Abstract
We are investigating the use of Alpha Fetoprotein (AFP) as a tumor rejection antigen for hepatocellular carcinoma (HCC). We recently completed vaccination of 10 AFP+/HLA-A2.1+ HCC subjects with AFP peptide-pulsed autologous dendritic cells (DC). There were increased frequencies of circulating AFP-specific T cells and of IFNγ-producing AFP-specific T cells after vaccination. In order to better understand the lack of association between immune response and clinical response, we have examined additional aspects of the AFP immune response in patients. Here, we have characterized the cell surface phenotype of circulating AFP tetramer-positive CD8 T cells and assessed AFP-specific CD4 function. Before vaccination, HCC subjects had increased frequencies of circulating AFP-specific CD8 T cells with a range of naïve, effector, central and effector memory phenotypes. Several patients had up-regulated activation markers. A subset of patients was assessed for phenotypic changes pre- and post-vaccination, and evidence for complete differentiation to effector or memory phenotype was lacking. CD8 phenotypic and cytokine responses did not correlate with level of patient serum AFP antigen (between 74 and 463,040 ng/ml). Assessment of CD4+ T cell responses by ELISPOT and multi-cytokine assay did not identify any spontaneous CD4 T cell responses to this secreted protein. These data indicate that there is an expanded pool of partially differentiated AFP-specific CD8 T cells in many of these HCC subjects, but that these cells are largely non-functional, and that a detectable CD4 T cell response to this secreted oncofetal antigen is lacking.
Keywords: Alpha fetoprotein, Immunotherapy, T lymphocytes, Immunological monitoring, Cancer vaccine
Introduction
Hepatocellular carcinoma (HCC) is one of the main causes of cancer deaths with a global incidence of over 600,000 new cases per year. Once diagnosed, HCC has no effective systemic therapy and an overall 8% probability of 5 year survival. We are investigating the use of Alpha Fetoprotein (AFP) as a tumor rejection antigen for HCC. We previously vaccinated six AFP+/HLA-A2.1+ subjects with 4 immunodominant AFP-derived MHC class I-restricted peptides emulsified in Montanide and found that the frequency of circulating AFP-specific T cells increased (by MHC Tetramer assay) and the frequency of interferon-gamma (IFNγ)-producing AFP-specific T cells also increased after vaccination (by ELISPOT assay) [1].
We recently completed treatment of 10 AFP+/HLA-A2.1+ HCC subjects with AFP peptide-pulsed autologous dendritic cells (DC) [2]. As in the pilot peptide/Montanide trial, we have seen increased frequencies of circulating AFP-specific T (AFP T) cells and of IFNγ-producing AFP-specific T cells after vaccination. This vaccine did not result in objective clinical responses in that group of ten advanced stage patients. To better understand the biology of the pre-existing immunity to this antigen and the changes induced with vaccination, we now report further study of the peripheral blood cells of these subjects, including several who were enrolled but not fully treated.
AFP is an oncofetal antigen and is the most abundant serum protein in the fetus (with serum levels at 1–3 mg/ml). At birth, levels drop to 30–100 μg/ml and the adult level of AFP is 1–3 ng/ml [3]. These levels of soluble antigen present during development could have broad impact on the T cell repertoire, including deletion of high affinity T cells and maturation of T cells after priming in vivo. The ability to detect AFP T cells ex vivo [1, 4–7] argues against deletion. We have also tested AFP T cell avidity [8] and found that some AFP T cells are of sufficiently high avidity to recognize very low levels of peptide as well as AFP+ tumor cells, indicating that not all T cells remaining are of low avidity. There have been no studies investigating whether these T cells are naïve or differentiated effector or memory cells, what effects tumor-derived AFP has on these cells and whether vaccination efforts lead to full differentiation to effector cells capable of tumor recognition and/or long lived memory cells.
Many groups have identified cell surface markers associated with the function of T cells. These include markers associated with the naïve, effector or memory status (CD45RA, CD27, CD28, CCR7); markers associated with trafficking to central (CD62L, CCR7) or peripheral sites (CCR5, CCR6), with activation state (HLA-DR, CD25, CD69) and with stages of apoptosis (CD95, Annexin-V) [9–11]. We have investigated these markers on the surface of AFP peptide tetramer-positive CD8 T cells. Several studies have investigated the phenotype of melanoma antigen-specific cells, especially those specific to MART-1, which are often of higher frequency [12–14]. Phenotypic differentiation to activated memory cells after peptide-based vaccines has been observed [15, 16]. Recent phenotypic studies of antigen-specific T cells have uncovered potentially important associations between presence of these markers and functional status of cells including the finding that head and neck cancer patients have a preponderance of pre-apoptotic (Annexin-V+/CD95+) [17] CD8+CCR7- T cells [18] which rapidly turn over in the circulation. We have also extended our earlier study of the cytokine production of these CD8 and CD4 T cells upon recognition of AFP peptides and protein beyond IFNγ.
Here, we have characterized the cell surface phenotype of the circulating AFP tetramer-positive CD8 T cells to determine their expression of several markers. We wished to determine whether HCC subjects with high circulating levels of this oncofetal antigen have effector or memory CD8 T cells pre-vaccination, which would be indicative of in vivo priming. We also test whether these T cells are activated, or predisposed to apoptosis and have investigated the potential Type 1/Type 2 skewing of cytokine production. Central to the analysis of the CD8 T cell function is the question of CD4 T cell function. For the first time, we are testing CD4 T cells from these subjects before and after vaccination for a functional response to AFP. Lastly, in a subset of patients who were immunized in a peptide-pulsed DC trial, we investigated vaccine-induced phenotypic and functional changes. We find that there is an expanded pool of naïve and central memory AFP-specific CD8 T cells in many of these HCC subjects which co-express central and peripheral trafficking markers, indicating only partial differentiation. Peptide-based vaccination led to modest up-regulation of activation markers, but most subjects showed no clear shifts to fully differentiated effector or memory cells.
Materials and methods
Clinical trial design
Sixteen patients were enrolled and ten were fully treated in a phase I/II, dose-escalation, single site study to evaluate the safety and immunological effects of AFP peptide-pulsed autologous DC in HLA-A*0201 subjects with AFP expressing HCC which was recently reported [2]. Briefly, increasing doses of AFP peptide-pulsed DC (1 × 106, 5 × 106, 1 × 107) were given to groups of 3–4 patients intradermally (i.d.). Patients received three vaccinations every other week. All patients were required to express the HLA-A*0201 allele, have an AFP-producing HCC and demonstrate immune competence by a positive skin delayed hypersensitivity test to at least one recall antigen (candida, tetanus toxoid or mumps). All subjects provided signed informed consent. This trial underwent review and approval by the Institutional Review Board (IRB #00-01-026) and the Internal Scientific Peer Review Committee at UCLA, and the Food and Drug Administration (BB IND #9395).
Patient characteristics
The characteristics of each patient in the present study are shown in Table 1. A3, A4 and B10 were enrolled but not vaccinated (and were not analyzed previously) and B2 and B5 (2) did not have sufficient cells for the additional studies presented here. Patients were stage III (1), IVa (6) or IVb (4), and 9/11 were heavily pretreated, and of an average age of 57 (range 36–69). Pretreatment serum AFP averaged 44,866 ng/ml (range 74–463,040). Risk factors for HCC were HCV (6), HBV (1), alcohol (1), porphyria (1) or unknown (2). There were 10 males and 1 female. All had liver disease, three (A1, B6 and B8) also had extra-hepatic metastases.
Table 1.
Patient characteristics
| Patienta | DC doseb | Agec | Sexd | Racee | Risk factorf | Stageg | Previous treatmentsh | Sites of diseaseI | Pre-AFPj | Post-AFPk | Responsel | OSm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 1 × 106 | 36 | F | Caucasian | Unknown | IVb | Chemoembo, CDDP, adriamycin, 5-FU, xeloda, thalidomide | Liver, bone, lung | 2,811 | 2,748 (d28) | Progr | 4 |
| A2 | 1 × 106 | 66 | M | Asian | HBV | IVa | Chemoembo | Liver | 4,740 | 7053 (d35) | Progr | 20 |
| A3 | 1 × 106 | 69 | M | Caucasian | Alcohol | IVa | RFA | Liver | 3,080 | (No DC for vaccines) | – | 2 |
| A4 | 1 × 106 | 62 | M | Asian | HCV | IVb | None | Liver | 10,800 | (1 vaccine) early progr | – | 1 |
| B3 | 5 × 106 | 55 | M | Caucasian | HCV | IVa | ChemoemboRFA | Liver | 102 | 61 (d35) | NE | 35 |
| B6 | 5 × 106 | 53 | M | Hispanic | HCV | IVb | XRT | LiverBone | 712 | 5980.1 (d120) | Progr | 10 |
| B8 | 5 × 106 | 60 | M | Caucasian | HCV | IVb | Chemoembo | LiverNodes | 108 | 13 (d120) | Progr | 6 |
| B9 | 1 × 107 | 55 | M | Caucasian | HCV | IVa | None | Liver | 3,719 | 3909 (V3) | Progr | 2 |
| B10 | 1 × 107 | 59 | M | Caucasian | HCV | IVa | RFA | Liver | 463,040 | (no vaccines, early progr) | − | 0 |
| B11 | 1 × 107 | 60 | M | Caucasian | Porphyria | IVa | Chemoembo | Liver | 4,340 | 7,080(d56) | Prog | 3 |
| B12 | 1 × 107 | 52 | M | Caucasian | Unknown | III | Chemoembo RFA | Liver | 74 | 2,170(d120) | Progr | 9 |
aPatient designation
bNumber of DC per injection
cPatient age
dPatient gender
ePatient race
fRisk factor for HCC
gStage of disease
hPrevious treatments received (chemoembo, chemoembolization; CDDP, cis-platin; 5-FU, 5-flouro-uracil; RFA, radio-frequency ablation; XRT, radiation therapy)
iSites of HCC at enrollment
jBaseline serum AFP level on day of first vaccine, ng/ml
kLast tested serum AFP level (day listed), ng/ml
lClinical response, Progr progression, NE no evidence of disease, – vaccine response not evaluable
mOverall survival duration in months. At final follow up (June 2005), all patients were deceased due to disease progression
Peptides and protein
The AFP-derived peptides used in the vaccines, hAFP137–145 (PLFQVPEPV), hAFP158–166 (FMNKFIYEI), hAFP325–334 (GLSPNLNRFL) and hAFP542–550 (GVALQTMKQ), were synthesized at the UCLA Peptide Synthesis Facility (Dr. Joseph Reeve, Jr., Director). Research peptides used in the in vitro stimulations were prepared by the University of Pittsburgh Peptide Synthesis Facility. Cord blood derived purified AFP protein was obtained from CalBiochem (EMD Biosciences, San Diego, CA).
Peripheral blood mononuclear cells (PBMC)
PBMC were obtained from a leukapheresis at baseline or from peripheral blood obtained at later time points. PBMC were isolated from the leukapheresis product or blood by Ficoll-hypaque gradient separation and cryopreserved in aliquots.
Tetramer analysis
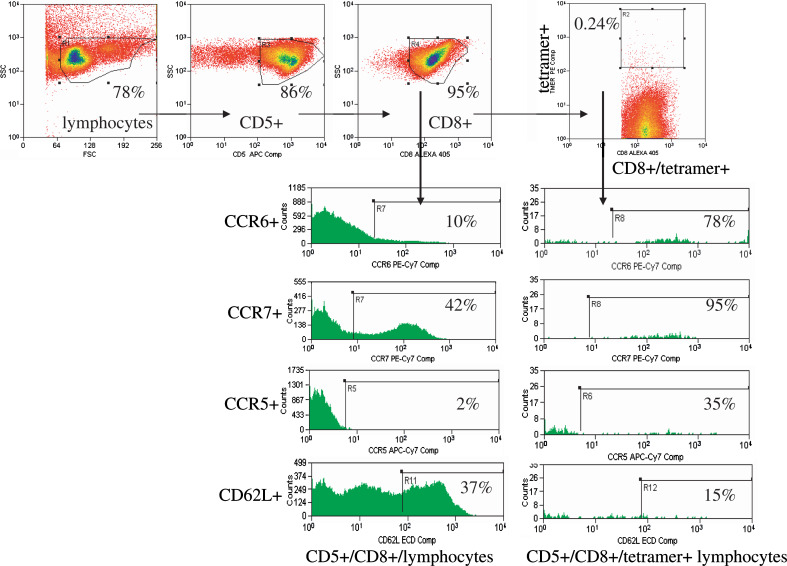
For the flow cytometric analysis, the gating strategy is shown in Fig. 1. Studies were performed in the UPCI Flow Cytometry Facility on a DakoCytomation CyAN 9-Color High Speed Analyzer using the Dako Summit analysis program, with compensation performed by facility staff (Dr. A. D. Donnenberg, Director). Unless otherwise noted, all antibodies were obtained from Coulter (Beckman Coulter, San Diego, CA) and were directly conjugated. In order to analyze a large number of cells quickly and with high purity, PBMC were first DNAse treated, then adherent cells were removed and CD8+ cells were magnetic bead-purified before being stained. To determine the phenotype of tetramer+ cells, CD5+ and CD8+ lymphocytes were selectively gated on. The phenotype of the tetramer+ cells was compared with that of the total CD8 population to identify tetramer+ cell-specific phenotypes. Compensation controls included PBMC stained with individual antibodies to CD8 conjugated to each flourochrome used and flow beads stained with the same panel of antibodies used to test patient cells. The range of lymphocytes events acquired was 19,951–236,009 (average 64,407). The range of CD8+ events acquired was 8,927–195,198 (average 36,841). Three healthy donors were also studied and used for comparisons with HCC patients.
Fig. 1.
Diagram of the gating strategy used for multi-parameter flow cytometric analysis. Patient cells were thawed, treated with DNAse, and the adherent cells were removed. The CD8+ cells were purified and stained with MHC tetramers and additional antibodies. For analysis, lymphocytes are gated on by FSC/SSC, then CD5+ and CD8+ cells are selected. The additional markers were compared for % positivity and MFI (mean fluorescence intensity) for both total CD8+ and CD8+/tetramer+ cells. The example is for patient B10, whose AFP137 specific T cells are shown for central (CCR7, CD62L) and peripheral (CCR5, CCR6) trafficking markers
Tetramers were obtained from Immunomics (Beckman Coulter). AFP542 would not fold properly into the A2.1 tetramer, nor would an anchor-substituted version [19]. 106 PBMC (or 3 × 105 CD8+ purified cells, Miltenyi Biotec, Auburn, CA) were stained with each individual tetramer plus CD8 (Caltag, Burlingame, CA) and additional antibodies to obtain phenotyping data from tetramer-stained cells. Staining was performed at room temperature (RT) for 30 min in the dark. The cells were then washed and analyzed immediately. The lymphocytes were gated on by forward and side scatter, CD8 positive cells were gated on, and the AFP peptide-specific cells were a distinct population of CD8+/tetramer+ cells. This MHC tetramer gating strategy has been used for sorting AFP-specific cells in other studies and results in a highly enriched, AFP-specific, functional T cell population (unpublished, L. Butterfield, 2005). When sufficient cells were available, MART-127–35 tetramers or negative tetramers (Coulter/Immunomics) were used as negative controls for comparison. When sufficient cells remained after the initial analysis (consistently for pre-vaccine leukapheresis, rarely for post-vaccine time points), tetramer assays were repeated.
Cytokine ELISPOT
The ELISPOT technique was used as previously described [5, 20, 21]. PBMC were thawed as above, then T cell restimulation was performed overnight with 2 × 105 CD8+ cells and 1 × 105 K562/A2.1 cells pulsed with AFP peptides, no peptide or control peptide (MART-127–35). K562/A2.1 cells without CTL also served as a negative control. CD4 ELISPOT responses were detected with AFP protein-pulsed DC, in which autologous DC (prepared by 7 day culture of adherent cells in 800 U/ml GM-CSF + 500 U/ml IL-4) were pulsed for 1 h at 37°C with 10 μg/ml AFP protein before plating with CD4+ T cells. The IFNγ, IL-5, IL-2, IL-10 or TNFα antibody (Pharmingen, San Diego, CA) coated plates (Millipore, Bedford, MA) were incubated with restimulated T cells (in duplicate at three dilutions) at 37°C. The colored spots, representing cytokine producing cells, were counted under a dissecting microscope and counts confirmed in an automated ELISPOT counter (Zeiss or CTL technologies). Background spots to the restimulator cells without peptide were subtracted.
Luminex
Cell free supernatants from ELISPOT assays (after 24 h incubations) were stored at −80°C until assayed for GM-CSF, IFNγ, IL-2, IL-4, IL-5, IL-10 and TNFα by Luminex bead array (Biosource) at the UPCI Luminex Core Facility (Dr. Anna Lokshin, Director).
Statistical analysis
Spearman’s test of correlation was used to test for any statistically significant correlation between the percentages of the different T cell phenotypes and ELISPOT frequency results and serum AFP level. No significant (P < 0.05) correlations were detected.
Results
From the immunological monitoring of AFP-specific CD8 T cell responses in two peptide-based vaccines trials, we found that most subjects were successfully immunized (by AFP-specific tetramer frequency increases and/or AFP-specific IFNγ ELISPOT frequency increases) [2]. In order to learn more about the biology of these cells and to better understand the state of immunity in HCC patients with variable levels of circulating serum AFP antigen, we focused on AFP137, AFP158 and AFP325 peptide-specific CD8+ T cell populations and used additional antibodies to determine the expression patterns of other cell surface proteins associated with functional status. We first studied the phenotype of pre-vaccine cells.
Tetramer frequencies and phenotype
There was a broad range of AFP peptide-specific CD8 T cell frequencies determined pre-vaccination, as shown in Table 2. Healthy donors had AFP T cells near the level of detection (0.01–0.06% CD5+/CD8+ lymphocytes, average 0.02%), and the HCC subjects generally had higher frequencies of these cells (average 0.08%). Some were not elevated (B8), but others were quite high (B10 0.12–0.24%, B12 0.07–0.16%). This may indicate that the subjects with higher levels of AFP-specific T cells have been primed by the circulating tumor-derived antigen to promote CD8 T cell proliferation. However, the level of expansion was not directly related to serum AFP level; lower tetramer frequency patients B8 and B9 had 108 and 3,719 ng/ml serum AFP, respectively, while higher frequency patients B10 and B12 had 463,040 and 74 ng/ml, respectively, indicating a lack of simple linear correlation.
Table 2.
Baseline AFP MHC class I tetramer frequencies
| Subject | AFP 137 (%) | AFP 158 (%) | AFP 325 (%) |
|---|---|---|---|
| A1 | 0.20 | 0.01 | 0.11 |
| A2 | 0.07 | 0.05 | 0.09 |
| A3 | 0.07 | 0.10 | 0.16 |
| A4 | 0.03 | 0.12 | 0.13 |
| B3 | 0.10 | 0.05 | 0.04 |
| B6 | 0.04 | 0.00 | 0.04 |
| B8 | 0.02 | 0.01 | 0.02 |
| B9 | 0.08 | 0.02 | 0.06 |
| B10 | 0.24 | 0.12 | 0.17 |
| B11 | 0.05 | 0.08 | 0.04 |
| B12 | 0.16 | 0.07 | 0.14 |
| Donor AFP | 0.03 | 0.04 | 0.03 |
| Donor MART1 | 0.04 | ||
| Donor Flu | 0.55 |
We next examined the naïve/effector/memory phenotype of these cells by the criterion of CD45RA/CCR7 expression (proposed by Sallusto et al. [10, 11]) (Fig. 2a). These data show that in 4 patients (B3, B8, B11, B12) the majority AFP-specific cells has a naïve (CD45RA+/CCR7+) phenotype in the peripheral blood. In 5 other subjects (A1, A3, B6, B9, B10) central memory (CM) phenotype predominates, and in A2 and A4 the phenotypes are mixed. There was little evidence for AFP-specific effector cells in the blood (A2 AFP137, B6 AFP158 and B8 AFP325 were the only notable examples). The CD45RA/CCR7 expression of these cells did not correlate with any of the patient characteristics examined (Table 1). The low frequency of AFP T cells detected in healthy donor (HD) peripheral blood was similarly divided between naïve and CM. However, due to the few events detected, HD AFP T cells were not analyzed further. As expected, the HD Flu-specific cells were of higher frequency (0.25–0.85%) and mostly effector/memory (EM) phenotype while HD MART-1 (melanocyte lineage antigen) specific cells were slightly elevated (relative to AFP) and largely naive.
Fig. 2.
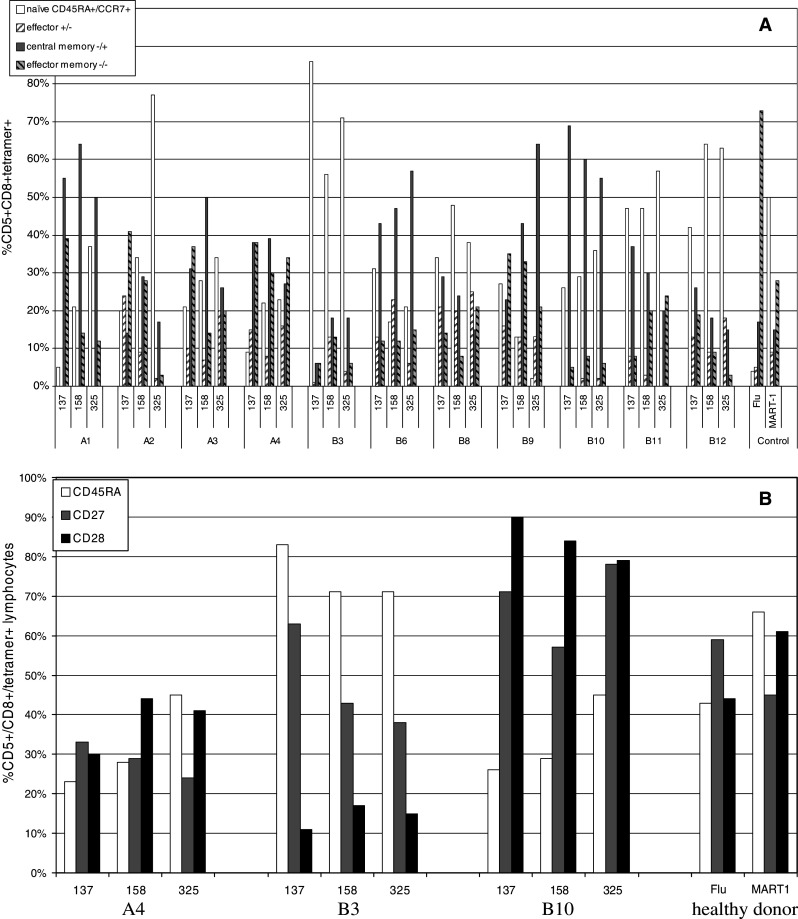
AFP tetramer positive T cell frequencies and phenotype. The percentage of tetramer positive cells detected pre-vaccination is shown. a Tetramer positive cells were stained for CD45RA and CCR7 and were divided into CD45RA+/CCR7+ (naïve), CD45RA+/CCR7- (effector memory), CD45RA-/CCR7+ (central memory), and CD45RA-/CCR7- (effector) groups. b CD45RA was compared with CD27 and CD28 to further examine differentiation. The representative HCC patients A4, B3 and B10 are shown, as well as healthy donor controls
Detailed phenotype
We next utilized additional markers to further address the question of antigen-experienced and naïve phenotype. We tested tetramer positive cells for CD27 and CD28 costimulatory receptors in combination with CD45RA isoform also used to define naïve and differentiated cells [9]. Several studies have shown that CD27 and CD28 are down-regulated with antigen exposure [22, 23]. This has also been observed in studies of human viral infection [9]. It has also been suggested that CD28 is down-regulated first, followed by CD27, with fully differentiated effectors having a CD45RA+/CD27-/CD28- phenotype [24]. All patients were tested and data are shown for A4, B3, B10 (who were representative of the common phenotypic patterns observed and had different levels of serum AFP) and a healthy donor in Fig. 2b. Patient A4 had the highest levels of EM and CM by CD45RA/CCR7 (Fig. 2a). Consistent with this state of differentiation, the levels of CD27 and CD28 were reduced. B3 had a high percentage of naïve cells and CD27 was at a differentially reduced level for each T cell specificity (63%, 43%, 38%+), with CD28 also reduced (11%, 17%, 15%+), which indicates that the majority (71–83%) CD45RA+ (83–94%) CCR7+ cells had become partially differentiated. In the subject with the highest serum AFP levels (B10) and largely CD45RA-/CCR7+ CM cells, CD27 and CD28 were expressed at high levels. While this CD28 might have become re-expressed, the high CD27 indicates that even in the case of high serum AFP antigen, there was no evidence of complete differentiation of AFP-specific CD8 T cells. A proportion of the tetramer+ cells (2–86%, average 36%) remain naïve and on average 40–41% continue to express CD27 and CD28. Therefore, taken together, each tested patient had evidence that a percentage of their AFP-specific T cells had down-regulated CD27 and CD28, sometimes in an epitope-specific manner, indicating that a percentage of these cells had undergone at least partial differentiation.
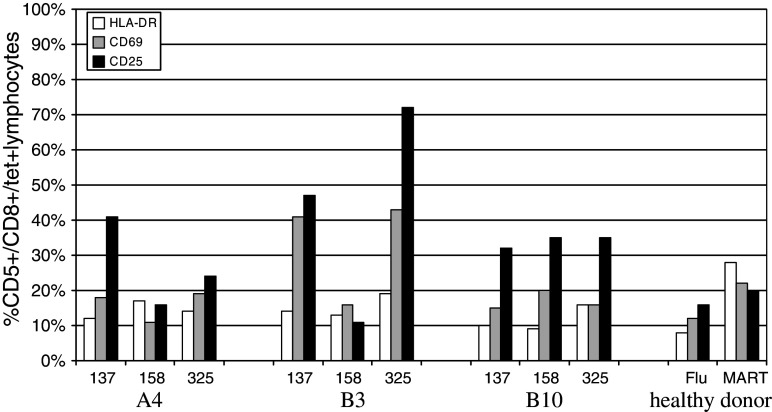
Activation status
Next, we wished to examine the activation state of the AFP-specific CD8 T cells by 3 measures: HLA-DR, which is present on antigen-experienced cells, and two markers transiently up-regulated, CD25 and CD69 (Fig. 3). The HCC subjects had a variable percentage of AFP-specific T cells that expressed these activation markers, 21–28% positive on average (all 3 markers). Figure 3 again shows A4, B3 and B10. Here, A4 and B10 are representative of most patients, with 10–41% activation marker expression on AFP-specific T cells. In contrast, B3 is representative of the highest levels, having 25–72% CD25+ and 16–43% CD69+, indicating more recent T cell activation. Interestingly, B3 responded clinically to chemoembolization and radio-frequency ablation, and then enrolled in the AFP peptide-pulsed DC vaccine protocol in the early post-chemoembolization period, while his AFP was still in a downward trend, which could explain this activation status, in agreement with recent reports on the immune activation inherent in some surgical and chemotherapy therapies [25]. Interestingly, the healthy donor MART-1 and Flu-specific T cells expressed activation markers only on a minority of cells, at a lower frequency than detected on HCC patient AFP T cells. Overall, many of the AFP T cells are partially activated, from less than 10 to >70%, indicating that many of these cells had encountered AFP antigen and become activated.
Fig. 3.
AFP tetramer-positive T cell activation phenotype pre-vaccination. The expression of HLA-DR, CD69, CD25 on AFP137–145, AFP158–166, and AFP325–334 tetramer positive cells is shown. The representative HCC patients A4, B3 and B10 are shown, as well as healthy donor controls
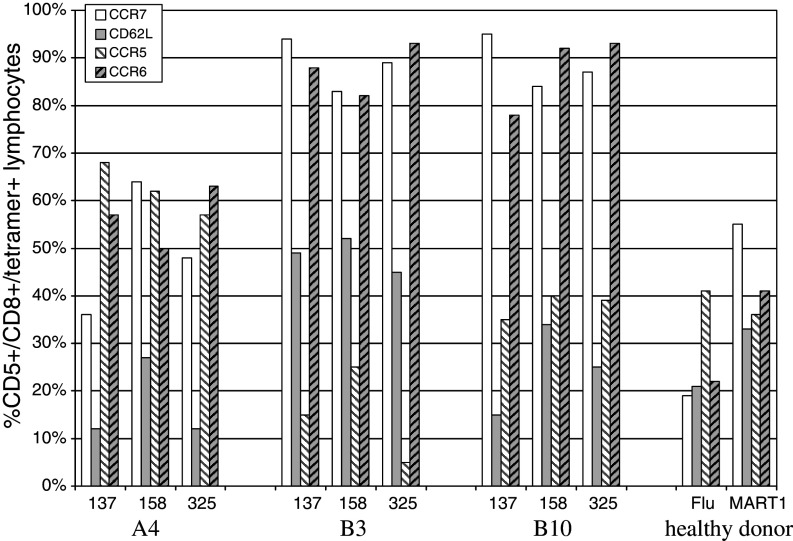
Trafficking markers
We next examined the trafficking potential of these cells. This was assessed with two markers for central trafficking, CCR7 (required for lymph node entry) and CD62L or l-Selectin (required for extravasation). We also tested two markers for peripheral trafficking, CCR5 (associated with Th1 cells and cells trafficking to the liver [26]) and CCR6 (peripheral marker). We hypothesized that naïve cells, which had not yet encountered antigen, would express only central trafficking markers CCR7 and CD62L, while any fully differentiated CD8 T cells would have down-regulated the central markers and up-regulated CCR5 and/or CCR6.
We found that most tetramer + cells co-expressed markers for both central and peripheral trafficking. The same three patients, again representing the patterns detected, are shown in Fig. 4. A patient with low levels of serum AFP (B3) had the highest levels of CCR7 and CD62L central markers on the three peptide-specific T cells (48 to >90% of T cells) of all tested patients. A4, with both CM and EM cells, had CCR7 on 36–64%, but had reduced CD62L to 12–28%. Peripheral markers were co-expressed, with CCR5 on 62–68% and CCR6 on 50–57%. Therefore, these memory cells co-expressed these central and peripheral markers. Because we were able to use CD62L and CCR6 in the same staining mix, we could confirm co-expression of these two markers on the same population of cells by back-gating. In B3, in which 88% AFP137 cells were CCR6+ and 49% were CD62L+, 27% of these CCR6+ cells co-expressed CD62L. Similarly, 51% of B3 CCR6+ AFP158 cells were also CD62L+ and 18% of B3 CCR6+ AFP325 cells were also CD62L+, again indicating co-expression. This pattern was present on a majority of patient T cells. This may indicate a partial state of differentiation, for which central markers are still expressed, and peripheral markers are also becoming expressed.
Fig. 4.
AFP tetramer-positive T cell trafficking phenotype pre-vaccination. The expression of central trafficking markers CCR7 and CD62L and peripheral trafficking markers CCR5 and CCR6 on AFP137–145, AFP158–166, and AFP325–334 tetramer positive cells is shown. The representative HCC patients A4, B3 and B10 are shown, as well as healthy donor controls
HD MART-1 and Flu cells showed the least evidence for trafficking marker co-expression, and only 6% of the Flu-specific CCR6+ population also expressed CD62L.
Apoptosis phenotype
Lastly, we examined the cells for markers for early apoptosis (Annexin-V) and ability to be killed by FasL-expressing cells (FasR, CD95) (data not shown). These markers have been observed on T cells from cancer patients [17, 18] and may indicate cells which are destined to apoptose and are less effective antitumor cells. We found that these HCC patient AFP-specific T cells expressed >10% and up to 45% of at least one of these two markers. B10 and B12 expressed higher levels of both markers, while A4 and B9 expressed some of the lowest levels. On average, 11% of cells expressed Annexin-V and 12% expressed CD95. These values are not as elevated as those observed by others [17] and are, therefore, also not a confounding factor in our assessment of other markers and function.
Changes in phenotype with peptide-pulsed DC vaccination
In four subjects (B6, B9, B11 and B12), there were sufficient peripheral blood cells available from time points during and after vaccination to follow the same markers. This enabled us to determine any effects of AFP peptide-pulsed DC-based vaccination on the functional phenotype of these T cells.
In the clinical trial report [2], we found that 6 of 10 patients had statistically significant increases in the frequency of tetramer+ cells, including B9 and B11 (strongest increases). B6 and B12 did not have increases which met the criteria of multiple consecutive increases of greater than twofold, but had single time point increases. There were also six patients with statistically significant increases in the frequency of IFNγ-producing cells by ELISPOT, including B6 and B9. Based on these findings, we expected B6 and B9 to be particularly important to analyze for changes in phenotype following vaccination.
Examining the CD45RA/CCR7 expression, B6 AFP137 and AFP325 specific cells remained primarily naïve at day + 28 and day + 112, showing only transient increases in %EM cells (not shown). Only AFP158 specific cells showed an increase from 4% EM (pre-vaccine) to 29% EM (day + 28) to 45% EM at day + 112, becoming the majority. This epitope-specific differentiation was the strongest seen for the 4 patients examined over time. B9 T cells remained primarily naïve pre-vaccine and day + 35. Overall, despite peptide-pulsed DC vaccination, the strongest evidence for effector or memory differentiation was the increase in %EM cells to a single epitope in one patient, B6.
In Fig. 5, the changes in activation phenotype are shown for all four patients and all time points with sufficient cells for analysis. In general, there were fluctuations in the expression levels of several markers, but there was a lack of change towards a fully activated phenotype in the majority of the cells. B6 showed transient CD69 increases for all 3 AFP T cell specificities, as well as CD25 increases for AFP137 and AFP325, with HLA-DR increases for AFP158 only. Surprisingly, B9 showed a noticeable increase only in CD69 for AFP325 specific T cells. The changes in HLA-DR expression in B12 were not evident until day + 56 and day + 112. This indicates that one limitation of this part of the analysis is the availability of cells from later time points (B9 had only day + 35 cells available). Together, Fig. 5 indicates that AFP-based vaccines can also lead to increased activation marker expression. However, the increases were modest, even in those patients with increased AFP-specific IFNγ production.
Fig. 5.
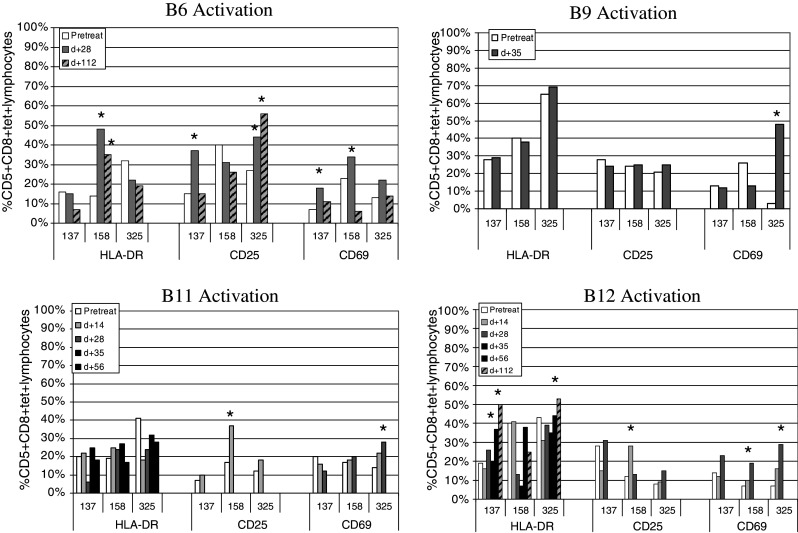
HCC patient changes in activation markers with vaccination. Patients treated with AFP peptide-pulsed autologous DC were examined before, during (B11, B12) and after vaccination (all 4 patients) for alterations of activation markers HLA-DR, CD69, CD25. Markers on AFP137–145, AFP158–166, and AFP325–334 tetramer positive cells are shown. Increases >10% of baseline value are marked with an asterisk
These post vaccine cells were also tested for changes in other markers. While there were many modest fluctuations in the percent of tetramer+ cells expressing these markers, the other consistent changes included up-regulation of CCR6 in B9 AFP137, AFP158 and AFP325 and increased CD95 expression on B12 AFP137, AFP158 and AFP325 with Annexin-V also increased on AFP137 and AFP325 (data not shown).
CD8 T cell cytokine analysis
In a subset of patients, there were sufficient cells to assess not only IFNγ but also to investigate production of other cytokines. Specifically, we tested patients A3, A4, B6, B9 and B10 for IL-5 by ELISPOT (to detect evidence for Th1/Th2 skewing [27]) and B6 and B9 for IL-10 by ELISPOT (to detect any antigen-specific suppressor function). A few background spots were detected in response to K562/A2.1 antigen presenting cells and all tested patients responded to the PHA mitogen stimulus, but no AFP peptide-specific spots were detected (data not shown). In addition, we tested supernatants from the ELISPOT plates for IFNγ, TNFα, GM-CSF, IL-2, IL-4, IL-5 and IL-10 for a broader panel of cytokines by the sensitive Luminex assay. We were unable to detect any additional cytokines, other than in response to the positive PHA stimulus (data not shown). Direct cytotoxicity was not tested here due to limited cells available and our focus on cytokine production.
CD4 T cell cytokine analysis
The AFP peptide-pulsed DC vaccine was designed to directly activate CD8 T cells, and the short peptides employed would not be expected to activate CD4 T cells. However, in order to determine whether there was any pre-existing AFP-specific CD4 T cell immunity before vaccination which might have had an impact of the ability to expand and differentiate CD8 T cells, we tested magnetic-bead purified CD4 T cells from pre-vaccine leukaphereses from 9 patients for evidence of cytokine production by ELISPOT. We prepared autologous DC and pulsed these immature DC with purified AFP protein (or medium alone) and tested for IFNγ, TNFα, IL-2 (to detect memory cells), IL-5, and IL-10. These experiments were designed to mimic the in vivo state of tumor-derived serum AFP antigen presentation via immature DC.
As shown in Fig. 6, we did not detect any significant responses to AFP in the patient cells (DC + AFP protein compared to DC alone). The majority of patients responded to the mitogen PHA with secretion of IFNγ, IL-2 or both cytokines. A minority also secreted IL-5 and TNFα. B3 was the exception, but this subject did mount an IFNγ ELISPOT response to MHC Class-I peptides. Several patients had a non-antigen-specific cytokine response to the autologous DC and there were also examples of cytokine release attributable to DC alone (or contaminating lymphocytes in the unpurified DC preparations). The largest frequency of AFP-specific CD4 T cells was observed in patient B6 (the only patient with pre-vaccine IFNγ-producing CD8 T cells), with a modest 13/105 CD4 T cells secreting IL-2.
Fig. 6.
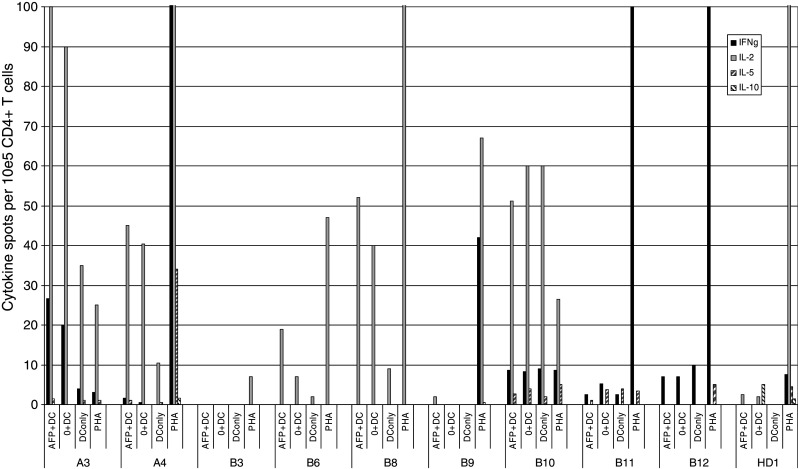
AFP-specific CD4 multi-cytokine ELISPOT analysis. ELISPOT assays were performed to detect IFNγ, IL-2, IL-5 and IL-10 from CD4 purified T cells stimulated with autologous DC pulsed for 1 h with AFP protein (AFP + DC). These were compared to unpulsed DC (0 + DC) and PHA positive control. Cytokines produced by DC plated alone (DC only) are also shown
Because we have observed determinant spreading from vaccine-induced CD8 T cell responses to spontaneous CD4 T cell responses to related tumor antigens in melanoma patients [28, 29], we tested patients B6, B9, B11 and B12 for any changes in AFP-specific CD4 T cell responses over time. These additional ELISPOT studies did not reveal any positive responses (data not shown). Luminex cytokine arrays were again performed on ELISPOT supernatants to repeat (IFNγ, TNFα, IL-2, IL-5, IL-10) and extend (GM-CSF, IL-4) the cytokines tested. Again, we did not detect any evidence for AFP-specific cytokine responses by Luminex (data not shown). AFP protein-pulsed DC are able to expand AFP-specific CD4 T cells after 1–3 rounds of in vitro stimulation in HCC patients [30] therefore, this mode of antigen presentation is able to activate CD4 T cells.
Lastly, we tested whether the T cell phenotype and functional data correlated with the patient serum AFP levels shown in Table 1. Due in part to the small number of subjects, none of the T cell phenotypic or functional characteristics was significantly correlated to serum AFP.
Discussion
Immunotherapy vaccination efforts are designed to provide adequate antigen presentation and to overcome the lack of presentation or inhibitory presentation provided by tumor. One might predict that constant exposure to high dose systemic antigen would quickly lead to exhaustion and deletion of antigen-specific T cells. In order to activate effective antitumor lymphocytes, we must first characterize the natural state of immunity to the target antigen and test the ability of lymphocytes to become activated, differentiate to effectors and respond to tumor. In the case of AFP, humans are exposed to high levels of this antigen during fetal development, and the majority of HCC patients are again exposed to high circulating levels of this antigen, in this case, tumor-derived (and potentially having an altered glycosylation pattern [31]. In addition, in two clinical trials, we have immunized AFP+ HCC patients with AFP-derived MHC class I-restricted peptides, presented either in adjuvant [1] or on autologous DC [2].
In order to better understand the successful expansion of IFNγ-producing AFP-specific CD8+ T cells and a lack of objective clinical responses, we have further investigated the biology of the AFP-specific CD8 T cells from HCC subjects by MHC tetramer and phenotyping, and both CD8 and CD4 T cells by ELISPOT and Luminex cytokine assay. In some cases, we have been able to follow the function and phenotype after vaccination with AFP peptides. We found pools of AFP-specific CD8 T cells which are both naïve and CM phenotype and lack of complete differentiation to effector and EM cells.
The continued presence of CCR7 on the majority of these T cells indicates their ability to traffic to lymph nodes, however, many of these cells co-expressed the liver-tropic molecule CCR5, peripheral marker CCR6 and reduced expression of the second lymph node trafficking molecule, CD62L. This may point to the partial state of differentiation of these cells. Continued presence of CCR7 despite reduced CD62L was recently observed in subjects with liver disease [32]. This study found CCR7 ligands CCL19 and CCL21 on vessels in the liver, and suggested that the CCR7+/CD62L- populations could be involved in both liver and regional lymph node trafficking. This new data might indicate the AFP-specific CCR7+/CD62L- cells we identified in the peripheral blood could also traffic to the liver and participate in localized trafficking there.
Our data are in agreement with recent data analyzing T cell phenotype in mice immunized with either immature or fully mature peptide-pulsed DC [33]. This group observed that immature DC immunization led to partial activation of peptide-specific T cells that were capable of cytokine production (IFNγ and TNFα) and a low level of proliferation, but limited down-regulation of CD62L and CCR7, which kept these T cells from leaving secondary lymph nodes. Lee et al. [12] also observed dysfunctional, partially differentiated tumor antigen-specific T cells in a melanoma patient which had reduced levels of activation marker expression and very limited function. In other investigations of pre- and post-melanoma peptide vaccination, differentiation towards memory cells was observed in a minority of patients, and effector differentiation was more limited [16, 34].
We also investigated cytokine production by these cells to extend our earlier studies of IFNγ ELISPOTs. We specifically sought evidence for Th1-Th2 skewing and IL-10-producing antigen-specific regulatory cells. We found no evidence for AFP-specific production of other tested cytokines by the CD8+ T cells by either ELISPOT or Luminex. Given the presence of CM cells phenotypically, the lack of IL-2 was unexpected. In conjunction with the lack of significant ex vivo AFP-specific CD4 T cell activity, the data support a scenario of CD8 T cell expansion and IFNγ production activity in conjunction with partial differentiation of CD8 T cells, but lack of acquisition of full effector phenotype or memory phenotype and IL-2 production, potentially due to lack of CD4 help. In a recent examination of the impact of chronic, high-level antigen exposure in human T cell responses (HIV [35]), it was shown that such viral antigen-specific T cells produced IFNγ exclusively. This is supporting a potential role for chronic antigen exposure in the limited cytokine response we observed. It should be noted that some antigen presentation and DC defects have been observed in patients with HBV and HCV in some [36, 37] but not all studies [38].
There have been two reports indicating that there may be AFP-specific help in a subset of HCC patients. One suggests the presence of IFNγ-expressing AFP-specific CD4 cells [7] and another reports circulating AFP IgG immune complexes (which recognize denatured AFP, not native AFP [39]) in 20% of tested HCC subjects. In our group of patients, the finding that spontaneous CD8 cell responses to AFP exist pre-vaccination (at a level detectable ex vivo by MHC tetramers) indicates that there is some level of endogenous presentation of AFP, however, the minimal level of functional CD4 responses found to date by us (by ELISPOT) suggests that lack of help may lead to dysfunctional CD8 T cells. This characterization of the AFP T cells is supported by the detailed phenotypic characterization indicating lack of effector phenotype and only partial trafficking differentiation. Such partial activation of T cells under suboptimal conditions [40, 41] can lead to proliferation without full effector function, for example in cases of signal 1 or signals 1 and 2 without 3 (IL-12, IFNα). In our ongoing immunotherapy efforts, our focus on immunization with full-length antigen, to directly activate CD8 and CD4 T cells, may allow for more potent T cell activation and full effector and memory differentiation. In addition, use of adenovirus to deliver antigen can lead to a more mature DC phenotype [42], which may also improve T cell responses.
Acknowledgments
This work was supported by NIH/NCI ROI CA 77623, RO1 CA 79976 (JSE), and by a Scientist Development Grant from the American Heart Association (#0330102N), the University of Pittsburgh Cancer Institute, the Pittsburgh Foundation and the Henry L. Hillman Foundation (LHB). We wish to acknowledge the expertise of the UPCI Flow Cytometry Facility and Erin McClelland and especially Drs. Albert D. and Vera S. Donnenberg for several helpful discussions. Also our thanks to the UPCI Luminex Facility, Dr. Anna Lokshin, Director.
Abbreviations
- AFP
Alpha fetoprotein
- AFP+
AFP-expressing
- HCC
Hepatocellular cancer
- DC
Dendritic cell(s)
- PBMC
Peripheral blood mononuclear cells
References
- 1.Butterfield LH, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9(16 Pt 1):5902–5908. [PubMed] [Google Scholar]
- 2.Butterfield LH, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12(9):2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 3.Ruoslahti E. Alpha-fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/S0065-230X(08)60849-0. [DOI] [PubMed] [Google Scholar]
- 4.Alisa A, et al. Analysis of CD4+ T-Cell responses to a novel alpha-fetoprotein-derived epitope in hepatocellular carcinoma patients. Clin Cancer Res. 2005;11(18):6686–6694. doi: 10.1158/1078-0432.CCR-05-0382. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield LH, et al. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59(13):3134–3142. [PubMed] [Google Scholar]
- 6.Butterfield LH, et al. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol. 2001;166(8):5300–5308. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 7.Hanke P, et al. Cirrhotic patients with or without hepatocellular carcinoma harbour AFP-specific T-lymphocytes that can be activated in vitro by human alpha-fetoprotein. Scand J Gastroenterol. 2002;37(8):949–955. doi: 10.1080/003655202760230928. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, et al. Hierarchy of AFP-specific T cell responses in subjects with AFP-positive hepatocellular cancer. J Immunol. 2006;177(1):712–721. doi: 10.4049/jimmunol.177.1.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamann D, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Lee PP. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5(6):677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar PR, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J Immunol. 2000;165(11):6644–6652. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 14.Pittet MJ, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190(5):705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speiser DE, Cerottini JC, Romero P. Can hTERT peptide (540–548)-specific CD8 T cells recognize and kill tumor cells? Cancer Immun. 2002;2:14. [PubMed] [Google Scholar]
- 16.Pittet MJ, et al. Expansion and functional maturation of human tumor antigen-specific CD8+ T cells after vaccination with antigenic peptide. . Clin Cancer Res. 2001;7(3 Suppl):796s–803s. [PubMed] [Google Scholar]
- 17.Kim JW, et al. Expression of pro- and antiapoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(15):5101–5110. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Ferris RL, Whiteside TL. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res. 2005;11(21):7901–7910. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- 19.Meng WS, et al. alpha-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61(24):8782–8786. [PubMed] [Google Scholar]
- 20.Herr W, et al. Detection and quantification of blood-derived CD8+ T lymphocytes secreting tumor necrosis factor alpha in response to HLA-A2.1-binding melanoma and viral peptide antigens. J Immunol Meth. 1996;191(2):131–142. doi: 10.1016/0022-1759(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 21.Mayer S, et al. A sensitive proliferation assay to determine the specific T cell response against HLA-A2.1-binding peptides. J Immunol Meth. 1996;197(1–2):131–137. doi: 10.1016/0022-1759(96)00124-X. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28(3):258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appay V, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 24.Smith CL, et al. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J Immunol. 2005;175(12):8431–8437. doi: 10.4049/jimmunol.175.12.8431. [DOI] [PubMed] [Google Scholar]
- 25.Lake RA, Robinson BW. Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer. 2005;5(5):397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 26.Ajuebor MN, Carey JA, Swain MG. CCR5 in T cell-mediated liver diseases: what’s going on? J Immunol. 2006;177(4):2039–2045. doi: 10.4049/jimmunol.177.4.2039. [DOI] [PubMed] [Google Scholar]
- 27.Tatsumi T, et al. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63(15):4481–4489. [PubMed] [Google Scholar]
- 28.Butterfield LH, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9(3):998–1008. [PubMed] [Google Scholar]
- 29.Ribas A, et al. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27(5):354–367. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Evdokimova VN et al (2007) AFP specific CD4+ helper T cell responses in healthy donors and HCC patients. J Immunother (in press) [DOI] [PMC free article] [PubMed]
- 31.Seregni E, Botti C, Bombardieri E. Biochemical characteristics and clinical applications of alpha-fetoprotein isoforms. Anticancer Res. 1995;15(4):1491–1499. [PubMed] [Google Scholar]
- 32.Heydtmann M, et al. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006;177(1):729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- 33.Dumortier H, et al. Antigen presentation by an immature myeloid dendritic cell line does not cause CTL deletion in vivo, but generates CD8+ central memory-like T cells that can be rescued for full effector function. J Immunol. 2005;175(2):855–863. doi: 10.4049/jimmunol.175.2.855. [DOI] [PubMed] [Google Scholar]
- 34.Speiser DE, et al. In vivo activation of melanoma-specific CD8(+) T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur J Immunol. 2002;32(3):731–741. doi: 10.1002/1521-4141(200203)32:3<731::AID-IMMU731>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Harari A, et al. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174(2):1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 36.Beckebaum S, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104(2):138–150. doi: 10.1006/clim.2002.5245. [DOI] [PubMed] [Google Scholar]
- 37.Ninomiya T, et al. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31(2):323–331. doi: 10.1016/S0168-8278(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 38.Piccioli D, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42(1):61–67. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Bei R, et al. Cryptic epitopes on alpha-fetoprotein induce spontaneous immune responses in hepatocellular carcinoma, liver cirrhosis, and chronic hepatitis patients. Cancer Res. 1999;59(21):5471–5474. [PubMed] [Google Scholar]
- 40.Curtsinger JM. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175(7):4392–4399. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- 41.Curtsinger JM, et al. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174(8):4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher L, et al. Human dendritic cell maturation by adenovirus transduction enhances tumor antigen-specific T-cell responses. J Immunother. 2004;27(3):191–200. doi: 10.1097/00002371-200405000-00003. [DOI] [PubMed] [Google Scholar]