Abstract
We evaluated tumor cell growth modulation by bee venom secretory phospholipase A2 (bv-sPLA2) and phosphatidylinositol-(3,4)-bisphosphate as well as potential cooperative effects. In addition, the immunomodulatory impact of tumor cell treatment was examined by monitoring changes in phenotype and function of monocyte-derived dendritic cells (moDCs) cocultured with pretreated tumor cells. Bv-sPLA2 or phosphatidylinositol-(3,4)-bisphosphate alone displayed moderate effects on the proliferation of A498 renal cell carcinoma cells, T-47D breast cancer cells, DU145 prostate cancer cells and BEAS-2B transformed lung cells. However, when bv-sPLA2 was coadministered with phosphatidylinositol-(3,4)-bisphosphate a potent inhibition of [3H] thymidine incorporation into all tested cell lines occurred. This inhibition was due to massive cell lysis that reduced the number of cells with proliferative capacity. Importantly, tumor cell lysates generated with bv-sPLA2 plus phosphatidylinositol-(3,4)-bisphosphate induced maturation of human moDCs demonstrated by enhanced expression of CD83 and improved stimulation in allogeneic mixed leukocyte reactions. Our data demonstrate that bv-sPLA2 and phosphatidylinositol-(3,4)-bisphosphate synergistically generate tumor lysates which enhance the maturation of immunostimulatory human monocyte-derived dendritic cells. Such tumor lysates which represent complex mixtures of tumor antigens and simultaneously display potent adjuvant properties meet all requirements of a tumor vaccine.
Keywords: Dendritic cells; Bee venom PLA2; Phosphatidylinositol-(3,4)-bisphosphate; Tumor cell lysate; Cancer immunotherapy
Introduction
The immunity-initiating potential of monocyte-derived dendritic cells (moDCs) is currently exploited in various clinical settings to treat diseases like cancer. In such studies moDCs are generated from patient peripheral blood precursor cells in vitro, loaded with tumor lysates or other antigen preparations, matured and injected back into the patient to induce antitumor immune responses [7, 15, 44, 52]. Tumor lysates are frequently generated by repetitive freeze and thaw cycles to disrupt cells and to prepare tumor-associated antigens for loading onto moDCs [16 19]. Such lysates comprise the complete antigenic repertoire of a specific tumor and can further be supplemented with helper antigens like keyhole limpet hemocyanin (KLH) or adjuvants to enhance immunogenicity.
Recently, it has been demonstrated that bee venom secretory phospholipase A2 (bv-sPLA2) acts as an adjuvant by triggering the maturation of moDCs [35, 38]. Bv-sPLA2 represents a member of the phospholipases A2 (PLA2, phosphatidylcholine-2-acylhydrolase, EC 3.1.1.4) family of enzymes that catalyze the hydrolysis of the sn-2 fatty acyl–ester bond of membrane glycero-3-phospholipids resulting in diverse biological effects [10, 30, 40, 45, 49]. Hydrolysis of these compounds generates lysophospholipids. Interestingly, lysolipids and in particular synthetic derivatives thereof have been proposed for anticancer therapies due to their antiproliferative effects and due to cytotoxicity [3, 41, 42]. These data suggest that bv-sPLA2 action may exert inhibitory effects on tumor cells, while concomitantly promoting immunostimulatory capacities of moDCs.
Increasing evidence also suggests a modulatory role of phospholipids in the differentiation process of moDCs thus affecting the immunogenic potential of antigen-presenting cells [1, 2, 9]. Phospholipids like phosphatidylinositols (PtdIns) and phosphorylated forms thereof exert their role either as precursors of second messengers, or directly by interacting with proteins to orchestrate the spatio-temporal organization of key intracellular signal transduction pathways [34, 48]. 3-Phosphorylated forms of PtdIns have been implicated in cell survival, control of proliferation and regulation of the cell cycle [13, 22, 34]. They are produced in response to agonist-mediated cell activation [34, 50] and therefore can be expected in a tumor environment characterized by elevated phosphoinositide 3-kinase (PI 3-kinase) activity and agonist stimulation via auto- and paracrine loops. These data indicate a modulatory role of PtdIns derivatives on cellular growth of tumor cells and the differentiation of immune cells.
In the present work we found a strong and unexpected synergy between bv-sPLA2 and phosphatidylinositol-(3,4)-bisphosphate (PtdIns(3,4)2) that mediates tumor cell inhibition and lysis. The resulting tumor lysates promoted maturation of immunostimulatory moDCs.
Materials and methods
Reagents
Secretory phospholipase A2 (Type III) from bee venom (bv-sPLA2) was purchased from Cayman Chemical (Ann Arbor, MI, USA). sPLA2 activity was routinely monitored using the colorimetric sPLA2 assay kit (from Cayman Chemical) which is based on the synthetic substrate diheptanoyl thiophosphorylcholine. Phosphatidylinositol-3,4-bisphosphate (1,2-dipalmitoyl, ammonium salt) (PtdIns(3,4)P2) was purchased from Cayman Chemical and was prepared according to the manufacturerȁ9s instructions.
Cell culture
The human kidney carcinoma cell line A498 [11], the human breast carcinoma cell line T-47D [23], the human prostate carcinoma cell line DU145 [47] and the human bronchial epithelium cell line BEAS-2B [24] were propagated at 37°C and 5% CO2 in the presence of 10% fetal calf serum (FCS, heat-inactivated, 30 min, 56°C; HyClone, UT, USA) in medium consisting of RPMI 1640 supplemented with 10 mM Hepes, non-essential amino acids (1×), 1 mM Na-pyruvate (all from Cambrex Bio Science, Verviers, Belgium), Glutamax (1×) (Invitrogen, Paisley Scotland, UK), 100 U/ml penicillin and 100 µg/ml streptomycin (PAA Laboratories, Linz, Austria).
Measurement of tumor cell proliferation
Before each experiment tumor cells were replated and stimulated with medium containing 10% FCS for 24 h in order to synchronize cells. A number of 20,000 cells were then plated in 200 µl medium without serum in 96-well flat-bottomed tissue culture plates (Falcon, BD, Franklin Lakes, NJ, USA). Cells were incubated for 32 h in the presence or absence of bv-sPLA2 and PtdIns(3,4)P2. During the last 16 h, cells were pulsed with 50 µl fresh medium containing 10% FCS and 1 μCi/well (37 kBq/well) [3H] thymidine (ICN Biomedicals, Eschwege, Germany). Cells were harvested with a Tomtec harvester (Hamden, CT, USA) and liquid scintillation counting was performed with a Chameleon Multi label reader (HVD-Life Science, Vienna, Austria). Results are mean cpm ± SEM of triplicate wells. Values were normalized by setting controls to 100%. Typical values for A498 cells in this setup ranged from 16,331 to 25,543 cpm. Concentrations used in this study were selected after titration experiments and were established based on a compound to cell ratio, for instance 10 µg/ml sPLA2 per 20,000 A498 cells. Bv-sPLA2 at 100 µg/ml in this setup was toxic for A498 cells, whereas PtdIns(3,4)P2 in the range from 10 to 100 µM was slightly dose-dependent, however, differences in [3H] thymidine uptake were statistically not significant.
7-Amino-actinomycin D (7-AAD) staining
A498 cells were replated and cultured in medium containing 10% FCS for 24 h in order to synchronize cells. Cells (100,000) were then plated in 100 µl medium without serum in 96-well round-bottomed tissue culture plates (Costar, Corning Inc. NY, USA). Cells were incubated for 45 min in the presence or absence of PtdIns(3,4)P2 and bv-sPLA2. Subsequently, FCS was added to a final concentration of 10% to attenuate bv-sPLA2 action. Cells were transferred to PBS buffer (Cambrex, Verviers, Belgium) and were incubated with 5 µg/ml of the fluorescent DNA stain 7-AAD (Sigma-Aldrich, Vienna, Austria). The percentage of 7-AAD+ cells was determined with a FACSCalibur flow cytometer (BD) and CellQuest software (BD).
Generation of moDCs
At the local Institute for Blood Transfusion, healthy individuals underwent a standard leukapheresis procedure performed with the Cobe Spectra cell separator (Gambro BCT, Lakewood, CO, USA). The study was approved by the Institutional Review Board and all donors gave written informed consent. Using the MNC program at a continuous whole-blood inlet flow rate ranging from 50 to 70 ml/min, 3 to 5 l of whole blood were processed. ACD-A solution (Baxter, Vienna, Austria) at a 1:12 ratio was used for anticoagulation. Subsequently, apheresis products (50–100 ml) were transferred to a cell culture flask (Costar, Corning, NY, USA) and adjusted to 200 ml using CliniMACS PBS/EDTA Buffer (Miltenyi Biotec, Bergisch Gladbach, Germany) supplemented with 2% heat-inactivated human AB serum from the local Institute of Blood Transfusion.
CD14+ monocytes were separated from apheresed cells by positive selection using the CD14-Reagent, the CliniMACS Tubing Set 600 (for up to 20×109 cells) and the CliniMACS Instrument [36]. All steps were carried out according to the manufacturerȁ9s instructions.
CD14+ cells (50×106 in 50 ml) were cultured in 162 cm2 cell culture flasks (Costar, Corning, NY, USA) in AIM-V (Invitrogen, Grand Island, NY, USA) containing 1% heat-inactivated human AB serum, 10 mM HEPES and 50 µM 2-mercaptoethanol (Merck, Darmstadt, Germany) as well as a combination of recombinant human GM-CSF (1,000 U/ml; Leucomax, Novartis, Basel, Switzerland) and recombinant human IL-4 (1,000 U/ml; CellGenix, Freiburg, Germany). After 2 days of culture, 50 ml of fresh medium containing supplements were added. Sterility testing of day-5 moDCs was performed at the local Institute of Hygiene. All tests were negative (100% sterility). Day-5 moDCs were harvested and frozen in liquid nitrogen using a standard protocol (50% AIM-V, 40% human AB serum, 10% DMSO).
Day-5 moDCs were thawed, counted and replated in 6-well plates at 1.8×106 cells per well in 3 ml of fresh medium consisting of RPMI 1640 supplemented with 10 mM Hepes, non-essential amino acids (1×), 1 mM Na-pyruvate , Glutamax (1×), 100 U/ml penicillin and 100 µg/ml streptomycin, GM-CSF (2,000 U/ml) and IL-4 (500 U/ml). Subsequently, moDCs were mixed with tumor lysates and controls.
Generation of tumor cell lysates
For the generation of PtdIns(3,4)P2-bv-sPLA2 lysate 300,000 A498 cells were seeded in 3 ml medium without serum in 6-well flat-bottomed tissue culture plates (Costar, Corning Inc. Corning, NY, USA). Lysis was induced by the addition of 10 µg/ml bv-sPLA2 and 10 µM PtdIns(3,4)P2. For control purposes, freeze–thaw lysates were generated by seeding 300,000 A498 cells in 3 ml medium without serum in 6-well flat-bottomed tissue culture plates. Lysis was induced by three cycles of freezing (−80°C, 2 h) and thawing (37°C incubator, 1 h).
Treatment of moDCs with tumor cell lysates
Tumor cell lysates were generated as described above. Control treatments with single substances were also performed. The lytic activity of lysates was quenched by the addition of 20% FCS or, alternatively, by lipoprotein-deficient FCS (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, 3 ml of lysate preparations were added to 3 ml of moDCs cultures resulting in a final concentration of 10% FCS. No additional maturation stimuli were added. After 48 h at 37°C, moDCs were harvested, washed with PBS and prepared for analysis (flow cytometry and T cell proliferation assay). MoDCs and A498 cells can be discriminated on the basis of adherence, as A498 cells attach to tissue culture plates, while moDCs do not. Thus, harvesting of moDCs with PBS in the absence of trypsin does not detach A498 cells as verified by measuring [3H] thymidine incorporation of appropriate controls (not shown).
Flow cytometry (FACS)
MoDCs treated with lysate preparations were subjected to FACS analysis using anti-CD83-PE- and anti-IgG1k-PE-antibodies (isotype control), a FACSCalibur and CellQuest software (all from BD, Mountain View, CA, USA).
T cell proliferation assay
The immunostimulatory capacities of the differentially treated moDCs were determined in an allogeneic mixed leukocyte reaction (MLR). MoDCs were used as stimulator cells of allogeneic peripheral blood mononuclear cells (PBMCs) derived from healthy individuals depleted of CD14+ cells (= CD14+-depleted PBMCs). CD14+-depleted PBMCs (106/ml) were stimulated with moDCs at a ratio of 40:1 in flat-bottomed 96-well plates in AIM-V (Invitrogen, Grand Island, NY, USA). Cultures were pulsed during the last 16 h of a day-5 culture at 37°C and 5% CO2 with 1 μCi/well (37 kBq/well) [3H] thymidine. Cells were harvested with a Tomtec harvester and liquid scintillation counting was performed with a Chameleon Multi label reader. Results are mean cpm ± SEM of triplicate wells.
Statistical analysis
Statistics were performed by analyzing data with the studentȁ9s t test by utilizing SPSS software. Results were considered statistically significant at P values ≤0.05.
Limulus amebocyte lysate (LAL) assay
To test for potential endotoxin (LPS) contamination, bv-sPLA2 was subjected to the LAL assay from Biowhittaker. Endotoxin concentration of bv-sPLA2 at the maximum final concentration used in the experiments (10 µg/ml) corresponded to 4.1 pg LPS/ml (i.e. 2.8×10−2 EU/ml). Endotoxin levels of PtdIns(3,4)P2 at 10 µM corresponded to 0.11 pg LPS/ml (7.6×10−4 EU/ml). At these concentrations, LPS fails to induce moDC maturation (own unpublished titration experiments).
Results
Bv-sPLA2 acts synergistically with PtdIns(3,4)P2 to inhibit [3H] thymidine uptake of renal cancer cells
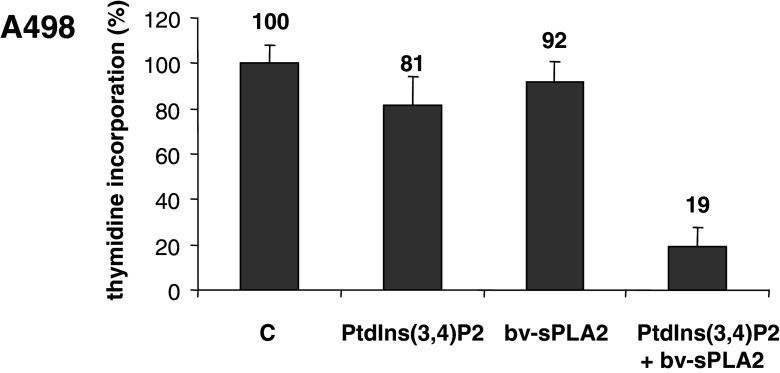
We investigated the effects of bv-sPLA2 and the PI 3-kinase product PtdIns(3,4)P2 on the proliferation of the well-characterized kidney cancer cell line A498 [11, 14, 21]. Cells were treated with bv-sPLA2 (10 µg/ml) and PtdIns(3,4)P2 (10 µM) either alone or in combination. After 32 h of incubation A498 cells were pulsed with [3H] thymidine and cell proliferation was measured as [3H] thymidine incorporation. Figure 1 demonstrates that either substance alone had little effect on the proliferation of A498 cells. The differences compared to untreated controls were statistically not significant (P>0.2). However, when bv-sPLA2 was present in combination with PtdIns(3,4)P2 a potent reduction of [3H] thymidine incorporation occurred. PtdIns(3,4)P2 clearly synergized with bv-sPLA2 in blocking the [3H] thymidine uptake in A498 cells (P<0.003).
Fig. 1.
Combined treatment of A498 kidney cancer cells with bv-sPLA2 and PtdIns(3,4)P2 inhibits proliferation. A498 cells were left untreated (C control), were treated with bv-sPLA2 (10 µg/ml), with PtdIns(3,4)P2 (10 µM) or a combination of both, bv-sPLA2 and PtdIns(3,4)P2. Proliferation was determined by assessing [3H] thymidine incorporation. Shown are normalized mean values ± SEM of seven independent experiments
Inhibition of renal cancer cells is due to the induction of cell lysis
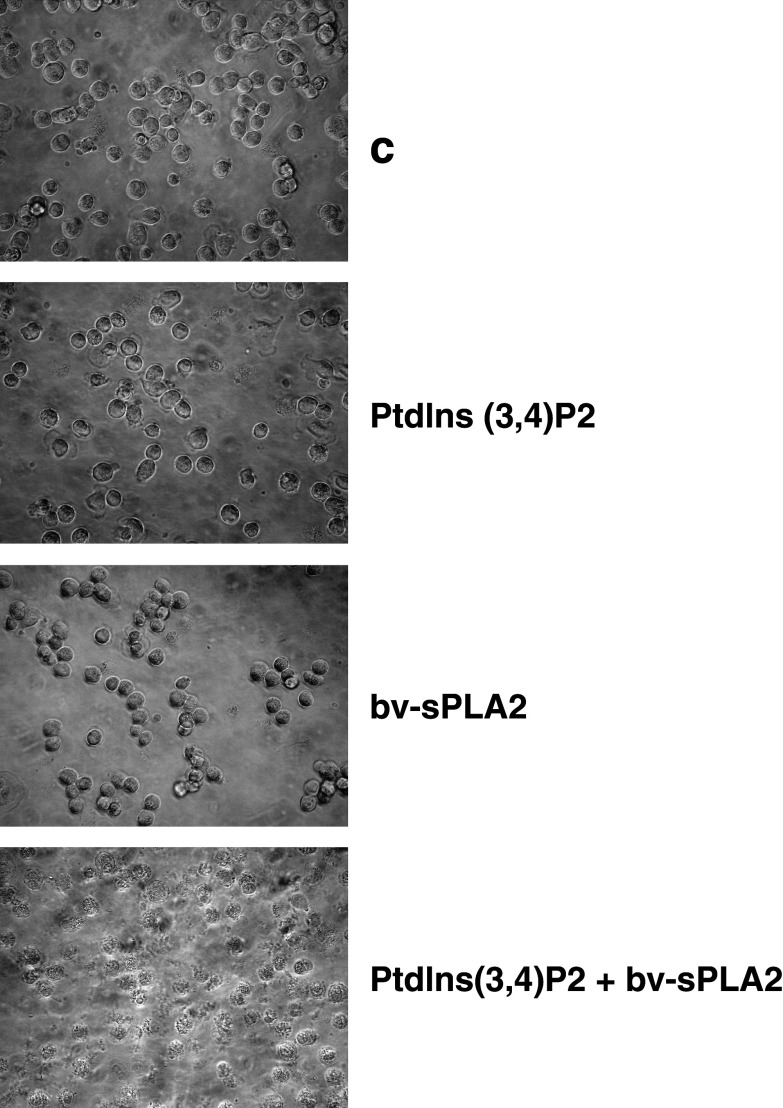
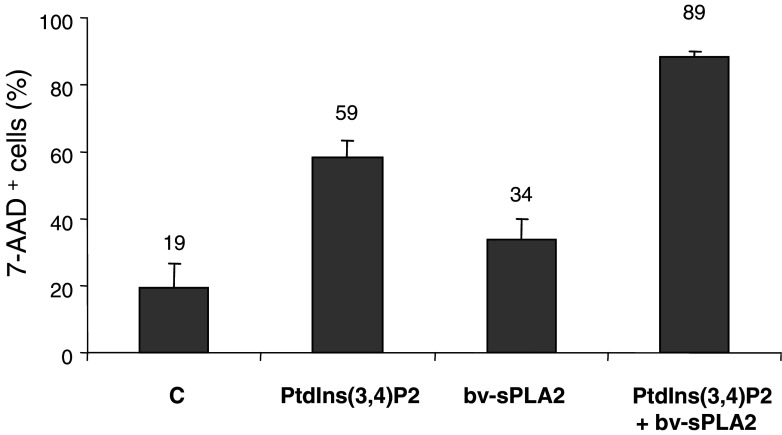
Figure 2 depicts phase contrast microscopy images which demonstrate that the combination of bv-sPLA2 and PtdIns(3,4)P2 induced lysis of A498 cells, while either substance alone had virtually no effect. Cell lysis only occurred under serum (protein)-free conditions and was visible within 2 h. Lysis affected the majority of the cells and was in accordance with data obtained by measuring [3H] thymidine incorporation (Fig. 1). Although [3H] thymidine uptake does not directly quantitate lytic capacity, it represents a sensitive method to detect proliferation of minimal numbers of residual unlysed cells surviving the combined treatment with bv-sPLA2 and PtdIns(3,4)P2. Such a treatment also affected susceptibility of A498 cells for staining with 7-AAD, which intercalates into double-stranded DNA by penetrating disrupted cell membranes but is excluded by intact cells. Hence, 7-AAD staining represents a useful measure for cell lysis. Treatment of A498 cells with bv-sPLA2 or PtdIns(3,4)P2 alone enhanced the number of 7-AAD+ cells from 19 to 34 and 59%, respectively (Fig. 3). Of note, the combination of bv-sPLA2 with PtdIns(3,4)P2 resulted in 89% 7AAD+ cells (P<0.003). Thus, the combined treatment disrupted cell integrity allowing access of 7-AAD to bind DNA in the majority of the cells. In order to exclude putative effects mediated by endogenous PLA2 activity in A498 cells, a pretreatment of cells for 1 h with the inhibitor methyl arachidonyl fluorophosphonate (MAFP, 1–10 µM) was performed [5]. The cPLA2 and iPLA2 inhibitor MAFP did not prevent synergistic inhibition of [3H] thymidine uptake and cell lysis induced by PtdIns(3,4)P2 and bv-sPLA2 (data not shown). However, inactivation of bv-sPLA2 at 100°C for 1 h completely abrogated lysis (data not shown). In conclusion, the lytic effect was indeed mediated by exogenous PLA2 enzymatic activity.
Fig. 2.
Combined treatment of A498 cells with bv-sPLA2 and PtdIns(3,4)P2 induces cell lysis. A498 cells were left untreated (C control), or treated with PtdIns(3,4)P2 (10 µM), with bv-sPLA2 (10 µg/ml), or the combination of both, PtdIns(3,4)P2 and bv-sPLA2. Cell cultures were examined under a phase contrast microscope 2 h after administration of the reagents
Fig. 3.
Combined treatment with PtdIns(3,4)P2 and bv-sPLA2 enhances the number of 7-AAD+ cells. A498 cells were left untreated (C control), or treated with PtdIns(3,4)P2, with bv-sPLA2, or the combination of both, PtdIns(3,4)P2 and bv-sPLA2. Shown are mean values ± SEM of 7-AAD+ cells from quadruplicate measurements
Bv-sPLA2 acts synergistically with PtdIns(3,4)P2 to inhibit various cancer cell lines
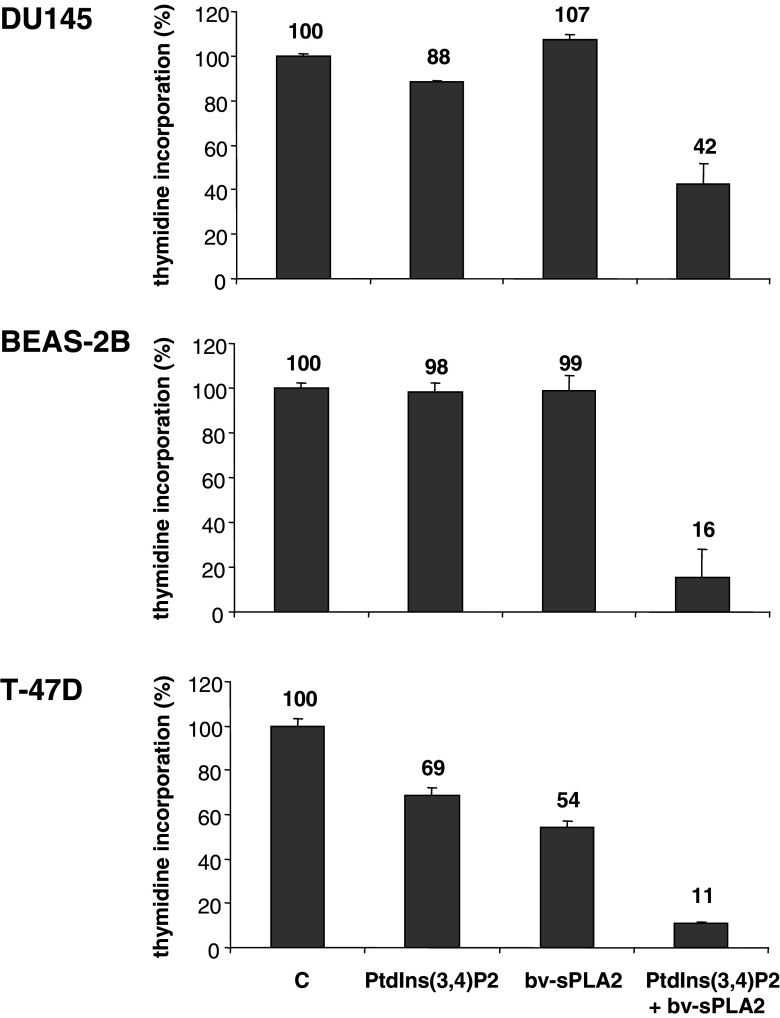
To investigate whether the observed inhibition was cell type-specific, other cell lines derived from different tissues were also tested. Figure 4 indicates that bv-sPLA2 and PtdIns(3,4)P2 exhibited a similar synergistic action affecting [3H] thymidine incorporation in DU145 prostate cancer cells (P<0.02), in immortalized BEAS-2B bronchial epithelial cells (P<0.008) and in T-47D breast cancer cells (P<0.007). T-47D cells were also sensitive to bv-sPLA2 activity alone (P<0.002) and single treatment with PtdIns(3,4)P2 (P<0.007). Similar lytic effects as demonstrated in Fig. 2 for A498 have been observed in all tested cell lines (data not shown). Hence, lysis was accompanied by a potent reduction of the number of cancer cells with proliferative capacity. In summary, bv-sPLA2 and PtdIns(3,4)P2 synergistically induced the lysis of transformed cells derived from various tissues (kidney, prostate, lung and breast).
Fig. 4.
Combined treatment of DU145, BEAS-2B and T-47D cells with bv-sPLA2 and PtdIns(3,4)P2 inhibits [3H] thymidine incorporation. Cells were left untreated (C control), were treated with bv-sPLA2 (10 and 5 µg/ml for T-47-D), with PtdIns(3,4)P2 (10 µM) or a combination of both, bv-sPLA2 and PtdIns(3,4)P2. Shown are normalized mean values ± SEM of a representative of at least two independent experiments
Tumor cell lysates generated through combined treatment with bv-sPLA2 and PtdIns(3,4)P2 induce the maturation of human moDCs
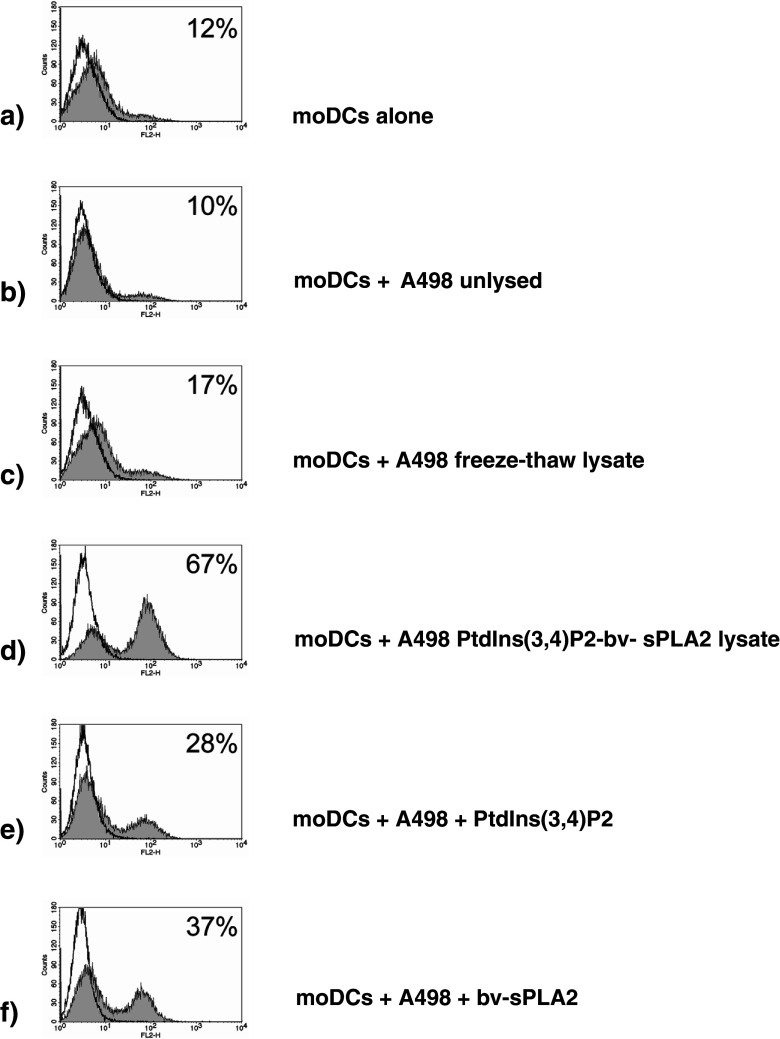
As a next step we examined the immunomodulating properties of cell lysates generated through the combined treatment with PtdIns(3,4)P2 and bv-sPLA2 (henceforth referred to as PtdIns(3,4)P2-bv-sPLA2 lysate). For this purpose, the A498 PtdIns(3,4)P2-bv-sPLA2 lysate was added to cultures of immature moDCs. A conventional lysate of A498 cells which was generated through three cycles of freezing at −80°C and rapid thawing at 37°C was used as a control. Freeze–thaw cycles represent an established procedure for the preparation of cell lysates [43] and resulted in complete cell lysis as assessed by trypan blue exclusion (data not shown). After 48 h of treatment of immature moDCs with the A498 cell lysates, moDCs were harvested and CD83 expression, which is one of the most reliable markers of moDC maturation [39] was determined by flow cytometry. While only 12% of untreated moDCs expressed the CD83 marker (Fig. 5a), 67% of the moDCs treated with the PtdIns(3,4)P2-bv-sPLA2 lysate were CD83-positive (Fig. 5d). In contrast, only 17% of the moDCs treated with the freeze–thaw lysate expressed CD83 (Fig. 5c), and coculture of moDCs with viable, non-lysed A498 cells even appeared to reduce CD83 expression (10% positive cells, Fig. 5b). Either substance alone induced the maturation of 28% (PtdIns(3,4)P2) and 37% (bv-sPLA2) of the moDCs (Fig. 5e, f). Treatments of moDCs solely with PtdIns(3,4)P2 (data not shown) or bv-sPLA2 alone [38] were basically similar in the presence or absence of A498 cells. For comparison, a cocktail consisting of prostaglandin E2, TNFα, IL-1β and IL-6 usually resulted in ≥85% CD83+ moDCs [19]. These results indicate that the PtdIns(3,4)P2-bv-sPLA2 lysate induces CD83 expression in moDCs by additive action of PtdIns(3,4)P2 and bv-sPLA2.
Fig. 5.
Treatment of moDCs with lysates generated by the combined administration of PtdIns(3,4)P2 and bv-sPLA2 matures moDCs. Immature day-5 moDCs were treated with A498 cell lysates generated by conventional freezing and thawing (freeze–thaw lysate) or by the combined actions of PtdIns(3,4)P2 (10 µM) and bv-sPLA2 (10 µg/ml). Control treatments were performed by treating moDCs with viable intact A498 cells or A498 cells treated with either PtdIns(3,4)P2 or bv-sPLA2 alone. After 2 days of incubation FACS analysis of CD83 expression was performed to determine the maturation status of moDCs. Shown is a representative of three independently performed experiments
MoDCs treated with the PtdIns(3,4)P2-bv-sPLA2 lysate have increased T cell stimulatory capacity
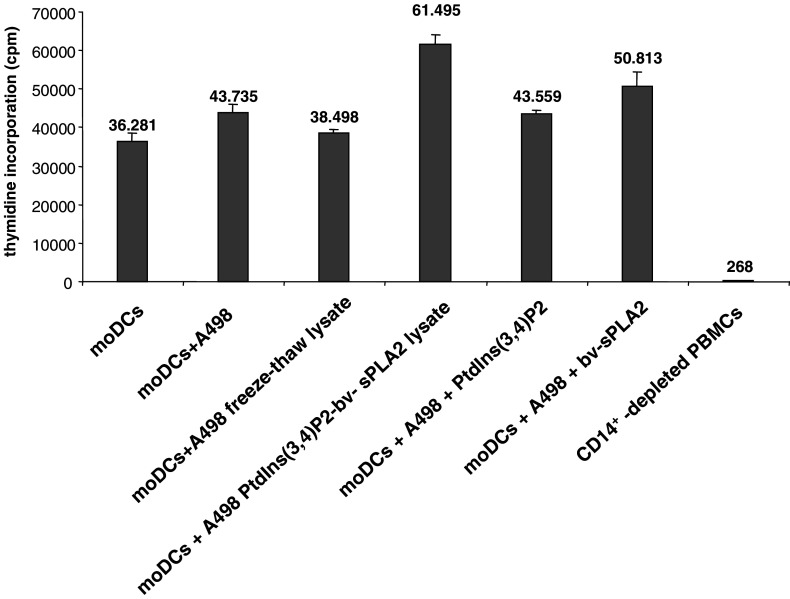
The purpose of moDC maturation is to increase T cell stimulatory capacity. We therefore compared the T cell stimulation of moDCs treated either with the conventional freeze–thaw A498 lysate or the PtdIns(3,4)P2-bv-sPLA2 lysate in the allogeneic MLR. Figure 6 demonstrates that moDCs treated with the PtdIns(3,4)P2-bv-sPLA2 lysate were always more potent in inducing the proliferation of allogeneic T cells than moDCs treated with the conventional freeze–thaw lysate. Thus, the T cell stimulatory capacity of moDCs was in line with CD83 expression shown in Fig. 5. A statistically significant augmentation of T cell stimulation by moDCs was achieved exclusively by bv-sPLA2 treatment alone (P<0.05) and with the PtdIns(3,4)P2-bv-sPLA2 lysate (P<0.005). Exposure of moDCs to PtdIns(3,4)P2-bv-sPLA2 lysate almost doubled T cell stimulation. Although, treatment of moDCs with PtdIns(3,4)P2 moderately enhanced T cell stimulation (P<0.07), its actual effect was an additive promotion of moDC maturation together with bv-sPLA2. In summary, moDCs treated with cell lysates generated by the combined administration of bv-sPLA2 and PtdIns(3,4)P2 displayed increased T cell stimulatory capacity.
Fig. 6.
MoDCs treated with lysates generated by the combined administration of PtdIns(3,4)P2 and bv-sPLA2 display enhanced T cell stimulatory capacity. Lysate-treated moDCs were used as stimulators of CD14+-depleted PBMCs in allogeneic mixed leukocyte reactions. After 5 days of coincubation, proliferation was determined by assessing [3H] thymidine incorporation. Shown are mean values (cpm) ± SEM of triplicate measurements of one representative experiment of three independently performed experiments
Discussion
In the present work, we demonstrate that the combined treatment with bv-sPLA2 and PtdIns(3,4)P2 lyses tumor cells and synergistically reduces the number of cells with proliferative capacity. The resulting tumor lysates enhanced the immunostimulatory capacity of moDCs.
Although the detailed mechanism for the synergistic action of PtdIns(3,4)P2 and bv-sPLA2 needs further clarification, the inhibitory effect on tumor cell lines is apparent. Several modes of cooperation can be envisaged. One obvious possibility for synergistic interaction is that PtdIns(3,4)P2 serves as a substrate of bv-sPLA2 which is specific for fatty acids attached to the sn-2 position. This would result in the massive generation of lyso-phospholipids. Interestingly, for structurally related lyso-phospholipids, such as lyso-phosphatidylcholin (lyso-PC) or synthetic alkyl-lysophospholipids, cytotoxic effects have been reported frequently. Lyso-PC, which is the product of PLA2 action on PC, can induce cytotoxicity by mechanisms related to programmed cell death or alternative modes of action which are independent from apoptosis. Among the latter are detergent-like properties [51]. Lysophosphoglycerides such as lyso-PC form micelles that disrupt membrane structure and impair the function of macromolecules and cellular receptors embedded in the membrane [20]. In addition, lyso-PC is related to activation of Ca2+ channels [46] and the activation of intracellular signaling cascades linked to the generation of oxygen-centered radicals which may cause membrane damage [33]. The plasma membrane also has been identified as the primary site of action of synthetic alkyl-lysophospholipids [32, 41]. These compounds structurally resemble sPLA2-reaction products and have also been suggested for tumor therapies.
Another possibility for the combined lytic action is that PtdIns(3,4)P2 enhances bv-sPLA2 activity. Such a mechanism has been proposed for PtdIns(4,5)P2 and a cytoplasmic (non-secretory) form of PLA2 [27]. However, the addition of PtdIns(3,4)P2 to bv-sPLA2 in our cellular system did not result in a significant increase of sPLA2 enzymatic activity as measured by a commercial sPLA2 assay kit (data not shown). This suggests additional modes of interaction between PtdIns(3,4)P2 and bv-sPLA2. Intriguingly, in our study PtdIns(3,4)P2 was very potent in synergizing with bv-sPLA2 under absolutely serum-free conditions. It is well established that sPLA2 requires the presence of FCS to act on membranes of living cells [28]. FCS is a very potent survival stimulus for cells and activates the PI-3 kinase which generates the 3-phosphorylated forms of PtdIns. Our findings therefore suggest that the underlying mechanism of the serum dependence of sPLA2 is due to serum-mediated regulation of the PI-3 kinase pathway resulting in the production of PtdIns(3,4)P2 which then cooperates with sPLA2.
Importantly, the lysates generated with this method are immunogenic since they induce the maturation of moDCs with increased immunostimulatory capacity. The method described here allows the efficient generation of tumor cell lysates which can be loaded onto moDCs. The moDCs become activated and mature as a consequence of the stimulatory activity of the lysate mediated by additive action of bv-sPLA2 and PtdIns(3,4)P2 on CD83 expression. Thus such lysates display features of a vaccine with adjuvant properties. In case of a cancer cell, the cell lysate itself contains the complete spectrum of potentially relevant target tumor antigens. For vaccination purposes antigens are admixed with commercial adjuvants which serve to enhance immunogenicity. Such adjuvants usually contain hydrophobic substances which are known to augment both antibody production and cell-mediated immunity [8]. In addition, adjuvants frequently contain components which induce local inflammation at the site of administration. Today it is known that inflammation serves to recruit immune cells to the site of vaccine administration and to promote DC maturation which favors the induction of the desired immune responses [6, 26]. Influence on inflammation and immune responses has also been attributed to phospholipids and related compounds [25]. The rapid cell lysis induced by the combined administration of PtdIns(3,4)P2 and bv-sPLA2 indicates the presence of detergent activity previously attributed to lyso-phospholipids generated by sPLA2 enzyme activity [29]. Lysophospholipids also act as chemoattractants recruiting phagocytic antigen-presenting cells to the site of vaccine application [37]. These cells pick up and present vaccine components to the effector cells of the immune system to promote immunogenicity. Recently, it has been demonstrated that bv-sPLA2 can be utilized as a fusion protein to enhance immunity [4]. In that study, a catalytically inactivated recombinant bv-sPLA2 fused to a CMV epitope was effective to provide cross presentation of that epitope by the membrane binding activity of bv-sPLA2. Our data are in line with reports that support the concept of bv-sPLA2-augmented immunity. However, in our experimental model a catalytically active bv-sPLA2 is required for the lytic effect on tumor cells and the promotion of CD83 expression on moDCs.
A novel finding in our study was the direct maturation-promoting effect of PtdIns(3,4)P2 on moDCs (Fig. 5e). Hence, elevated levels of PtdIns(3,4)P2 may exhibit a “danger” signaling function and facilitate moDC activation [12]. The observed effect of the PI 3-kinase product PtdIns(3,4)P2 on moDCs is in line with previous data that demonstrate a role of PI 3-kinase signaling in antigen-presenting cells [2]. PI 3-kinase has been shown to act in concert with mitogen-activated protein kinase pathways to regulate LAG-3-induced activation and maturation of moDCs [1], whereas DCs from PI 3-kinase-deficient mice displayed reduced migratory and immunostimulatory capacities [9].
Taken together, bv-sPLA2 in combination with PtdIns(3,4)P2 displayed antitumor effects with concomitant stimulation of the immune system. This intriguing observation strongly suggests the further exploration of the therapeutic potential of these compounds in cancer immunotherapy.
Acknowledgment
This work was supported by a grant to MT of the kompetenzzentrum medizin tirol (kmt), a center of excellence.
References
- 1.Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223) Blood. 2003;102:2130–2137. doi: 10.1182/blood-2003-01-0273. [DOI] [PubMed] [Google Scholar]
- 2.Appel S, Rupf A, Weck MM, Schoor O, Brummendorf TH, Weinschenk T, Grunebach F, Brossart P. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin Cancer Res. 2005;11:1928–1940. doi: 10.1158/1078-0432.CCR-04-1713. [DOI] [PubMed] [Google Scholar]
- 3.Ashagbley A, Samadder P, Bittman R, Erukulla RK, Byun HS, Arthur G. Synthesis of ether-linked analogues of lysophosphatidate and their effect on the proliferation of human epithelial cancer cells in vitro. Anticancer Res. 1996;16:1813–1818. [PubMed] [Google Scholar]
- 4.Babon A, Almunia C, Boccaccio C, Beaumelle B, Gelb MH, Menez A, Maillere B, Abastado JP, Salcedo M, Gillet D. Cross-presentation of a CMV pp65 epitope by human dendritic cells using bee venom PLA2 as a membrane-binding vector. FEBS Lett. 2005;579:1658–1664. doi: 10.1016/j.febslet.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Balsinde J, Dennis EA. Function and inhibition of intracellular calcium-independent phospholipase A2. J Biol Chem. 1997;272:16069–16072. doi: 10.1074/jbc.272.26.16069. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.den Brok MH, Nierkens S, Figdor CG, Ruers TJ, Adema GJ. Dendritic cells: tools and targets for antitumor vaccination. Expert Rev Vaccines. 2005;4:699–710. doi: 10.1586/14760584.4.5.699. [DOI] [PubMed] [Google Scholar]
- 8.Cox JC, Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15:248–256. doi: 10.1016/S0264-410X(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 9.Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, Bernasconi S, Sironi M, Santoro A, Garlanda C, Facchetti F, Wymann MP, Vecchi A, Hirsch E, Mantovani A, Sozzani S. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. Embo J. 2004;23:3505–3515. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 11.Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 13.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 14.Grunebach F, Muller MR, Nencioni A, Brossart P. Delivery of tumor-derived RNA for the induction of cytotoxic T-lymphocytes. Gene Ther. 2003;10:367–374. doi: 10.1038/sj.gt.3301901. [DOI] [PubMed] [Google Scholar]
- 15.Grunebach F, Muller MR, Brossart P. New developments in dendritic cell-based vaccinations: RNA translated into clinics. Cancer Immunol Immunother. 2005;54:517–525. doi: 10.1007/s00262-004-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höltl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M. CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal-cell cancer. Lancet. 1998;352:1358. doi: 10.1016/S0140-6736(05)60748-9. [DOI] [PubMed] [Google Scholar]
- 17.Höltl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777–782. doi: 10.1016/S0022-5347(01)61767-1. [DOI] [PubMed] [Google Scholar]
- 18.Höltl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- 19.Höltl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2004;54:663–670. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CC, Yen MH, Liu HW, Lau YT. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL. Atherosclerosis. 2000;151:481–491. doi: 10.1016/S0021-9150(00)00453-6. [DOI] [PubMed] [Google Scholar]
- 21.Johrer K, Zelle-Rieser C, Perathoner A, Moser P, Hager M, Ramoner R, Gander H, Holtl L, Bartsch G, Greil R, Thurnher M. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005;11:2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 23.Keydar I, Chen L, Karby S, Weiss FR, Delarea J, Radu M, Chaitcik S, Brenner HJ. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979;15:659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- 24.Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Cult Methods. 1985;9:43–48. doi: 10.1007/BF01797773. [DOI] [Google Scholar]
- 25.Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 27.Mosior M, Six DA, Dennis EA. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J Biol Chem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M, Kuwata H, Amakasu Y, Shimbara S, Nakatani Y, Atsumi G, Kudo I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J Biol Chem. 1997;272:19891–19897. doi: 10.1074/jbc.272.32.19891. [DOI] [PubMed] [Google Scholar]
- 29.Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima S, Ikeno Y, Yokoyama T, Kuwana M, Bolchi A, Ottonello S, Kitamoto K, Arioka M. Secretory phospholipases A2 induce neurite outgrowth in PC12 cells. Biochem J. 2003;376:655–666. doi: 10.1042/BJ20030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakashima S, Kitamoto K, Arioka M. The catalytic activity, but not receptor binding, of sPLA(2)s plays a critical role for neurite outgrowth induction in PC12 cells. Brain Res. 2004;1015:207–211. doi: 10.1016/j.brainres.2004.04.069. [DOI] [PubMed] [Google Scholar]
- 32.Noseda A, White JG, Godwin PL, Jerome WG, Modest EJ. Membrane damage in leukemic cells induced by ether and ester lipids: an electron microscopic study. Exp Mol Pathol. 1989;50:69–83. doi: 10.1016/0014-4800(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 33.Ohara Y, Peterson TE, Zheng B, Kuo JF, Harrison DG. Lysophosphatidylcholine increases vascular superoxide anion production via protein kinase C activation. Arterioscler Thromb. 1994;14:1007–1013. doi: 10.1161/01.atv.14.6.1007. [DOI] [PubMed] [Google Scholar]
- 34.Payrastre B, Missy K, Giuriato S, Bodin S, Plantavid M, Gratacap M. Phosphoinositides: key players in cell signalling, in time and space. Cell Signal. 2001;13:377–387. doi: 10.1016/S0898-6568(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 35.Perrin-Cocon L, Agaugue S, Coutant F, Masurel A, Bezzine S, Lambeau G, Andre P, Lotteau V. Secretory phospholipase A2 induces dendritic cell maturation. Eur J Immunol. 2004;34:2293–2302. doi: 10.1002/eji.200324797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putz T, Gander H, Ramoner R, Zelle-Rieser C, Rahm A, Nussbaumer W, Bartsch G, Höltl L, Thurnher M. Generation of clinical grade monocyte-derived dendritic cells using the CliniMACS system. Methods Mol Med. 2004;24:653–663. doi: 10.1385/1-59259-862-5:071. [DOI] [PubMed] [Google Scholar]
- 37.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramoner R, Putz T, Gander H, Rahm A, Bartsch G, Schaber C, Thurnher M. Dendritic cell activation by secretory phospholipase A2. Blood. 2005;105:3583–3587. doi: 10.1182/blood-2004-08-3001. [DOI] [PubMed] [Google Scholar]
- 39.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo MT, Nguyen E, Aldo-Benson M, Lambeau G. Secreted phospholipase A(2) induces vascular endothelial cell migration. Blood. 2000;96:3809–3815. [PubMed] [Google Scholar]
- 41.Ruiter GA, Verheij M, Zerp SF, van Blitterswijk WJ. Alkyl-lysophospholipids as anticancer agents and enhancers of radiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2001;49:415–419. doi: 10.1016/S0360-3016(00)01476-0. [DOI] [PubMed] [Google Scholar]
- 42.Samadder P, Bittman R, Byun HS, Arthur G. Synthesis and use of novel ether phospholipid enantiomers to probe the molecular basis of the antitumor Effects of alkyllysophospholipids: correlation of differential activation of c-Jun NH(2)-terminal protein kinase with antiproliferative effects in neuronal tumor cells. J Med Chem. 2004;47:2710–2713. doi: 10.1021/jm0302748. [DOI] [PubMed] [Google Scholar]
- 43.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 45.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 46.Stoll LL, Spector AA. Lysophosphatidylcholine causes cGMP-dependent verapamil-sensitive Ca2+ influx in vascular smooth muscle cells. Am J Physiol. 1993;264:C885–C893. doi: 10.1152/ajpcell.1993.264.4.C885. [DOI] [PubMed] [Google Scholar]
- 47.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 48.Toker A. Phosphoinositides and signal transduction. Cell Mol Life Sci. 2002;59:761–779. doi: 10.1007/s00018-002-8465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentin E, Lambeau G. What can venom phospholipases A(2) tell us about the functional diversity of mammalian secreted phospholipases A(2)? Biochimie. 2000;82:815–831. doi: 10.1016/S0300-9084(00)01168-8. [DOI] [PubMed] [Google Scholar]
- 50.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 51.Weltzien HU, Richter G, Ferber E. Detergent properties of water-soluble choline phosphatides. Selective solubilization of acyl-CoA:lysolecithin acyltransferase from thymocyte plasma membranes. J Biol Chem. 1979;254:3652–3657. [PubMed] [Google Scholar]
- 52.Wierecky J, Mueller M, Brossart P (2005) Dendritic cell-based cancer immunotherapy targeting MUC-1. Cancer Immunol Immunother (in press) [DOI] [PMC free article] [PubMed]