Abstract
We have investigated the tumour therapeutic efficacy of homologous and heterologous prime-boost vaccine strategies against the 5T4 oncofoetal antigen, using both replication defective adenovirus expressing human 5T4 (Ad5T4), and retrovirally transduced DC lines (DCh5T4) in a subcutaneous B16 melanoma model (B16h5T4). In naïve mice we show that all vaccine combinations tested can provide significant tumour growth delay. While DCh5T4/Adh5T4 sequence is the best prophylactic regimen (P > 0.0001), it does not demonstrate any therapeutic efficacy in mice with established tumours. In active therapy the Adh5T4/DCh5T4 vaccination sequence is the best treatment regimen (P = 0.0045). In active therapy, we demonstrate that B16h5T4 tumour growth per se induces Th2 polarising immune responses against 5T4, and the success of subsequent vaccination is dependant on altering the polarizing immune responses from Th2 to Th1. We show that the first immunization with Adh5T4 can condition the mice to induce 5T4 specific Th1 immune responses, which can be sustained and subsequently boosted with DCh5T4. In contrast immunisation with DCh5T4 augments Th2 immune responses, such that a subsequent vaccination with Adh5T4 cannot rescue tumour growth. In this case the depletion of CD25+ regulatory cells after tumour challenge but before immunization can restore therapeutic efficacy. This study highlights that all vaccine vectors are not equal at generating TAA immune responses; in tumour bearing mice the capability of different vaccines to activate the most appropriate anti-tumour immune responses is greatly altered compared to what is found in naïve mice.
Keywords: Heterologous prime boost, Cancer vaccine, Oncofoetal antigens, CD25 regulatory T cells, T helper response
Introduction
Cancer vaccination may be defined as specific active immunotherapy of cancer as opposed to adoptive or passive immunotherapy, which entails immunising patients against antigens that are expressed in cancer with the goals of eradicating these cancer cells and distant metastases by the generation of robust cellular immune responses and immunological memory. The tumour employs several mechanisms to escape recognition by the immune system including; down regulation of components of the antigen-processing and antigen-presentation ‘machinery’ [1, 2], the production of cytokines that inhibit or divert productive effector responses [3], and the induction of tumour antigen specific T cell tolerance through normal pathways of self-tolerance generation [4–6]. Thus, a successful vaccine must be capable of channelling tumour antigens to appropriate dendritic cell (DC) (abbreviations see Appendix) subsets, and providing the optimal conditions for the maturation of DC into potent immunostimulatory antigen presenting cell (APC), generating antitumour immune responses capable of overcoming or reversing the level of T cell tolerance.
The choice of tumour rejection antigen is another important consideration in the optimal design of cancer vaccine strategies. Various classes of tumour associated antigens (TAA) are being exploited including those derived from viral and mutated genes, differentiation or embryonic antigens. In principle tumour antigens corresponding to fetal gene products or products that are expressed in immunoprivileged sites, will have triggered little or no tolerance therefore making them excellent tumour rejection antigens [7]. The human 5T4 oncofoetal antigen was originally defined by a monoclonal antibody made against human trophoblast glycoproteins [8]. 5T4 is an attractive tumour target showing only low expression in normal tissues but is frequently expressed by carcinomas of diverse origin [8, 9]. In colorectal, gastric and ovarian cancer the tumour associated expression of this molecule has been shown to correlate with poorer clinical outcome [10–15]. Several approaches to developing immunotherapies against this target are under development; homologous prime-boost vaccination with modified vaccinia ankara (MVA) h5T4 have shown efficacy in animal models of human 5T4 expressing tumour protection and active therapy [16]. This and the establishment of a repertoire of human CD8 T cell responses in normal individuals has supported ongoing clinical trials in colorectal cancer patients [17].
We have investigated the potential of replication defective recombinant adenoviruses (Ad) as vectors for a 5T4 cancer vaccines used in combination with a DC line (DC2.4) constitutively expressing the 5T4 oncofoetal antigen. Such prime heterologous boost approaches can obviate the limitations generated by antivector immunity and maximise the anti-TAA response [18–21]. Several studies have demonstrated the advantages of DC or adenovirus vectors for delivery of TAA to the immune system and elicitation of tumour antigen specific CD8 T cell responses [22–28]. The DC2.4 cell line is an immortalised dendritic cell line, which can be as effective as bone marrow derived DCs in providing increased tumour survival in prophylactic and therapeutic model of MK16 tumour transplants [29–31]. The availability of DC cells expressing 5T4 offers the opportunity for better in vitro restimulation of lymphocytes from immunised animals when analysing the generation of CD4+ and CD8+ specific responses.
We utilize the B16 melanoma tumour model (B16F10) [32] stably expressing the human 5T4 antigen under neomycin selection to evaluate the efficacy of these 5T4 vaccines. Such a genetically engineered model of tumour immunity directed at human 5T4 is a first step in preclinical evaluation of different vaccination regimes. The B16 melanoma is an attractive tumour model for evaluation of immunotherapies for several reasons; principally because of its relative resistance to immunotherapy. In this study we have compared homologous and heterologous prime-boost vaccine strategies with first generation adenovirus vectors and DC lines expressing h5T4, for the optimal induction of h5T4 immunity, and tumour protection against 5T4 positive B16 melanoma in both prophylaxis and active therapy.
Materials and methods
Mice
Six to 8 week female C57Bl/6 mice were obtained from Charles River. The mice were bred and housed under specific pathogen free conditions. All experimental procedures were conducted in accordance with the British Home office guidelines.
Cell lines
B16F10 are melanoma cells derived from spontaneous melanoma tumours in C57Bl/6 mice [32]. A B16 cell line expressing human 5T4 (= h5T4, B16h5T4 was isolated by transfection using a PCMVα neo vector encoding h5T4 cDNA [33]. B16.neo was generated by transfecting with empty vector. DC2.4 (H-2b) is a DC line established by transducing bone-marrow derived DCs from C57Bl/6 with GM-CSF, followed by super transfection with myc and raf oncogenes (kindly provided by K.Rock, Dana Farber Cancer Institute, Boston). The 293 cell line (ATCC), the Hela cell line (ATCC) and the Cre8 (provided by S. Hardy, Somatix, Alamada, CA), were used for recombinant adenovirus amplification and purification. All these cell lines were cultured in DMEM, supplemented with 10% FCS, 2 mM glutamine, 100 U/ml penicillin and 100 U/ml streptomycin. The transfected B16 cell lines were routinely cultured in medium supplemented with G418 (100 μg/ml). All media was obtained from Sigma (Dorset, UK) and all supplements from Life Technologies (Paisley, UK).
Hybridomas
For CD4+depletion rat anti CD4 (ATCC TIB207) was used. The hybridoma was cultured in Iscoves medium supplemented with sodium bicarbonate (1.5 g/l), 20% FBS and 100 U/ml penicillin and 100 U/ml streptomycin. For CD8+ depletion rat anti CD8 (ATCC TIB 105) was used. This hybridoma was cultured in RPMI supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 100 U/ml streptomycin. For depletion of CD25+ rat anti CD25 clone PC615.3 (ECACC 88041902) was used and this was cultured in RPMI supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 100 U/ml streptomycin The anti CD4, anti-CD8 and anti-CD25 antibodies were purified from hybridoma supernatant by Sepharose G purification. Protein concentration was assessed by spectrophotometry.
Generation of Recombinant adenovirus expressing h5T4 oncoprotein
Recombinant replication defective E1-E3 deleted ψ5 viruses were constructed as previously described [34] by co transfection of Cre 8 with ψ5 viral DNA and Sfi 1 digested pAdloxh5T4. Recombinant viruses were passaged twice in Cre8 cells to reduce contamination of with ψ5 adenovirus. The Adh5T4 was expanded on Cre8 cells and purified by cesium chloride gradient centrifugation. The virus titre was determined by end-point dilution, a cytopathic effect assay, and spectrophotometry (Abs 630 nm, 1 mg/ml protein = 3.4 × 1012particles/ml = 3.4 × 1010 pfu/ml). Stocks of Adh5T4 had titres of 3.7 × 1010 pfu/ml. AdGFP was used as a control and this virus was grown in 293 cells [35]. Expression of h5T4 by Adh4T4 was confirmed by Facscan analysis of BHK cells infected with Ad.h5T4 (MOI 300) using the mab5T4 antibody.
Retroviral transfection and cloning of DC2.4h5T4
For retroviral expression, the h5T4 was cloned as a BamH1 fragments into the retroviral pLX plasmid [36]. Briefly, the full length h5T4 was amplified by PCR from the plasmid pBS2.1, using the following conditions; 5% DMSO, 2 mm MgSO4, 1 ml × 10 mM dNTPs, 1 ml × 50 pmol primers, and 1 × Taq polymerase. The primers used; forward = GACTCGGATCCAGCCGCGATGCCTG M/H Fret, which has a start codon BamHI site, and the reverse primer used was the following; TTGGTGGATCCTCTAATATTTCTCCAG HRRET, which contains BamHI site and the stop codon. Positive pLX-h5T4 clones were then transfected into amphotrophic GP-AM12 cells. Five milliliter of filtered supernatant from the viral producer cells and 2 mg/ml polybrene was used to transduce 2 × 105 DC2.4 cells. Three rounds of FACS sorting for 5T4 positive cells was followed by dilution cloning. Several DC2.4h5T4 clones were stained with a panel of RPE- or FITC-conjugated monoclonal antibodies against typical DC surface markers. Isotype matched monoclonal antibodies were used as negative controls. RPE labelled antibodies were against CD80, CD86 and CD40 (Serotec, Oxford, UK). FITC labelled antibodies included CD11C, I-Ab, H2Kb (BD Pharmingen, Heidelberg, Germany). Immunostained cells were analysed on a Facsan flow cytometer (BD Biosciences) using PCLysys Software (BD Biosciences). A clone with stable high h5T4 expression and good expression of DC surface markers was selected. The DCh4T4 expresses high levels of MHCI, MHCII, and high levels of the co-stimulatory antigen B7.2, but low levels of B7.1 and CD40. This phenotype is consistent with a more mature DC with antigen presenting capabilities. Treatment with mitomycin C does not change the phenotype.
In-vivo tumour assays
Protection
Groups of 13 mice were primed with either 1 × 109 pfu Adh5T4 (sc and im) or 1 × 106 DC2.4h5T4 on day 0. On day 7 mice were boosted with either homologous, Adh5T4/Adh5T4 or DCh5T4/DCh5T4; or heterologous Adh5T4/DCh5T4 or DCh5T4/Adh5T4 vector combinations. Control vaccinations used appropriate AdGFP and DC2.4 combinations. Twenty-one days after boost vaccination, seven mice were challenged with 5 × 105 B16.h5T4 cells/200 μl/sc. The product of perpendicular tumour dimensions was determined essentially every other day until reaching 1.24 cm2. At least two separate experiments were performed in each tumour challenge scenario. The remaining mice were used to characterise the immune responses to h5T4 after priming and boosting.
Active therapy
Groups of seven mice were challenged with, 1 × 105 B16h5T4 cells/200 μl/sc on day 0. Following tumour challenge the mice were immunised on day 7 with either 1 × 109pfu Adh5T4 (sc and im) or 1×106 DCh5T4 or the appropriate controls. On day 14 the mice were boosted with either the homologous, Adh5T4/Adh5T4 or DCh5T4/DCh5T4; or heterologous Adh5T4/DCh5T4 or DCh5T4/Adh5T4 vaccines. Tumour growth analysis in these experiments, were measured from day 3, and then every alternative day until reaching 1.24 cm2.
Antibody depletion of T cell subsets
Following immunisation, T cell subsets were depleted in-vivo using rat antibodies to CD4, CD8 or CD25 starting 3 days before tumour challenge on day 25, using 0.3 mg/0.5 ml, and then on day 30 followed by once weekly depletion with 0.3 mg/0.5 ml antibody thereafter. In the active therapy, mice were depleted of CD25 lymphocytes with 0.5 mg/ml on days 4 and day 11 following tumour challenge, 3 days before each vaccination. Deletion efficacy was confirmed by FACs analysis of splenocytes 5 days after the antibody treatment were the target subsets were essentially undetectable.
Statistical analysis
Survival in protective and active treatments was analyzed with standard Kaplan–Meier plots. The log rank test was used to analyse the statistical differences between the vaccine groups versus controls. In all experiments a P < 0.05 was considered significant.
The one-way ANOVA test with the Turkeys multiple range test was used to compare whether there were any statistical differences between the cytokine release obtained with each vaccine treatment.
Immune analysis
In naïve mice immune responses to single and prime-boost vaccinations were measured by harvesting spleens 7 days post prime and 7 and 21 days post prime-boost on days 14 and 28, respectively. For each vaccination two spleens were pooled and the splenocytes harvested tested simultaneously in all assays described below. Each of the immune assays was repeated two or more times.
In tumour challenged mice immune responses were characterised following a single and prime-boost vaccination by harvesting blood and spleen 7 days post prime and 7 days post prime boost on days 14 and 21, respectively. In this instance three mice were used per treatment and the spleens harvested tested as individual responses to treatment using the assays described. This was repeated twice.
Immune assays
ELISA
Flexible ELISA plates were coated with 0.5 mg/ml of h5T4-Fc [37] overnight at 4°C, washed and blocked with 2% Marvel in PBS for 1 h at 37°C. Doubling dilution of sera were added for 2 h at 37°C and following washing incubated for 1 h at 37°C with rabbit anti mouse HRP labelled secondary antibody diluted at 1:1,000 in PBS, 2% Marvel (DAKO, Glostrup, Denmark). To further delineate the isotype response the following HRP labelled antibodies were used: Goat anti-mouse IgG1 (γ) polyclonal, Goat anti-mouse IgG2a (γ) polyclonal, Goat anti-mouse IgG2b (γ) polyclonal (AMS Biotechnology, Abingdon, UK). The reactions were developed using 100 μl of a 0.1 mg ml−1 solution of 3,3′,5,5′,-tetramethylbenzidine (TMB) (Sigma, MO, USA) in 50 mM phosphate–citrate buffer, pH 5. The reaction was stopped by the addition of 50 μl of 1 M H2SO4, and absorbance was read at 450–650 nm on the E max precision microplate reader (Molecular Devices, CA, USA).
Cytokines in culture supernatants were measured by ELISA using kits for INF-γ (Th1 type), IL–5, and IL-10 (Th2 type) (OptEIA; BD PharMingen). Briefly, 96-well plates were coated with the appropriate anti-cytokine antibodies overnight. After the plates were blocked with bovine serum albumin (BSA), plates were incubated for 2 h with culture supernatant or standard, the plates were developed with biotin-conjugated anti-cytokine Abs. Horseradish peroxidase-conjugated streptavidin was added before development with ELISA substrate solution (TMB) (Sigma, MO, USA).
Lymphocyte restimulation in vitro
Splenocytes were plated in six well plates (1 × 107 cells), along with DCh5T4 (1 × 106 cells) at a ratio of 10:1 in 5 ml complete medium. DC2.4 cells when used as stimulators were treated with mitomycin-C (50 μg ml−1, 30 min incubation). Two milliliter of fresh medium was added on day 3. After 5 days, splenocytes were used in a cytotoxicity assays. For characterisation of cytokine profiles, splenocytes (4 × 106 cells) were plated in 12 well plates along with DCh5T4 or DC2.4 (2 × 105 cells) at a 20:1 ratio in 2 ml of complete medium. After 96 h supernatant was harvested and analysed using INF-γ, IL-5, and Il-10 specific ELISA.
51Cr cytotoxic assay
The B16 cells were pre-treated with 1 (g ml−1 murine INF-( (R&D Systems, Abingdon, UK) to maximise MHC class I expression. Target cells (B16neo, B16h5T4) were labelled with Cr51 20 ml (100 μCi) (ICN, Belgium) for 1.5 h at 37°C washed and distributed as 1 × 103 cells/well/50 μl in V-96-well plates. Effector cells were then added at various E:T ratios (100:1, 50:1, 25:1 etc.) to a final volume of 150 μl with each ratio tested in triplicates. Maximum 51Cr release from targets was determined from supernatants of cells lysed with 100 μl PBS 2% Tween, whilst spontaneous release was obtained by incubating target cells in medium alone. The plates were incubated at 37°C/5% CO2 for 4 h. A total of 90 μl of supernatant was collected from each well and placed on 96 well LumaPlates (Yttrium Silicate scintillator coated plates, Packard Bioscience, Groningen, Netherlands), left to dry overnight, and then counted by a Packard TopCount instrument. Percentage of specific lysis was calculated by comparing the radioactive counts relative to the maximum and background counts according to the following formula:
 |
In order to characterise the lymphocyte population responsible for cell cytotoxity, effector cells (same source as above) were incubated for 30 min with anti-rat CD4 or anti-rat CD8 monoclonal antibodies before targets were added to an E:T ratio of 100:1.
Results
Immunogenicity of Adh5T4 and DCh5T4 vaccine combinations in naïve mice
In the first instance we were interested in characterising the range of 5T4 specific immune responses generated following homologous and heterologous prime-boost immunisation with Adh5T4 and DCh5T4 vaccines. The dose for each vaccine used was predetermined by previous studies [22] and pilot experiments established that 109 pfu Adh5T4 given via the sc + im was the optimal single dose for the induction of 5T4 specific antibody and CTL responses (data not shown). A dose of 1 × 106 DCh5T4 given sc was used based on previous studies [30].
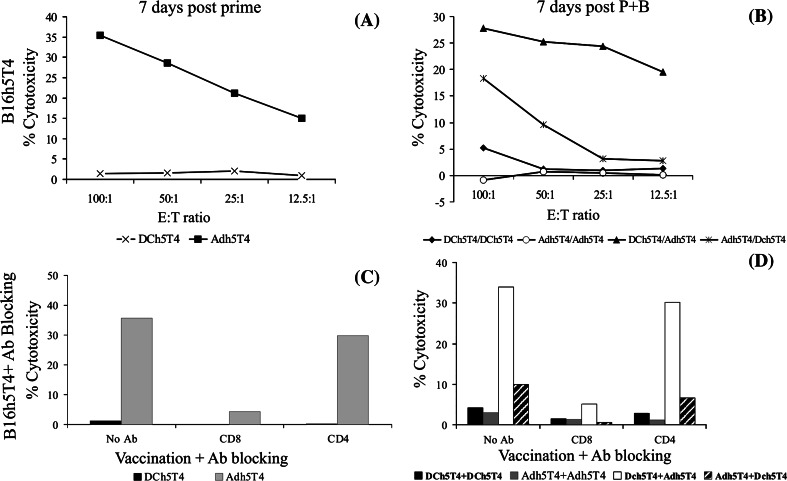
The induction of cytotoxic T cell responses is considered important for the control of tumour growth. Splenocytes from vaccinated mice were restimulated in vitro for 5 days with DCh5T4 and subsequently assessed for their ability to lyse B16h5T4 using a standard 4 h chromium release assay. Splenocytes harvested following a single Adh5T4 vaccination but not DCh5T4 could specifically lyse B16h5T4 (Fig. 1a), whilst these failed to show any specific lysis of B16neo control tumours (data not shown). Following boost vaccination, cytotoxic killing could be reproducibly demonstrated in mice vaccinated with DCh5T4/Adh5T4 (E:T=100:1; range of killing 25–60%) or Adh5T4/DCh5T4 (E:T=100:1; range of killing 15–60%). It was more difficult to demonstrate reproducible cytotoxic killing (greater than 10% at E:T 100:1), with splenocytes treated with Adh5T4/Adh5T4 and DCh5T4/DCh5T4 (Fig 1b). We show that the cytotoxic killing is CD8+ T cell mediated as co-culture of splenocytes with CD8 specific antibody prior to incubation with B16h5T4 completely abrogates cytotoxic killing, while co-culture with CD4 antibody has little or no effect (Fig. 1c, d).
Fig. 1.
Cytotoxic cellular response measured by 51CR release from B16h5T4 targets. a, b Cytotoxic killing of B16h5T4 by splenocytes harvested 7 days after a single vaccination with Adh5T4 or DCh5T4 (a) or by splenocytes harvested 7 days after prime-boost (b) (pooled from two mice). c, d cytotoxic killing of B16h5T4 by the same splenocytes harvested after single vaccination (c) or prime-boost (d) immunization but in the presence of antibody blocking. Specific cytotoxic killing of B16h5T4 targets after prime only occurs following vaccination with Adh5T4, whilst after boost significant killing is observed with the treatments Adh5T4/DCh5T4, and DCh5T4/Adh5T4. Cytotoxic killing is CD8+ specific in all cases as pre-incubation of splenocytes with monoclonal antibodies against CD8+ completely abrogate cytotoxic killing, whilst CD4 blocking has little or no effect (c, d). Data from one of four experiments is presented
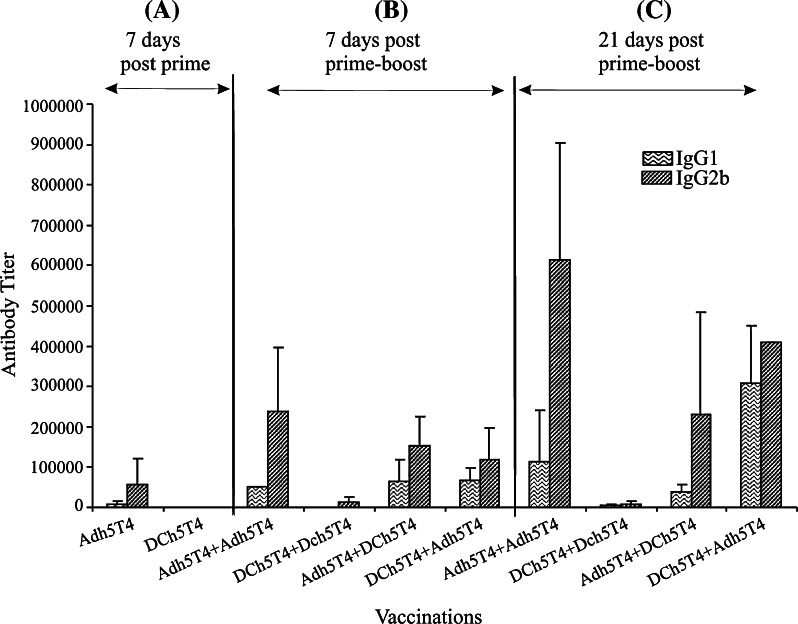
The induction of T helper responses polarising toward Th1 or Th2 was demonstrated by characterising antibody isotypes (IgG2b = Th1 versus IgG1 = Th2) in sera and measuring cytokine release (INF-γ = Th1, or IL-5 and IL-10 = Th2) from splenocytes restimulated in vitro with either DCh5T4 or DC2.4. Analysis of 7 day post boost sera, shows that with each treatment the antibody isotype profiles are IgG2b > IgG1, but with differential induction. Increasing antibody titres are observed with time, with maintenance of antibody isotype polarisation except for DCh5T4/DCh5T4 where the titres are consistently low (Fig. 2 and legend).
Fig. 2.
T helper responses indicated by isotype antibody responses (IgG1 vs. IgG2b) to the different Adh5T4 and DCh5T4 vaccine regimens, following a 7 days post prime, b 7 days post P + B and c 21 days post P + B. There is significant induction of IgG2b (115,200) versus IgG1 (25,600) responses following a single vaccination with Adh5T4 indicating a Th1 bias. Seven days following homologous or heterologous P + B, the isotype responses for all treatments is IgG2b > IgG1, but with differential induction. Following homologous or heterologous boost 5T4 specific isotype antibodies are increased with a bias of IgG2b versus IgG1 for all treatments including an Adh5T4 immunisation (Fig. 1a) [IgG1(10,000–150,000) : IgG2b(200,000–500,000) titre range] . The homologous DC vaccinations produce significantly less antibodies (titre IgG1(800) IgG2b(26667)), but are still biased towards IgG2b. Further increases in antibody titre are observed 21 days post boost, where the IgG2b/IgG1 bias is maintained for those vaccinations with Adh5T4 as the priming immunisation whereas the DCh5T4 + Adh5T4 showed increased levels of both IgG1 and IgG2b; antibody responses to homologous DCh5T4 + DCh5T4 vaccinations stay low. The figure represents average titres (±SE) from four independent experiments carried out on serum samples immunized with the vaccine combinations on four separate occasions. Analysis of the individual independent experiments shows the trends (antibody polarization) remain the same for each experiment, although the antibody titre measured differs each time
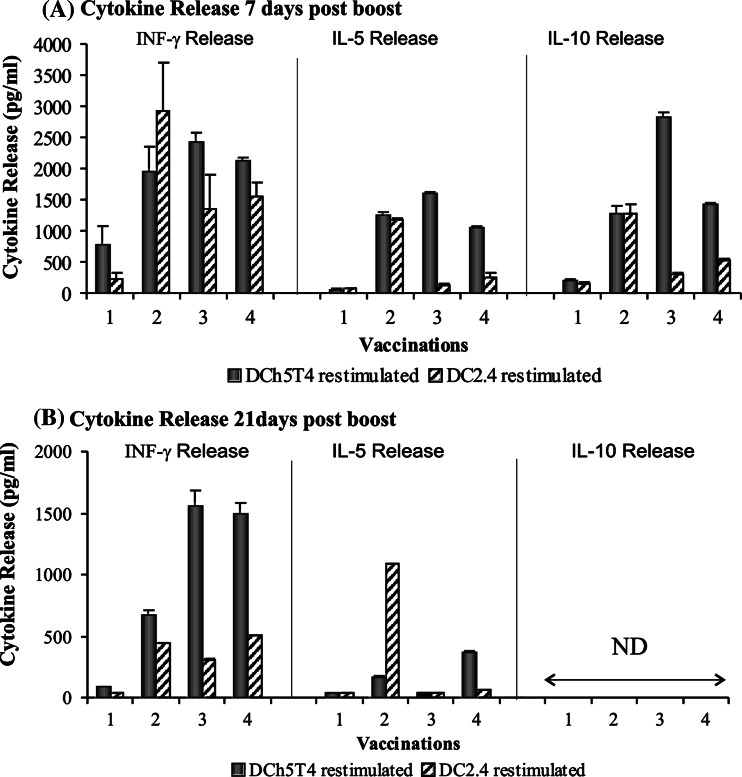
In terms of cytokine release (Fig. 3a), vaccination with Adh5T4/Adh5T4 generates a Th1 type response with significant production of INF-γ compared with control vaccinations and little or no IL-5 or IL-10. Vaccination with Adh5T4/DCh5T4 or DCh5T4/Adh5T4 induces a mixed Th1/ Th2 response, with significant production of INF-γ, IL-5 and IL-10. The interpretation of Th1/Th2 bias from these data is partly compromised by the significant release of INF-γ with the control DC vaccinations. Polarising immune responses to DCh5T4/DCh5T4 vaccination were difficult to assess because there is similar production of cytokines by therapeutic and control vaccinated splenocytes. This probably reflects immune responses to FCS antigens which may be presented by the DCs used as the vaccine component. We used the one way ANOVA test with Turkeys comparison to test whether the cytokine release for each treatment was statistically different to each other at day 7. Statistical comparison of INF-γ release between Adh5T4/Adh5T4, Adh5T4/DCh5T4, DCh5T4/Adh5T4, illustrates that there is no difference between Adh5T4/DCh5T4 and DCh5T4/Adh5T4, but both of these are significantly different from Adh5T4/Adh5T4 (P = 0.006). In terms of IL-5 secretion between Adh5T4/Adh5T4, Adh5T4/DCh5T4, DCh5T4/Adh5T4 treatments, each of the treatments are significantly different to each other (P<0.0005). In terms of IL-10 secretion between Adh5T4/Adh5T4, Adh5T4/DCh5T4, DCh5T4/Adh5T4 treatments, each of the treatments are significantly different to each other (P<0.0005). Cytokine release from DCh5T4/DCh5T4 restimulated splenocytes was not compared because of the non-specific background.
Fig. 3.
T helper responses indicated by cytokine Release of INF-γ, IL-5, IL-10 from splenocytes harvested a 7 days post prime-boost and b 21 days post prime-boost, restimulated in vitro with DCh5T4. Cytokine release determined from culture supernatant from splenocytes restimulated in vitro for 96 h with DCh5T4 at an E;T ratio of 20:1 was tested using the cytokine Elisa kits from BD Pharmingen. Early cytokine responses represent a mixture of Th1 and Th2 cytokines. Splenocytes vaccinated Adh5T4/Adh5T4 only secrete INF-γ, whilst splenocytes vaccinated with DC5T4/Adh5T4 or Adh5T4/DCh5T4 secrete all three cytokines. It is difficult to interpret cytokine profiles from the DCh5T4/DCh5T4 as there is non-specific release of cytokines with DC2.4 restimulation in vitro. 21 days following boost there is no IL-10 secretion by splenocytes. IFN-γ secretion is no longer detectable in the Adh5T4/Adh5T4 vaccination group. Interestingly the mixed Th1/Th2 cytokine profile is sustained with DCh5T4/Adh5T4 vaccination, whilst Adh5T4/DCh5T4 appears polarised to a Th1 biased response. (1 Adh5T4/Adh5T4 vs. AdGFP/AdGFP, 2 DCh5T4/DCh5T4 vs. DC2.4/DC2.4, 3 Adh5T4/DCh5T4 vs. AdGFP/DC2.4, 4 DCh5T4/Adh5T4 vs. DC2.4/AdGFP)
Analysis of cytokine production by splenocytes harvested 21 days after boost, showed no IL10 secretion following any treatment. INF-γ was no longer detectable in the Adh5T4/Adh5T4 vaccination group. Interestingly the mixed Th1/Th2 cytokine profile is sustained with DCh5T4/Adh5T4 vaccination, whilst Adh5T4/DCh5T4 appears polarised to a Th1 response (Fig. 3b). Statistically the Adh5T4/DCh5T4 and DCh5T4/Adh5T4 are similar in terms of INF-γ release, but are both different from Adh5T4/Adh5T4 (P<0.0005), whilst for IL-5 secretion there is no statistical difference between Adh5T4/Adh5T4 and Adh5T4/DCh5T4, but these are both different from DCh5T4/Adh5T4 restimulated splenocytes (P<0.0005). This cytokine polarisation data appears consistent with the Th1 or Th2 profiles reflected from the antibody isotype analysis for the different vaccine combinations.
In conclusion, the different vaccine combinations can engage a broad range of immune effectors, with differential induction and persistence of antibodies, CD4+ and CD8+ T cell responses reactive against the h5T4 antigen. The heterologous prime-boost regimens are more efficient in inducing and sustaining 5T4 specific CD8+ T cells with cytokine and antibody isotype ratios consistent with a bias to a Th1- type response.
Efficacy of prophylactic vaccination against B16h5T4 (tumour protection)
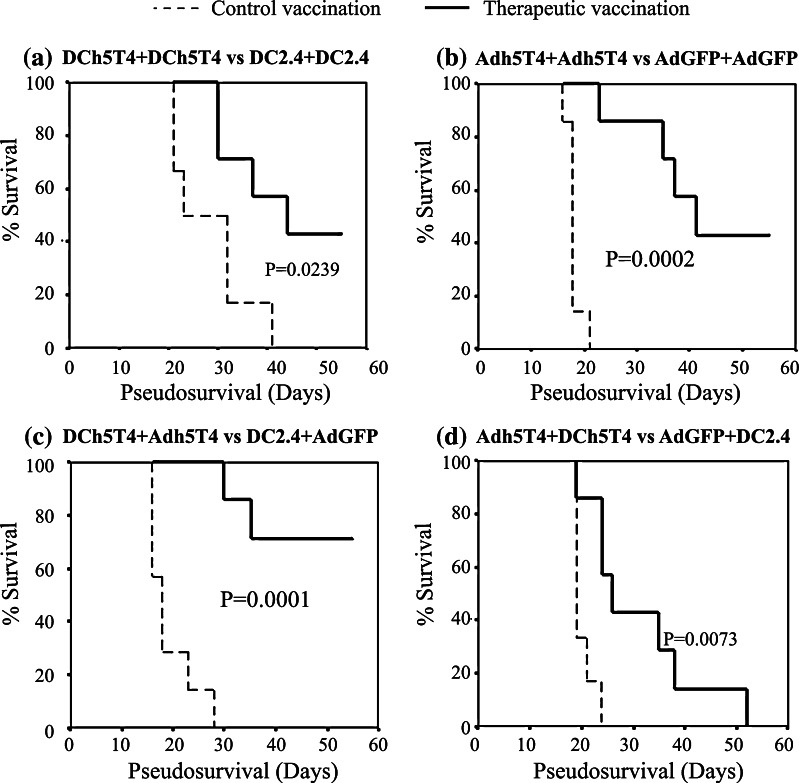
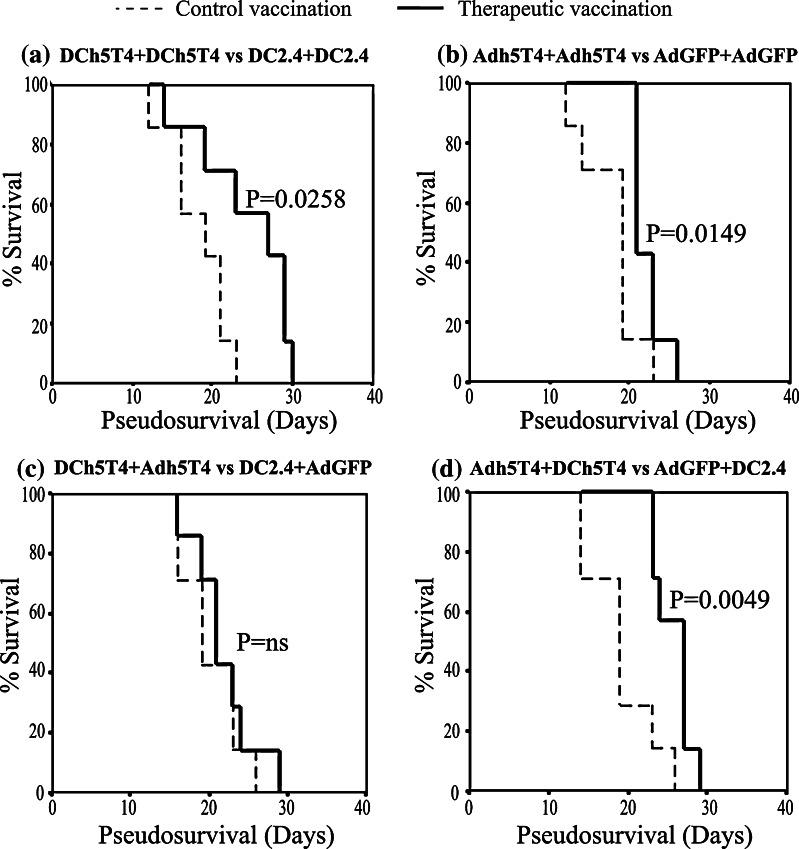
Next we tested the efficacy of the different prime-boost vaccine combinations to provide tumour protection. Mice were pre-immunised with the following treatment combinations; Adh5T4/Adh5T4, DCh5T4/DCh5T4, Adh5T4/DCh5T4, DCh5T4/Adh5T4 or appropriate vector controls and then challenged with a lethal dose of B16h5T4 melanoma. Essentially all treatment combinations tested can provide a statistically significant delay in tumour growth compared to control vector vaccinations (P<0.05) (Fig. 4a–d). In prophylaxis, vaccination with DCh5T4/Adh5T4 provided the best protection as 5/7 mice were tumour free at the end of the study followed by, Adh5T4/Adh5T4 (3/7 tumour free), DCh5T4/DCh5T4 (3/7 tumour free), and lastly Adh5T4 /DCh5T4 (0/7). Thus despite differences in magnitude and persistence of immune effectors reactive against h5T4 that can be measured in vitro, all treatment combinations are able to induce protective tumour immunity.
Fig. 4.
Vaccinations with DCh5T4/DCh5T4, Adh5T4/Adh5T4, DCh5T4/Adh5T4 and Adh5T4/DCh5T4 induce protective tumour immunity to B16h5T4 tumour challenge. Groups of seven mice were primed on day 0 and then boosted on day 7 with the above vaccine combinations. Mice were challenged with 5 × 105 B16h5T4 21 days post boost and tumour growth monitored until the product of perpendicular tumour diameter reached 1.24 cm2. The data is presented as a Kaplan–Meier survival curves and representative two independent studies. Log Rank test was used to determine whether vaccine mediated survival was significant versus respective control vaccinations
Immune protection: in-vivo mechanisms
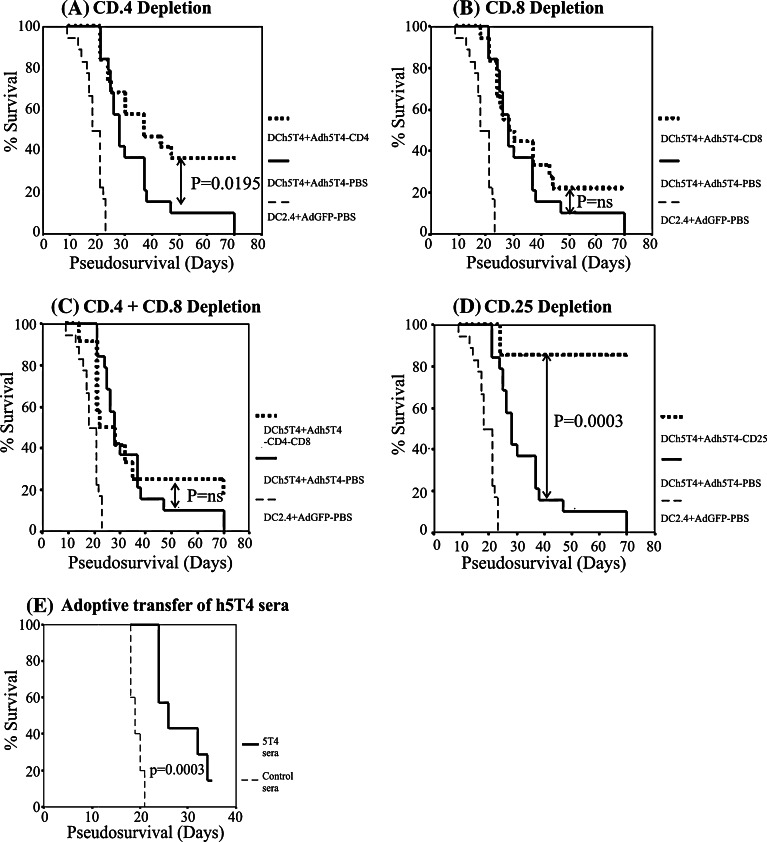
A range of immune effectors can be measured in vitro following vaccination. In order to gain more mechanistic insight into the role of different immune effectors in delaying B16h5T4 tumour growth following the best vaccination, DCh5T4/Adh5T4, groups of immunised mice were selectively depleted with rat anti mouse monoclonal antibodies against CD4, CD8, CD4/CD8 populations starting 3 days before tumour challenge. In Fig. 5a depletion of CD4+ T cells resulted in a statistically significant enhancement in tumour growth delay (P = 0.012) with more mice tumour free at the end of the study. By contrast, depletion of CD8 T cells made no impact on the vaccination induced protection (Fig. 5b), while removal of both CD4 and CD8 effectors obviates the improved protection offered by CD4 depletion alone, suggesting that CD8 maybe important for the anti-tumour effect (Fig. 5c). However, the combined depletion of CD4+CD8 lymphocytes does not alter the level of tumour protection compared with DCh5T4/Adh5T4 (undepleted) suggesting that antibodies and other innate immune effectors may also contribute to tumour growth delay. In support of this we have shown that adoptive transfer of sera from immunised mice to naïve mice can provide tumour protection against subsequent challenge with B16h5T4 (Fig. 5e)
Fig. 5.
In-vivo lymphocyte depletion and adoptive transfer for characterisation of tumour immune mechanism. Following vaccination with DCh5T4/Adh5T4 groups of mice were depleted of, a CD4, b CD8, c CD4 + CD8, and d CD25 starting 3 days before tumour challenge. In the absence of CD8 cells or combined depletion of both CD8 and CD4 mice are still protected. Depletion of CD4 enhances protective efficacy but depletion of CD25 provides the greatest tumour protection. Adoptive transfer of sera from vaccinated mice can also delay tumour growth significantly compared to nonvaccinated sera (e). Data from separate depletion studies (3 × CD4 and CD8, 2 × CD4/CD8, 1 × CD25) which gave the same basic result in the repeats but provided increased statistical power when pooled (n = 7–18 for each group), are presented as Kaplan–Meier survival plots, and the log rank test was used to compare influence of DCh5T/Adh5T4 (depleted) versus DCh5T4/Adh5T4 (undepleted)
The potentiation of immune protection by CD4 depletion suggests that vaccination with DCh5T4/Adh5T4 induces cells within the CD4 population with a suppressor phenotype. To study whether “conventional” CD4+CD25+ regulatory cells are involved in the suppression of vaccine-mediated immunity, groups of immunised mice were depleted of CD25+ cells. In Fig. 5d, the depletion of CD25+ cells leads to a statistically significant increase in tumour free survival.
Anti-tumour efficacy of therapeutic vaccination (active therapy)
Therapeutic immunity following vaccination in tumour-bearing mice is much more difficult to establish. With the knowledge that both positive and negative immune effectors may be generated following prophylactic vaccination, we tested the relative efficacy of the different prime-boost regimens in mice with established B16h5T4 tumours (Tumour size is 0.3 cm2 at day 7, with median survival 19 days to reach tumour limit of 1.24 cm2). Surprisingly, in active therapy DCh5T4/Adh5T4, the regimen that afforded the best protection against tumour challenge, showed no effect compared to control treatment (median survival 21 vs. 19 days, P = ns). By contrast, the reciprocal order of prime boost vaccination Adh5T4/ DCh5T4 provided the greatest delay in tumour growth compared to control treatment (median survival 26 vs. 19 days, P = 0.005). The homologous immunisations were also somewhat effective compared to controls with DCh5T4/DCh5T4 (median survival 27 vs. 19 days, P = 0.026) or Adh5T4/Adh5T4 (median survival 21 vs. 19 days, P = 0.015) (Fig. 6a–d). In a repeat experiment the same rank order of therapeutic effect was obtained except with homologous DC vaccination which failed to provide any significant tumour delay compared to control.
Fig. 6.
Comparing vaccine efficacy of DCh5T4/DCh5T4, Adh5T4/Adh5T4, DCh5T4/Adh5T4 and Adh5T4/DCh5T4 in active therapy. Adh5T4/DCh5T4, DCh5T4/DCh5T4 and Adh5T4/Adh5T4 can delay tumour progression, but DCh5T4/Adh5T4 provides no survival advantage. Groups of seven mice were challenged with tumour on day 0 and then treated on day 7 and 14 with the above treatment and tumour growth monitored until the product of perpendicular tumour diameter reached 1.24 cm2. The data is presented as a Kaplan–Meier survival curves and is representative two independent studies. Log Rank test was used to determine whether vaccine mediated survival was significant versus respective control vaccinations
Characterisation of h5T4 immunity in tumour bearing mice following heterologous prime-boost vaccination
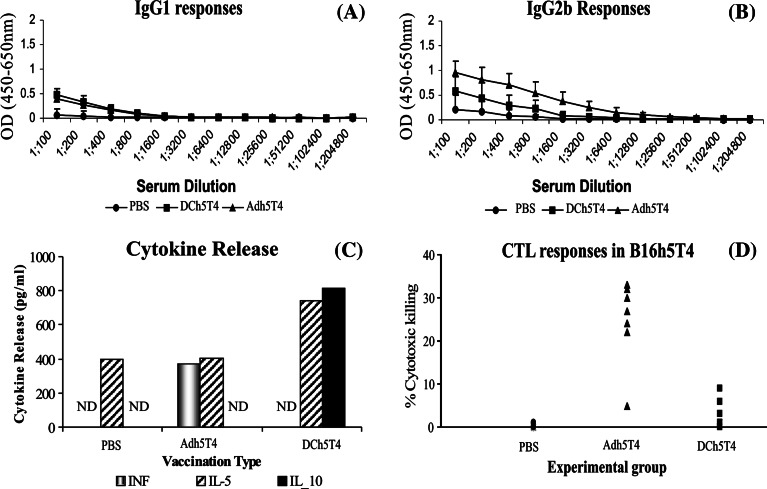
To understand the differences in efficacy between DCh5T4/Adh5T4 versus Adh5T4/DCh5T4 in tumour bearing mice, the effect of therapeutic vaccination on 5T4 immune responses (Th1/Th2) was tested by examining the 5T4 specific IgG isotype, cytokine profile and CTL generation 7 days following a single (Fig. 7a–d) and prime-boost immunisations. Importantly we show that B16h5T4 tumour growth in the absence of therapeutic vaccination is not immunologically ignored. Low levels of 5T4 specific IgG1 and IgG2b antibodies can be detected in the sera. Consistent with this we show that splenocytes from these mice secrete IL-5 when restimulated in vitro with DCh5T4 and restimulated splenocytes are unable to lyse B16h5T4 tumour in vitro, suggesting that tumour growth polarises to a Th2 phenotype.
Fig. 7.
Comparing 5T4 immune responses following a single vaccination with either, Adh5T4, DCh5T4, or PBS in mice with pre-existing tumour. a, b represents isotype antibody response. c Cytokine release by splenocytes after re-stimulation with DCh5T4 versus DC2.4 (results are presented as cytokine release after DCh5T4 restimulation—release after DC2.4 restimulation). ND is not detected. d Cytotoxic killing of B16h5T4 measured by 51CR release. In terms of antibody responses there is production of both IgG1 and IgG2b in sera from mice treated with PBS and DCh5T4, whilst vaccination with Adh5T4 shows a polarization of IgG2b > IgG1 (a, b). Statistical analysis of the cytokine release from splenocytes treated with either PBS/Adh5T4/DCh5T4, shows there is no difference in IL-5 secretion between the different groups using the one-way ANOVA test (P = 0.138). Adh5T4 treated splenocytes secrete significant amounts of INF-γ and are able to lyse B16h5T4 targets. In contrast DCh5T4 treated splenocytes secrete in addition to IL-5 significant amount of IL-10 and are unable to lyse B16h5T4 targets
A single vaccination with Adh5T4 in tumour bearing mice polarises the immune response from Th2 to Th1. This is demonstrated by the antibody isotypes which are biased IgG2b > IgG1 (Fig. 7a, b); the splenocytes restimulated in vitro secrete in addition to IL-5 statistically significant amounts of INF-γ compared to PBS and DCh5T4 treated splenocytes (P = 0.023) (Fig. 7c) and are able to specifically lyse B16h5T4 tumour in vitro (Fig. 7d). In contrast, the DCh5T4 immunisation potentiates the Th2 phenotype which is reflected by the induction of mixed IgG1/IgG2b antibody isotypes, secretion of in addition to IL-5 statistically increasing amounts of IL-10 by splenocytes restimulated in vitro compared with PBS and Adh5T4 treated splenocytes (P ≤ 0.0005). Consistent with this DCh5T4 treated splenocytes demonstrate a complete absence of cytolytic T cell activity. Statistically there is no difference in the levels of Il-5 secreted between the three treatments.
Analysis post heterologous boost immunisation at day 21 showed the same Th1/Th2 profiles were maintained (data not shown). Thus the different efficacy of vaccination in prophylaxis and therapy models of B16h5T4 tumours may relate to the naïve or antigen/tumour exposed status of the animals. In tumour-bearing mice, the induction of Th1 polarising immune responses is pivotal in the control of 5T4+ tumour growth, and the choice of vaccine used to immunise after tumour challenge is critical for determining efficacy of subsequent immunisations.
Role of regulatory T cells in modulating tumour regression in active therapy
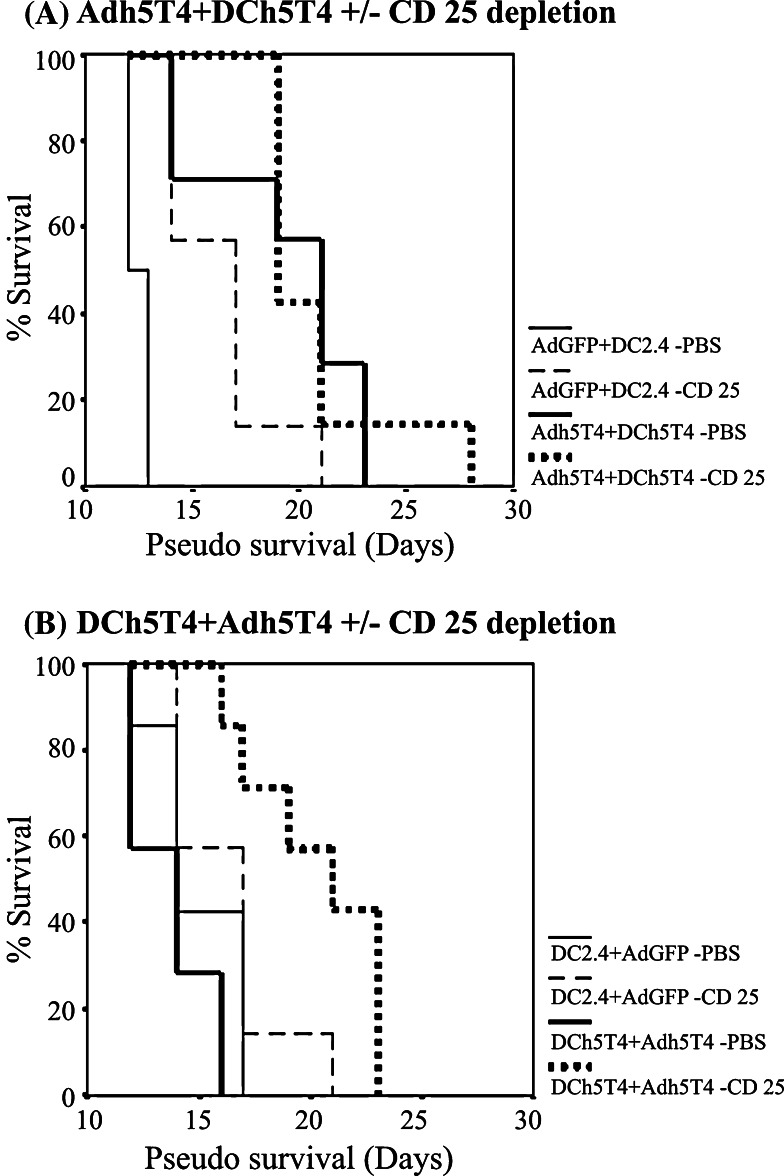
As the tumour growth polarises 5T4 immune reactivity to a Th2 phenotype it is possible that this may induce regulatory T cells. We hypothesised that because both positive and negative immune effectors are generated following vaccination even in naïve mice, the same vaccinations in the context of therapy may selectively expand the number and activity of regulatory T cells. Thus the difference in vaccine efficacy in active therapy between DCh5T4/Adh5T4 versus Adh5T4/DCh5T4 may reflect differential induction of regulatory cells as a consequence of tumour growth and the priming vaccination. To test this hypothesis, tumour bearing mice were depleted of CD25+ cells with 1 mg/0.5 ml anti CD25 antibody starting 4 days prior to treatment with Adh5T4/DC5T4 or DC5T4/Adh5T4. Figure 8a shows that the specific therapeutic activity of the Adh5T4+DCh5T4 vaccination is not enhanced by CD25 depletion although the control vaccination is potentiated. Importantly Fig. 8b, shows that the ineffective DCh5T4/Adh5T4 treatment is dramatically improved by the depletion CD25 cells. This suggests that the removal of CD25 cells can be used to improve suboptimal immunisation regimens in cancer vaccine immunotherapy.
Fig. 8.
Investigating the synergistic effects of vaccination Adh5T4/DCh5T4 versus DCh5T4/Adh5T4 with in-vivo depletion of CD25+ lymphocytes in mice with pre-existing tumours. Depletion of CD25+ cells does not alter survival when combined with Adh5T4/DCh5T4 (a), but dramatically improves the efficacy of DCh5T4/Adh5T4 vaccinations (b)
Discussion
The goal of this study was to evaluate the immune response in protective and active tumour therapy to homologous and heterologous prime boost vaccinations consisting of first generation adenoviral vectors and DC2.4 cells encoding 5T4 oncofoetal antigen. In naïve mice, there were differences in the magnitude of immune responses and polarising potential (Th1 vs. Th2) of the different regimens tested but all provided significant tumour protection against lethal challenge with B16h5T4. The prime heterologous combination DCh5T4/Adh5T4 was the best prophylactic treatment but did not provide any therapeutic benefit while Adh5T4/ DCh5T4 was efficacious. Critically, these alternative vaccine regimens elicited very different immune responses in mice with pre-existing tumours with the demonstration of CTL and Th1 polarisation following priming with Adh5T4 and not DCh5T4. Further experiments showed the important role for CD25+ regulatory cells in both prophylaxis and active therapy with their depletion yielding enhancement of tumour therapy.
The activation of immune effectors to target antigens is dependent on several factors including the choice of the vaccine vector, dose and route used for immunisation [38]. In naïve mice we show that each of the homologous and heterologous prime-boost vaccine regimens, comprising Adh5T4 and DCh5T4 can elicit a broad range of immune effectors reactive against the h5T4 antigen in naïve mice, including antibodies, CD4+ and CD8+ T cells, which can be readily measured in vitro using classical immune assays. However we show that each vaccine combination is not equal at inducing or sustaining similar levels of 5T4 immunity. The heterologous prime-boost combinations are the most efficient at sustaining 5T4 specific CTL responses, with cytokine and antibody isotype responses consistent with a polarisation to Th1, which has been reported for other targets [18–21].
The failure to reproducibly to demonstrate CTL activity to homologous vaccinations with Adh5T4/Adh5T4 may reflect competition between and selected expansion of CTLs to more immunogenic viral vector proteins such that specific target antigen CTLs are not appropriately activated and/or become anergised [20] or may lead to their rapid functional exhaustion and deletion [39]. The failure to elicit CTLs with the DCh5T4/DCh5T4 was surprising as other studies with similar dosing schedule have used DC2.4 successfully to induce CTLs to alternative target antigens [25, 40]. It is possible that further optimisation of the dose and time of vaccinations together with the optimisation of DC phenotype [41, 42] in terms of activation status is required to properly evaluate the efficacy of DC vaccines as vectors for 5T4. However despite these differences in magnitude and persistence of 5T4 immune responses each vaccine regimen is able to provide tumour growth delay to a subsequent lethal challenge with B16h5T4.
The evaluation of the role of the different immune effectors following vaccination with the best preventative vaccine sequence DCh5T4/Adh5T4 using antibody depletion and adoptive transfer of specific immune effectors before tumour challenge, shows that each of the 5T4 specific immune effectors generated including antibodies, CD4+, CD8+ T cells as well as innate mechanisms can provide some level of tumour protection. The induction of antibodies may be useful clinically and it is significant that 5T4 specific antibodies are induced following MVAh5T4 immunisations in cancer patients [43].
Importantly our depletion studies demonstrate induction of 5T4 specific CD4+ cells which can have dual effects. We show that these CD4 responses may be required to support the efficient function of CD8+ cells (Fig. 5b), but simultaneously there is evidence of a suppressor population which maybe regulating the overall immune reactivity against 5T4 (Fig. 5a). We demonstrate that these CD4 suppressors are classical “CD4+CD25+” regulatory cells, and their depletion significantly potentiates anti-tumour immunity (Fig. 5d). Thus it can be concluded that the differential induction of 5T4 immune responses with each vaccination treatment is a representation of the integrated sum of both positive and negative immune responses generated. The demonstration that vaccine regimens may induce cells with a regulatory phenotype, even in naïve mice is important, as the potentiation of these negative immune responses clearly needs to be avoided in effective cancer vaccine protocols. While the results presented derive from a genetically engineered model of h5T4 tumour expression, the amino acid sequences of h- and m5T4 are 81% identical and so it is possible that induction of T cells with h5T4 vaccination may cross react with m5T4 targets although this was not seen for antibody induction against m5T4 using MVAh5T4 vaccination [16].
There has been limited vaccine success in patients with large cancer burdens tested in the last 10 years un humans, despite successful pre-clinical efficacy [44–47] [http://www.clinicaltrials.gov/]. Most studies test immunotherapies in tumour models which are either slowly growing, or soon after tumour challenge, which makes it much easier to achieve therapeutic success in these models as opposed to models with established tumour burdens and tumour immune phenotypes. In the active therapy setting we established an aggressive tumour model, to rigorously test the efficacy of our vaccination protocols. In the current study although the treatments tested only provide modest tumour growth delay, they highlight important immunological differences between the vaccines Adh5T4 versus DCh5T4 used for immunisation. The consequences of generating positive and negative 5T4 immune responses are not so critical or important in prophylactic tumour models, but the generation of negative immune responses becomes more important in tumour bearing mice, which are no longer antigen naïve.
We show in tumour bearing mice that, the best prophylactic treatment DCh5T4/Adh5T4 is no longer effective, whilst significant tumour growth delay can be achieved with the Adh5T4/DCh5T4. Analysis of the immune responses generated against h5T4, shows that the B16h5T4 tumour growth per se is not immunologically ignored, driving a Th2 response against h5T4, with the induction of CD25+ regulatory cells. Similar results have been obtained in other studies of B16 melanoma tumour immunity, and have shown that the outgrowth of tumours is frequently not associated with strong “danger signals” which are required to alert the immune system via the activation of DCs, favouring the induction of tolerance [48–51]. The difference in therapeutic efficacy between DCh5T4/Adh5T4 and Adh5T4/DCh5T4 is correlated with the ability of the latter to drive a Th1 polarised 5T4+ immune response with CTL activity, which can be induced soon after vaccination with Adh5T4, and sustained by subsequent boost with DCh5T4. The efficacy of adenoviral vectors may be associated with differences in the duration and level of 5T4 expression, and the differential activation of DCs by virus associated TLRs and subsequent antigen presentation driving efficient induction and expansion of relevant antitumour immunity [52, 53]. In contrast the DCh5T4 priming vaccination augments Th2 immune responses and expansion of CD25+ T regulatory cells, such that tumour growth of an aggressively growing tumour cannot be rescued by subsequent vaccination with Adh5T4, but may be if CD25+ cells are depleted in-vivo. Similar studies exploiting the potential of DC vaccines have demonstrated CD25+ T cells as important barrier to their efficacy [5, 54].
In conclusion this study highlights that active tumour therapy models are more relevant for the evaluation of cancer vaccines than prophylactic models. Clearly the sequence of the vaccine vectors can offer differential tumour growth delay in prophylaxis and therapy presumably reflecting different tumour specific responses in the two immunological settings. This is seems to derive from the immune activating capabilities of the vectors together with maintenance of appropriate tumour immune phenotypes in the context of immune modulation by the tumour. The use of antigen naïve mice can be used to indicate the immunogenicity of the vectors in terms of evaluating both positive and negative TAA immune responses. In tumour bearing mice some therapeutic vaccinations may expand tumour induced regulatory cells. The potentiation of CD25+ T regulatory cells represents one mechanism of tumour and vaccine induced immunosuppression. Immunotherapy using anti CD25 monoclonal antibodies to deplete these represents one approach to increasing TAA specific immune responses. This might be avoided if appropriate vaccination regimens are used for immunisation in the first instance. Several recent studies have demonstrated that classical CD4+CD25+ regulatory T cells are not the only barrier to effective cancer vaccines, and acquisition of regulatory T cell function by other CD4 T cells may occur concomitantly with the induction of anti-tumour effector T cells in the same lymph nodes [55–57], and act to promote tumour growth through the inhibition of tumour specific CD8+ T cell responses [58]. The presence of alternative regulatory T cell populations, together with the stage of tumour growth challenged in the current study may explain the modest vaccine responses observed. Any future studies, together with clinical trial protocols should be designed to address both positive and negative influences of vaccination. A more detailed analysis of the negative T cell populations would need to consider at least three distinct types of T regulatory cells which have distinct cytokine profiles and probable modes of action. These include CD4+/CD25 + /Foxp3 + T lymphocytes (Treg) of thymic origin, TR1 lymphocytes able to release IL-10, and TGF-beta producing TH3 lymphocytes all of which could influence tumour growth by inhibiting the immune responses. Future studies will focus on understanding the modulation of vaccine induced immune responses to 5T4 together with the optimisation of dosing and scheduling of vaccinations such that better tumour-free survival can be obtained.
Ultimately, the development of potent immunotherapeutic vaccination strategies, which increase the frequency and function of tumour-specific T cells, in conjunction with strategies to inactivate or remove regulatory T cell populations and the immunosuppressive nature of the tumours are required. The choice of vaccine vector used for immunisation is clearly pivotal in terms facilitating optimal antigen presentation by DCs and the induction of effective anti-tumour immune responses that can challenge tumour growth in-vivo.
Acknowledgements
The research was supported by Cancer Research UK and Christie Hospital Endowments. We would also like to thank Ric Swindell for critical appraisal of the statistics used in the study.
Appendix: Abbreviations
- Ad
- Adenovirus
- AdGFP
- First generation E1/E3 deleted adenovirus expressing green fluorescent protein
- APC
- Antigen presenting cell
- B16h5T4
- B16 melanoma cells stably expressing human 5T4
- CTL
- Cytotoxic T lymphocyte
- CpG motif
- Adenovirus
- Ad
- DNA sequences which can act as adjuvants to activate immune responses
- Th1/Th2
- T helper type 1/2 cells
- DC
- Dendritic cell
- DCh5T4
- DC2.4 cells expressing human 5T4
- E:T
- Effector to target ratio
- FCS
- Fetal calf serum
- h5T4
- Human 5T4
- h5T4-FC
- Fusion protein of FC conjugated to the exogenous region of 5T4 protein
- INF-γ
- Interferon gamma
- IL-5
- Interleukin 5
- IL-10
- Interleukin 10
- I.P
- Intraperitoneal
- LPS
- Lipopolysaccharide
- ND
- Not detected
- MVA
- Modified Vaccinia Ankara
- h5T4
- Human 5T4
- SC
- Subcutaneous
- Sc and im
- Subcutaneous and intramuscular
- TAA
- Tumour Associated Antigen
References
- 1.Hui K, Grosveld F, Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984;311:750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 3.Beck C, Schreiber H, Rowley D. Role of TGF-beta in immune-evasion of cancer. Microsc Res Tech. 2001;52:387–395. doi: 10.1002/1097-0029(20010215)52:4<387::AID-JEMT1023>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 6.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 7.Dermime S, Armstrong A, Hawkins RE, Stern PL. Cancer vaccines and immunotherapy. Br Med Bull. 2002;62:149–162. doi: 10.1093/bmb/62.1.149. [DOI] [PubMed] [Google Scholar]
- 8.Hole A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239–246. doi: 10.1038/bjc.1988.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61:89–95. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulder WM, Stern PL, Stukart MJ, de Windt E, Butzelaar RM, Meijer S, Ader HJ, Claessen AM, Vermorken JB, Meijer CJ, Wagstaff J, Scheper RJ, Bloemena E. Low intercellular adhesion molecule 1 and high 5T4 expression on tumor cells correlate with reduced disease-free survival in colorectal carcinoma patients. Clin Cancer Res. 1997;3:1923–1930. [PubMed] [Google Scholar]
- 11.Naganuma H, Kono K, Mori Y, Takayoshi S, Stern Tasaka PL K, Matsumoto Y. Oncofetal antigen 5T4 expression as a prognostic factor in patients with gastric cancer. Anticancer Res. 2002;22:1033–1038. [PubMed] [Google Scholar]
- 12.Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899–902. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzynska T, Rahi V, Stern PL. The expression of 5T4 antigen in colorectal and gastric carcinoma. Br J Cancer. 1992;66:867–869. doi: 10.1038/bjc.1992.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzynska T, Wiechowska-Kozlowska A, Marlicz K, Bromley M, Roberts SA, Lawniczak M, Kolodziej B, Zyluk A, Stern PL. 5T4 oncofetal antigen in gastric carcinoma and its clinical significance. Eur J Gastroenterol Hepatol. 1998;10:479–484. doi: 10.1097/00042737-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Wrigley E, McGown AT, Rennison J, Swindell R, Crowther D, Starzynska T, Stern PL. 5T4 oncofetal antigen expression in ovarian carcinoma. Int J Gynecol Cancer. 1995;5:269–274. doi: 10.1046/j.1525-1438.1995.05040269.x. [DOI] [PubMed] [Google Scholar]
- 16.Mulryan K, Ryan MG, Myers KA, Shaw D, Wang W, Kingsman SM, Stern PL, Carroll MW. Attenuated recombinant vaccinia virus expressing oncofetal antigen (tumor-associated antigen) 5T4 induces active therapy of established tumors. Mol Cancer Ther. 2002;1:1129–1137. [PubMed] [Google Scholar]
- 17.Smyth L, Elkord E , Taher TEI, Jiang h-R, Burt DJ, Clayton A, van Veelen PA, de Ru A, Ossendorp F, Melief CJM, Drijfhout JW, Dermime S, Hawkins RE, Stern PL (2006) CD8 T cell recognition of human 5T4 oncofoetal antigen Int J cancer (in press) [DOI] [PubMed]
- 18.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–768. doi: 10.1016/S0264-410X(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 19.Irvine KR, Chamberlain RS, Shulman EP, Surman DR, Rosenberg SA, Restifo NP. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J Natl Cancer Inst. 1997;89:1595–1601. doi: 10.1093/jnci/89.21.1595. [DOI] [PubMed] [Google Scholar]
- 20.Palmowski MJ, Choi EM, Hermans IF, Gilbert SC, Chen JL, Gileadi U, Salio M, Van Pel A, Man S, Bonin E, Liljestrom P, Dunbar PR, Cerundolo V. Competition between CTL narrows the immune response induced by prime-boost vaccination protocols. J Immunol. 2002;168:4391–4398. doi: 10.4049/jimmunol.168.9.4391. [DOI] [PubMed] [Google Scholar]
- 21.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8 + T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol. 2003;171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong AC, Dermime S, Allinson CG, Bhattacharyya T, Mulryan K, Gonzalez KR, Stern PL, Hawkins RE. Immunization with a recombinant adenovirus encoding a lymphoma idiotype: induction of tumor-protective immunity and identification of an idiotype-specific T cell epitope. J Immunol. 2002;168:3983–3991. doi: 10.4049/jimmunol.168.8.3983. [DOI] [PubMed] [Google Scholar]
- 23.Chen PW, Wang M, Bronte V, Zhai Y, Rosenberg SA, Restifo NP. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J Immunol. 1996;156:224–231. [PMC free article] [PubMed] [Google Scholar]
- 24.Dranoff G, Mulligan RC. Gene transfer as cancer therapy. Adv Immunol. 1995;58:417–454. doi: 10.1016/S0065-2776(08)60624-0. [DOI] [PubMed] [Google Scholar]
- 25.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardoll DM. Tumor reactive T cells get a boost. Nat Biotechnol. 2002;20:1207–1208. doi: 10.1038/nbt1202-1207. [DOI] [PubMed] [Google Scholar]
- 27.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmerman JM, Caspar CB, Lambert SL, Syrengelas AD, Levy R. Idiotype-encoding recombinant adenoviruses provide protective immunity against murine B-cell lymphomas. Blood. 2001;97:1370–1377. doi: 10.1182/blood.V97.5.1370. [DOI] [PubMed] [Google Scholar]
- 29.Mendoza L, Bubenik J, Simova J, Jandlova T, Vonka V, Mikyskova R. Prophylactic, therapeutic and anti-metastatic effects of BMDC and DC lines in mice carrying HPV 16-associated tumours. Int J Oncol. 2003;23:243–247. doi: 10.3892/ijo.23.1.243. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza L, Bubenik J, Simova J, Korb J, Bieblova J, Vonka V, Indrova M, Mikyskova R, Jandlova T. Tumour-inhibitory effects of dendritic cells administered at the site of HPV 16-induced neoplasms. Folia Biol (Praha) 2002;48:114–119. [PubMed] [Google Scholar]
- 31.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 32.Winkelhake JL, Nicolson GL. Determination of adhesive properties of variant metastatic melanoma cells to BALB/3T3 cells and their virus-transformed derivatives by a monolayer attachment assay. J Natl Cancer Inst. 1976;56:285–291. doi: 10.1093/jnci/56.2.285. [DOI] [PubMed] [Google Scholar]
- 33.Myers KA, Rahi-Saund V, Davison MD, Young JA, Cheater AJ, Stern PL. Isolation of a cDNA encoding 5T4 oncofetal trophoblast glycoprotein. An antigen associated with metastasis contains leucine-rich repeats. J Biol Chem. 1994;269:9319–9324. [PubMed] [Google Scholar]
- 34.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Tew SR, Russell AM, Gonzalez KR, Hardingham TE, Hawkins RE. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 2004;10:575–584. doi: 10.1089/107632704323061933. [DOI] [PubMed] [Google Scholar]
- 36.Fairbairn ES. Retroviral gene transfer into haemopoietic cells. In: Testa N, Molineux G, editors. Haemopoiesis: a practical approach. New York: Oxford University Press; 1993. pp. 175–187. [Google Scholar]
- 37.Shaw DM, Woods AM, Myers KA, Westwater C, Rahi-Saund V, Davies MJ, Renouf DV, Hounsell EF, Stern PL. Glycosylation and epitope mapping of the 5T4 glycoprotein oncofoetal antigen. Biochem J. 2002;363:137–145. doi: 10.1042/0264-6021:3630137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinkernagel RM. Localization dose and time of antigens determine immune reactivity (discussion 257–344) Semin Immunol. 2000;12:163–171. doi: 10.1006/smim.2000.0253. [DOI] [PubMed] [Google Scholar]
- 39.Krebs P, Scandella E, Odermatt B, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J Immunol. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- 40.Okada N, Tsujino M, Hagiwara Y, Tada A, Tamura Y, Mori K, Saito T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Administration route-dependent vaccine efficiency of murine dendritic cells pulsed with antigens. Br J Cancer. 2001;84:1564–1570. doi: 10.1054/bjoc.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camporeale A, Boni A, Iezzi G, Degl’Innocenti E, Grioni M, Mondino A, Bellone M. Critical impact of the kinetics of dendritic cells activation on the in vivo induction of tumor-specific T lymphocytes. Cancer Res. 2003;63:3688–3694. [PubMed] [Google Scholar]
- 42.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 43.Reinis M. Technology evaluation: TroVax, Oxford BioMedica. Curr Opin Mol Ther. 2004;6:436–442. [PubMed] [Google Scholar]
- 44.Adamina M, Daetwiler S, Rosenthal R, Zajac P. Clinical applications of recombinant virus-based cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1211–1224. doi: 10.1517/14712598.5.9.1211. [DOI] [PubMed] [Google Scholar]
- 45.Morse MA, Chui S, Hobeika A, Lyerly HK, Clay T. Recent developments in therapeutic cancer vaccines. Nat Clin Pract Oncol. 2005;2:108–113. doi: 10.1038/ncponc0098. [DOI] [PubMed] [Google Scholar]
- 46.Reichardt VL, Brossart P, Kanz L. Dendritic cells in vaccination therapies of human malignant disease. Blood Rev. 2004;18:235–243. doi: 10.1016/j.blre.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Salazar LG, Disis ML. Cancer vaccines: the role of tumor burden in tipping the scale toward vaccine efficacy. J Clin Oncol. 2005;23:7397–7398. doi: 10.1200/JCO.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Cuenca A, Cheng F, Wang H, Brayer J, Horna PGuL, Bien H, Borrello IM, Levitsky HI, Sotomayor EM. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63:9007–9015. [PubMed] [Google Scholar]
- 49.Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur J Immunol. 2003;33:2645–2654. doi: 10.1002/eji.200324341. [DOI] [PubMed] [Google Scholar]
- 50.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O’Carroll K, Levitsky H. I. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.V98.4.1070. [DOI] [PubMed] [Google Scholar]
- 51.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, Chen SJ, Varnavski AN, LeClair C, Raper SE, Wilson JM. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 54.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4 + CD25 + regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.den Boer AT, van Mierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE. CD4 + T cells are able to promote tumor growth through inhibition of tumor-specific CD8 + T-cell responses in tumor-bearing hosts. Cancer Res. 2005;65:6984–6989. doi: 10.1158/0008-5472.CAN-04-3344. [DOI] [PubMed] [Google Scholar]
- 56.Hiura T, Kagamu H, Miura S, Ishida A, Tanaka H, Tanaka J, Gejyo F, Yoshizawa H. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J Immunol. 2005;175:5058–5066. doi: 10.4049/jimmunol.175.8.5058. [DOI] [PubMed] [Google Scholar]
- 57.Zhou G, Drake CG, Levitsky HI (2005) Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood [DOI] [PMC free article] [PubMed]
- 58.Huang Y, Obholzer N, Fayad R, Qiao L. Turning on/off tumor-specific CTL response during progressive tumor growth. J Immunol. 2005;175:3110–3116. doi: 10.4049/jimmunol.175.5.3110. [DOI] [PubMed] [Google Scholar]