Abstract
Survivin, a member of the inhibitor of apoptosis protein family, is expressed in many malignant tumors including urothelial cancer but is hardly detectable in normal, differentiated adult tissues. Previously we reported CD8-positive cytotoxic T-lymphocytes (CTLs) were successfully induced by stimulation with survivin-2B80-88 peptide in vitro. We started a phase I clinical study of survivin-2B80-88 peptide vaccination for advanced urothelial cancer patients to assess the safety and efficacy of this vaccination. Nine patients were received vaccination and were evaluated for immunological evaluation, adverse events, and clinical responses. A total of 46 vaccinations were carried out. There was no severe adverse event. HLA-A24/survivin-2B80-88 peptide tetramer analysis revealed a significant increase in the peptide-specific CTL frequency after the vaccination in five patients. Slight reduction of the tumor volume was observed in one patient. Survivin-2B80-88 peptide-based vaccination is safe and should be further considered for potential immune and clinical efficacy in urothelial cancer patients.
Keywords: Immunotherapy, Survivin, Peptide vaccination, Urothelial cancer
Introduction
Increasing numbers of T-lymphocyte epitopes derived from various cancer-associated antigens have been reported, and they have been proved to play significant roles in cytotoxic T-lymphocyte (CTL)-based immunotherapy [1]. Survivin, a member of the inhibitor of apoptosis protein family, is expressed in various malignant tumors but is undetectable in normal and differentiated adult tissues [2–4]. Because of its cancer-specific expression, survivin might be an attractive target for immunotherapy via CTL responses.
We previously reported that survivin and its splicing variant survivin-2B were expressed abundantly in various cancer tissues and cancer cell lines, including urothelial cancer, and were suitable as target antigens for active-specific anticancer immunization [5]. Subsequently, we identified the human leukocyte antigen (HLA)-A24-restricted antigenic peptide survivin-2B 80-88 (AYACNTSTL) derived from the exon 2B-encoded region and recognized by CTLs in the context of HLA-A24 molecules. In addition, we reported further evidence that the survivin-2B80-88 peptide might serve as a potent immunogenic cancer vaccine for various cancers, including those of the colon, lung and breast [6]. On the basis of these studies, we started a phase I clinical study using survivin-2B80-88 peptide vaccination for colorectal and breast cancers. These studies revealed that survivin-2B80-88 peptide vaccination was safe and well tolerated without severe side effects and could induce survivin-2B80-88 peptide-specific CTLs [7, 8].
With respect to urogenital cancers, we previously reported that survivin was selectively expressed in 87.5% of bladder cancers and survivin-specific CTLs were successfully induced from peripheral blood mononuclear cells of a bladder cancer patient [9]. On the basis of these studies, we therefore started a phase I clinical study assessing the safety and efficacy of survivin-2B80-88 peptide vaccination in patients with advanced or recurrent urothelial cancer expressing survivin. Herein we show that survivin-2B80-88 peptide-based vaccination is safe and should be further considered for potential immune and clinical efficacy in HLA-A24+ survivin-expressing patients with urothelial cancer.
Materials and methods
Patient selection
The study protocol was approved by the Clinical Institutional Ethical Review Board of the Medical Institute of Bioregulation, Sapporo Medical University, Japan. All the patients gave informed consent before being enrolled. Patients enrolled in this study were required to conform to the following criteria: (1) to have histologically proven urothelial cancer, (2) to be HLA-A*2402 positive, (3) to have survivin- and HLA class I-positive carcinomatous lesions on the primary site by immunohistochemistry, (4) to be between 20 and 85 years old, (5) to have received surgical excision of the primary tumor and (6) to have Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 3. Exclusion criteria included (1) prior cancer therapy such as chemotherapy, radiation therapy, steroid therapy, or other immunotherapy within the previous 4 weeks, (2) the presence of other cancers that might influence the prognosis, (3) immunodeficiency or a history of splenectomy, (4) severe cardiac insufficiency, acute infection, or hematopoietic failure, and (5) unsuitability for the trial based on clinical judgment.
Nine patients with refractory recurrent urothelial cancer were initially enrolled in this study (Table 1). Of the nine patients, eight (cases 2–9) had recurrent advanced bladder cancer and one (case 1) had ureteral cancer. Four patients (cases 3, 5, 6 and 9) had lung metastasis and three (cases 1, 2 and 7) had regional and/or distant lymph node metastasis. All patients had previously received systemic chemotherapy such as MVAC (methotrexate, vinblastine, adriamycin and cisplatin), GC (gemcitabine and cisplatin) and/or TIN (paclitaxel, ifosfamide and nedaplatin). Survivin and HLA class I expression on the metastatic sites were confirmed histologically in five patients (cases 1, 2, 4, 7 and 8).
Table 1.
Summary of clinical characteristics of patients enrolled in the study
| Age | Sex | Primary | Recurrence site | |
|---|---|---|---|---|
| 1 | 57 | F | Rt ureteral Ca. | Pelvic LNa |
| 2 | 51 | F | Bladder Ca. | Cervival LNa |
| 3 | 67 | M | Bladder Ca. | Lung |
| 4 | 65 | F | Bladder Ca. | Pelvic soft tissuea |
| 5 | 65 | M | Bladder Ca. | Lung |
| Bone | ||||
| 6 | 64 | M | Bladder Ca. | Lung |
| 7 | 38 | F | Bladder Ca. | Inguinal LNa |
| Mesenchymal LN | ||||
| 8 | 59 | F | Bladder Ca. | Pelvic soft tissuea |
| 9 | 57 | M | Bladder Ca. | Lung |
| Liver |
LN lymph node
ahisologically survivin and HLA class I expression confirmed
Peptide preparation
The survivin-2B80-88 peptide with the sequence AYACNTSTL, was prepared under good manufacturing practice conditions by Multiple Peptide Systems (San Diego, CA, USA). The identity of the peptide was confirmed by mass spectrometry analysis, and the purity was shown to be more than 98% as assessed by high pressure liquid chromatography analysis. The peptide was supplied as a freeze-dried, sterile white powder. It was dissolved in 1.0 ml of physiological saline (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) and stored at −80°C until just before use.
Incomplete Freund’s adjuvant preparation
Montanide ISA 51 (SEPPIC Inc., NJ, USA) was used as an incomplete Freund’s adjuvant (IFA).
Vaccinations
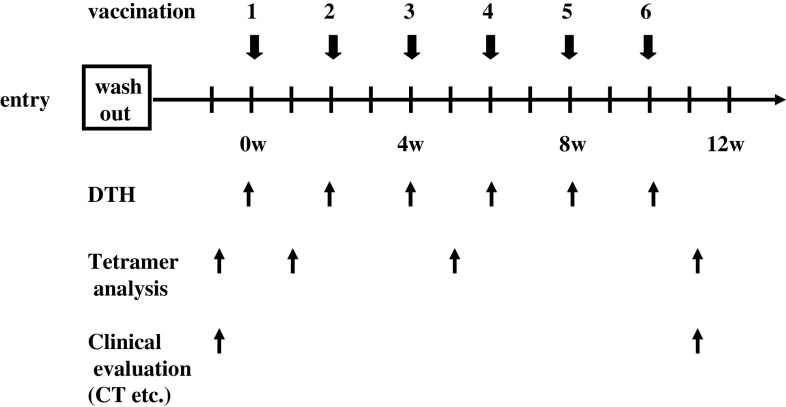
We previously showed that the peptide doses of 0.1 and 1 mg had efficacy with little adverse events [7]. On the basis of these data, we set two arms of which peptide dosage has 0.1 and 1.0 mg. Vaccinations with survivin-2B80-88 peptide (0.1 or 1.0 mg) vaccines with IFA serving as a carrier for water-in-oil emulsion [10] were given subcutaneously six times at 14-day intervals (Fig. 1).
Fig. 1.
Vaccination, immunological and clinical evaluation schedule. The vaccination with survivin-2B peptide (0.1 or 1.0 mg) vaccines with incomplete Freund’s adjuvant (IFA) were carried out. Patients received vaccinations every 2 weeks, and A DTH skin test was performed at each vaccination. Patients were examined closely for signs of toxicity during and after vaccination. Before and 1-week after the third and sixth vaccination, HLA-A24/survivin-2B80-88 peptide tetramer analysis were evaluated. Tumor size was evaluated by computed tomography (CT) before treatment and at the end of the study period
Delayed-type hypersensitivity skin test
A delayed-type hypersensitivity (DTH) skin test was performed at each vaccination. The peptide (10 μg) solution in physiological saline (0.1 ml) or physiological saline alone (0.1 ml) was separately injected intradermally (i.d.) into the forearm. A positive reaction was defined as more than 4 mm diameter area of erythema and induration 48 h after the injection.
Toxicity evaluation
Patients were examined closely for signs of toxicity during and after vaccination. Adverse events were recorded using the National Cancer Institute Common Toxicity Criteria (NCI-CTC).
Tetramer staining
HLA-A24/survivin-2B80-88 peptide tetramers were constructed according to the procedure described by Altman et al. [11]. Briefly, recombinant HLA-A24 heavy chain [12] and human β2-microgloblin were refolded with survivin-2B80-88 peptides as described [13]. The resulting HLA-A24-peptide monomer was biotinylated by incubation with the BirA enzyme (Avidity, Denver, CO) for 17 h at room temperature and purified using fast protein liquid chromatography. A tetrameric HLA-peptide complex was produced by incubating streptavidin-phycoerythrin (PE) (Vector Laboratories, Burlingame, CA, USA) with the biotinylated monomer at a molar ratio 1:4. For flow cytometric analysis, peripheral blood mononuclear cells were isolated from blood samples by Ficoll-Conray density gradient centrifugation. Then, they were incubated in the presence of 40 μl/ml survivin-2B peptide in AIM-V medium containing 10% human serum, followed by addition of interleukin-2 at a final concentration of 50 U/ml at 1 h, 2, 4 and 6 days after the addition of the peptide. On day 7 of culture, the Peripheral blood mononuclear cells (PBMCs) were stained with the PE-labeled tetramer at 37°C for 20 min, followed by staining with a fluorescein isothiocyanate (FITC)-conjugated anti-CD8 monoclonal antibody (mAb) (Beckton Dickinson Biosciences) at 4°C for 30 min. Cells were washed twice with phosphate-buffered saline (PBS) before fixation in 1% formaldehyde. Flow cytometric analysis was performed using FACSCalibur and Cell Quest software (BD Biosciences). As a negative control, a tetramer with an HLA-A*2402-restricted human immunodeficiency virus (HIV) peptide (RYLRDQQLL) was used. The frequency of CTL precursors was calculated as the number of tetramer-positive cells over the number of CD8-positive cells.
Clinical response evaluation
Physical examinations and hematological examinations were conducted before and after each vaccination. Tumor size was evaluated by computed tomography (CT) before treatment and at the end of the study period. A complete response (CR) was defined as complete disappearance of all measurable diseases. A partial response (PR) was defined as a ≥30% decrease from the baseline in the size of all measurable lesions (sum of products of maximal perpendicular diameters) lasting for a period of at least 4 weeks. Progressive disease (PD) was defined as an increase in the sum of the bidimensional measurements of all known disease sites by at least 20% or by the appearance of new lesions. No change (NC) was defined as the absence of matched criteria for CR, PR, or PD.
Results
Vaccinations
Altogether, 46 vaccinations were carried out in nine patients. Three patients (cases 3, 5 and 7) discontinued halfway through the protocol because of disease progression. Six patients (cases 1, 2, 4, 6, 8 and 9) received the complete regimen including six vaccinations and were evaluated. Table 2 summarizes the immunological and clinical outcomes of the nine patients.
Table 2.
Summary of clinical courses of patients administered survivin-2B80-88 peptide vaccine
| Peptide dose | Adverse events (Grade)a | DTH skin test | Tetramer staining assay % of HLA-A24/survivin-2B80-88 peptide tetramer-positiv e CTL (post-/pre-vaccination) | Reduction rate (judgment) | |
|---|---|---|---|---|---|
| 1 | 0.1 mg × 6 | Induration at the injection site (Grade 1) | (–) | Increased (1.29/0.04) | 19.4% (SD) |
| 2 | 0.1 mg × 6 | Induration at the injection site (Grade 1) | (–) | Increased (1.34/0.07) | 17.6% (SD) |
| 3 | 0.1 mg × 2 | None | (–) | NA | NA |
| 4 | 0.1 mg × 6 | Induration at the injection site (Grade 1) | (–) | No change (0.28/0.06) | −52.5% (PD) |
| 5 | 1.0 mg × 3 | None | (–) | NA | NA |
| 6 | 1.0 mg × 6 | Induration at the injection site (Grade 1) | (–) | No change (0.06/0.10) | −68.0% (PD) |
| 7 | 1.0 mg × 5 | Induration at the injection site (Grade 1) | (–) | Increased (0.60/0.15)b | NA |
| 8 | 1.0 mg × 6 | Induration at the injection site (Grade 1) | (–) | Increased (0.78/0.07)b | −87.6% (PD) |
| 9 | 1.0 mg × 6 | None | (–) | Increased (1.42/0.13)b | −81.4% (PD) |
NA not available
aAdverse events were recorded using the National Cancer Institute Common Toxicity Criteria (NCI-CTC)
bEvaluation after third vaccination
Safety
Peptide vaccination was well tolerated in all nine patients. As shown in Table 2, no hematologic, cardiovascular, hepatic, or renal toxicity was observed. No other severe adverse events such as fever and fatigue were observed during or after vaccination in any patient. As minor side effect, six patients (cases 1, 2, 4, 6, 7 and 8) developed grade 1 local skin reactions with redness and swelling at the injection sites.
DTH skin test
A DTH skin test was performed at each vaccination and assessed 48 h later. No positive DTH reaction was observed in any patient.
Tetramer staining assay
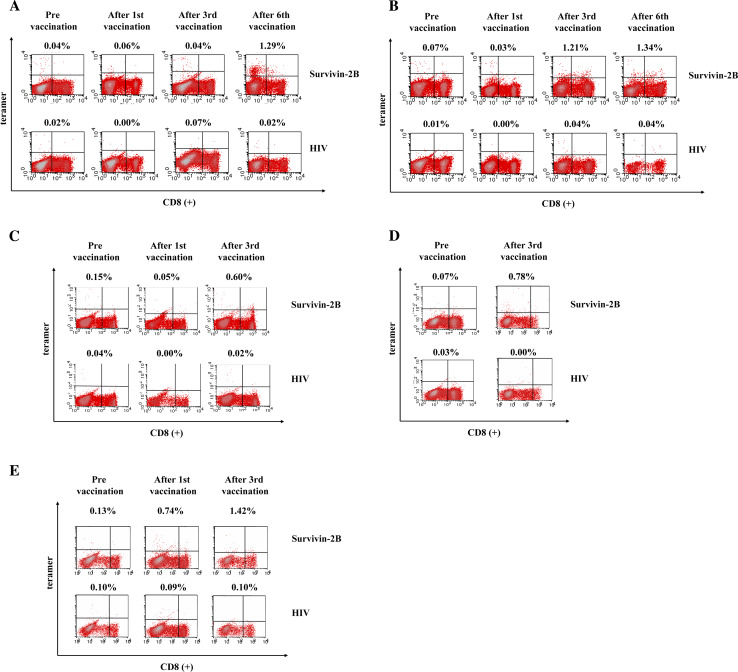
Peripheral blood lymphocytes of patients using HLA-A24/survivin-2B80-88 peptide tetramers were available from seven patients (cases 1, 2, 4, 6, 7, 8 and 9). HLA-A24/survivin-2B80-88 peptide tetramer analysis revealed a significant increase in the peptide-specific CTL frequency of CD8-positive T cells after the vaccination in five patients (cases 1, 2, 7, 8 and 9) (Table 2). In cases 1 and 2, the frequency of HLA-A24/survivin-2B80-88 peptide tetramer-positive CTLs was increased from 0.04 to 1.29% and 0.07 to 1.34%, respectively, after the sixth vaccination (Fig. 2a, b). In case 7 who could not receive the complete regimen due to disease progression, HLA-A24/survivin-2B80-88 peptide tetramer analysis after the third vaccination revealed an increase in the peptide-specific CTL frequency from 0.15 to 0.60% (Fig. 2c). In cases 8 and 9, peptide-specific CTL frequency increased from 0.07 to 0.78% and 0.13 to 1.42%, respectively, after the third vaccination (Fig. 2d, e). In these five patients, these CTLs did not show any increases of frequency with the HLA-A24/HIV peptide tetramer.
Fig. 2.
Frequency of peptide-specific CTLs analyzed by HLA-A24/survivin-2B80-88 peptide tetramer analysis (a case 1, b case 2, c case 7, d case 8 and e case 9). Lymphocytes were collected from peripheral blood of the patients before and after the first, third and sixth vaccinations, stained with an FITC-labeled anti-CD8 mAb and PE-labeled HLA-A24/survivin-2B80-88 peptide tetramer, and analyzed by flow cytometry. As a negative control, a tetramer with an HIV peptide was used. The frequency of CTL precursors was calculated as the number of tetramer-positive cells over the number of CD8-positive cells. The frequencies of HLA-A24/survivin-2B80-88 peptide tetramer -positive CTLs were increased from 0.04 to 1.29%, and 0.07 to 1.34% after the sixth vaccination in cases 1 and 2, respectively (a and b). In case 7, 8 and 9 peptide-specific CTL frequency increased from 0.15 to 0.60%, 0.07 to 0.78% and 0.13 to 1.42%, respectively, after the third vaccination (c–e)
Clinical responses
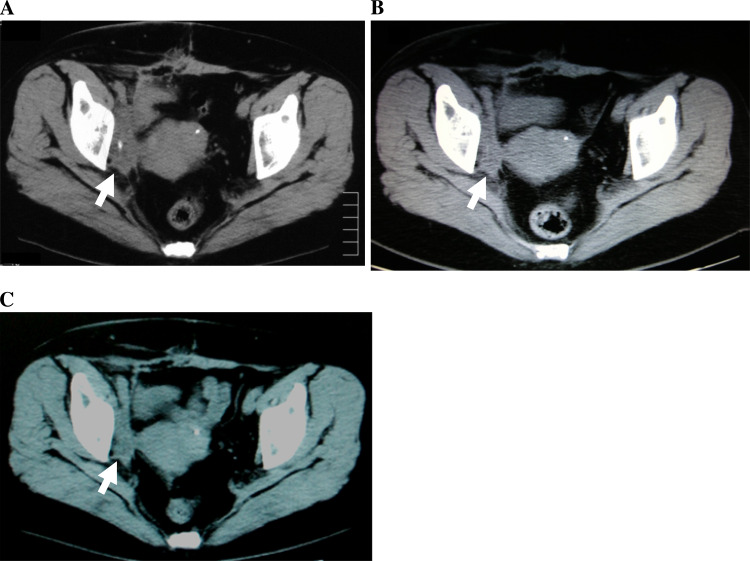
As indicated in Table 2, the two patients (cases 1 and 2) who showed increased frequencies of tetramer-positive CTLs showed slight reduction of the tumor volume after six-vaccination therapy. One responder (case 1) with right ureteral cancer who developed chemotherapy-refractory obturator lymph node metastasis chose to continue vaccination after finishing this phase I study because there were no severe adverse events. Before this study, as shown in Fig. 3a, pelvic CT showed that the recurrent right obturator node metastasis was 60 × 25 mm in size. The metastatic nodal disease was decreased to 46 × 15 mm after the sixth vaccination (arrow, Fig. 3b) and to 45 × 14 mm 21 months after first vaccination (arrow, Fig. 3c). In this patient with advanced urothelial cancer, slight reduction of the tumor volume was observed for 2 years, which was considered as minor response.
Fig. 3.
Pelvic CT findings of case 1 patient. Pelvic CT shows slight reduction of recurrent right obturator node tumor size after the sixth vaccination. The vaccinations were continued and, after 21 months, the size of the recurrent right obturator node tumor was almost unchanged. Arrows, recurrent right obturator node masses and changes of the tumor size (mm) are shown as follows: pre (a 60 × 25), after sixth vaccination (b 46 × 15), and after 21 months (c 45 × 14)
Discussion
High-throughput gene expression profiling of cancer has led to the discovery of many novel genes associated with it. An increasing number of T-cell epitopes derived from these various tumor-associated antigens have been reported and proved to play significant roles in CTL-based immunotherapy. Survivin was originally identified as a member of the inhibitor of apoptosis protein family. It has the capability to inhibit caspase-3, -7, and -9 in cells receiving an apoptotic stimulus and may lead to tumor initiation, progression, and therapeutic resistance [4]. It is expressed in colorectal, breast, and urothelial cancers but is hardly detectable in normal, differentiated adult tissues [2–5]. Moreover, overexpression of survivin in cancer cells is associated with unfavorable clinicopathologic variables such as poor prognosis and shorter patient survival rates.
Because of its cancer-specific expression and function of protecting cancer cells from apoptotic stimuli, survivin might be an ideal target for CTL-based immunotherapy. We focused on a survivin-derived peptide carrying the HLA-A24 binding motif. This HLA-A24 genotype is predominant in Japanese (about 60%) and less abundant in Caucasians (17%), Blacks (9%), and Hispanics (27%) [14]. Previously we reported that survivin-2B80-88, a survivin-derived peptide carrying the HLA-A24 binding motif, was established and CD8-positive CTLs were successfully induced by stimulation with this peptide in vitro [5]. In addition, we demonstrated that survivin was expressed in a large proportion of bladder cancer specimens, and this survivin-2B-derived peptide could induce CTL responses in the context of HLA-A24 [9]. On the basis of these observations, we started a phase I clinical study of survivin-2B80-88 peptide vaccination for patients with advanced or recurrent urothelial cancer.
The primary end point in the current clinical trial was to evaluate the safety and toxicity of survivin-2B80-88 peptide vaccination. In this study, there was no severe adverse event during or after vaccination in either the 0.1 or 1.0 mg group. Thus, we concluded that the survivin-2B80-88 peptide vaccine was safe and could be repeatedly injected into patients without serious side effects.
The secondary aims of our study were to evaluate vaccine-induced specific immune reactions and clinical responses. With respect to the immunological response, by objective scientific HLA-A24/peptide tetramer analysis, we could confirm that the peptide-based vaccines activated peptide-specific CTLs in some patients. Under conditions in which HIV peptide-specific CTL frequencies in PBMCs remained at background levels (less than 0.1%), the frequencies of tetramer-positive CTLs were increased after the vaccination in five cases. However, no positive DTH reaction was observed in any vaccination of the nine patients. A previous study reported that there was a positive correlation between DTH and clinical responses [15]. The CTL response induced by the survivin-2B peptide vaccine might not be sufficient to induce cytotoxic activity against bulky recurrent masses and induce dramatic clinical regression in patients with an advanced urothelial cancer.
HLA-A24/survivin-2B80-88 peptide tetramer analysis revealed tetramer-positive cells were detected in CD8-positive population (Fig. 2a–e), however, they were considered to be non-specific binding since they could be eliminated after titration of the tetramer. In order to confirm if CD8±/tetramer± cells represent the survivin2B80-88-specific CTL, CD8±/tetramer± T-cells were cloned from PBMCs of vaccinated patients by single cell sorting and analyzed for tetramer reactivity and specific killing activity. The CTL clones that were approximately 98% positive for the survivin2B80-88 tetramer showed the peptide-specific killing activity, indicating that CD8±/tetramer± cells represent the survivin2B80-88-specific CTLs (data not shown).
This survivin-2B80-88 peptide vaccination therapy may have limitations to induce clinically relevant results because of using only single HLA class I restricted peptides. Recently, a number of survivin epitopes restricted to several additional HLA molecules have been identified [16], and several clinical trials of immunotherapy based on survivin-derived peptides have been initiated. It would be interesting to identify and use other HLA class I or HLA class II survivin epitopes to induce more survivin-specific CTLs. Furthermore, it is possible that vaccination with the peptide in combination with some cytokines may be able to lead to stronger immune responses both in the induction and effector phases [17, 18]. On the basis of the information obtained from this study, further studies are required to evaluate the efficacy of the survivin-2B peptide vaccine in combination with various adjuvant drugs such as granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-2 and interferon (IFN). Our preliminary clinical study suggested that survivin-2B80-88 peptide vaccination in conjunction with IFN-α was more effective than the peptide alone in colon and pancreas cancer patients. Moreover, heat shock protein (HSP)-peptide complexes elicited antitumor responses in studies on immunization protocols. In our laboratories, we have found that HSPs such as Hsp70 and Hsp90 could be subjected to receptor-mediated uptake by antigen-presenting cells with subsequent representation of the HSP-associated peptides to HLA class I molecules on antigen presenting cells, facilitating efficient cross-presentation [19]. Toll-like receptors (TLR) have an essential role in the innate immune recognition of antigens [20]. Thus, it should be effective to use TLR-mediated signaling pathways to induce more survivin-specific CTLs.
Although our study consisted of only a limited number of patients, these preliminary data seem to suggest that survivin-2B peptide vaccination is safe without serious adverse events. As the first step, this study revealed that survivin-2B peptide-based vaccines activated peptide-specific CTLs and may be considered for potential immune and clinical efficacy in HLA-A24 positive/survivin-expressing patients with urothelial cancer. In the future, if the efficacy and safety of this vaccination therapy are established, we might be able to use this vaccine as an adjuvant therapy for high-risk non-immune-suppressed patients before systemic chemotherapy.
Conclusion
This phase I clinical study indicates that survivin-2B80-88 peptide-based vaccination is safe and should be further considered for potential immune and clinical efficacy in HLA-A24+ survivin-expressing patients with urothelial cancer.
Acknowledgments
Kumiko Shimozawa and Emiri Nakazawa provided technical assistance. This study was supported in part by a grant-in-aid for Clinical Cancer Research from the Ministry of Health, Labor and Welfare and a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 17390441 to T. Tsukamoto), and research grants from the Stiftelsen Japanese-Swedish Research Foundation, and Gotaro Sugawara Research Fund for Urological Diseases.
Abbreviations
- CR
Complete response
- CT
Computed tomography
- CTL
Cytotoxic T-lymphocyte
- DTH
Delayed-type hypersensitivity
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- HSP
Heat shock protein
- IFA
Incomplete Freund’s adjuvant
- IFN
Interferon
- NC
No change
- PBMC
Peripheral blood mononuclear cell
- PD
Progressive disease
- PR
Partial response
References
- 1.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/S1074-7613(00)80028-X. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki H, Altieri DC, Lu CD, et al. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–5074. [PubMed] [Google Scholar]
- 4.Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 5.Hirohashi Y, Torigoe T, Maeda A, et al. An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res. 2002;8:1731–1739. [PubMed] [Google Scholar]
- 6.Idenoue S, Hirohashi Y, Torigoe T, et al. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res. 2005;11:1474–1482. doi: 10.1158/1078-0432.CCR-03-0817. [DOI] [PubMed] [Google Scholar]
- 7.Tsuruma T, Hata F, Torigoe T, et al. Phase I clinical study of anti-apoptosis protein, survivinderived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19–29. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuruma T, Iwayama Y, Ohmura T, et al. Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med. 2008;6:24. doi: 10.1186/1479-5876-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura H, Torigoe T, Honma I, et al. Expression and antigenicity of survivin, an inhibitor of apoptosis family member, in bladder cancer: implications for specific immunotherapy. Urology. 2006;67:955–959. doi: 10.1016/j.urology.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 10.Koh YT, Higgins SA, Weber JS, et al. Immunological consequences of using three different clinical/laboratory techniques of emulsifying peptide-based vaccines in incomplete Freund’s adjuvant. J Trans Med. 2006;4:42. doi: 10.1186/1479-5876-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Sahara H, Tsukahara T, et al. Improved generation of HLA class/peptide tetramers. J Immunol Methods. 2002;20:177–184. doi: 10.1016/S0022-1759(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Nabeta Y, Tsukahara T, et al. Detection and induction of CTLs specific for SYT-SSX-derived peptides in HLA-A24(+) patients with synovial sarcoma. J Immunol. 2002;169:1611–1618. doi: 10.4049/jimmunol.169.3.1611. [DOI] [PubMed] [Google Scholar]
- 14.Date Y, Kimura A, Kato H, et al. DNA typing of the HLA-A gene: population study and identification of four new alleles in Japanese. Tissue Antigens. 1998;47:93–101. doi: 10.1111/j.1399-0039.1996.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 15.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 16.Andersen MH, Pedersen LO, Capeller B, et al. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 17.Ojima T, Iwahashi M, Nakamura M, et al. The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen. Int J Oncol. 2006;28:947–953. [PubMed] [Google Scholar]
- 18.Svane IM, Nikolajsen K, Walter MR, et al. Characterization of monocyte-derived dendritic cells maturated with IFN-alpha. Scand J Immunol. 2006;63:217–222. doi: 10.1111/j.1365-3083.2006.01728.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurotaki T, Tamura Y, Ueda G, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179:1803–1813. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 20.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60:795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]