Abstract
Active hexose correlated compound (AHCC) is a mixture of polysaccharides, amino acids, lipids and minerals derived from cocultured mycelia of several species of Basidiomycete mushrooms. AHCC has been implicated to modulate immune functions and plays a protective role against infection. However, the potential role of AHCC in tumor immune surveillance is unknown. In this study, C57BL/6 mice were orally administered AHCC or water, followed by tumor cell inoculation. We showed that compared to pure water-treated mice, AHCC treatment significantly delayed tumor development after inoculation of either melanoma cell line B16F0 or lymphoma cell line EL4. Treatment with AHCC enhanced both Ag-specific activation and proliferation of CD4+ and CD8+ T cells, increased the number of tumor Ag-specific CD8+ T cells, and more importantly, increased the frequency of tumor Ag-specific IFN-γ producing CD8+ T cells. Interestingly, AHCC treatment also showed increased cell number of NK and γδ T cells, indicating the role of AHCC in activating these innate-like lymphocytes. In summary, our results demonstrate that AHCC can enhance tumor immune surveillance through regulating both innate and adaptive immune responses.
Keywords: Nutrition food, T cells, Tumor immunity, IFN-γ, Tumor therapy
Introduction
Active hexose correlated compound (AHCC) is a mixture of polysaccharides, amino acids, lipids and minerals derived from fungi [1]. The chemical analysis has revealed that oligosaccharides are the major components of AHCC, consisting about 74% of the mixture. Nearly 20% of this fraction is composed of the α-1,4-glucans and their acetylated forms with an average molecular weight of 5,000. Consequently, these oligosaccharides may account for the biological activities associated with AHCC.
As a therapeutic agent, AHCC is well tolerated and largely free of adverse effects [2, 3]. This mixture has been reported to have the following effects: increase detoxification enzymes in the liver and protect the liver from CCl4-induced injury [4], prevent the onset of diabetes induced by streptozotocin in animal models [1], suppress thymic apoptosis induced by dexamethasone [5], decrease ferric nitrilotriacetate (Fe-NTA)-mediated excretion of 8-hydroxy-2′-deoxyguanosine (8-OhdG) in rat urine [6], and act as an antioxidant to ameliorate endocrine disorders [7]. Overall, these data suggest that AHCC can act as a potential modifier of biological responses. In terms of immunity and cancer, studies have revealed that AHCC enhanced the activity of natural killer cell in cancer patients [8, 9], induced the production of IL-12 and IFN-γ [10, 11], enhanced spleen cell proliferation and cytokine production, as well as nitric oxide and cytokine production in peritoneal cells [12], and reduced the metastasis rate of rat mammary adenocarcinomas [8].
Using tumor transplantation model, it has been demonstrated that T cells and IFN-γ are critical elements for tumor immune surveillance [13, 14]. These animal models have also been used to study the effect of the adoptive immunotherapy by CD8+ T cells and by other mechanisms [15–17]. Therefore, studies using tumor cells in animal models may provide good indications for searching novel therapeutic agents to treat cancer patients. Although these investigations suggest that AHCC may modulate the immune response to tumors, the precise mechanism by which AHCC regulates the immune function has not been studied systematically. In this study, we demonstrate that AHCC enhances tumor immune surveillance against both melanoma and lymphoma formation by regulating both innate and adaptive immune responses. Our results suggest that AHCC may be a useful complementary therapy for treating certain cancers.
Materials and methods
Mice
C57BL/6 (B6) mice were purchased from National Cancer Institute and were used at 6–8 weeks of age in all experiments. All mice were maintained under specific pathogen-free conditions at Yale University.
Administration of AHCC
AHCC (provided by Amino Up, Japan) was dissolved in water with concentration of 60 mg/ml. Mice were administered with AHCC or water orally (200 μl/mouse) once a day for 2 weeks. Oral administration was achieved by gavage to ensure all mice received the entire dose.
Reagents
Recombinant murine IL-2 was purchased from R&D Systems (Minneapolis, MN, USA). Anti-mouse antibodies (CD3, NK1.1, CD4, CD8, CD62L, CD44, anti-α β, anti-γδ and IFN-γ) used for phenotypic and cytokine analysis were all purchased from BD Biosciences (San Jose, CA, USA). Dye 5-(and –6) carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Molecular Probes (Paisley, UK). PE SIIFEKL H2Kb tetramer was purchased from Beckman Coulter (Fullerton, CA, USA). Formaldehyde 37% solution and saponin were purchased from J.T. Baker Inc. (Phillipsburg, NJ, USA) and Sigma-Aldrich Co. (St. Louis, MO, USA), respectively.
Tumor models
B16 F0 melanoma cell line was kindly provided by Dr. Mark Mamula, (Yale School of Medicine, New Haven, CT). EL4 and its derivative OVA-expressing EG7 cell lines were purchased from ATCC (Manassas, VA, USA). For tumor induction, 1×105 B16 F0 melanoma cells or 1.5×106 EL 4 cells were injected subcutaneously and tumor growth was monitored and recorded daily for over 3 weeks as described in our previous studies [14]. For some experiments, tumor cells (EL-4 or EG7) were also administered intraperitoneally (1.5×106 tumor cells/mouse).
Tumor antigen-specific CD8+ T cell IFN-γ production
Mice were treated with AHCC or water for 2 weeks as described above. These treated mice (n=5 for each group) were immunized with 200 μg of B16 F0 tumor lysate emulsified in CFA in the hind footpad, as described in our previous studies [14]. On day 7 post-immunization, draining lymph node cells were cultured with complete Click’s medium containing 200 μg/ml B16F0 tumor lysate for 24 h, in the presence of brefeldin A for the last 3 h. Cells were then used for intracellular cytokine staining as described below.
Intracellular cytokine staining
Cultured draining lymph node cells were stained with FITC-anti-CD8 followed by fixation with 2% formaldehyde and permeabilization with 0.5% saponin (w/v) for intracellular IFN-γ staining, using PE anti-IFN-γ as described [18]. PE-conjugated rat IgG2a (BD Pharmingen) was used as an isotype control. Gating was performed on CD8+ T cells and the percentage of IFN-γ+ cells was reported.
In vivo proliferation assay
C57BL/6 mice were treated with AHCC or water for 2weeks. CFSE-labeled OT-IICD4+ or OT-ICD8+ T cells were transferred into AHCC-treated mice or control mice (n=5), followed by administration of OVA (200 μg /mouse) on next day. Four days later, CFSE-positive cells from splenocytes were analyzed by flow cytometry.
Detecting tumor antigen-specific CD8+ T cells
Both AHCC-treated mice (n=4) and control mice (n=4) were inoculated intraperitoneally with 1×106 EG7 tumor cells. On day 10, splenocytes from these mice were isolated and stained with FITC-anti-CD8 and PE-tetramer for OVA. Percentage of tetramer positive CD8+ T cells was reported with FACS analysis.
Analysis of cell composition and activation
Both AHCC-treated mice (n=4) and control mice (n=4) were inoculated intraperitoneally with 1×106 EL-4 or EG7 tumor cells. On day 10, splenocytes from these mice were isolated and stained with one of the following antibody combinations: FITC anit-CD3 and PE anti-NK1.1; FITC anti-γδ and PE anti-αβ; PE anti-CD62L, CyChrome anti-CD44, FITC anti-CD8α and APC anti-CD4. Percentages of different cell sub-populations were reported with FACS analysis.
Antigen-specific CD8 T cell response
Age- and sex-matched AHCC-treated mice (n=4) and control mice (n=4) were inoculated intraperitoneally with 1×106 EG7 tumor cells. On day 10, splenocytes from these mice were isolated and stained with PE SIIFEKL-H2Kb tetramer, FITC anti-CD8 and CyChrome anti-CD3. Gating on CD8 T cells, the percentage of SIIFEKL-H2Kb positive CD8+ T cells were reported.
Statistics
Statistical significance was evaluated by two-tailed unpaired Student’s test or non-parameter analysis if SDs were significantly different between two compared groups using software InState 2.03 for Macintosh (Graph Pad Software). The incidence of tumor development was compared and analyzed using the log rank test, performed by GraphPad prism Version 3.0a for Macintosh (GraphPad Software). Throughout the text, figures and legends, *P<0.01 and **P<0.05 were used to denote statistical significance.
Results
Oral administration of AHCC enhances tumor immune surveillance
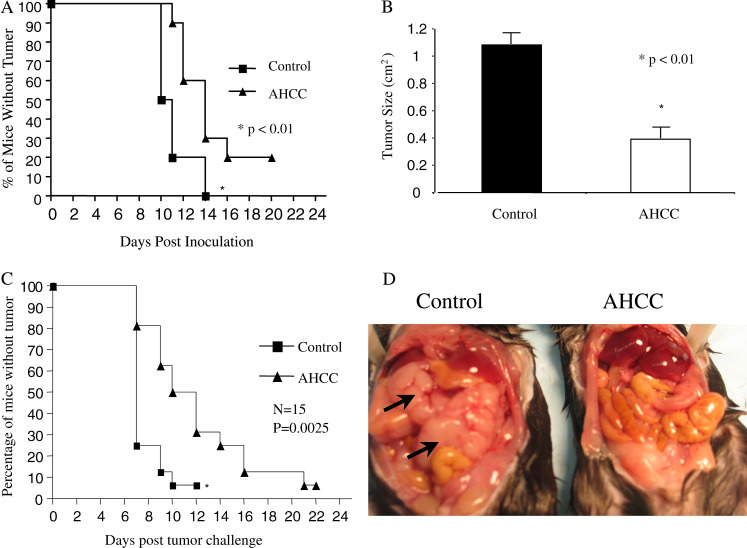
Based on the findings that AHCC reduces the metastasis of rat mammary adenocarcinomas and its ability to modulate immune functions [8, 12], we hypothesized that AHCC might enhance tumor immune surveillance. To assess the effect of AHCC on tumor development, AHCC (or water) was administered to sex- and age-matched C57BL/6 mice by gavage daily for 2 weeks, followed by subcutaneous inoculation of B16F0 melanoma tumor cells (1×105 cells/mouse). Tumor growth was observed and recorded daily as previously described [14, 19]. Compared to control group (water-treated), AHCC-treated mice showed delayed tumor development (Fig. 1a) as well as reduced tumor size (Fig. 1b). To define whether the effect of AHCC on tumor development is melanoma specific, AHCC- or water-treated mice were inoculated subcutaneously in the flank or intraperitoneally with thymoma EL-4 cells. Similar to results observed with B16F0 melanoma cell challenge, AHCC-treated mice were much more resistant to tumor cell growth upon subcutaneous tumor inoculation (Fig. 1c) and tumor formation after intraperitoneal tumor injection (Fig. 1d). Our results indicate that AHCC significantly enhances tumor surveillance.
Fig. 1.
AHCC enhances tumor surveillance. a AHCC treatment delays B16F0 melanoma tumor formation. Sex- and age-matched B6 mice were administered orally either with 12 mg AHCC or equivalent volume of water (control) daily for 14 days (n=20 for each group), followed by subcutaneous inoculation of B16F0 melanoma tumor cells (1×105/mouse) on day 7 after the initiation of AHCC or water treatment. Tumor growth was recorded daily. Tumor size >4×4 mm was considered positive. Data represents three independent experiments. *P<0.01. b AHCC treatment inhibits tumor growth. The mean tumor size from AHCC and pure water-treated mice at day 20 is shown in this figure. *P<0.01. c AHCC treatment delays EL4 tumor development. B6 mice were administered orally with AHCC or water (n=15 for each group) as above for a total of 14 days, followed by subcutaneous inoculation with EL4 tumor cells at day 7 of initial AHCC treatment (1.5×106 cells/mouse). Tumor growth was observed and recorded daily. Data represents three independent experiments. *P<0.01. d AHCC treatment significantly reduces intraperitoneal tumor formation. B6 mice were treated with AHCC or water followed by EL-4 tumor cell inoculation intraperitoneally (n=20 for each group) as described above, and tumor growth was monitored. A representative example of tumor formation is provided. Arrows point to intraperitoneal tumor
AHCC increases tumor-specific activation of CD4+ and CD8+ T cells
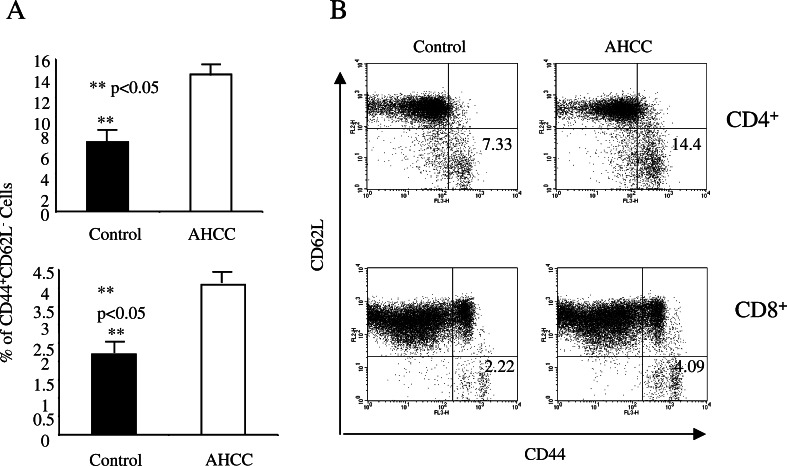
To define the underlying molecular mechanisms of AHCC-mediated anti-tumor immune response, we first tested the effect of AHCC on the adaptive immune response. Sex- and age-matched B6 mice were given AHCC or water daily for 2 weeks, followed by intraperitoneal inoculation with EG7 tumor cells (EL-4 tumor cells expressing OVA, 1×106 cells/mouse [20]). On day 10 post-inoculation, harvested splenocytes were used for analysis of CD4+ and CD8+ T cell activation using specific activation markers. AHCC treatment significantly increased the number of activated CD4+ and CD8+ T cells. According to our finding, the percentage of CD62LloCD44hi population in the spleen for each T cell subset was significantly higher in AHCC-treated mice as compared to those treated with water [14.5±3.3 vs 7.28±3.75 for CD4+ T cells, and 4.28±1.39 vs 2.39±1.08 for CD8+ T cells, respectively (Fig. 2a, P<0.05)]. An example of the FACS profile for CD4+ and CD8+ T cells from AHCC- or water-treated mice is provided in Fig. 2b.
Fig. 2.
AHCC enhances CD4+ and CD8+ T cell activation. Sex- and age-matched B6 mice were administered orally with 12 mg AHCC or equivalent volume of water daily for 14 days (n=5 for each group), and on day 7, received an intraperitoneal inoculation with EG7 tumor cells (1×106 cells/mouse). Ten days post-inoculation, splenoctyes were stained with antibodies against different surface molecules and analyzed by FACS. The percentage of activated CD4+ and CD8+ T cells (mean ± SD) is shown (a) **P<0.05. An example of the FACS profile for CD4+ and CD8+ T cells is given (b)
AHCC administration enhances antigen-specific CD4+ and CD8+ T cell proliferation
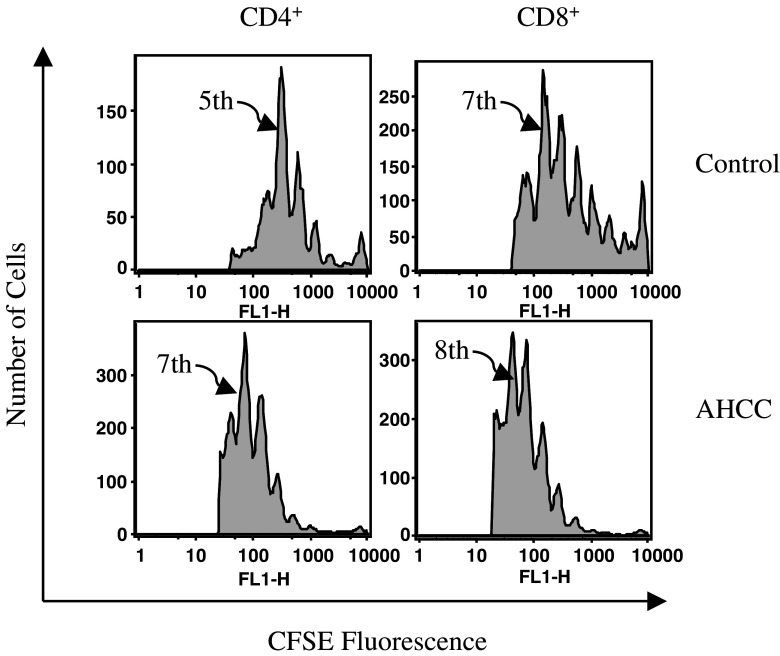
CD4+ and CD8+ T cells play a key role in the adaptive anti-tumor immune response. To define the effect of AHCC on the proliferation of CD4+ and CD8+ T cells, sex- and age-matched B6 mice were administered AHCC or pure water for 2 weeks as above, followed by adoptive transfer of 2×106 CFSE-labeled OT-II CD4+ or OT-I CD8+ T cells (n=5 for AHCC or control group for each T cell subset). On the following day, these mice were administered with 200 μg OVA protein intravenously, and splenocytes were analyzed by flow cytometry 3 days post-injection. After gating on CFSE-positive CD4+ or CD8+ T cells, the number of cell divisions is expressed as the dilution of CFSE fluorescence. AHCC treatment clearly enhanced both CD4+ and CD8+ T cell proliferation over that of water, with the highest peak of cells in seventh cell division compared to fifth division for CD4+ T cells, respectively, and the highest peak of cells in eighth division versus seventh division for CD8+ T cells, respectively (Fig. 3). More importantly, a significantly greater proportion of cells appear on the far right of histogram for the control group as compared to those from AHCC-treated mice (Fig. 3), indicating that fewer T cells have divided in the control group. These results indicated that AHCC has an important impact on the proliferative response of both CD4+ and CD8+ T cells.
Fig. 3.
AHCC enhances both CD4+ and CD8+ T cell proliferation. Sex- and age-matched B6 mice were administered orally with 12 mg AHCC or equivalent volume of water daily (n=5 for each group) for 10 days, followed by intravenous injection of CFSE-labeled OT-II CD4+ or OT-I CD8+ T cells (2×106 cells/mouse). Then mice were challenged with intravenous injection of OVA (200 μg/mouse) on the following day. Three days later, CFSE-positive cells from splenocytes were analyzed by FACS. One representative example is shown. Arrows point to cell division number with the highest peak
AHCC promotes IFN-γ production in CD8+ T cells
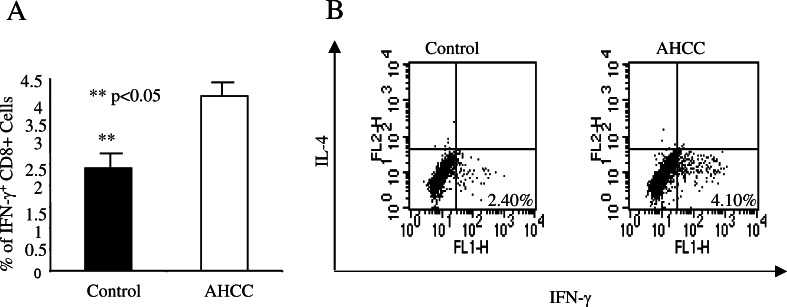
IFN-γ has been shown previously to be a critical cytokine in tumor immunosurveillance [21]. To define the effect of AHCC on tumor antigen-specific IFN-γ production, AHCC (or water) was administered to sex- and age-matched B6 mice (n=5 for each group) as mentioned above for 2 weeks, and then immunized in the hind footpad with B16 tumor lysate in CFA. Eight days post-immunization, lymphocytes from the draining lymph nodes were isolated, cultured with 200 μg/ml tumor lysate for 24 h, with brefeldin A added during the last 3 h of culture. These cells were then fixed and permeabilized with 0.5% saponin for intracellular cytokine staining. The percentage of IFN-γ producing CD8+ T cells from AHCC-treated mice (mean ± SD) was significantly higher than that of pure water-treated mice (4.25±1.3 and 2.28±1.09, respectively) (P<0.05, Fig. 4a). An example of FACS analysis is provided in Fig. 4b. In the same cultures, the percentage of IFN-γ-producing CD4+ T cells from AHCC-treated mice was also higher than those from water-treated mice, although it did not reach the significance (data not shown). AHCC treatment did not enhance the percentage of IFN-γ-producing CD8+ T cells in responding to control tumor lysate (different tumor cell line, data not shown). Our results demonstrate that AHCC not only enhances T cell activation and proliferation, but also increases their capacity to produce IFN-γ.
Fig. 4.
AHCC enhances tumor antigen-specific CD8+ T cell IFN-γ production. Sex- and age-matched B6 mice were administered orally with 12 mg AHCC or equivalent volume of water daily (n=5 for each group) for 14 days, followed by immunization with 200 μg of B16 F10 tumor lysate emulsified in CFA. After 7 days, lymphocytes recovered from draining lymph nodes of immunized mice were cultured with 200 μg/ml of tumor lysate for 24 h, with the addition of brefeldin A to the culture for the remaining 3 h. Cells were then fixed with 2% formaldehyde and permeabilized with 0.5% saponin for intracellular IFN-γ staining. The percentage of IFN-γ producing cells (mean ± SD) from CD8+ T cells is shown (a). **P<0.05. An example of intracellular cytokine staining upon gating on CD8+ T cells is shown (b)
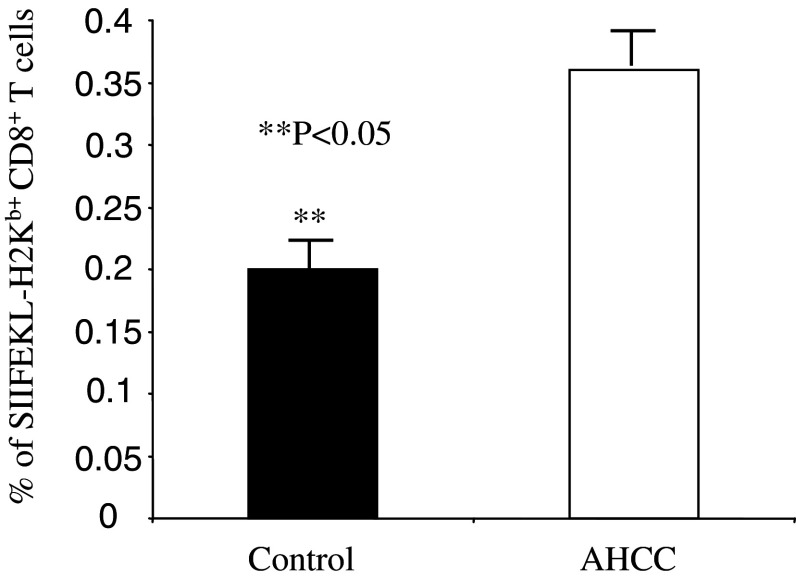
AHCC increases the percentage of antigen-specific CD8+ T cells
An efficient anti-tumor immune response requires that enough number of CD8+ T cells be activated and quickly expanded. To test whether AHCC could increase the number of antigen-specific CD8+ T cells, we adapted a tumor cell line EG7, which could express full-length of OVA protein. AHCC- or water-treated mice were challenged with EG7 (1×106 cells/mouse) intraperitoneally and 10 days later, percentage of OVA-specific CD8+ T cells were determined by H2Kb bound with OVA SIIFEKL peptide tetramer. The percentage of tetramer positive CD8+ T cells in AHCC-treated mice were significantly higher than that in water-treated control mice (Fig. 5). This result indicated that AHCC could increase the number of antigen-specific CD8+ T cells in tumor-bearing mice.
Fig. 5.
AHCC treatment increases the percentage of antigen-specific CD8+ T cells. Sex- and age-matched B6 mice were administered orally with 12 mg AHCC or equivalent volume of water daily (n=4 for each group) for 14 days, followed by inoculation with EG7 tumor cells intraperitoneally. Ten days post-inoculation, splenocytes were used for analysis of tetramer positive cells. The percentage of SIIFEKL-H2Kb+ CD8+ T cells (mean ± SD) is shown. **P<0.05
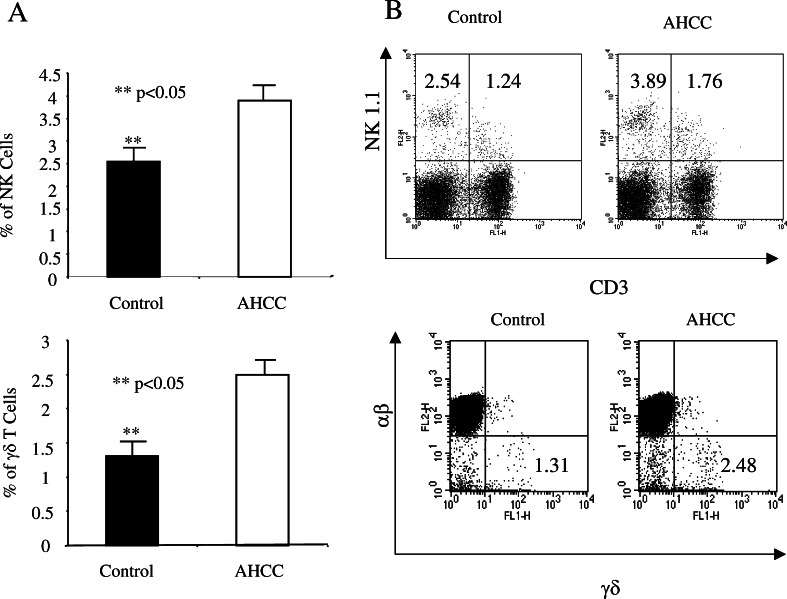
AHCC increases the number of both NK and γδ T cells
Both NK cells and γδ T cells play a critical role in tumor immune surveillance. To test the effect of AHCC on these cell types, sex- and age-matched B6 mice were treated with AHCC or water (n=5 for each group) as above for 2 weeks, and the number of NK and γδ T cells in the spleen was analyzed by flow cytometry. Treatment with AHCC significantly increased the number of splenic NK cells and γδ T cells, with the percentage (mean ± SD) of NK1.1+ cells in AHCC-treated mice vs control being 3.98±0.90 and 2.58±0.97, respectively, and the percentage of CD3+γδ+ cells for AHCC vs control being 2.57±0.79 and 1.45±0.69, respectively (Fig. 6, left panel, P<0.05). An example of FACS analysis for CD3 and NK1.1 as well as αβ/γδ T cell staining is provided (Fig. 6, right panel). Splenocytes from AHCC- or water-treated mice were cultured with anti-CD3 and anti-CD28 in the presence of brefeldin A for 6 h, and the production of IFN-γ by γδ T cells was analyzed by intracellular cytokine staining after gating TCR γδ-positive cells. The percentage of IFN-γ producing γδ T cells from these two groups of mice did not reach significant difference (data not shown). NKT cells (CD3+NK1.1+) were also higher in AHCC-treated mice compared to controls, although it did not reach significance. These results indicate that AHCC could enhance the anti-tumor immune response through modulating not only the adaptive immune response, but also innate immunity.
Fig. 6.
AHCC treatment increases the number of NK and γδ T cells. Sex- and age-matched B6 mice were administered orally with 12 mg AHCC or equivalent volume of water daily (n=5 for each group) for 14 days, followed by inoculation with EL-4 tumor cells intraperitoneally. Ten days post-inoculation, splenocytes were used for analysis of NK, NKT, αβ, and γδ T cells. The percentage of NK and γδ T cells (mean ± SD) is shown (a). **P<0.05. An example of the FACS analysis is given (b)
Discussion
AHCC is a nutritional food and supplement ingredient that has been widely sold in Japan and Asia for the past 15 years, and increasingly in the US and Europe. Consumers often use AHCC as a complementary therapy for cancer treatment, and in some cases, as an alternative to conventional cancer treatment [22, 23]. Although it has been suggested to have potential anti-cancer and immune modulating function, there are no well-controlled systemic studies to define the effect of AHCC in tumor immunity. In this study, we demonstrate that AHCC enhances tumor immune surveillance against both melanoma and lymphoma by regulating both innate and the adaptive immune responses.
We first demonstrated that oral administration of AHCC prior to tumor inoculation significantly delayed melanoma formation and reduced tumor size (Fig. 1a, b). The anti-tumor effect of AHCC is not limited to melanoma, since similar results were obtained using EL-4 lymphoma cell line (Fig. 1c). Even more impressively, upon intraperitoneal injection of EL-4 tumor cells, four out of 20 AHCC-treated mice (4/20) were tumor free, whereas all mice in control group developed large tumors and died within 2 weeks; examples are shown in Fig. 1d. Our results firmly establish a protective role of AHCC against tumor formation.
Both lymphocytes and IFN-γ have been shown to be essential components of tumor immune surveillance [21, 24, 25]. Different subsets of lymphocytes contribute to anti-tumor immune responses at different stages. Both CD4+ and CD8+ T cells are critical elements for the adaptive anti-tumor immune response. CD4+ T cells, especially Th1 cells, produce IFN-γ and facilitate both innate and adaptive immune responses. These cells also provide help for CD8+ T cells to develop memory response, whereas CD8+ T cells provide both cytokines (IFN-γ and TNF-α) and direct cytotoxicity. To define the molecular mechanisms by which AHCC could mediate anti-tumor immune response, we sought to determine whether AHCC modulates the adaptive immune response. We demonstrate that AHCC treatment enhances activation and proliferation of both CD4+ and CD8+ T cells (Figs. 2, 3). Moreover, administration of AHCC significantly increases the frequency of tumor antigen-specific CD8+ T cells, and their ability to produce IFN-γ (Fig. 4). Finally, AHCC treatment can make antigen-specific CD8+ T cells to expand more actively. The increased CD8+ T cell number partially contributes to the tumor resistance of AHCC-treated mice. However, it is unclear how AHCC enhances the function of these T cells. It is possible that the mixture of polysaccharides in AHCC may activate the innate immune response through undefined signaling pathways, such as toll-like receptors and the down-stream NF-κB pathway, which in turn helps to regulate the adaptive immune response. Consistently, it has been reported that AHCC enhances IL-12 production from macrophages [10] and increases nitric oxide production [12]. Further studies are needed to clarify the underlying mechanisms that mediate the effect of AHCC on the adaptive immune response.
A potential target of AHCC modulation within the innate immune system may be γδ T cells. These cells are a unique subset of T cells. They recognize protein or peptide independent of antigen presentation and function as innate-like cells [26]. Our earlier studies have demonstrated that γδ T cells predominantly produce IFN-γ upon activation [18, 27] and play a critical role in tumor immune surveillance by providing an early source of IFN-γ [14]. Interestingly, AHCC treatment significantly increases the number of γδ T cells compared to those of water-treated mice (Fig. 5). Since AHCC was given orally, it is possible that the effective components in AHCC might directly encounter γδ T cells lining the epithelial layer of the intestine resulting in their activation. In addition to γδ T cells, it has been well established that NK cells play an essential role in tumor immune surveillance. A previous study has reported that AHCC increases the number of NK cells in aged mice [28]. Interestingly, we found that treatment with AHCC also significantly increases the number of NK cells upon tumor inoculation. Although the changes of NKT cells did not reach significance, the trend is clear that AHCC also increases the number of NKT cells. These results indicate that AHCC has multiple effects on the immune system.
Given the enhancing effects of AHCC for several immune parameters (CD4+, CD8+ and γδ T cells), which were shown previously to be important for tumor immune surveillance, it is likely that AHCC mediates its potentiating effects for tumor surveillance, at least in part, by enhancing these particular parameters. Future studies using different T cell subset deficient mice will help to clarify these issues.
In summary, we have presented clear evidence that as a nutritional food, AHCC enhances tumor immune surveillance by regulating both the innate and the adaptive immune responses. Future studies are needed to further define the molecular mechanisms mediating the effect of AHCC, and to define the effect of AHCC in eradicating the formed tumors.
Acknowledgements
We thank Dr. Kim Bottomly from Yale Immunobiology for providing C57BL/6 OT-1 and OT-II transgenic mice. We thank Dr. Fotios Koumpouras and Dr. Bohdan Harvey for critical review of the manuscript. This work was supported by an Arthritis Foundation Investigator Award, NIH (NIAMS) K01 AR 02188 and NIH (NIAID) R01 (R01 AI56219) grant (Z.Y.), and partially supported by the Amino Up, Japan (Z.Y). D.Z. is supported by National Science Foundation of China (No. 30471593), Shanghai Leading Academic Discipline Project (T 0206). Authors from Yale have no financial conflict of interest.
Abbreviations
- CFSE
5-(and –6) Carboxyfluorescein diacetate, succinimidyl ester
- AHCC
Active hexose correlated compound
References
- 1.Wakame K. Protective effects of active hexose correlated compound (AHCC) on the onset of diabetes induced by Streptozotocin in the rat. Biomed Res. 1999;20:145–152. [Google Scholar]
- 2.Ghoneum M, Wimbley M, Salem F, Mcklain A, Attallah N, Gill G. Immunomodulatory and anticancer effects of active hemicellulose compound (AHCC) Int J Immunother. 1995;11:23–28. [Google Scholar]
- 3.Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. [PubMed] [Google Scholar]
- 4.Sun B, Wakame K, Mukoda T, Toyoshima A, Kana-zawa T, Kosuna K. Protective effects of AHCC on carbon tetrachloride induced liver injury in mice. Nat Med. 1997;51:310–315. [Google Scholar]
- 5.Burikhanov RB, Wakame K, Igarashi Y, Wang S, Matsuzaki S. Suppressive effect of active hexose correlated compound (AHCC) on thymic apoptosis induced by dexamethasone in the rat. Endocr Regul. 2000;34:181–188. [PubMed] [Google Scholar]
- 6.Shuyi Wang, Ichimura K, Wakame K. Preventive effects of active hexose correlated compound (AHCC) on oxidative stress induced by ferric nitrilotriacetate in the Rat. Dokkyo J Med Sci. 2001;28:745–752. [Google Scholar]
- 7.Ye SF, Wakame K, Ichimura K, Matsuzaki S. Amelioration by active hexose correlated compound of endocrine disturbances induced by oxidative stress in the rat. Endocr Regul. 2004;38:7–13. [PubMed] [Google Scholar]
- 8.Matsushita K, Kuramitsu Y, OhiroY, Obara M, Kobayashi M, Li YQ, Hosokawa M. Combination therapy of active hexose correlated compound plus UFT significantly reduces the metastasis of rat mammary adenocarcinoma. Anticancer Drugs. 1998;9:343–350. doi: 10.1097/00001813-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Mamdooh G, Phyllis P, Yasuo N, Mabrouk G, Gus G (1992) Enhancement of NK cell activity in cancer patients by active hemicellulose compound (AHCC). In: Adjuvant Nutrition in Cancer Treatment Symposium, Tulsa, OK
- 10.Yagita A, Matsuzaki S, Wakasugi S, Sukegawa Y. H-2 haplotype-dependent serum IL-12 production in tumor-bearing mice treated with various mycelial extracts. In Vivo. 2002;16:49–54. [PubMed] [Google Scholar]
- 11.Ye SF, Ichimura K, Wakame K, Ohe M. Suppressive effects of active hexose correlated compound on the increased activity of hepatic and renal ornithine decarboxylase induced by oxidative stress. Life Sci. 2003;74:593–602. doi: 10.1016/j.lfs.2003.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G. Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions. J Appl Physiol. 2004;97:1437–1444. doi: 10.1152/japplphysiol.00259.2004. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFN gamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/S1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 16.Dobrzanski MJ, Reome JB, Dutton RW. Therapeutic effects of tumor-reactive type 1 and type 2 CD8+ T cell subpopulations in established pulmonary metastases. J Immunol. 1999;162:6671–6680. [PubMed] [Google Scholar]
- 17.Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D, Chapoval A, Lu J, Kasperbauer JL, Padley D, Vile R, Gastineau D, Wettstein P, Chen L. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62:1884–1889. [PubMed] [Google Scholar]
- 18.Yin Z, Zhang DH, Welte T, Bahtiyar G, Jung S, Liu L, Fu XY, Ray A, Craft J. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-gamma: failure to down-regulate IL-12 receptor beta 2-chain expression. J Immunol. 2000;164:3056–3064. doi: 10.4049/jimmunol.164.6.3056. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Tao J, Li MO, Zhang D, Chi H, Henegariu O, Kacch SM, Davis RJ, Flavell RA, Yin Z. JNK1 is essential for CD8+ T cell-mediated tumor immune surveillance. J Immunol. 2005;175(9):5783–5789. doi: 10.4049/jimmunol.175.9.5783. [DOI] [PubMed] [Google Scholar]
- 20.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 22.deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B (2004) Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer Urology 63:259–263 [DOI] [PubMed]
- 23.deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology. 2002;60:640–644. doi: 10.1016/S0090-4295(02)01856-3. [DOI] [PubMed] [Google Scholar]
- 24.Dunn GP, Old LJ, Schreiber RD, Bruce AT, Ikeda H. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old Schreiber LJ RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 26.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 27.Yin Z, Chen C, Szabo SJ, Glimcher LJ, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 28.Ghoneum M, NY, Torabi M, Gill G, Wojdani A (1992) Active hemicellulose compound (AHCC) enhance NK cell activity of aged mice in vivo. FASEB J 6:A1213 (Abstract)