Abstract
Despite advances in surgical technology and radiation therapy, the prognosis in the patients with malignant glioma remains poor. Recent studies show that interleukin-13 receptor {alpha}2 chain (IL-13Ra2), a brain tumor-associated receptor for IL-13, may play a role in immunotherapy for glioblastoma. We thus amplified human IL-13Ra2 gene from the human glioblastoma cell line using RT-PCR and cloned the target gene into the pET-28a, a prokaryotic expressing plasmid. After transformation, the recombinant plasmid expressed a soluble protein induced by IPTG. The purified recombinant protein was shown to be a single band on the SDS-PAGE with a predicated molecular weight of human IL-13Ra2 gene, suggesting that the recombinant protein of human IL-13Ra2 was successfully expressed. Recombinant IL-13Ra2 protein can be used as an anti-tumor vaccine, which may provide a promising new strategy for the treatment of brain malignant gliomas.
Keywords: Malignant gliomas, Interleukin-13 receptor a2, Prokaryotic expression, Plasmid, Immunotherapy
Introduction
It is widely accepted that the malignant gliomas are highly invasive, aggressive and neurologically destructive tumors. Despite the adoption of advanced treatments such as surgical resection, radiotherapy, chemotherapy, most patients with malignant glioma are still unoptimistic [1, 2]. The interleukin-13 receptor {alpha}2 (IL-13Ra2)chain is a primary IL-13 binding and internalization component of the IL-13R system. Recent studies show that the IL-13Ra2 is specifically overexpressed in glioblastoma [3–5]. Some experts reported that IL-13Ra2 chain did not seem to form a complex with any other known chain and did not mediate signaling through STAT6 pathway, even though it binded with IL-13 with high affinity [6–8]. Therefore, it was thought to have a intimate relation with the occurrence and development of brain glioblastoma. The human leukocyte antigen (HLA)-A2.1-restricted CD8+ T-cell IL-13Ra2 derived from the glioma-associated antigen was identified [9]. So IL-13Ra2 can be used as the target in glioblastoma immunotherapy. The objectives of the present study were to generate an IL-13Ra2 antigen using prokaryotic expression technique and to provide the groundwork for immunotherapy of glioblastoma.
Material and methods
Cell lines and cell culture
U251 cell lines were purchased from Shanghai institute of biochemistry and cell biology, China. The glioma cell line transfected with IL-13Ra2 plasmid was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10 mM l-glutamine (all reagents from Bio Basic Inc., Canada) at 37°C in a humidified incubator with 5% CO2.
IL-13Ra2 gene isolation
The RNA was extracted from U251 using Trizol reagent and the human IL-13Ra2 gene was amplified by the reverse-transcription PCR method. The primers used for human IL-13Ra2 gene (GeneBank accession number: NM_000640) were 5′-GGCCTCGAGATGGCTTTCGTTTGCTT-3′ (forward) and 5′-GCGAAGCTTTCATGTATCACAGAAAAATTC-3′ (reverse), containing XhoI and HindIII restriction sites underlined, respectively. The PCR reaction was performed with the Ex taq DNA polymerase (Takara Co., Japan) in three steps: (1) 94°C predenaturation for 5 min, (2) 35 amplification cycles 94°C predenaturation for 30 s, 59°C primer annealing for 45 s, then primer extension at 72°C for 1 min, and (3) 72°C for 5 min. The 1,142 base pair (bp) fragment was generated. A total of 10 μl of the production was used for the analysis on 1.5% agarose gel containing EB and then the photographs were taken. After purification using gel purify kit (Bio Basic Inc.), the obtained sequence was analyzed and matched to Gene Bank sequence.
Cloning of human IL-13Ra2 gene
The IL-13Ra2 gene was cloned according to the cloning manufacture protocol as follows. The purified IL-13Ra2 DNA fragment was inserted into the cloning plasmid pMD-19 simple T vector(Takara Co., Japan) in water bath 42°C for 30 min. Then 100 μl of the connected product was put into the competence E. coli, DH5α. The conditions were set at heat shock at 42°C for 42 s and iced bath for 1 min, followed by shaking culture in 890 ml SOC medium with 200 rpm at 37°C for 1 h and cultivation in solid LB medium with 100 mg/ml ampicilin, 24 mg/ml IPTG and 20 mg/ml X-Gal at 37°C overnight. The white colony proved the successful clone through the identification by the restriction enzyme digestion and sequencing analysis.
Construction of the expression plasmid
The extracted plasmid from cloning was digested by the restriction enzymes, XhoI and HindIII (Takara Co.). The digested product was then inserted into the expression plasmid pET-28a with T4 DNA ligase at 16°C for 24 h. The pET-28a was also digested by XhoI and HindIII. The connected product was transformed into the E. coli BL21(DE3) (Novagen Inc., USA) and cultured in LB media (1% tryptone, 0.5% yeast extract, 1% NaCl) with 50 μg/ml kanamycin at 37°C overnight, followed by the restriction enzyme digestion and sequencing analysis.
Expression and purification of IL-13Ra2 antigen
The cells containing pET-28a–IL-13Ra2 were picked from the LB media and then in shaking culture at 37°C overnight. Then 100 ml of the cells was respectively added into five tubes (labeled as 1, 2, 3, 4, and 5) with 5 ml LB media containing 50 μg/ml kanamycin. 50 μl of isopropyl-β-d-thiogalactoside (IPTG) (24 μg/ml) was added into tubes 2–4, respectively. after shaking the culture at 37°C for 3 h. After culturing for 1, 2, 3, and 4 h, 1 ml of the cells was then analyzed using SDS-PAGE with 12% polyacrylamide gel at different time points, respectively. After we confirmed that the objective antigen was highly expressed at the condition of inducing temperature 35°C and at 0.8 mM final concentration of IPTG, we induced lots of cells which contain pET-28a–IL-13Ra2 at this condition. In addition, the induced cells were then harvested by centrifugation at 6,000×g for 10 min and resuspended in 30 ml buffer B(pH 8.0, 8 mM urea). The cells were broken by turning on and off an ultrasound equipment (KS-1200, Haishu ultrasound equipment Inc., China) with 20 cycles and a power of 300 W for 5 s. Inclusion bodies containing rh IL-13Ra2 were recovered by centrifugation at 2,000×g, washed for three times, and then resuspended in buffer B. The inclusion bodies were then filtered by Chromatography columns with Ni-NTA his-bind resin (Novagen Inc.). The depurated inclusion bodies washed down by buffer E (pH 4.5, 8 mM urea) were collected and analyzed by SDS-PAGE using 12% polyacrylamide gels. Moreover, the depurated product was the objective protein.
Results
Cell culture and amplification of IL-13Ra2 gene
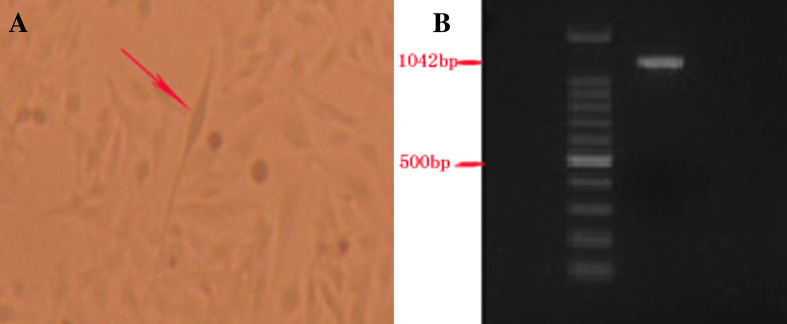
The three-forth of the total U251 cells had adhered to the fusiform-liked plate. A large cell body and multi-macula were obtained after 3 days of culturing (Fig. 1a).The IL-13Ra2 Gene was amplified successfully by reverse-transcription PCR from U251 glioma cells. The results showed a 1,142 bp sequence on DU 800 Nucleic Acid Analyzer and the homology was 100% after sequencing and contrast with the GeneBank CDS (accession NM_000640) (Fig. 1b).
Fig. 1.
The U251 glioma cell lines and the IL-13Ra2 gene. a The U251 cell with fusiform-liked cell body after 3 days of culturing (arrow). b The IL-13Ra2 gene amplified by RT-PCR from the U251 cells is 1,142 bp using DU 800 Nucleic Acid Analyzer
Construction of cloning plasmid
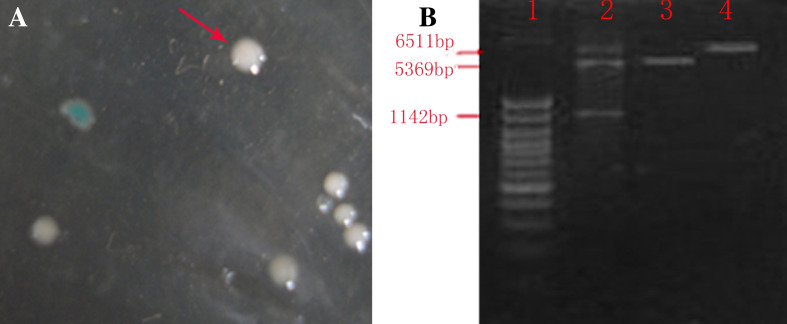
Thirty colonies of white and blue growth were shown on the LB plate after culturing for one night (Fig. 2a). The white colony, the positive transfection colony, was picked and identified by the analysis of PCR, enzyme and sequencing. As a result, the homology was 100% after sequencing and contrast with GeneBank, which means the cloning plasmid was constructed successfully.
Fig. 2.
The IL-13Ra2 gene is cloned and constructed to obtain the recombination plasmid pET-28a–IL-13Ra2. a The IL-13Ra2 gene is transferred to E. coli DH5α and screened by blue–white patch, the white colony (arrow) is the positive transfection colony. b The recombination plasmid pET-28a–IL-13Ra2 is identified by the enzymes, XhoI and HindIII. 1 marker, 2 the recombinant plasmid after digestion, 3 vacant plasmid, and 4 recombinant plasmid
Construction of expression plasmid
It was observed that ~40 colonies grew on the LB plate. One of the colonies was selected and identified by PCR, sequencing and double digestion with XhoI and HindIII. All the results proved that the construction was successful. Figure 2b shows the digestion result.
Expression and purification of IL-13Ra2 antigen
The IL-13Ra2 antigen was expressed successfully with inclusion body form and the peak expression appeared after 3 h inducing (Fig. 3a).The molecular weight of the IL-13Ra2 antigen was analyzed using SDS-PAGE as equal to approximately 60 kDa. The purity was >95% after purifying in Chromatography columns with Ni-NTA his-bind resin (Fig. 3b).
Fig. 3.
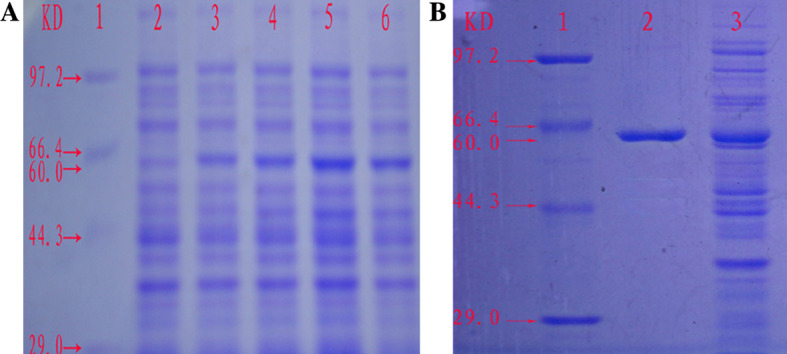
The IL-13Ra2 antigen is expressed and purified by SDS-PAGE. a SDS-PAGE analysis of IL-13Ra2 antigen expression. 1 marker, 2 inducing without IPTG, 3 inducing with IPTG for 1 h, 4 inducing with IPTG for 2 h, 5 inducing with IPTG for 3 h, and 6 inducing with IPTG for 4 h. It is shown that IL-13Ra2 antigen expression reaches the peak after 3 h of induction. b The IL-13Ra2 antigen after purifying. 1 indicates marker, 2 IL-13Ra2 antigen after purifying, and 3 the control without purifying
Discussion
Malignant glioma is considered as one of the most malignant tumors in brain. Despite significant advances in modern microsurgery, radiotherapy and chemotherapy, the prognosis for patients with malignant glioma remains poor. Immunotherapy for glioma is an attractive alternative treatment because activated antitumor immune cells have potentials to migrate into the central nervous system (CNS) and to selectively destroy the malignant cells that have infiltrated normal CNS tissues [10]. The IL-13Ra2 chain, a primary IL-13 binding and internalization component of the IL-13R system, was restricted and expressed in high level in most human malignant gliomas. Therefore, IL-13Ra2 might be a specific antigen to target on malignant gliomas in human beings.
The major goal of our study was to obtain a specific and effective antigen of IL-13Ra2 by prokaryotic expression technique, which could make a big contribution to the immunotherapy of human malignant gliomas. The primer with the two restriction enzymes XhoI and HindIII of the IL-13Ra2 gene could be designed according to the Genebank IL-13Ra2 CDS. Furthermore, the IL-13Ra2 gene could also be amplified by reverse-transcription PCR method from glioma cells U251. After purification by gelatin recovery, the IL-13Ra2 gene could be connected to the cloning plasmid pMD-19 simple T vector by connecting T–A and transfecting the E. coli DH5α with high efficiency. The IL-13Ra2 gene was well cloned, which was identified by the analysis of PCR, enzyme and sequencing. It was then digested from the pMD-19- IL-13Ra2 and connected to the expression plasmid pET-28a which was also double-digested by XhoI and HindIII. In addition, the recombinant plasmid of pET-28a–IL-13Ra2 was then transfected to the E. coli BL21(DE3), and also identified by the analysis of PCR, enzyme and sequencing. Moreover, the IL-13Ra2 antigen was successfully highly expressed after 3 h of induction by IPTG with a final concentration of 0.8 mM, and the main form of the IL-13Ra2 antigen was inclusion body. Finally, the IL-13Ra2 antigen was purified by Chromatography columns with Ni-NTA his-bind resin conveniently and analyzed by the SDS-PAGE. It is well reported that prokaryotic expression is a perfect and well-rounded technique [11, 12], so we can use it to obtain our objective IL-13Ra2 antigen conveniently and successfully.
Some studies have demonstrated that IL-13Ra2 chain has been thought to play a role in immunosurveillance in cancer-bearing hosts [13]. It does not seem to form a complex with any other known chain and does not mediate signal transduction through the Janus-activated kinase-signal transducers and activators of transcription (STAT) pathway, even though it binds with IL-13 with high affinity. So it is known as a decoy receptor in high-grade malignant glioma [14–17].
Recent studies have well reported that the IL-13Ra2 chain is abundantly and specifically overexpressed by glioblastomas while normal brain cells do not express this protein or express it in a very low level [18–20]. In addition, our previous studies also showed that the IL-13Ra2 chain was highly and specifically expressed in high-grade gliomas, but no or low-level expression was found in normal or low-grade gliomas tissues [21]. In recent years, the immunotherapy on brain glioblastomas has a good progression, and most of the vaccines target on glioblastomas were tumor cell RNA or cell lysates [22–26], but these vaccines had some negative side effects such as immunizing cephalitis, vitiligo and others [27]. However, Eguchi et al. 28 and Jiang et al. 29 proved that the IL-13Ra2 protein could be an attractive vaccine target and their results showed that the effective anti-tumor immune responses could be induced in preclinical models using dendritic cell-based vaccines. However, the vaccines they used were short peptide, which was part of the IL-13Ra2 protein. In contrast, our objectives are to express a total and effective vaccine IL-13Ra2 that targets on brain malignant glioma and thus to prevent the immune escape. Most importantly, it is well documented that IL-13Ra2 antigen in HLA-A0201 CD8+ T cells could induce specific T-cell response [30, 31]. Therefore, the IL-13Ra2 could be a HLA-A0201-restricted CTL antigen in immunotherapy of malignant gliomas.
The present study showed that recombinant expression plasmid pET-28a–IL-13Ra2 could be successfully constructed in vitro and IL-13Ra2 antigen could also be well expressed by the prokaryotic expression technique. It is possible that IL-13Ra2 antigen is considered as a special vaccine to target on malignant gliomas and then will make a great contribution to the immunotherapy of brain malignant gliomas.
Contributor Information
Weiming Zheng, Email: zhwm61@126.com.
Yanjun Zeng, Email: yjzeng@bjut.edu.cn.
References
- 1.Stupp R, Dietrich PY, Ostermann KS, Pica A, Maillard I, Maeder P, Meuli R. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas. Curr Neurol Neurosci Rep. 2004;4:218–227. doi: 10.1007/s11910-004-0042-4. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami M, Kawakami K, Takahashi S. Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer. 2004;101:1036–1042. doi: 10.1002/cncr.20470. [DOI] [PubMed] [Google Scholar]
- 4.Joshi BH, Plautz GE, Puri RK. Interleukin-13receptor alpha chain: a novel tumor-associated ransmembrane protein in primary explants of human malignantgliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- 5.Caput D, Laurent P, Kaghad M. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor a chain. J Biol Chem. 1996;271:16921–16926. doi: 10.1074/jbc.271.28.16921. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami K, Taguchi J, Murata T. The interleukin-13 receptor a2 chain: an essential component for binding and internalization but not for IL-13 induced signal transduction through STAT6 pathway. Blood. 2001;97:2673–2679. doi: 10.1182/blood.V97.9.2673. [DOI] [PubMed] [Google Scholar]
- 7.Murata T, Taguchi J, Puri RK. Interleukin-13 receptor a2 chain but not a chain: a functional component of interleukin-4 receptor. Blood. 1998;91:3884–3891. [PubMed] [Google Scholar]
- 8.Wood N, Whitters MJ, Jacobson BA. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor a2. J Exp Med. 2003;197:703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okano F, Storkus WJ, Chambers WH, Pollack IF, Okada H. Identification of a novel HLA-A*0201 restricted cytotoxic T lymphocyte epitope in a human glioma associated antigen, interleukin-13 receptor 2 chain. Clin Cancer Res. 2002;8:2851–2855. [PubMed] [Google Scholar]
- 10.Holladay FP, Heitz T, Chen YL, Chiga M, Wood GW. Successful treatment of a malignant rat glioma with cytotoxic T lymphocytes. Neurosurgery. 1992;31:528–533. doi: 10.1097/00006123-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Busuttil BE, Turney KL, Frauman AG. The expression of soluble, full-length recombinant human TSH receptor in a prokaryoutic system. Protein Expr Purif. 2001;23:369–373. doi: 10.1006/prep.2001.1519. [DOI] [PubMed] [Google Scholar]
- 12.Leandro P, Lechner MC, Almerda H. Glycerol increases the yield and activity of human phenylalanine hydroxylase mutant enzymes produced in a prokaryotic expression system. Mol Genet Metab. 2001;73:173–178. doi: 10.1006/mgme.2001.3172. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami K, Kawakami M, Snoy PJ. In vivo over-expression of IL-13 receptor a2 chain inhibits tumorigenicity of human breast and pancreatic tumors in immunodeficient mice. J Exp Med. 2001;194:1743–1754. doi: 10.1084/jem.194.12.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard J, Treton D, Vermot-Desroches C. Expression of interleukin 13 receptor in glioma and renal cell carcinoma: IL13Ra2 as a decoy receptor for IL13. Lab Invest. 2001;81:1223–1231. doi: 10.1038/labinvest.3780336. [DOI] [PubMed] [Google Scholar]
- 15.Wills-Karp M, Luyimbazi J, Xu X. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 16.Rahaman SO, Sharma P, Harbor PC. IL-13Ra2, a decoy receptor for IL-13 act as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62:1103–1109. [PubMed] [Google Scholar]
- 17.Chiaramonte MG, Mentink-Kane M, Jacobson BA. Regulation and function of the interleukin 13 receptor a2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson DD, Whitters MJ, Fitz LJ. The murine IL-13 receptor a2: molecular cloning, characterization, and comparison with murine IL-13 receptor a1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 19.Debinski W, Gibo DM, Slagle B, Powers SK, Gillespie GY. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int J Oncol. 1999;15:481–486. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 20.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W, Zhang J, Li J. The relation between IL-13Rα2 and prognosis in astrocytoma. Chin J Neurosurg. 2006;22:324. [Google Scholar]
- 22.Zhan R, Tong Y, Zhou Y. Experimental study of treatment of intracranial G422 glioblastoma with bone marrow2 derived dendritic cells pulsed with tumor RNA. Chin J Neurosurg. 2003;19:99–102. [Google Scholar]
- 23.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpentier AF, Meng Y. Recent advances in immunotherapy for human glioma. Curr Opin Oncol. 2006;18:631–636. doi: 10.1097/01.cco.0000245321.34658.f4. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami K, Terabe M, Kawakami M. Characterization of a novel human tumor antigen interleukin-13 receptor a2 chain. Cancer Res. 2006;66:4434–4442. doi: 10.1158/0008-5472.CAN-05-1265. [DOI] [PubMed] [Google Scholar]
- 26.Liau LM, Black KL, Martin NA. Treatment of a glioblastoma patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I–matched tumor peptides. Neurosurg Focus. 2000;9:1–5. doi: 10.3171/foc.2000.9.6.9. [DOI] [PubMed] [Google Scholar]
- 27.Liau LM, Black KL, Prins RM, Sykes SN, DiPatre PL, Cloughesy TF, Becker DP. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90:1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi J, Hatano M, Nishimura F. Identification of interleukin-13 receptor A2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Lu X, Liu R. Induction of cytotoxic T lymphocytes specific to malignant glioma using T2 cells pulsed with HLA-A2-restricted interleukin-13 receptor α2 peptide in vitro. Acta Biochim Biophys Sin. 2007;39:641–648. doi: 10.1111/j.1745-7270.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Lu X, Liu R, Zhang F, Zhao H. HLA tetramer based artificial antigen-presenting cells efficeenfly stimulate CTLs specific for malignant glioma. Clin Cancer Res. 2007;24:7329–7334. doi: 10.1158/1078-0432.CCR-07-1025. [DOI] [PubMed] [Google Scholar]
- 31.kikuchi T. Novel immunotherapeutic approach. Gan To Kaqaku Ryoho. 2005;34:453–457. [PubMed] [Google Scholar]