Abstract
Background: Ligands for CXCR3 chemokines [IFN-γ-inducible protein of 10 kD (IP-10/CXCL10), monokine induced by IFN-γ (Mig/CXCL9), IFN-inducible T cell α chemoattractant (I-TAC/CXCL11)] and those for CCR4 [macrophage-derived chemokine (MDC/CCL22), thymus- and activation-regulated chemokine (TARC/CCL17)] have been shown to play the central roles for T helper-cell recruitment into the tissues. To examine the role of these chemokines in tumor progression of lung cancer, we investigated their expression in human lung cancer tissues to determine the possible relationship between their expression and the prognosis of patients. Methods: Total RNA was prepared from lung cancer tissues of 40 patients (24 adenocarcinoma and 16 squamous cell carcinoma). We measured gene expression levels of chemokines (IP-10, Mig, I-TAC, MDC and TARC) by real-time quantitative RT-PCR. Results: Higher gene expression of MDC in lung cancer was significantly correlated with longer disease-free survival time and lower risk of recurrence after tumor resection. We could not find any significant relationship of IP-10, Mig, I-TAC and TARC gene expression with disease-free survival or lower risk of recurrence after surgery. Conclusions: These results suggest that increased gene expression of MDC in tumor tissues may be a predictive marker for improving the prognosis of lung cancer.
Keywords: Lung cancer, Macrophage-derived chemokine (CCL22), Prognosis, Real-time quantitative RT-PCR
Introduction
In spite of recent advances in new chemotherapy agents or molecular-targeted medicine, lung cancer is still one of the leading causes of death [1]. Although surgical resection is generally recognized as the most effective initial treatment for the early stage of non-small cell-lung cancer (NSCLC), local recurrence or distant metastasis frequently occurs after surgery. The current TNM classification for lung cancer has been widely used in order to evaluate the prognosis and to decide the most appropriate management. However, the overall 5-year survival rate for patients with stage I NSCLC is approximately 70–80%, and TNM staging is not sufficient to predict the prognosis of the disease [2]. Therefore, it is important to determine which patients are at a higher risk for relapse. For this purpose, a better understanding of the fundamental nature and dynamics of various immuno-inflammatory cells in lung cancer tissues and the search for new molecular markers are required.
Chemokines are small chemotactic cytokines that participate in pleiotropic functions, including migration of various kinds of inflammatory cells or regulation of tumor growth [3]. Recent studies have demonstrated that chemokine is a useful molecule for cancer immunotherapy because it regulates tumor cell growth by the mechanism of chemoattraction for specific immune cells including T cells, which play a critical role in establishing anti-tumor immunity [4, 5]. However, the more recent data indicated that tumor-derived chemokines and chemoattracted leukocytes may provide selective advantage for specific tumors [6]. Depending on the cancer types and on the types of recruited cells, the prognostic outcome may vary considerably. Certain types of chemokine receptors are selectively expressed in specific immune cells [7]. CXCR3 is expressed mainly in type-1 helper T cells (Th1 cells), and ligands for CXCR3 (IP-10/CXCL10, Mig/CXCL9 and I-TAC/CXCL11) are specific chemoattractants for Th1 CD4+ T cells [8–10]. CCR4 is expressed in type-2 helper T cells (Th2 cells) [7], and ligands for CCR4 (MDC/CCL22 and TARC/CCL19) are thought to be characteristic for Th2 CD4+ T cell-mediated immunity [11]. Although polarization of CD4+ T cells to either Th1 or Th2 has a great impact on T cell-mediated tumor immunity [12], the role of Th1/Th2 balance in malignant tumor tissue is not fully understood, especially in lung cancer tissue.
We hypothesized that the expression of CXCR3 ligands (IP10, Mig and I-TAC) and CCR4 ligands (MDC and TARC) in tumor tissue might reflect the immune-inflammatory status of Th cell polarization to Th1 or Th2, and it could be a useful marker for prediction of prognosis of lung cancer patients. In this study, we investigated IP-10, Mig, I-TAC, MDC and TARC gene expressions in tumor tissues obtained from lung cancer patients who underwent surgical resection. We analyzed the possible association between the level of gene expression of each chemokine and clinico-pathologic factors including disease-free survival.
Materials and methods
Patients and sample collections
Tumor tissues were collected from 40 patients undergoing lobectomy or pneumonectomy for the treatment of lung cancer including 24 adenocarcinoma and 16 squamous cell carcinoma at Nagoya University Hospital between March 2000 and July 2001. Normal lung tissues distal to tumor tissue were used as controls. Specimens were irrigated with physiological saline immediately after excision. Some of the specimens were frozen immediately with liquid nitrogen and kept at −80°C until analysis. The remaining tissues were fixed with 10% formalin. All the patients provided written informed consent, and tissue was obtained with the approval of the Nagoya University Institutional Review Board. None of the patients received adjuvant chemotherapy after surgery. None of these subjects had atopic diseases such as bronchial asthma or atopic dermatitis. The characteristics of the 40 lung cancer patients are shown in Table 1.
Table 1.
Characteristics of 40 patients with lung cancer
| Number of patients (men/women) | 40 (27/13) | |
| Mean age (±SD) | 66.2±9.0 | |
| Adenocarcinoma/Squamous cell carcinoma | 24/16 | |
| Stage | Adenocarcinoma | Squamous cell carcinoma |
| Ia | 9 | 4 |
| Ib | 5 | 4 |
| IIa | 3 | 2 |
| IIb | 3 | 3 |
| IIIa | 4 | 3 |
| Differentiation | ||
| Well | 13 | 4 |
| Moderate | 8 | 7 |
| Poor | 3 | 5 |
Real-time quantitative reverse transcriptase polymerase chain reaction
Total RNA was extracted using ISOGEN (Nippon Gene, Kanazawa, Japan) from human lung cancer tissue and normal lung tissue according to the manufacturer’s instructions. Total RNA (5 μg) was reverse-transcribed using random primers (Promega, Madison, WI) and Superscript II reverse transcriptase (Life Technologies Inc., Gaithersburg, MD) according to the manufacturer’s protocol. One microliter of each cDNA sample, corresponding to 250 ng total RNA, was used for real-time PCR. The sequences of PCR primers and TaqMan probes were as follows: IP-10 (CXCL10 gene; NCBI accession No. NM001565) forward primer, 5′-TCCAAGGTCTTTAGAAAAACTTGAAAT-3′/location in the sequence 189–215; reverse primer, 5′-CTTTTTCATTGTAGCAATGATCTCAAC-3′/244–270; probe, 5′-ATTCCTGCAAGCCAATTTTGTCCACG-3′/217–242; Mig (CXCL9 gene; NCBI accession No. BC095396) forward primer, 5′-GGAGTGCAAGGAACCCCAGTA-3′/115–135; reverse primer, 5′-TGTTTAAGGTCTTTCAAGGATTGTAGGT-3′/ 185–212; probe, 5′-CGCTGTTCCTGCATCAGCACCAAC-3′/148–171; I-TAC (CXCL11 gene; NCBI accession no. NM005409) forward primer, 5′-GACATTATTACTGGAGTCAAGCCCTTA-3′/ 863–889; reverse primer, 5′-GGAAAGAAGTGTGTATTTGCATGAA-3′/936–960; probe, 5′-AAGTCAAAAGCATCTATGTGTCGTAAAGCATTCCT-3′/891–925; MDC (CCL22 gene; NCBI accession no. NM002990) forward primer, 5′-GCGTGGTGTTGCTAACCTTCA-3′/202–222; reverse primer, 5′-AAGGCCACGGTCATCAGAGT-3′/301–320; probe, 5′-CTGTGCCGATCCCAGAGTGCCC-3′/236–257; TARC (CCL17 gene; NCBI accession No. NM002987) forward primer, 5′-TCCAGGGATGCCATCGTTT-3′/301–319; reverse primer, 5′-AACTGCATTCTTCACTCTCTTGTTGT-3′/359–384; probe, 5′-TGCAGGGCAGGGCCATCTGTTC-3′/329–350; glyceraldehyde-3- phosphate dehydrogenase (GAPDH) (GAPDH gene) forward primer, 5′-CCAGGTGGTCTCCTCTGACTTC-3′/912–933; reverse primer, 5′-GTGGTCGTTGAGGGCAATG-3′/975–993; probe, 5′-ACACCCACTCCTCCACCTTTGACGCT-3′/ 941–966. TaqMan probes for IP-10, and GAPDH were purchased from PE Applied Biosystems (Foster City, CA) and probes for Mig, I-TAC, MDC and TARC were purchased from NIPPON EGT (Toyama, Japan). Real-time PCR was performed in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) to quantify IP-10, Mig, I-TAC, MDC, TARC and GAPDH mRNA. Real-time PCR was performed in a total volume of 50 μl with the following components: 2× reaction buffer (qPCR Mastermix Plus; Eurogentec, Seraing, Belgium), 0.3 μM of each primer and 0.1 μM of TaqMan probe. Cycle parameters were 50°C for 2 min to activate UNG, 95°C for 10 min to activate Taq, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The threshold cycle (C T) was recorded for each sample to reflect the mRNA expression levels and ΔC T was calculated by subtracting C T (control:GAPDH) from C T (target gene). IP-10, Mig, I-TAC, MDC, and TARC mRNAs were indexed to the GAPDH mRNA using the following formula:
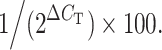 |
1 |
Cell lines and cultures
A549 (alveolar cell carcinoma), H1299 (lung adenocarcinoma) and H441 (papillary lung adenocarcinoma) were purchased from American Type Culture Collection (Manassas, VA). EBC-1 (lung squamous cell carcinoma) was purchased from RIKEN Cell Bank (Tsukuba, Ibaragi, Japan). All cell lines were maintained in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 100 U/ml of penicillin, 100 μg/ml of streptomycin, 0.25 μg/ml of amphotericin B, sodium pyruvate 1 mM, non-essential amino acids 0.1 mM (all from Gibco BRL, Carlsbad, CA), 2 mM glutamine (Cosmo Bio, Tokyo, Japan) and 10% fetal calf serum (Gibco BRL). Cells were cultured at 37°C in a humidified 5% CO2 incubator. These cells were seeded into a 6-well culture dish (Nunc A/S, Rosklide, Denmark), (106 per well) and cultured for 24 h with or without TNF-α and/or IFN-γ. Recombinant human TNF-α was purchased from Pepro Tech EC Ltd. (London, UK). Recombinant human IFN-γ was purchased from Invitrogen Corporation (Carlsbad, CA). Supernatants from each well were collected to measure chemokine concentration. After centrifugation for 5 min at 4°C, cell-free supernatant was aliquoted and preserved at −70°C until chemokine concentrations were measured.
Enzyme-linked immunosorbent assay
The concentration of chemokines was measured by enzyme-linked immunosorbent assay (ELISA) method as described previously [13]. Briefly, the aliquoted samples of supernatant from cell culture were thawed only once and tested to avoid repeated freeze–thaw cycles. Concentrations of chemokines were analyzed in specific ELISAs with matched antibody pairs (R&D Systems Inc., Minneapolis, MN), according to the manufacturer’s recommendations. Flat-bottomed 96-well microtiter plates (Nunc-Immunoplate, Nunc, Roskilde, Denmark) were coated with 50 μl/well of the monoclonal anti-chemokines antibody [2 μg/ml in phosphate-buffered saline (PBS)] for 24 h at 4°C and then washed with wash buffer (0.05% Tween in PBS pH 7.4) three times. The microtiter plates were blocked with blocking buffer (PBS containing 1% BSA, 5% sucrose, and 0.05% NaN3) for 1 h at room temperature. After washing three times, 50 μl of supernatant was added and followed by incubation for 2 h at room temperature. Plates were washed three times and a 50 μl/well of biotinylated anti-chemokines antibody (50 ng/ml) was added. Then the plates were incubated for 2 h at room temperature. After washing, streptoavidin–peroxidase conjugate was added and incubated at room temperature for 20 min. Then the plates were washed again and o-phenylendiamine substrate (Sigma-Aldrich Co., St. Louis, MO) was added, followed by incubation at room temperature to the desired extinction. The reaction was terminated with 25 μl/well of 1 M H2SO4 solution. Plates were read at 490 nm in an automated microplate reader (Wallac ARVO SX, PerkinElmer Inc., Wellesley, MA). Standard curves were generated using a dilution of recombinant chemokines serially (R&D Systems Inc., Minneapolis, MN). The lower limit of detection of the ELISA was 31.25 pg/ml for IP-10, 31.2 pg/ml for Mig and 15.6 pg/ml for I-TAC, MDC and TARC. No cross-reactivity was observed among IP-10, Mig, I-TAC, MDC and TARC. We also confirmed that our ELISA system for each chemokine had no cross-reactivity with recombinant human IL-8 (rhIL-8), rhMCP1, rhMCP2, rhMCP3, rhRANTES, rhI-309, rhMIP1α and rhMIP1β.
Immunohistochemistry
Immunohistochemistry for CCR4 and CXCR3 was performed on paraffin-embedded 5-μm-thick sections obtained from surgical specimens fixed in 10% formalin. Samples were immunostained with anti-human CCR4 antibody (clone 1G1; BD Biosciences Pharmingen, San Diego, CA) or anti-human CXCR3 antibody (clone 1C6; BD Biosciences Pharmingen, San Diego, CA). In brief, sections were dewaxed in xylene and dehydrated through graded concentrations of ethanol. Endogenous peroxidase was blocked by incubation in peroxidase blocking solution (S2023; Dako Japan Inc., Kyoto, Japan). Antigen retrieval was performed for 20–40 min at 95°C in a water bath, incubating with target retrieval solution (S1700 or S2368; Dako Japan Inc., Kyoto, Japan). After these procedures, sections were washed in TBST (50 mM Tris–HCL buffer, pH7.6, including 0.1% Tween 20 and 0.15 M NaCl) for 5 min, followed by incubation of the primary antibodies (anti-human CXCR3 antibody or anti-human CCR4 antibody) for 30 min. After washing three times with TBST, the sections were incubated with Envision+ (K4061; Dako Japan Inc., Kyoto, Japan) for 30 min in a humidified box at room temperature. Sections were washed again in TBST for 5 min, three times and then developed with DAB+ (K3467, Dako Japan Inc., Kyoto, Japan) for 5 min, and counter-stained with hematoxyline solution for 1 min (S2020; Dako Japan Inc., Koyto, Japan).
Statistical analysis
Differences of chemokine gene expression between lung cancer specimens and normal tissues were evaluated with the Mann–Whitney U test. For correlation analyses between chemokine gene expression levels with one another, we performed a Pearson’s correlation analysis to determine the degree of statistical significance. Associations between the chemokine expression level and clinical factors were analyzed with the Mann–Whitney U test and χ2 test. Disease-free survival curve was generated by the Kaplan–Meier method and verified by the log-rank (Mantel–Cox) and Breslow–Gehan–Wilcoxon tests. Cox’s proportional hazard model was used for univariate analysis of prognostic values. Statistical significance was considered when a P value <0.05 was obtained. All analyses were performed using the StatView 5 statistical package for Macintosh except for univariate Cox hazard analysis, which was done using JMP.
Results
Chemokine gene expression in lung cancer tissue
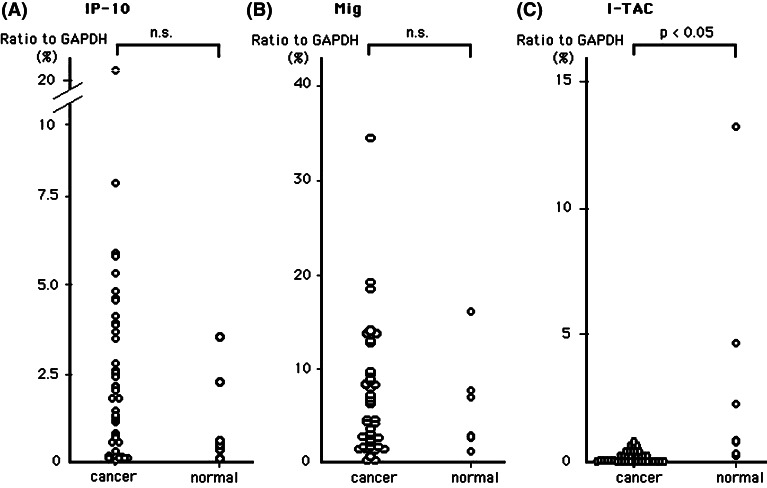
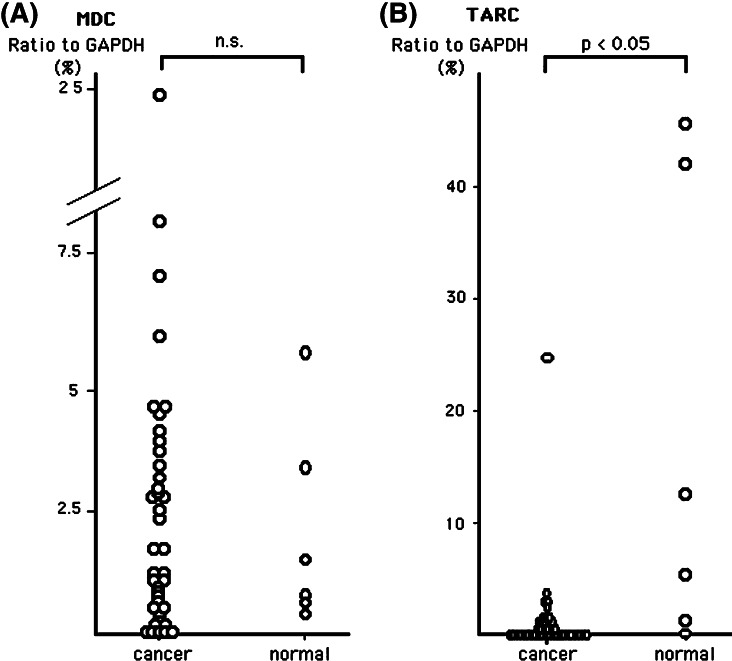
We investigated the gene expression of CXCR3 ligand chemokines (IP-10/CXCL10, Mig/CXCL9 and I-TAC/CXCL11) (Fig. 1) and CCR4 ligand chemokines (MDC/CCL22 and TARC/CCL17) (Fig. 2) in lung cancer tissues and normal lung tissues. As shown in Figs. 1 and 2, no significant difference was observed in the mean expression levels of IP-10, Mig and MDC genes between lung cancer and normal lung tissues. However, several cases of lung cancer tissues showed ‘higher expression’ gene levels of these three chemokines. I-TAC and TARC gene expression levels of lung cancer tissues were significantly lower than normal lung tissues (Figs. 1c, 2b).
Fig. 1.
Gene expression level of IP-10 (a), Mig (b), I-TAC (c) in lung cancer tissues (n=40) and normal lung tissues (n=6) evaluated by quantitative real-time RT-PCR. Total RNA was extracted from tumor tissues and normal lung tissue, and quantity of mRNA was measured using real-time RT-PCR. Quantitative RT-PCR analysis was performed in duplicate and repeated twice. Values are means of two independent experiments. Levels of each chemokine gene expression are expressed as a percentage of the GAPDH gene expression calculated as described in Materials and methods
Fig. 2.
Gene expression level of MDC (a) and TARC (b) in lung cancer tissues and normal lung tissues evaluated by quantitative real-time RT-PCR. Quantitative RT-PCR analysis was performed in duplicate and was repeated twice. Values are means of two independent experiments. Levels of each chemokine gene expression are expressed as a percentage of the GAPDH gene expression calculated as described in Materials and methods
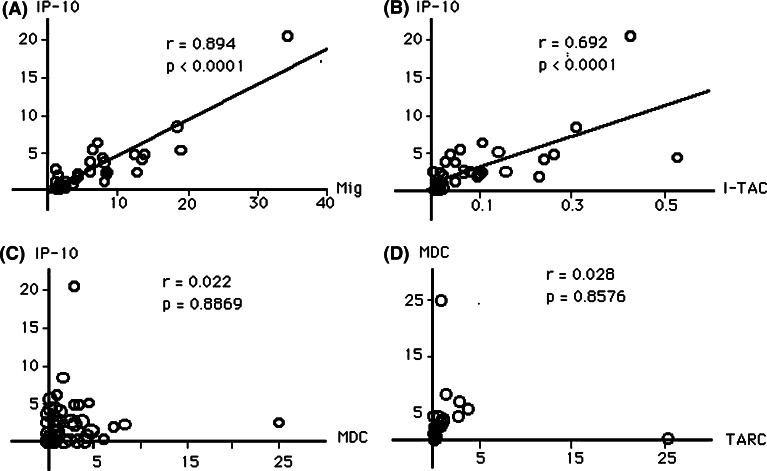
To investigate the balance of gene expression levels among these chemokines, we performed correlation analyses. As shown in Fig. 3, gene expression levels of IP-10 and Mig were highly correlated (Fig. 3a), and those of IP-10 and I-TAC were also highly correlated (Fig. 3b). We could not detect correlation of gene expression levels between IP-10 and MDC (Fig. 3c). These results indicate that CXCR3 ligand chemokines and MDC are differentially regulated in lung cancer tissues although CXCR3 ligand chemokines in tumor tissues were well correlated with each other. There was no significant correlation between IP-10 and TARC (data not shown). Further, MDC expression was not correlated with TARC (Fig. 3d), which indicates that these two chemokines may also be differentially regulated in lung cancer tissues although they share the same receptor, CCR4. We then analyzed the ratio of IP-10 gene expression to MDC gene expression. We could not detect any differences of IP10/MDC gene expression ratio between lung cancer and normal lung tissues (data not shown). These results indicated that the levels and the balances of gene expression of CXCR3 ligand chemokines and CCR4 ligand chemokines in lung cancer tissues might not be different from those of normal tissues.
Fig. 3.
Correlation between chemokines in lung cancer tissue. a–c In each tumor, gene expression levels of IP-10 are plotted on the vertical axis against the levels of chemokines indicated on the horizontal axis. Expression of IP-10 gene was highly correlated with Mig (a) and I-TAC (b), but not with MDC (c). d MDC expression levels are plotted on the vertical axis against TARC expression levels. Expression of MDC gene was not correlated with TARC
MDC/CCL22 gene expression and the prognosis of lung cancer
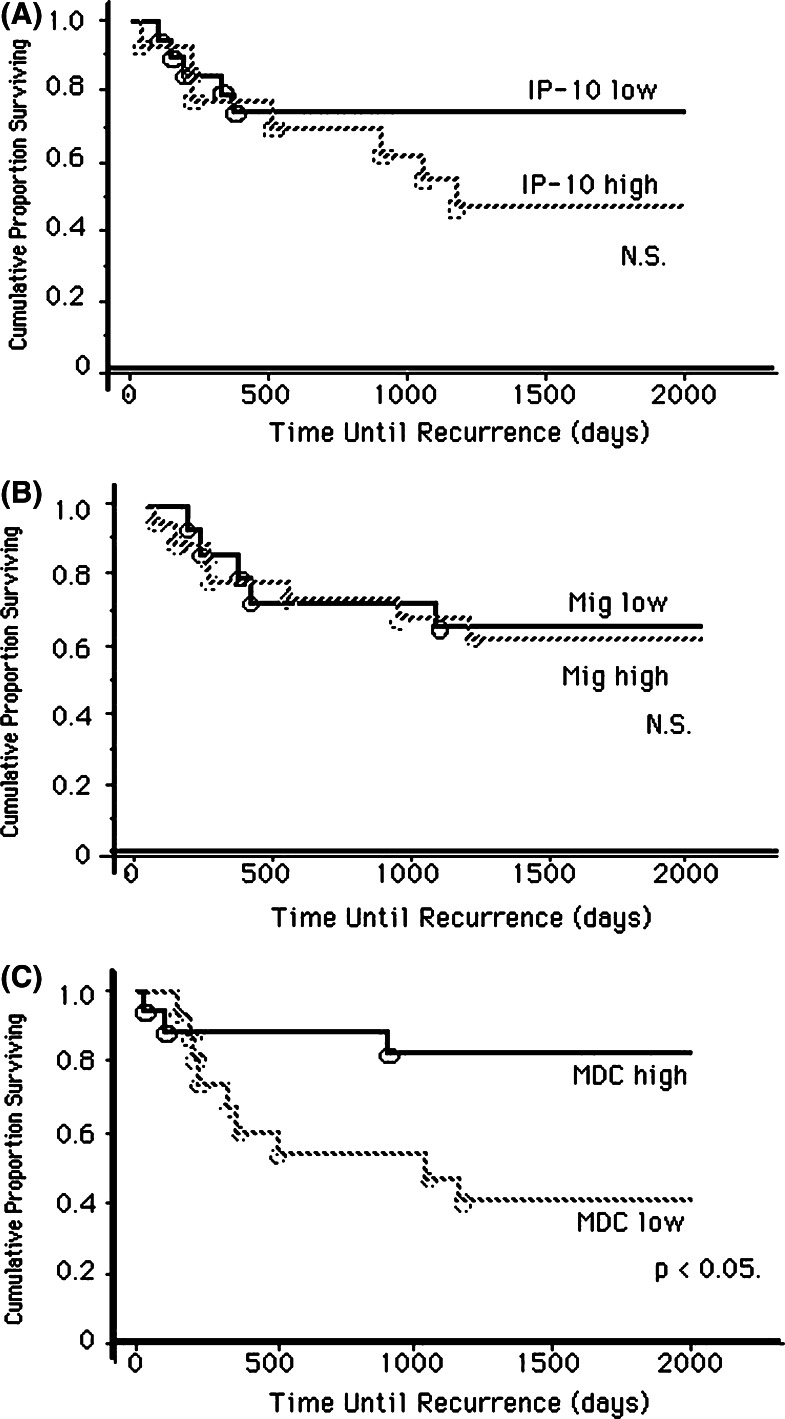
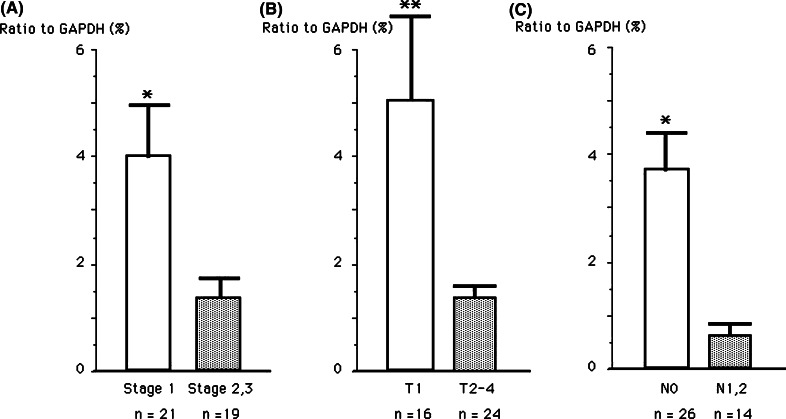
As we speculated that expression of chemokines in lung cancer tissue might influence tumor progression, we investigated the association between gene expression of CXCR3 ligand and CCR4 ligand chemokines in the cancer tissues, and the prognoses of lung cancer patients. We analyzed the association of IP-10, Mig and MDC expression levels and the recurrence of lung cancer patients after surgery in a univariate Cox proportional hazards model (Table 2). We found that MDC high gene expression showed significant inverse association with the recurrence of lung cancer (P=0.006); however, high IP-10 or Mig gene expression did not. We also analyzed disease-free survival according to gene expression levels of these chemokines. Higher expression of MDC was significantly correlated with longer disease-free survival time (P<0.05) although no significant difference was observed in high levels of IP-10 or Mig gene expression (Fig. 4). Then we analyzed the relationships between intra-tumor expression levels of MDC gene and other clinical factors of lung cancer patients. As shown in Fig. 5a, patients with stage I lung cancer showed significantly higher expression levels of MDC gene than those with stage II or III disease (P<0.02). In addition, patients with T1 disease compared with T2-4, and patients with N0 compared with N1-2 showed significantly higher MDC gene expression (Fig. 5b, c). We could not find any association between expression levels of other chemokines investigated in this study and clinical factors. These results demonstrate that MDC/CCL22 gene expression in cancer tissues may be a possible predictive marker for a favorable outcome of lung cancer.
Table 2.
Cox-proportional hazards model-derived relative risk of recurrence after resection of lung cancer based on chemokine level as a dichotomous variable
| Variable (chemokine expression level above the median) | Relative risk (95% CI) | P |
|---|---|---|
| CXCL10 (IP-10) high (>2.175) | 1.909 (0.527–7.308) | 0.329 |
| CXCL9 (Mig) high (>4.067) | 1.238 (0.341–4.563) | 0.744 |
| CCL22 (MDC) high (>1.172) | 0.117 (0.022–0.488) | 0.006 |
Groups of patients were stratified based on whether they had levels of corresponding chemokines above (high) or below the median
Fig. 4.
Kaplan–Meier curves describing disease-free survival. Patients with high levels of IP-10 (a) or Mig (b) gene expression showed no better disease-free survival compared to those with low levels of expression. However, patients with high level of MDC gene expression (c) showed significantly better disease-free survival compared to those with low levels of gene expression
Fig. 5.
Expression of MDC was significantly increased in cases with early stage. MDC gene expression quantified by real-time PCR was analyzed according to the TNM staging categories. a Stage I versus stages II and III, b T1 versus T2, T3 and T4, c N0 versus N1 and N2. *P<0.05; **P<0.02 for the difference between two groups
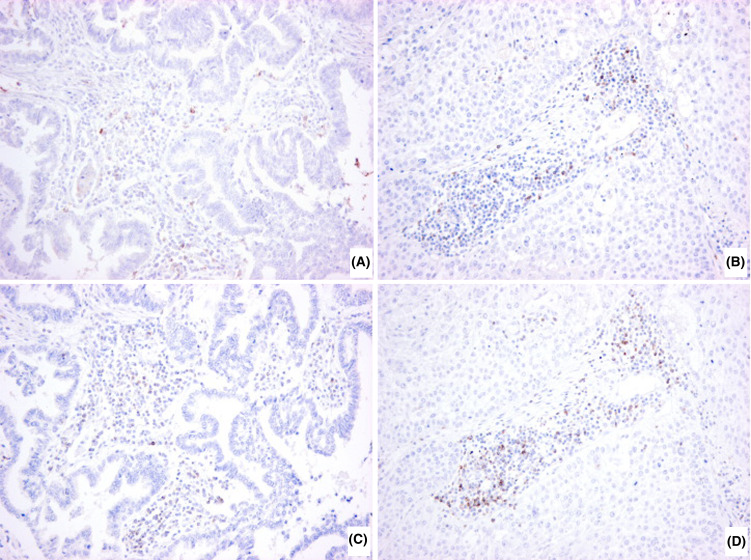
Histological examination of lung cancer tissues showed that many mononuclear cells or macrophages infiltrated into the tumor nest or peripheral non-tumor area in most cases. We could not find the association between MDC gene expression levels and intensity of inflammatory cell infiltrations (data not shown). Immunohistochemistry for CCR4 and CXCR3 showed that these receptors were expressed in infiltrated lymphoid cells, suggesting that these cells might be chemoattracted into cancer tissues (Fig. 6).
Fig. 6.
Representative immunohistochemistry of CCR4 (a, b) and CXCR3 (c, d) in lung cancer tissues. Paraffin sections of lung cancer tissues were immunostained with anti-human CCR4 antibody (1G1) or anti-human CXCR3 antibody (1C6). a and c adenocarcinoma; b and d squamous cell carcinoma. (×200 original magnification)
MDC/CCL22 production in lung cancer cell lines in vitro
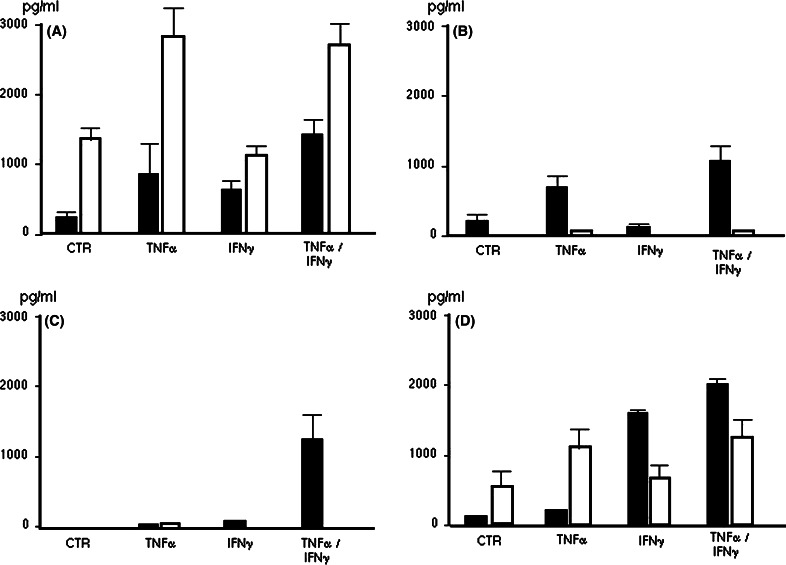
As lung cancer tissue contains various types of cells such as cancer cells, stromal cells, and infiltrating inflammatory cells, we tried to elucidate whether lung cancer cells could produce MDC/CCL22 and IP-10 or not. We measured the concentration of MDC (white column) and IP-10/CXCL10 (black column) in supernatant from cultured human lung cancer cell lines stimulated with or without pro-inflammatory cytokines (TNF-α and IFN-γ). We used lung cancer cell lines of H441 (papillary adenocarcinoma), A549 (alveolar cell carcinoma), H2099 (adenocarcinoma) and EBC1 (squamous cell carcinoma). As shown in Fig. 7a, H441 cells spontaneously produced a considerably high amount of MDC. The production of MDC was enhanced by the stimulation of TNF-α. A549 cells and H2099 cells produced little MDC even under the TNF-α stimulation (Fig. 7b, c). EBC1 (squamous cell carcinoma) produced MDC moderately, and TNF-α enhanced its production (Fig. 7d). In contrast to MDC, IP-10/CXCL10 production was observed in all cell lines under stimulation with TNF-α or TNF-α combined with IFN-γ (Fig. 7a–d). These results demonstrate that lung cancer cells could produce MDC in response to various immunological stimulations such as pro-inflammatory cytokines in the tumor microenvironment, and the level of its production varies widely with tumor cells.
Fig. 7.
MDC/CCL22 and IP-10/CXCL10 production by human lung cancer cell lines. a H441 human lung papillary adenocarcinoma, b A549 human alveolar cell carcinoma, c H2099 human adenocarcinoma and d EBC1 human squamous cell carcinoma. Each cell line was cultured in 6-well plates in normal RPMI medium or medium containing TNF-α (100 U/ml) and/or IFN-γ (100 U/ml) for 24 h. Supernatants from each well were collected to measure chemokines concentrations by ELISA (MDC white bar; IP-10 black bar). Values are means and standard error of the mean (SEM) for at least three independent experiments
Discussion
Primary functions of chemokines are chemoattraction and activation of specific leukocytes in various immuno-inflammatory responses [4]. Recent studies have shown that chemokines are also involved in growth, angiogenesis and metastasis of cancer cells, and therefore they are thought to be key players in cancer biology [4]. In addition, some investigators have shown that chemokines or expression of their receptors in cancer correlates with disease progression and prognosis [14–16].
In this study, we investigated gene expressions of five chemokines including IP-10, Mig and I-TAC (which bind CXCR3 receptor of Th1 cells), and MDC and TARC (which bind CCR4 receptor of Th2 cells) in lung cancer tissues. We showed that IP10, Mig and MDC gene expression levels in lung cancer tissues were not significantly different from those in normal tissues. However, a subset of lung cancer cases showed relatively increased gene expression of these chemokines. Among these chemokines, MDC gene expression showed a positive correlation with better prognosis after surgery. The result is rather unexpected as we had first hypothesized that increased gene expression of CXCR3 binding chemokines (chemoattractive factors for Th1 cells) might be associated with better prognosis. In general, it is thought that type-1 immunity (Th1-type immunity), which is regulated by IFN-γ and IL-12, is important for eradicating tumors, and type-2 immunity (Th2-type immunity) effector cells suppress Th1-dependent immunity by producing IL-4 or IL-13 [12]. However, IL-4 has also been reported to provide potent anti-tumor activity against various tumors [17, 18]. Thus, the precise role of Th1 and Th2 cells in anti-tumor immunity has not been fully understood [12]. MDC/CCL22 is a chemotactic factor for a variety of immune cells including not only CD4+ T cells but also NK cells, monocytes or dendritic cells. Some investigators have reported the gene transfer of MDC using adenoviral vector into murine tumor cells, which brought successful results in the treatment of lung carcinoma [19, 20]. These reports suggested that dendritic cells or T cells (CD4 or CD8 T cells) play important roles for establishing anti-tumor activity by MDC. Interestingly, Guo et al. [19] observed a significant increase of IL-4 production early after the MDC administration into tumor site followed by the increase of IL-12 and IL-2 production. They showed the anti-tumor response induced by MDC was markedly impaired in IL-4 knock out mice, suggesting an important role of IL-4 in the induction of anti-tumor immunity by MDC. In precursor B cell lymphoblastic leukemia, MDC is secreted by tumor cells following CD40 ligation, and mediates the chemoattraction of tumor-specific effector T cells [21]. Our immuno-histochemical study illustrated that CCR4 positive lymphocytes infiltrated into lung cancer tissues. This might suggest that MDC could enhance infiltration of immune cells to tumor environment. However, we could not find the association between intensity of mononuclear cell infiltration into tumor tissues and gene expression levels of MDC. The infiltration of inflammatory cells into tumor tissue is influenced by many factors. So, it may be difficult to assess the association between inflammatory cells recruitment and the single chemokine (MDC). Recently, several research groups have shown that an inflammatory reaction in tumor tissue could foster tumor development [22]. Lin et al. [23] reported that macrophages could promote both the development of breast cancer and their metastases. They showed this by knocking out the gene for colony-stimulating factor 1 (CSF-1), a cytokine that attracts macrophages in a mouse genetically engineered to develop breast cancer. Daniel et al. [24] also showed reduced tumor development of skin cancer in a mouse strain lacking CD4+ T cells. These reports suggest that inflammatory reactions including expression of chemokines at tumor environment may play dual roles in cancer development. Although chemokines may recruit anti-tumor immune cells into tumor site, they may also induce inflammation that could promote invasion and metastasis of tumor [6]. Further studies are needed to elucidate the precise relationship among chemokines’ expression, inflammatory cells’ recruitment and anti-tumor effect.
IP-10 and Mig are important angiostatic factors as well as chemoattractants for Th1 lymphocyte recruitment [25]. In renal cell carcinoma, IP-10 and Mig expressions in tumor tissue have been reported to be associated with better prognosis [26]. In contrast, we could not demonstrate the association between gene expression of these chemokines and prognosis of lung cancer patients. As reported previously, tumor-suppressive activities are greatly influenced by the kind of tumor and activation state of the host’s immune system in chemokine-gene-based immunotherapy [27]. Therefore, the discrepancies may be due to the difference of tumor biology between lung carcinoma and renal cell carcinoma.
In the present in vitro study, we showed that lung adenocarcinoma cell line H441 produced a large amount of MDC with or without pro-inflammatory cytokine stimulation. EBC-1 (squamous cell carcinoma cell line of the lung) also produced MDC with TNF-α stimulation. In contrast, we could not detect any significant production of MDC from A549 cells even with TNF-α–IFN-γ stimulation, which was consistent with previous reports that bronchial epithelial cell lines (A549 and BEAS-2B) produced no MDC even in cultivation with TNF-α, IL-4 or IFN-γ [11, 28]. In addition, a previous report showed that human ovarian carcinoma cells as well as microenvironmental macrophages produce a large amount of MDC in vivo [29]. Taken together, we speculated that lung cancer cells by themselves could produce MDC, and its production might depend on the characteristics of tumor cells. To investigate the effect of autocrine on MDC-producing cells, we examined the expression of CCR4 at mRNA level, and the blocking of CCR4 using anti-CCR4 antibody. The experiment showed no CCR4 expression and no influence on cell growth or growth inhibition in vitro (data not shown). Further studies are needed to determine the precise mechanism of MDC production in lung cancer tissue and how intra-tumoral MDC influences the prognosis in lung cancer.
Recently, increased evidences have shown the benefit of adjuvant chemotherapy for lung cancer patients who underwent surgery at stages IB or II [30, 31]. However, for patients with stage IIIA disease, the role of chemotherapy at adjuvant settings is still controversial. In such cases, precise prediction of prognosis or recurrence of tumors after tumor resection may be useful for adding the next therapeutic modality. Our study indicates that measurement of MDC gene expression in tumor tissue specimen obtained by biopsy or surgical procedures would give important information to predict the tumor recurrence or the relapse. To confirm our results prospectively, a protocol study for the treatment of lung cancer patients using the stratification dependent on MDC expression status needs to be validated.
In summary, we have found that a subset of lung cancer tumors express increased levels of MDC/CCL22 gene expression, which correlated with decreased risk of the tumor recurrence after tumor resection. Our study also showed that lung cancer cell lines produced MDC with or without chemokine stimulation, which suggested that tumor cells could produce MDC as well as intra-tumoral inflammatory cells. Although additional prospective studies are needed to confirm this hypothesis, we suggest that the intra-tumoral gene level of MDC may be a useful marker for predicting the prognosis of lung cancer.
Acknowledgements
The authors thank Ms. Ayako Asai, Emi Kusama, Hiromi Isejima and Keiko Shimamoto for their continuous technical assistance. This work was supported in part by a Grant-in-Aid for the 21st Century COE Program “Integrated Molecular Medicine for Neuronal and Neoplastic Disorders” from the Ministry of Education, Culture, Sports, Science and Technology and a Grant-in-Aid for the Scientific Research from the Japan Society for the Promotion of Science.
Abbreviations
- MDC
Macrophage-derived chemokine
- IP-10
Interferon-inducible protein of 10 kD
- Mig
Monokine induced by interferon-gamma
- I-TAC
Interferon-inducible T cell α chemoattractant
- TARC
Thymus- and activation-regulated chemokine
- RT-PCR
Reverse transcription-polymerase chain reaction
- TNF-α
Tumor necrosis factor-α
- IFN-γ
Interferon γ
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
Footnotes
Toru Nakanishi and Kazuyoshi Imaizumi equally contributed to this work.
References
- 1.Winterhalder RC, Hirsch FR, Kotantoulas GK, Franklin WA, Bunn PA., Jr Chemoprevention of lung cancer—from biology to clinical reality. Ann Oncol. 2004;15:185–196. doi: 10.1093/annonc/mdh051. [DOI] [PubMed] [Google Scholar]
- 2.Rea F, Marulli G, Callegaro D, Zuin A, Gobbi T, Loy M, Sartori F. Prognostic significance of main bronchial lymph nodes involvement in non-small cell lung carcinoma: N1 or N2? Lung Cancer. 2004;45:215–220. doi: 10.1016/j.lungcan.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Frederick MJ, Clayman GL (2001) Chemokines in cancer. Expert Rev Mol Med 2001:1–18 [DOI] [PubMed]
- 4.Arya M, Patel HRH, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin. 2003;19:557–564. doi: 10.1185/030079903125002216. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme J, Struyf S, Opdenakker G. Chemokine-protease interactions in cancer. Semin Cancer Biol. 2004;14:201–208. doi: 10.1016/j.semcancer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Opdennakker G, van Damme J. The countercurrent principle in invasion and metastasis of cancer cells. recent insights on the roles of chemokines. Int J Dev Biol. 2004;48:519–527. doi: 10.1387/ijdb.041796go. [DOI] [PubMed] [Google Scholar]
- 7.D’Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–1275. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- 8.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 9.Qiu B, Frait KA, Reich F, Komuniecki E, Chensue SW. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am J Pathol. 2001;158:1503–1515. doi: 10.1016/S0002-9440(10)64101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlo ED, Cappello P, Sorrentino C, D’Antuono T, Pellicciotta A, Giovarelli M, Forni G, Musiani P, Triebel F. Immunological mechanisms elicited at the tumour site by lymphocyte activation gene-3 (LAG-3) versus IL-12: sharing a common Th1 anti-tumour immune pathway. J Pathol. 2005;205:82–91. doi: 10.1002/path.1679. [DOI] [PubMed] [Google Scholar]
- 11.Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol. 2000;165:2205–2213. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95:697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi M, Imaizumi K, Kawabe T, Wakayama H, Horio Y, Sekido Y, Hara T, Hashimoto N, Takahashi M, Shimokata K, Hasegawa Y. Induction of antitumor immunity by transduction of CD40 ligand gene and interferon-gamma gene into lung cancer. Cancer Gene Ther. 2001;8:421–429. doi: 10.1038/sj.cgt.7700320. [DOI] [PubMed] [Google Scholar]
- 14.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–860. [PubMed] [Google Scholar]
- 15.Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, Di Carlo V, Allavena P, Piemonti L. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- 16.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 17.Tepper RI, Pattengale PK, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 18.Nagai S, Toi M. Interleukin-4 and breast cancer. Breast Cancer. 2000;7:181–186. doi: 10.1007/BF02967457. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Wang B, Zhang M, Chen T, Yu Y, Regulier E, Homann HE, Qin Z, Ju DW, Cao X. Macrophage-derived chemokine gene transfer results in tumor regression in murine lung carcinoma model through efficient induction of antitumor immunity. Gene Ther. 2002;9:793–803. doi: 10.1038/sj.gt.3301688. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Merritt RE, Mahtabifard A, Yamada R, Kikuchi T, Crystal RG, Korst RJ. Intratumoral expression of macrophage-derived chemokine induces CD4+ T cell-independent antitumor immunity in mice. J Immunother. 2003;26:117–129. doi: 10.1097/00002371-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ghia P, Transidico P, Veiga JP, Schaniel C, Sallusto F, Matsushima K, Sallan SE, Rolink AG, Mantovani A, Nadler LM, Cardoso AA. Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells. Blood. 2001;98:533–540. doi: 10.1182/blood.V98.3.533. [DOI] [PubMed] [Google Scholar]
- 22.Marx J. Inflammation and cancer: the link grows stronger. Science. 2004;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 23.Lin EY, Nguyen AV, Russell GR, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel D, Meyer-Morse N, Bergsland EK, Dehne K, Coussens LM, Hanahan D. Immune enhancement of skin carcinogenesis by CD4+ T cells. J Exp Med. 2003;197:1017–1028. doi: 10.1084/jem.20021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo T, Ito F, Nakazawa H, Horita S, Osaka Y, Toma H. High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. J Urol. 2004;171:2171–2175. doi: 10.1097/01.ju.0000127726.25609.87. [DOI] [PubMed] [Google Scholar]
- 27.Okada N, Gao JQ, Sasaki A, Niwa M, Okada Y, Nakayama T, Yoshie O, Mizuguchi H, Hayakawa T, Fujita T, Yamamoto A, Tsutsumi Y, Mayumi T, Nakagawa S. Anti-tumor activity of chemokine is affected by both kinds of tumors and the activation state of the host’s immune system: implications for chemokine-based cancer immunotherapy. Biochem Biophys Res Commun. 2004;317:68–76. doi: 10.1016/j.bbrc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Berin MC, Eckmann L, Broide DH, Kagnoff MF. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am J Respir Cell Mol Biol. 2001;24:382–389. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]
- 29.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 30.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 31.Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]