Abstract
Dendritic cells (DCs) are promising antigen presenting cells for cancer treatment. Previously, we showed that the combination of monophosphoryl lipid A (MPLA) with IFNγ generates mature DCs that produce IL-12 and polarize CD4+ T cells towards a Th1 phenotype. Here, we extended these observations by showing that the DCs generated with the clinical grade maturation cocktail of MPLA/IFNγ induce superior tumour antigen-specific CD8+ CTL responses compared to the cytokine cocktail matured DCs that are currently used in the clinic. MPLA/IFNγ DCs can induce CTL responses in healthy individuals as well as in melanoma patients. The CTL induction was mainly dependent on the IL-12 produced by the MPLA/IFNγ DCs. The high amounts of induced CTLs are functional as they produce IFNγ and lyse target cells and this cytolytic activity is antigen specific and HLA restricted. Furthermore, the CTLs proved to kill tumour cells expressing endogenous tumour antigen in vitro. Therefore, MPLA/IFNγ DCs are very promising for the use in future cancer immunotherapy.
Keywords: Cytotoxic T lymphocyte; Dendritic cells; IL-12; Immunotherapy; Monophosphoryl lipid A, MART-1
Introduction
As professional antigen presenting cells, dendritic cells (DCs) play a major role in the generation of an effective immune response. Because of their central role in the induction of immunity, ex vivo generated monocyte-derived DCs have been applied as a cellular vaccine for the treatment of cancer [1–3]. Most patients with cancer do not or hardly mount an immune response to tumours cells. The aim of vaccinating the patient with tumour-specific DCs is to generate an anti-tumour-specific T cell response that will help to fight the cancer by tipping the immune balance in favour of effective anti-tumour immunity. In order to induce a good cytotoxic T response against the cancer, the functional characteristics of the monocyte-derived DC type used is of great importance.
The injected DCs should be capable to migrate to the lymphoid organs in order to activate the residing naïve T cells via TCR-peptide MHC interaction. Furthermore, the DCs should give proper co-stimulation to the T cells via the expression of costimulatory molecules and secreting inflammatory cytokines [4–6]. Of these, IL-12 is a hallmark cytokine that has a positive effect on the induction of cytotoxic CD8 responses [7–11], but moreover also induces Th1 polarization of the CD4 T cells [12]. These polarized CD4 T cells again are important helper cells for the induction of specific CTLs, which are the key effector cells in the acquired immune response against tumours.
The method used to generate the monocyte-derived DCs ex vivo will be crucial for their capacity to induce an anti-tumour response. Until recently the DCs used in the clinical trials were generated with a cytokine cocktail described by Jonuleit and co-workers [13]. These cytokine cocktail matured DCs (CC-DCs) migrate well due to their exposure to PGE2, but seem to be less optimal programmed to induce CTL responses, as they do not produce IL-12 and therefore tend to generate Th2 responses [14, 15]. Therefore, lately several studies have investigated ways to generate DCs that induce superior anti-tumour CTL responses using maturation cocktails which will be clinically applicable [5, 16–20]. From these studies it is apparent that the inclusion of a TLR ligand to the DC maturation cocktail is essential for the induction of IL-12 production.
One of the promising TLR ligands is monophosphoryl lipid A (MPLA), also called detoxified LPS. MPLA has been shown to exhibit potent adjuvant activity, while exhibiting essentially no toxicity. It has been safely used as an adjuvant in various vaccine trials in humans (as reviewed in [21]). MPLA is therefore nearing regulatory approval and likely to be the first vaccine adjuvant to be approved since alum. We have previously shown that the combination of MPLA with IFNγ generates DCs which are mature, still have an intermediate migratory capacity, produce IL-12 and polarize CD4 T cells towards a Th1 phenotype [22]. These characteristics make the combination of MPLA with IFNγ very promising as a clinical applicable maturation cocktail for monocyte-derived DCs for cancer immunotherapy. In this paper, we show that the MPLA/IFNγ-matured DCs indeed, as was expected from the previously generated data, induce superior CTL responses compared to the currently used CC-DCs.
Materials and methods
Reagents, media and cell lines
Cellgro DC serum-free medium and IL-4, GM-CSF, IL-1β, TNFα were all obtained from CellGenix (Freiburg, Germany). PGE2 and MPLA were obtained from Sigma (Steinheim, Germany). IFNγ (Immunkine) was obtained from Boehringer Ingelheim (Alkmaar, The Netherlands). IL-7 was obtained from R&Dsystems (Minneapolis, USA). Yssel’s medium was made by adding 1% human serum, penicillin (100 U/ml), streptomycin (100 mg/ml), linolic acid (2 μg/ml), oleic acid (2 μg/ml), ethanolamine (2 μg/ml), insuline (5 μg/ml) and transferrine (30 μg/ml) to Iscoves modified Dulbecco’s medium (IMDM, Bio Whittaker, Verviers, Belgium). All monoclonal antibodies (mAb) used for flow cytometry were obtained from Becton–Dickinson (BD, San Jose, USA). HLA-A2/MART-1 (ELAGIGILTV) tetramer was made as previously described [23]. HLA-A2 gp100-209 (IMDQVPFSV) and HLA-A2 gp100-280 (YLEPGPVTV) tetramers were kindly provided by Dr. John Haanen (Netherlands Cancer Institute, Amsterdam, The Netherlands). Flowcytometric analysis of tetramer binding was performed as previously described [24]. The cell lines Mel-AKR and Mel-JKO were described previously [25]. JY, T2, Mel-AKR, Mel-JKO and J558 cells were cultured in IMDM with 10% FCS.
DC generation and phenotyping
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh buffy coats or aphaereses [Sanquin Blood Bank North West (Amsterdam, The Netherlands)], obtained from healthy HLA-A2 positive volunteers upon informed consent, by separation over a Ficoll gradient. Monocytes were isolated from the PBMC fraction by positive selection using CD14 microbeads and a magnetic cell separator (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes were cultured at a concentration of 1 × 106 cells/ml in 20 ml Cellgro medium supplemented with GM-CSF (1,000 IU/ml) and IL-4 (800 IU/ml) in a 80 cm2 cell culture flask (Nunc, Roskilde Denmark). At day 6, the immature DCs were matured with the cytokine cocktail [IL-1β (10 ng/ml), TNFα (10 ng/ml) and PGE2 (1 μg/ml)] or with the combination of MPLA (2.5 μg/ml) and IFN-γ (1,000 U/ml). After 2 days of maturation the cells were harvested. The adherent DCs were harvested after a few minutes’ incubation with a 0.25% solution of trisodiumcitrate and washed.
For phenotyping, the DCs were washed with PBS containing 0.5% bovine serum albumin (PBA) and incubated with 50 μl mAb or appropriate isotype controls diluted in PBA with 3 mg/ml human gamma globulin for 30 min. Cells were washed twice and resuspended in PBA. DAPI was added to the cells before analysis to assess cell viability and exclude dead cells from analysis. Cells were analysed on an LSRII flow cytometer (BD) and analysed with FACS Diva software (BD).
Cytokine analysis
DCs were harvested, washed and cultured in 96-well flat bottom plates at a concentration of 1 × 104 per well in culture medium. To mimic the interaction with CD40L-expressing CD4+ cells, CD40L-transfected J558 cells (a kind gift from Dr. P. Lane, University of Birmingham, UK) were added at a concentration of 5 × 104 per well. After 24 h the supernatant was harvested. The production of IL-12p70, IL-23, IL-6 and IL-10 during maturation and upon CD40L restimulation was determined by ELISA. For the detection of IL-10 and IL-6, the PeliKine-compact ELISA kit was used (Sanquin Reagents). For the detection of IL-12p70, a combination of B-T21 mAb (Diaclone, Besançon, France) and C8.6 (BD) was used in an ELISA. For the detection of IL-23, a combination of a monoclonal IL-23p19 (eBioscience, San Diego, CA, USA) and C8.6 (BD) was used.
Real-time PCR
Cytokine cocktail matured DCs and MPLA/IFNγ DCs were harvested, washed and lysed in peqGOLD Trifast (PeQlab, Erlangen, Germany) 6 h after maturation. GlycoBlue (Ambion, Austin, TX) was added as a carrier and total RNA was extracted according to the manufacturer’s instructions. The isolated RNA was quantified and reverse transcribed to complementary DNA (cDNA) using random hexamers [pd(N)6, Amersham Biosciences, Piscataway, NJ, USA] and SuperScript II, RNase H-reverse transcriptase kit (Invitrogen, Breda, The Netherlands). Gene expression levels were measured in the Applied Biosystems StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) using Fast universal mastermix (Applied Biosystems). Primer/probe sets for the following genes were used: GUS (forward, GAAAATATGTGGTTGGAGAGCTCATT; reverse, CCGAGTGAAGATCCCCTTTTTA; probe, FAM-CCAGCACTCTCGTCGGTGACTGTTCA-TAMRA), CCL22 (forward, CCCTGGGTGAAGATGATTCT; reverse, AATGCAGAGAGTTGGCACAG; probe, FAM-ATGACCGTGGCCTTGGCTCC-TAMRA), CXCL10 (forward, AAGTGGCATTCAAGGAGTACC; reverse, TGATCTCAACACGTGGACAA; probe, FAM-CCGTACGCTGTACCTGCATCAGC-TAMRA). All results were normalized to the internal control GUS, and are expressed relative to the other DC type.
Induction of MART-1 peptide-specific CTLs
CD8+ T cells from the same HLA-A2+ donor or HLA-A2-matched melanoma patients were isolated from the PBMCs by positive selection using CD8 microbeads (Miltenyi). Pre-treatment blood samples were obtained after informed consent from patients with advanced melanoma participating in experimental immunotherapy trials at the VU University Medical Center, approved by the local medical ethical committee. DCs were loaded with 1 μg/ml MART-1 peptide (ELAGIGILTV) for 2–4 h at room temperature in Yssel’s medium in the presence of 3 μg/ml β2-microglobulin (Sigma) and washed twice. Multiple cultures containing 1 × 105 peptide-loaded mature DCs, 1 × 106 autologous CD8+ T cells and 1 × 106 autologous irradiated (40 Gy) CD4+ T cells were set up in 1.5 ml Yssel’s medium in a 24-well plate (Nunc). 10 days later the cultures were restimulated with 1 × 105 thawed DCs loaded with 10 ng/ml MART-1 peptide in the presence of 5 ng/ml IL-7. 2 days later, 10 U/ml IL-2 was added. From now on the cultures were restimulated weekly with peptide-loaded (10 ng/ml) irradiated JY cells (HLA-A2+) in the presence of 5 ng/ml IL-7. IL-2 (10 U/ml) was added 2 days after stimulation. In case blocking antibodies (10 μg/ml) were used, those were added together with the peptide-loaded DCs. The following neutralizing antibodies were used; anti-IL-12p40 (C8.6), anti-IL-12p35 (B-T21, Diaclone), anti-IL-23p19 (B-Z23, Diaclone), anti-IL-6 (IL6.8 [26]).
CTL analysis
Before restimulation the CTLs were analysed for specificity using APC-labelled HLA-A*0201 tetramers presenting the MART-1-derived epitope (ELAGIGILTV). Tetramer staining was performed in PBA for 20 min at room temperature. CD8+ T cells were stained with a FITC-labelled antibody (Sanquin). DAPI was added just before analysis to exclude dead cells. In some cases the tetramer positive cells were further analysed by staining them with a combination of CD45RA and CD27, CD28 or CCR7. For intracellular staining of granzyme B (GB11, Sanquin) or IFNγ in the CTLs we stained the CTLs first with TM and CD8 as described above. Subsequently the CTLs were fixed with 4% paraformaldehyde and stained with the appropriate antibody in saponin staining buffer consisting of 0.5% saponin and 1% BSA in PBS for 30 min at room temperature.
Cytotoxicity assay
To determine the cytotoxic activity of primed CD8+ T cells, MART-1 peptide-loaded (1 μg/ml) T2 cells, Her2 peptide (KIF)-loaded T2 cells, and MART-1 expressing melanoma cell lines Mel-AKR (HLA-A2+) and Mel-JKO (HLA-A2−) were labelled with 100 μCi of 51Cr for 1 h at 37°C and then used as targets. 51Cr-labelled targets (2 × 103) were subsequently incubated with varying numbers of CD8+ T cells for 4 h at 37°C in IMDM with 10% FCS. The percentage-specific lysis was calculated from the following equation: [(experimental release × spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous and maximum releases were determined in the presence of medium or 2% sodium dodecyl sulphate, respectively.
Statistics
Wilcoxon matched pair t test was used for comparing the cytokine levels produced by the two types of DCs and comparing the mean fluorescence intensity (MFI) of the DC markers. Unpaired t test was used for comparing percentage Tm positive cells between two groups, while a one-way ANOVA was used to compare the percentage Tm positive cells between more groups.
Results
MPLA/IFNγ DCs induce a superior CTL response
Previously we have shown that the clinical grade maturation cocktail consisting of MPLA/IFNγ induced DCs which were phenotypically mature, produced high levels of IL-12p70 and induced mainly Th1 cells in vitro [22]. These data suggest that MPLA/IFNγ DCs could well be superior inducers of CTL responses, and with it very good candidate for immunotherapy of cancer.
In order to show that MPLA/IFNγ DCs are superior in inducing CTL responses, we generated cytokine cocktail matured DCs (CC-DCs), based on the cytokine cocktail described by Jonuleit and co-workers [13] and MPLA/IFNγ DCs. In line with previous experiments, both DC types were phenotypically mature as shown by expression of CD83 and the co-stimulatory molecules CD40, CD80 and CD86 (Fig. 1a). The CD80 and CD40 expression levels were significantly higher for the MPLA/IFNγ DCs compared to the CC-DCs, which is in accordance with our previous publication [22]. The CD83 expression level was significantly lower for the MPLA/IFNγ DCs compared to the CC-DCs. The MPLA/IFNγ DCs produced high levels of the Th1 polarizing cytokine IL-12p70 during culture as well as upon restimulation by CD40L triggering, while no production was detected for the CC-DCs (Fig. 1b). Besides IL-12p70, the MPLA/IFNγ DCs produced also significantly higher levels of IL-23 and IL-6 during culture and upon CD40L restimulation compared to CC-DCs (Fig. 1b). The CC-DCs produced higher levels of IL-10 during culture, while upon CD40L restimulation the MPLA/IFNγ DCs produced more IL-10 (Fig. 1b). We analysed the expression of effector-cell (CXCL10) and Treg-attracting chemokines (CCL22) and found that the MPLA/IFNγ DCs expressed very low levels of CCL22 and very high levels of CXCL10 compared to CC-DCs (Fig. 1c) as is previously also found for other Th1 polarizing DCs [27]. These data indicate that the MPLA/IFNγ DCs in vivo will preferentially activate inflammatory T cells and will likely not induce unwanted activation of Tregs during immunotherapy.
Fig. 1.
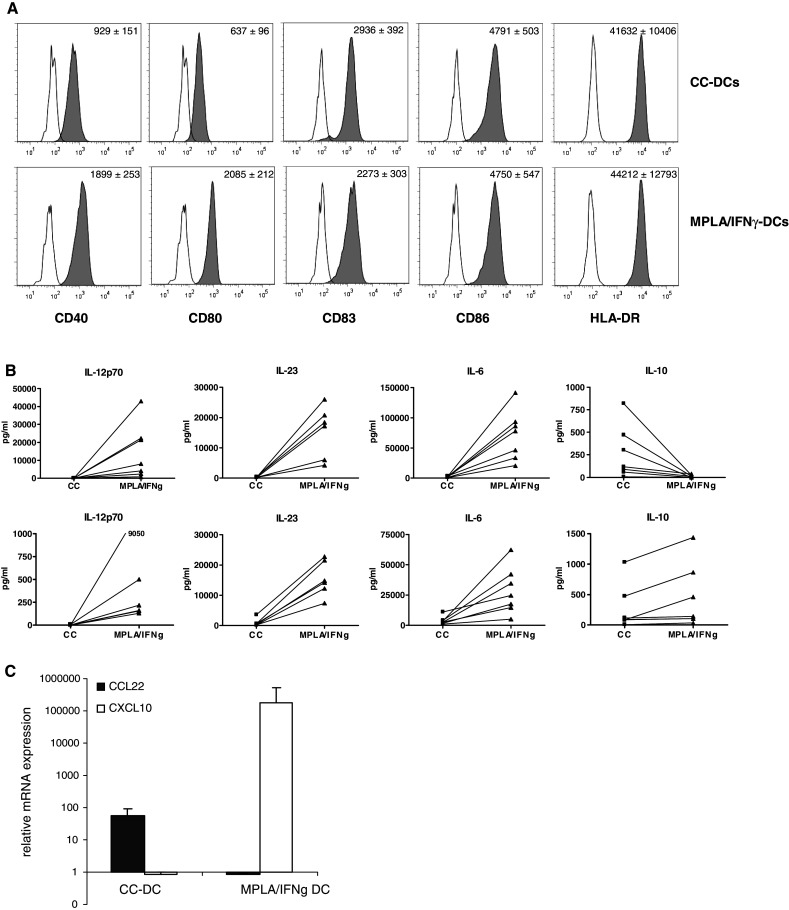
Phenotype, cytokine production, and chemokine expression of the matured monocyte-derived DCs. a DCs were harvested 2 days after maturation and stained for the different cell surface molecules with fluorescently conjugated mAbs and appropriate isotype controls. Histograms of the different markers are shown with the isotype matched controls as open graphs and the indicated marker Abs filled in grey. A representative experiment of eight experiments is shown. The mean fluorescent intensity ± SEM of all experiments in depicted in the upper right corner of the graphs. b IL-12p70, IL-23, IL-6 and IL-10 were determined in the culture supernatant 2 days after maturation. Harvested DCs were incubated with J558-cells expressing CD40L to determine the production of these cytokines upon CD40 restimulation. After 24 h the supernatant was harvested and cytokines were detected. The amount of cytokines produced by the two types of DCs from the same donor is connected by a line. A Wilcoxon matched pair t test was performed for statistical analysis; all cytokines produced were significantly different (P < 0.05) between the two DC types. c mRNA expression levels of CCL22 and CXCL10 were determined by real-time PCR 6 h after maturation. The expression of CCL22 in MPLA/IFNγ DCs was set to one. The expression of CXCL10 in CC-DCs was set to one. Relative mRNA expression (mean ± SEM) of four independent experiments is depicted
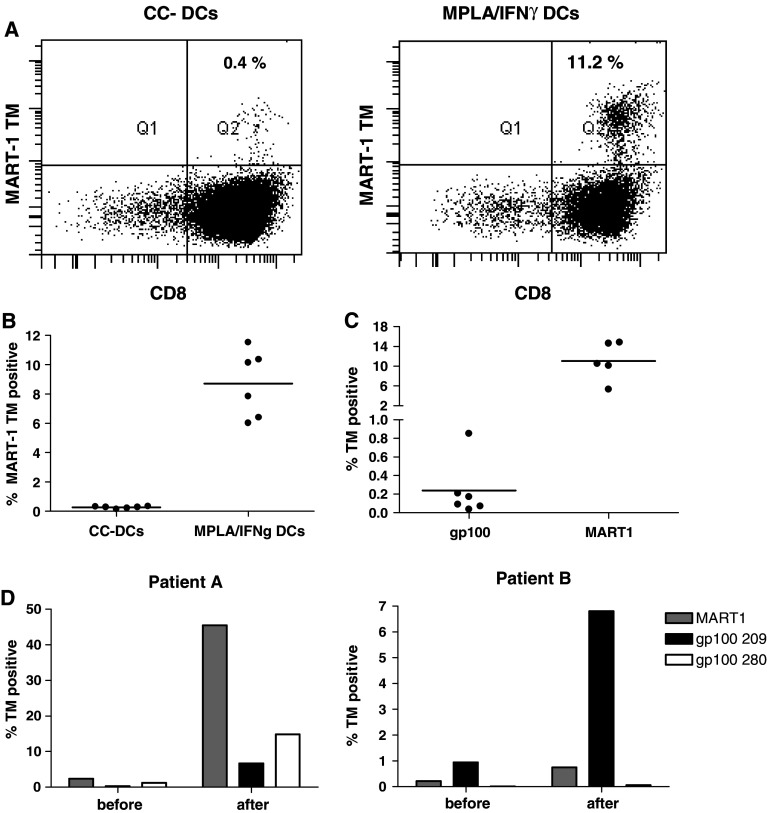
Two days after the start of the maturation the two types of DCs were harvested, loaded with MART-1 peptide and subsequently incubated with autologous CD8+ T cells. After 10 days, the CD8+ T cells were restimulated with the peptide-loaded DCs. 7 days after the second stimulation the CTLs were analysed for the expansion of MART-1 peptide-specific CTLs by the use of tetramer staining (Fig. 2a). While the CC-DCs, which currently are used for many DC trials, repeatedly induced only very low numbers of MART-1 peptide-specific CTLs, MPLA/IFNγ DCs induced consistently high percentages of MART-1 peptide-specific CTLs (Fig. 2b). From this we can conclude that MPLA/IFNγ DCs induce superior CTL responses compared to CC-DCs.
Fig. 2.
DCs matured with MPLA/IFNγ are inducing high numbers of melanoma-antigen-specific CTLs. a 7 days after the second stimulation of the CTLs with DCs (day 17), the CTLs were stained with the MART-1 tetramer (TM) and CD8-FITC. A representative graph of CTLs stimulated with CC-DCs and MPLA/IFNγ DCs is shown. b A graph showing the % MART-1 tetramer cells from six separate cultures from one donor. A representative experiment out of seven experiments with independent donors is depicted. An unpaired t test was performed and the two DC conditions significantly differed (P < 0.0001). c In one donor the CTLs were not only stimulated with MART-1 peptide-loaded MPLA/IFNγ DCs, but also with gp100-209 peptide-loaded DCs. Before culture and after the second stimulation the CTLs were stained with CD8-FITC and MART-1 or gp100 tetramer, respectively. A graph showing the % tetramer positive CTLs from the separate cultures after two stimulations are shown. Before culture the donor was 0.11% MART-1 TM positive, gp100 TM positive cells were undetectable. d CTLs of melanoma patients were stimulated once with HLA-A2-matched DCs from a healthy donor. The MPLA/IFNγ DCs were loaded with MART-1, gp100-209 or gp100-280 peptide, respectively. Before and 1 week after stimulation the cells were analysed with the respective tetramers, a graph is showing the % TM positive cells at both time points
Besides MART-1-specific CTL responses, we were also able to induce CTLs against a HLA-A2 restricted peptide of gp100, another melanoma-associated antigen, although in lower number than found for the MART-1 peptide (Fig. 2c). Furthermore, HLA-A2-matched MPLA/IFNγ DCs of a healthy donor were able to expand melanoma peptide-specific responses in CTLs of patients (Fig. 2d). After one stimulation of the patients CTLs with the peptide-loaded MPLA/IFNγ DCs, we observed the expansion of both MART-1 as well as gp100 peptide responses in patient A. In patient B, especially the response to peptide gp100-209 was observed, while the expansion of MART-1 peptide-specific CTLs was minor and no induction of CTLs specific for peptide gp100-280 was observed. These data show that MPLA/IFNγ DCs can induce CTL responses to multiple antigens in both healthy individuals and melanoma patients.
CTLs induced by MPLA/IFNγ DCs are GrB+, CD45RA−, CCR7−, CD27+, CD28+, and secrete IFNγ upon antigen-specific stimulation
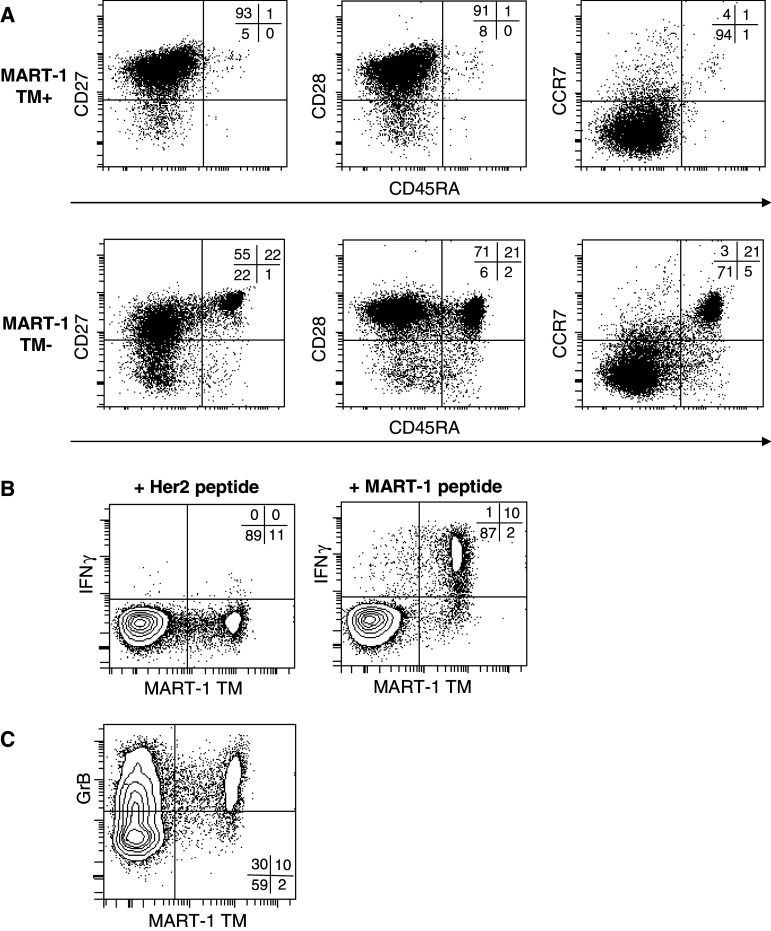
Characterization of the MART-1-specific CTLs induced by the MPLA/IFNγ DCs showed that these CTLs are mainly CD45RA−CCR7−CD27+CD28+, a subset by Romero et al. [28] defined as effector memory 1 (EM1) (Fig. 3a). In contrast, the tetramer negative CD8+ cells are, besides CD45RA−CCR7−CD27+CD28+ (EM1), mainly CD45RA+CCR7+CD27+CD28+ (naïve) and few CD45RA−CCR7−CD27−CD28− (EM3) (Fig. 3a). EM1 are described to be closely related to central memory cells and to have no lytic potential, e.g. because of low expression levels of granzyme B [28].
Fig. 3.
CTLs, induced by MPLA/IFNγ DCs, are CD45RA−, CCR7−, CD28+ CD27+ and GrB+ and secrete IFNγ upon antigen-specific stimulation. a The phenotype of the CTLs were analysed 7 days after the third stimulation by staining the CTLs for MART-1 TM and analysing the expression of CD27, CD28, CCR7 and CD45RA on the MART-1 TM positive and negative CTLs. b The CTLs of the same cultures were incubated with JY cells loaded with either Her2 peptide or MART-1 peptide in an E:T ratio of 2:1 for 5 h in the presence of BFA (10 μg/ml). Subsequently, the intracellular accumulation of IFNγ was determined by FACS. CD8+ cells are depicted. c CD8+ cells were also analysed for the presence of intracellular GrB in combination with MART-1 TM staining. A representative CTL culture out of multiple cultures of two independent experiments is shown
Therefore, we studied the functionality of the antigen-specific CTLs induced by MPLA/IFNγ DCs, by first examining the IFNγ response upon antigen stimulation. Contact with MART-1 peptide-loaded JY cells specifically upregulated IFNγ production in the MART-1-specific CTLs, while IFNγ was not induced in the tetramer negative CTL population or upon contact with JY cells loaded with a Her2-derived HLA-A2-specific peptide (Fig. 3b). We observed the same specific induction of IFNγ production for the peptide-specific CTLs of the patients described in Fig. 2d (data not shown). Thus, the MART-1-specific CTLs are antigen specific and functionally active. Furthermore, we determined that the MART-1-specific CTLs contain a high level of granzyme B (Fig. 3c), indicating that they also have a cytolytic potential, in contrast to what has previously been suggested for EM1 cells.
CTLs induced by MART-1-loaded MPLA/IFNγ DCs have a strong specific cytolytic activity towards a melanoma-specific tumour cell line
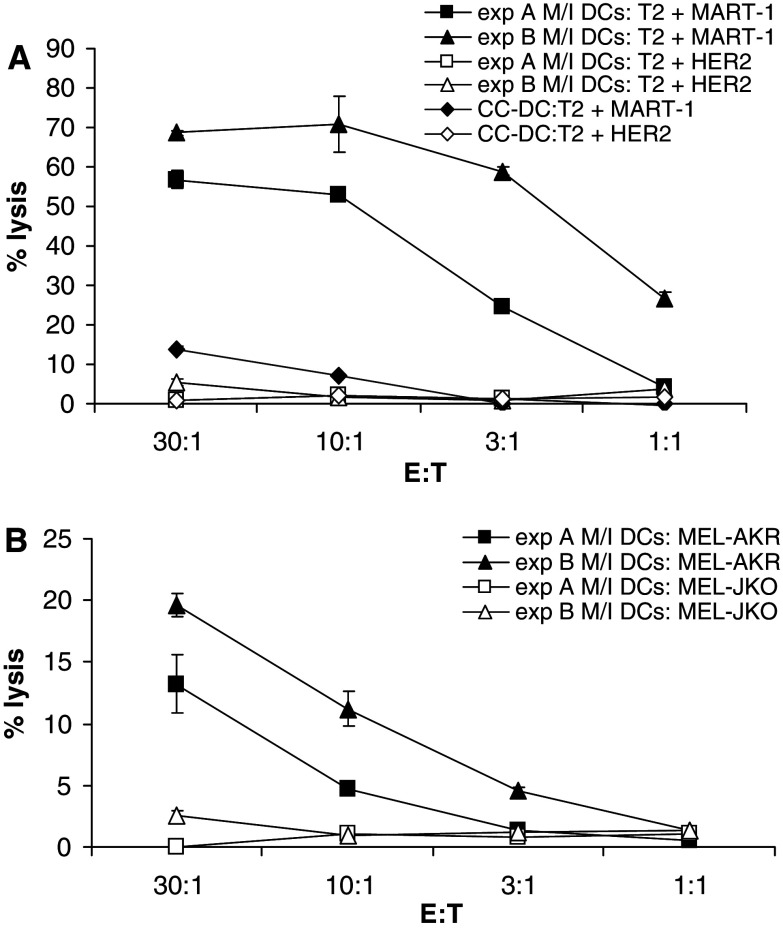
In order to investigate the cytotoxic functionality and the specificity of the MART-1-specific CTLs induced by the MPLA/IFNγ DCs in more detail, we studied the cytolytic activity towards T2 cells (HLA-A2+) which were either loaded with the MART-1 peptide or a Her2-derived HLA-A2-specific peptide. We tested various MPLA/IFNγ DC-induced CTL cultures which all displayed a different percentage of MART-1-specific CTLs as determined by tetramer staining. All cultures were able to specifically lyse the T2 cells loaded with MART-1 peptide, while the T2 cells loaded with the irrelevant Her2 peptide were not lysed (Fig. 4a). The CC-DCs induced CTL cultures induced only very low specific lysis at high E:T ratios, in line with the low percentage of MART-1-specific CTLs that were induced by the CC-DCs.
Fig. 4.
MART-1-specific CTLs, induced by MPLA/IFNγ DCs specifically and effectively lyse target cells. a 51Cr-loaded T2 cells either loaded with Her2 or MART-1 peptide were incubated in different ratio’s with CTLs in triplicate, 7 days after the third stimulation (day 24). Percentage of lysis by representative cultures from one experiment out of five experiments is shown. Mean ± SD of triplicates is depicted. MPLA/IFNγ DCs induced CTL culture A was 40% MART-1 TM positive and culture B was 20% MART-1 TM positive. CC-DC-induced CTL culture was 4.4% MART-1 TM positive. b The CTLs of the same cultures were also incubated with 51Cr-loaded MEL-AKR (MART-1+, HLA-A2+) and MEL-JKO (MART-1+, HLA-A2−) in different ratios. Percentage lysis is depicted as mean ± SD
As the MART-1 peptide concentration after the external loading of the T2 cells is very high, we were interested if the MPLA/IFNγ DC-induced CTL cultures would also have a specific cytolytic activity towards a cell line endogenously expressing MART-1, as it is likely to present lower concentrations of MART-1 peptide on its HLA-A2 molecules. In addition, this situation is a better reflection of the true anti-tumour potential of the CTLs. We compared the CTL-induced lysis of a MART-1 positive melanoma cell line that is HLA-A2+ (Mel-AKR) with one that is HLA-A2− (Mel-JKO). Only the Mel-AKR was lysed substantially, while the HLA-A2− melanoma line remained intact (Fig. 4b). These data show that the CTLs induced by MPLA/IFNγ DCs are effector cells with an effective antigen-specific and HLA-restricted cytolytic activity.
The superior induction of CTLs by MPLA/IFNγ DCs is dependent on IL-12, but not on IL-6 and IL-23
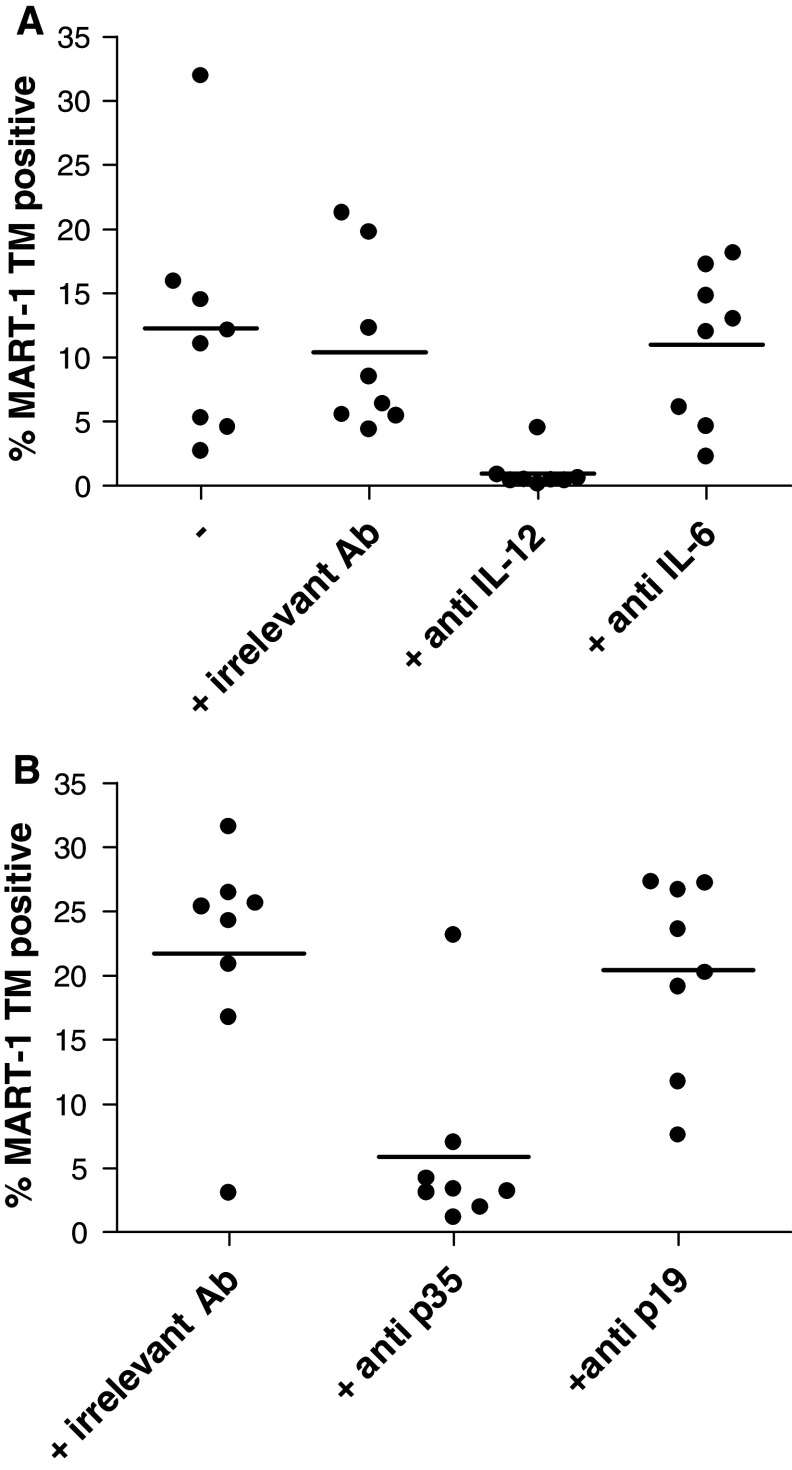
MPLA/IFNγ DCs produce higher amounts of IL-12, IL-23 as well as IL-6 compared to CC-DCs (Fig. 1b). For IL-12 and IL-6, a role in the induction of CD8+ CTLs has been described [9–11, 29–31]. To elucidate the role of both cytokines in the induction of CTLs by MPLA/IFNγ DCs, we added blocking antibodies specific for IL-12p40, and IL-6 at the initiation and restimulation stage of the co-culture of DCs and CD8+ T cells. In the presence of the blocking antibody directed against IL-6 no change in percentage of induced MART-1-specific CTLs was observed (Fig. 5a). Therefore, we can conclude that the IL-6 produced by the MPLA/IFNγ DCs does not play a dominant role in the induction of CTLs. In contrast, the addition of the blocking antibody directed against IL-12p40 induced a substantial and significant lower percentage of MART-1-specific CTLs (Fig. 5a). However, using this antibody we not only blocked the effect of IL-12, which is a heterodimer composed of a p35 and a p40 subunit, but also of IL-23, which is a heterodimeric cytokine consisting of the p19 and the p40 subunit. Therefore, to distinguish between an IL-12- and IL-23-mediated effect, the experiment was repeated with neutralizing antibodies specific for the p35 and p19 subunits (Fig. 5b). Specific neutralization of the p35 subunit resulted in an evident significant lower percentage of MART-1-specific CTLs compared to the conditions treated with the irrelevant control antibody. In contrast, the neutralization of the p19 subunit did not affect the percentage of MART-1-specific CTLs induced by MPLA/IFNγ DCs. This indicates that the IL-12 produced by MPLA/IFNγ DCs is the most important factor for the induction of the high percentage of specific tumour antigen-specific CD8+ CTLs.
Fig. 5.
The superior induction of CTLs by MPLA/IFNγ DCs compared to CC-DCs is dependent on IL-12 and not on IL-6 or IL-23. a Blocking antibodies specific for IL-12p40 and IL-6, and an irrelevant antibody were added to MPLA/IFNγ DC-CTL cocultures. 7 days after the second stimulation of the CTLs with DCs (day 17), the CTLs were stained with the MART-1 tetramer (TM) and CD8-FITC. A graph showing the % MART-1 TM cells for all separate cultures of one experiment is shown. The condition treated with anti-IL-12 significantly differed (one-way ANOVA, P < 0.05) from untreated or isotype control treated conditions. A representative experiment out of four is depicted. b Blocking antibodies specific for IL-12p35 and IL-23p19, and an irrelevant antibody were added to MPLA/IFNγ DC-CTL cocultures. 7 days after the third stimulation of the CTLs, the CTLs were stained with the MART-1 TM and CD8-FITC. A graph showing the % MART-1 TM cells for all separate cultures of one experiment is shown. The condition treated with anti-IL-12p35 significantly differed (one-way ANOVA, P < 0.01) from isotype control treated condition
Discussion
Previously we have shown that MPLA/IFNγ DCs have an intermediate migratory capacity, produce IL-12 and induce Th1 polarization. In this study, we extended these observations showing that DCs matured with clinical grade maturation cocktail of MPLA/IFNγ induce superior CD8+ CTL responses in vitro compared to CC-DCs. Furthermore, the CTLs induced by MPLA/IFNγ DCs have an antigen-specific and HLA-restricted cytolytic activity towards a tumour cell line endogenously expressing the antigen.
Phenotyping of the antigen-specific CTLs induced by MPLA/IFNγ DCs showed that these CTLs are granzyme B+, CD45RA−, CCR7−, CD27+ and CD28+. Classically, CD27+CD28+ effector memory cells (EM1) have a poor lytic capacity, produce high level of cytokines and are closely related to central memory cells (CD45RA−CCR7+) [28, 32], while CD27−CD28− effector memory (EM3) have a strong cytolytic activity. The CD45RA−CCR7−CD27+CD28+ CTLs induced by MPLA/IFNγ DCs in this study, however, do express high levels of granzyme B and furthermore we could demonstrate that these CTLs have an antigen-specific cytolytic activity even against cells endogenously expressing tumour antigen, showing that the CTLs induced by MPLA/IFNγ DCs do not fit the non-lytic potential suggested for EM1 [28]. In this respect, it is worth noting that DeBenedette and co-workers [30] have also described that their CTLs that were induced by CD40L-overexpressing DCs showed a similar phenotype to the ones we found and also exhibited a potent lytic potential. Recent clinical trials using adoptively transferred melanoma-specific effector memory CD27+CD28+CD8+ CTLs show that subjects receiving these cells were more likely to experience tumour regression than subjects receiving CD28−CD8+ cells [33], pointing to the importance of CD27 and CD28 expression on CTLs during tumour therapy.
DCs are crucial for the induction of primary antigen-specific CTLs. Studies in mice have shown that naïve T cells need a third signal, next to TCR triggering and co-stimulation to differentiate into functional effector cells. This third signal can be provided by IL-12 [34]. Also in human studies it has been shown that IL-12 is an important cytokine for the antigen-specific expansion of high avidity CD8+ CTLs [7–11]. Next to the important role of IL-12 in the induction of CTLs, IL-12-producing DCs can also activate NK effector cells [35], which may play an important role in the killing of tumour cells which have a loss of MHC class I antigens and are therefore not killed by tumour antigen-specific CTLs. As MPLA/IFNγ DCs produce significantly more IL-12 than CC-DCs, we investigated if the IL-12 produced by MPLA/IFNγ DCs is one of the major factors making the difference between the percentages of antigen-specific CTLs induced by the two types of DCs. By the use of a neutralizing antibody for IL-12p40, and later on also more specifically for IL-12p35, we showed that by blockade of IL-12 during the co-culture of DCs and CTLs the percentage of antigen-specific CTLs induced was dramatically lower. This indicates that IL-12 is indeed a very important factor in the induction of CTLs by MPLA/IFNγ DCs. IL-23 is also produced significantly more by MPLA/IFNγ DCs than by CC-DCs. By the use of a blocking antibody directed against IL-23p19, we showed that neutralization of IL-23 did not influence the percentage of specific CTLs. DeBenedette and co-workers [30] also found that IL-23, in contrast to IL-12, was not important for the induction of the CTLs. However, in mice models a potent effect for IL-23 in induction of long-lasting immunity has been described [36–38]. Furthermore, IL-6 has been described to improve the CTL induction by DCs [11] by reducing the TCR signalling threshold required to stimulate CD8+ T cells [31]. However, blockade of the IL-6 in our DC-CTL co-cultures did not reduce the amount of induction of tetramer positive CTLs.
The MPLA/IFNγ maturation cocktail has a high potential to be used for the maturation of DCs for cancer vaccination trials. Besides our promising in vitro results, MPLA is very well characterized, not toxic and used as an adjuvant in vaccines and therefore safe to be used as a maturation component for DC vaccination trials [21]. Furthermore, our DC culture with the MPLA/IFNγ maturation cocktail is already optimized for clinical use, e.g. we used GMP grade culture medium without use of serum.
Altogether, MPLA/IFNγ DCs induce high percentages of specific CTLs, in healthy individuals as well as in melanoma patients, with cytolytic activity to native antigens. This finding together with our previous results showing the Th1 polarizing capacity and intermediate migratory capacity of MPLA/IFNγ DCs [22] make MPLA/IFNγ DCs functionally superior to DCs matured with the cytokine cocktail described by Jonuleit and co-workers [13] in in vitro experimental setups. Therefore, MPLA/IFNγ DCs are very promising for the use in cancer immunotherapy.
Acknowledgments
We want to thank Miranda Dieker for performing the realtime PCR analysis.
References
- 1.Lesterhuis WJ, Aarntzen EH, de Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, Punt CJ. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–134. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 3.Palucka AK, Ueno H, Fay J, Banchereau J. Dendritic cells: a critical player in cancer therapy? J Immunother. 2008;31:793–805. doi: 10.1097/CJI.0b013e31818403bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alder J, Hahn-Zoric M, Andersson BA, Karlsson-Parra A. Interferon-gamma dose-dependently inhibits prostaglandin E(2)-mediated dendritic-cell-migration towards secondary lymphoid organ chemokines. Vaccine. 2006;24:7087–7094. doi: 10.1016/j.vaccine.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 6.Vujanovic L, Ranieri E, Gambotto A, Olson WC, Kirkwood JM, Storkus WJ. IL-12p70 and IL-18 gene-modified dendritic cells loaded with tumor antigen-derived peptides or recombinant protein effectively stimulate specific Type-1 CD4+ T-cell responses from normal donors and melanoma patients in vitro. Cancer Gene Ther. 2006;13:798–805. doi: 10.1038/sj.cgt.7700964. [DOI] [PubMed] [Google Scholar]
- 7.Bontkes HJ, Kramer D, Ruizendaal JJ, Kueter EW, Van Tendeloo VF, Meijer CJ, Hooijberg E. Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic T cells. Gene Ther. 2007;14:366–375. doi: 10.1038/sj.gt.3302874. [DOI] [PubMed] [Google Scholar]
- 8.Calderhead DM, DeBenedette MA, Ketteringham H, Gamble AH, Horvatinovich JM, Tcherepanova IY, Nicolette CA, Healey DG. Cytokine maturation followed by CD40L mRNA electroporation results in a clinically relevant dendritic cell product capable of inducing a potent proinflammatory CTL response. J Immunother. 2008;31:731–741. doi: 10.1097/CJI.0b013e318183db02. [DOI] [PubMed] [Google Scholar]
- 9.Bontkes HJ, Ruizendaal JJ, Kramer D, Meijer CJ, Schreurs MW, Hooijberg E. Interleukin-12 increases proliferation and interferon-gamma production but not cytolytic activity of human antigen-specific effector memory cytotoxic T lymphocytes: power of the effect depends on the functional avidity of the T cell and the antigen concentration. Hum Immunol. 2005;66:1137–1145. doi: 10.1016/j.humimm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Felzmann T, Huttner KG, Breuer SK, Wimmer D, Ressmann G, Wagner D, Paul P, Lehner M, Heitger A, Holter W. Semi-mature IL-12 secreting dendritic cells present exogenous antigen to trigger cytolytic immune responses. Cancer Immunol Immunother. 2005;54:769–780. doi: 10.1007/s00262-004-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajewski TF, Renauld JC, Van Pel A, Boon T. Costimulation with B7-1, IL-6, and IL-12 is sufficient for primary generation of murine antitumor cytolytic T lymphocytes in vitro. J Immunol. 1995;154:5637–5648. [PubMed] [Google Scholar]
- 12.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 14.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.V97.11.3466. [DOI] [PubMed] [Google Scholar]
- 15.Kubo S, Takahashi HK, Takei M, Iwagaki H, Yoshino T, Tanaka N, Mori S, Nishibori M. E-prostanoid (EP)2/EP4 receptor-dependent maturation of human monocyte-derived dendritic cells and induction of helper T2 polarization. J Pharmacol Exp Ther. 2004;309:1213–1220. doi: 10.1124/jpet.103.062646. [DOI] [PubMed] [Google Scholar]
- 16.Sakakibara M, Kanto T, Inoue M, Kaimori A, Yakushijin T, Miyatake H, Itose I, Miyazaki M, Kuzushita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Quick generation of fully mature dendritic cells from monocytes with OK432, low-dose prostanoid, and interferon-alpha as potent immune enhancers. J Immunother. 2006;29:67–77. doi: 10.1097/01.cji.0000183093.77687.46. [DOI] [PubMed] [Google Scholar]
- 17.Boccaccio C, Jacod S, Kaiser A, Boyer A, Abastado JP, Nardin A. Identification of a clinical-grade maturation factor for dendritic cells. J Immunother. 2002;25:88–96. doi: 10.1097/00002371-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Peng JC, Thomas R, Nielsen LK. Generation and maturation of dendritic cells for clinical application under serum-free conditions. J Immunother. 2005;28:599–609. doi: 10.1097/01.cji.0000175491.21099.04. [DOI] [PubMed] [Google Scholar]
- 19.Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez-Ribas D, Schreibelt G, van de Rakt MW, Scharenborg NM, de Boer A, Kramer M, Figdor CG, Punt CJ, Adema GJ, de Vries IJ. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immunother. 2008;57:1589–1597. doi: 10.1007/s00262-008-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C, Mason M, Adams M. A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine. 2009;27:107–115. doi: 10.1016/j.vaccine.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25:7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Kostense S, Ogg GS, Manting EH, Gillespie G, Joling J, Vandenberghe K, Veenhof EZ, van Baarle D, Jurriaans S, Klein MR, Miedema F. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::AID-IMMU677>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, Haanen JB, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 25.Verra NC, Jorritsma A, Weijer K, Ruizendaal JJ, Voordouw A, Weder P, Hooijberg E, Schumacher TN, Haanen JB, Spits H, Luiten RM. Human telomerase reverse transcriptase-transduced human cytotoxic T cells suppress the growth of human melanoma in immunodeficient mice. Cancer Res. 2004;64:2153–2161. doi: 10.1158/0008-5472.CAN-03-1339. [DOI] [PubMed] [Google Scholar]
- 26.Brakenhoff JP, Hart M, De Groot ER, Di Padova F, Aarden LA. Structure-function analysis of human IL-6. Epitope mapping of neutralizing monoclonal antibodies with amino- and carboxyl-terminal deletion mutants. J Immunol. 1990;145:561–568. [PubMed] [Google Scholar]
- 27.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 29.Latz E, Visintin A, Lien E, Fitzgerald KA, Espevik T, Golenbock DT. The LPS receptor generates inflammatory signals from the cell surface. J Endotoxin Res. 2003;9:375–380. doi: 10.1179/096805103225003303. [DOI] [PubMed] [Google Scholar]
- 30.DeBenedette MA, Calderhead DM, Ketteringham H, Gamble AH, Horvatinovich JM, Tcherepanova IY, Nicolette CA, Healey DG. Priming of a novel subset of CD28+ rapidly expanding high-avidity effector memory CTL by post maturation electroporation-CD40L dendritic cells is IL-12 dependent. J Immunol. 2008;181:5296–5305. doi: 10.4049/jimmunol.181.8.5296. [DOI] [PubMed] [Google Scholar]
- 31.Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J Immunol. 2008;180:7958–7968. [Google Scholar]
- 32.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538–5550. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 33.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 35.Bontkes HJ, Kramer D, Ruizendaal JJ, Meijer CJ, Hooijberg E. Tumor associated antigen and interleukin-12 mRNA transfected dendritic cells enhance effector function of natural killer cells and antigen specific T-cells. Clin Immunol. 2008;127:375–384. doi: 10.1016/j.clim.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Matsui M, Moriya O, Belladonna ML, Kamiya S, Lemonnier FA, Yoshimoto T, Akatsuka T. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J Virol. 2004;78:9093–9104. doi: 10.1128/JVI.78.17.9093-9104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, Yuan X, Belladonna ML, Ong JM, Wachsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 38.Ha SJ, Kim DJ, Baek KH, Yun YD, Sung YC. IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization. J Immunol. 2004;172:525–531. doi: 10.4049/jimmunol.172.1.525. [DOI] [PubMed] [Google Scholar]