl Cancer Inst. 1984;73:51–57. [PubMed] [Google Scholar]
- 36.Schonmann SM, Iyer J, Laeng H, et al. Production and characterization of monoclonal antibodies against human neuroblastoma. Int J Cancer. 1986;37:255–262. doi: 10.1002/ijc.2910370214. [DOI] [PubMed] [Google Scholar]
- 37.Takagi S, Fujikawa K, Imai T, et al. Identification of a highly specific surface marker of T-cell acute lymphoblastic leukemia and neuroblastoma as a new member of the transmembrane 4 superfamily. Int J Cancer. 1995;61:706–715. doi: 10.1002/ijc.2910610519. [DOI] [PubMed] [Google Scholar]
- 38.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7–H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morisaki T, Matsumoto K, Onishi H, et al. Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives. Hum Cell. 2003;16:175–182. doi: 10.1111/j.1749-0774.2003.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 40.Redlinger RE, Jr, Mailliard RB, Barksdale EM., Jr Neuroblastoma and dendritic cell function. Semin Pediatr Surg. 2004;13:61–71. doi: 10.1053/j.sempedsurg.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Fujii S. Exploiting dendritic cells and natural killer T cells in immunotherapy against malignancies. Trends Immunol. 2008;29:242–249. doi: 10.1016/j.it.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T, Berhanu A, Redlinger RE, Jr, et al. Watkins S, Lotze MT, Barksdale EM Jr. Interleukin-12 transduced dendritic cells induce regression of established murine neuroblastoma. J Pediatr Surg. 2001;36:1285–1292. doi: 10.1053/jpsu.2001.25796. [DOI] [PubMed] [Google Scholar]
- 44.Castriconi R, Dondero A, Cilli M, et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother. 2007;56:1733–1742. doi: 10.1007/s00262-007-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shurin GV, Shurin MR, Bykovskaia S, et al. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 46.Redlinger RE, Jr, Mailliard RB, Barksdale EM., Jr Advanced neuroblastoma impairs dendritic cell function in adoptive immunotherapy. J Pediatr Surg. 2003;38:857–862. doi: 10.1016/S0022-3468(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 48.Walker SR, Redlinger RE, Jr, Barksdale EM., Jr Neuroblastoma-induced inhibition of dendritic cell IL-12 production via abrogation of CD40 expression. J Pediatr Surg. 2005;40:244–250. doi: 10.1016/j.jpedsurg.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 49.Shurin GV, Gerein V, Lotze MT, Barksdale EM., Jr Apoptosis induced in T cells by human neuroblastoma cells: role of Fas ligand. Nat Immun. 1998;16:263–274. doi: 10.1159/000069452. [DOI] [PubMed] [Google Scholar]
- 50.Garrido F, Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res. 2001;83:117–158. doi: 10.1016/S0065-230X(01)83005-0. [DOI] [PubMed] [Google Scholar]
- 51.Slavin S, Aker M, Shapira MY et al (2003) Reduced-intensity conditioning for the treatment of malignant and life-threatening non-malignant disorders. Clin Transpl 275–282 [PubMed]
- 52.Stein J, Dini G, Yaniv I, Pediatric Diseases Working Party of the EBMT The hope and the reality of reduced intensity transplants in children with malignant diseases. Bone Marrow Transplant. 2005;35(Suppl 1):S39–S43. doi: 10.1038/sj.bmt.1704845. [DOI] [PubMed] [Google Scholar]