Abstract
In order to broaden the possibility for anti-HER-2/neu (HER-2) immune targeting, it is important to identify HLA-A24 restricted peptide epitopes derived from HER-2, since HLA-A24 is one of the most common alleles in Japanese and Asian people. In the present study, we have screened HER-2-derived, HLA-A24 binding peptides for cytotoxic T lymphocyte (CTL) epitopes. A panel of HER-2-derived peptides with HLA-A24 binding motifs and the corresponding analogs designed to enhance HLA-A24 binding affinity were selected. Identification of HER-2-reactive and HLA-A24 restricted CTL epitopes were performed by a reverse immunology approach. To induce HER-2-reactive and HLA-A24 restricted CTLs, PBMCs from healthy donors were repeatedly stimulated with monocytes-derived, mature DCs pulsed with HER-2 peptide. Subsequent peptide-induced T cells were tested for the specificity by enzyme linked immunospot, cytotoxicity and tetramer assays. CTL clones were then obtained from the CTL lines by limiting dilution. Of the peptides containing HLA-A24 binding motifs, 16 peptides (nine mers) including wild type peptides (IC50<1,000 nM) and substituted analog peptides (IC50<50 nM) were selected for the present study. Our studies show that an analog peptide, HER-2(905AA), derived from HER-2(905) could efficiently induce HER-2-reactive and HLA-A24 restricted CTLs. The reactivity of the HER-2(905AA)-induced CTL (CTL905AA) was confirmed by different CTL assays. The CTL905AA clones also were able to lyse HER-2(+), HLA-A24(+) tumor cells and cytotoxicity could be significantly reduced in cold target inhibition assays using cold targets pulsed with the HER-2(905) wild type peptide as well as the inducing HER-2(905AA) analog peptide. A newly identified HER-2(905) peptide epitope is naturally processed and presented as a CTL epitope on HER-2 overexpressing tumor cells, and an MHC anchor-substituted analog, HER-2(905AA), can efficiently induce HER-2-specific, HLA-A24 restricted CTLs.
Keywords: Substitution analog, HLA-A24, HER-2, Epitope, CTL
Introduction
It is now well established that small peptide epitopes which bind to MHC class I molecules on the surface of tumor cells can be recognized as antigens (Ags) by cytotoxic T lymphocyte (CTL). Tumor-specific CTL, adoptively transferred or activated in vivo by tumor-associated CTL epitopes, have therapeutic activity and can induce regression of established tumors or micrometastases [23, 27]. The development of immunotherapeutic methods to treat cancer is critically dependent on the identification of tumor-associated Ags. Several immunogenic peptide epitopes, recognized by CTL lines and clones, have been defined from human carcinomas [1, 4, 6, 11, 12, 33].
As an alternative to the genetic and biochemical approach for identifying tumor-associated CTL epitopes, a reverse immunology method has been developed [2, 12, 13, 34]. In this method, predicted MHC class I binding epitopes within a tumor Ag sequence are identified using algorithms of MHC anchor residue motifs and peptides corresponding to these epitopes are synthesized and tested to confirm binding to purified HLA molecules. Peptides demonstrating strong HLA binding affinity are screened further for their capacity to induce peptide- and tumor-specific CTL from healthy individuals or cancer patients. This approach has recently been used for the definition of several new CTL epitopes in different melanoma Ags [2, 12, 13, 34] as well as tumor Ags expressed on breast, colon and lung adenocarcinomas [15].
The HER-2/neu (HER-2) proto-oncogene encodes a 185-kDa transmembrane glycoprotein that contains an extracellular domain and an intracellular domain with tyrosine-specific kinase activity and has a similarity in structure and sequence to the epidermal growth-factor receptor [5]. HER-2 is amplified and overexpressed in approximately 30% of the human ovarian and breast tumors [29], and in 20% of gastric cancers [10], and is correlated with the stage progression of gastric cancer [19, 30]. In a previous study, we have provided evidence that HER-2-derived peptides are naturally processed as tumor-associated Ags in gastric cancer and can be recognized by tumor-specific, HLA-A2 restricted CTLs [18]. HLA-A2 restricted CTL epitopes derived from HER-2, that are recognized by ovarian [8, 17] and breast [22] cancer-specific CTLs, have previously been defined. Additional HLA-A2 restricted, CTL epitopes derived from HER-2 which can activate CTLs from healthy donors and patients with advanced ovarian carcinoma have also been reported [14, 26]. Based on the above reports, it may be speculated that anti-HER-2 immune targeting may be utilized as a common approach to immunotherapy of a variety of cancers.
HLA-A24 is one of the most common alleles in the Japanese population with more than 60% of this ethnic group expressing this HLA allele [7]. Therefore, in order to broaden the possibility for anti-HER-2 immune targeting, it is important to identify HLA-A24 restricted peptide epitopes derived from HER-2. Furthermore, in this study, we have synthesized analogs of HER-2-derived peptides which are substituted at one or both of the MHC anchor positions of the sequence to enhance HLA binding and immunogenicity. It has been shown that MHC anchor-substituted analogs derived from gp100 can more efficiently induce CTL response than wild type peptide epitopes [25].
In the present study, we describe the identification of a new HLA-A24 restricted, HER-2-derived anchor-substituted analog epitope which efficiently induces CTLs that respond to the native HER-2 wild type peptide epitope as well as to the endogenously processed epitope presented by HLA-A24(+) and HER-2(+) tumor cell lines.
Material and methods
Cell lines
MKN-7 (HER-2+, HLA-A26+ gastric cancer), KATOIII (HER-2+, HLA-A24+ gastric cancer), MRKnu-1 (HER-2+, HLA-A24+ gastric cancer), WiDr (HER-2+, HLA-A24+ colon cancer) and PC-9 (HER-2+, HLA-A24+ lung cancer) were obtained from the IBL cell bank (Gunma, Japan). HCT-15 (HER-2+, HLA-A24+ colon cancer), SKOV 3 (HER-2+, HLA-A3/A11+ ovarian cancer) and K562 (HER-2−, HLA-A24− lymphoma cell) were obtained from ATCC (Rockville, MD). TISI cells are human B-lymphoblastoid cell lines expressing HLA-A24. These cell lines were kept in RPMI 1640 with 5% FCS, 50 U/ml penicillin and 2 mM l-glutamine.
Peptide synthesis
Peptides were either synthesized at Epimmune, Inc. (San Diego, CA), as previously described [28], or, for large epitope libraries, purchased as crude material from Mimotopes (Clayton, Victoria, Australia). Peptides synthesized at Epimmune were purified to >95% homogeneity by reverse-phase HPLC. Purity was determined on an analytical reverse-phase column and the composition was ascertained by amino acid analysis and/or mass spectrometry analysis. In the present study, we have synthesized HER-2-derived peptides with HLA-A24 binding motifs and the corresponding analogs designed to enhance HLA-A24 binding affinity.
HLA-A24 binding assay
The peptide binding assay specific for HLA-A24 molecules has been described previously [16, 21]. Briefly, the assay is based on the inhibition of a radiolabeled standard peptide to detergent solubilized HLA molecules by unlabeled test peptides. The standard peptide, with the sequence AYIDNYNKF, was radiolabeled with 125I by the chloramine T method. HLA-A24 molecules were purified by affinity chromatography from detergent extracts prepared from the EBV-transformed cell line KT3, as previously described [16]. Purified human HLA-A*2402 molecules, at a concentration which bound approximately 10–20% of the total radioactivity (generally between 5 and 15 nM), were incubated with 1–10 nM of the 125I-radiolabeled probe peptide and varying doses of test peptide ranging from 120 μg/ml to 1.2 ng/ml. The binding reaction between HLA molecules, standard peptide and the competing test peptide was carried out in the presence of 1 μM human β2-microglobulin (Scripps Laboratories, San Diego, CA) and a cocktail of protease inhibitors for 48 h at room temperature. Class I peptide complexes were then separated from the free peptide by gel filtration on TSK2000 columns (Tosohaus, Montgomeryville, AL). Peptide binding was quantified by determining the concentration of peptide required to inhibit the binding of the radiolabeled standard peptide by 50% (IC50%). Peptides were tested in 2–4 independent experiments. The average IC50 level of the standard peptide was 6.0 nM.
Preparation of DCs
DCs were generated from PBMC from HLA-A24 healthy donors. Briefly, PBMCs were separated from peripheral blood by centrifugation over Ficoll-Paque (Pharmacia, Uppsala, Sweden) and monocytes were enriched by adherence to a plastic tissue culture flask (Corning, NY) for 90 min at 37°C. Adherent cells were cultured with 1,000 units/ml of granulocyte macrophage colony-stimulating factor (GM-CSF, Peprotech EC Ltd, London, UK) and 1,000 units/ml of IL-4 (Peprotech EC Ltd) in X-VIVO (Life Technologies, Inc., Gaithersburg, MD). On day 5, the DCs were matured with TNF-alpha (10 ng/ml, Peprotech EC Ltd), PGE2 (1 μg/ml), IL-1β (10 ng/ml), IL-6 (1,000 U/ml). On day 7, the cytokine-treated cells were used as mature DC.
Generation of HER-2-specific CTL lines and CTL clone
After 7 days of culture as described above, mature DCs were pulsed with HER-2 peptide (20 μg/ml), which included the wild type peptide and the substitution analog peptide, in the presence of β2-microglobulin (3 μg/ml) for 60 min at 37°C. Then, these peptide (wild type or substitution analog)-loaded mature DCs were co-incubated with autologous PBMCs, which were obtained from HLA-A24 healthy donors, at 1:10 in a 12-well plate in X-VIVO with 1% autologous serum, 100 IU/ml of IL-2 (Shionogi). Subsequent cultured cells were re-stimulated with these peptide-loaded, irradiated (25 Gy) mature DCs every 7 days. After four stimulations, the cultured CTL lines were tested for the reactivity with the enzyme linked immunospot (Elispot) analysis and cytotoxic assay. All CTL lines were generated from five different healthy donors.
CTL clone was then obtained from the CTL lines by limiting dilution. Briefly, the CTLs were isolated in 96-well U-bottom plates in X-VIVO with irradiated allogeneic PBMC (5×104 cells/well) from two different donors in the presence of HER-2 peptide (20 μg/ml) and 100 IU/ml of IL-2 (Shionogi). The CTL clones were expanded with irradiated allogeneic PBMC, HER-2 peptide and 100 IU/ml of IL-2.
Elispot analysis and cytotoxic assay
The HER-2-specific response was determined by the IFN-γ Elispot analysis and cytotoxic assay. Elispot analysis was performed with the Mabtech assay system (Nacka, Sweden). After 96-well plates with nitrocellulose membrane (Millipore) were pre-coated with a primary anti-IFN-γ antibody (1D1K) for 24 h, the plates were pre-treated with AIM-V containing 1% human serum albumin. Target cells (2×104 per well) and CTLs (2×103 per well) were incubated in 200 μl of AIM-V for 24 h in triplicate. Thereafter, a biotinylated secondary anti-IFN-γ antibody (7-B6-1) was added for 2 h and then the plates were incubated with the streptavidin-alkaline phosphatase reagent and stained with NBT and BCIP (Gibco). All Elispot analyses were performed in the same condition.
For the cytotoxic assay, a standard 4 h 51Cr release assay was performed. To assess the peptide-specificity of CTL, TISI cells were pulsed with HER-2-derived, HLA-A24 restricted peptide for 16 h at 37°C. Thereafter, peptide-pulsed TISI cells were washed and subjected to the cytotoxic assay as a target. After the target cells were labeled with 100 μCi 51Cr for 60 min, they (5×103 per well) and the effector cells at various effector/target ratios were co-incubated in 200 μl of X-VIVO medium in a 96-well U-bottom plate in triplicate for 4 h at 37°C. Subsequently, cold target inhibition was carried out using the non-radiolabeled TISI cells loaded with HER-2 peptide or with an irrelevant HIV peptide (as negative control) at various hot/cold target ratios. The supernatants were harvested and radioactivity was determined using a gamma counter. The percentage of 51Cr release was calculated according to the following formula:
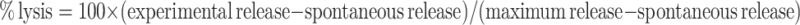 .
.
Flow cytometric analysis and tetramer assay
For the evaluation of HER-2 expression, a PE-labeled anti-HER-2 mAb (Becton Dickinson, San Jose, CA) and PE-labeled mouse IgG1 mAb (Beckman-Coulter, Miami, FL) as a negative control were used for immunostaining by flow cytometric analysis.
To evaluate the specificity of the CTL905AA clone, FITC-labeled anti-CD8 (MBL, Nagoya, Japan) and a PE-labeled HLA-A*2402-HER-2(905AA) tetramer (NH2–VYSYGVTVF–COOH:905AA peptide; MBL, Nagoya, Japan) were used for immunostaining, according to the manufacturer’s recommendations.
Statistics
To evaluate statistical differences between the two groups, a non-paired Student’s t test was performed. Statistically significant differences were considered to be P values<0.05.
Results
Identification of HLA-A*2402 binding epitopes and generation of HER-2-derived, epitope-specific CTL lines
HER-2-derived epitopes were identified on the basis of the presence of an HLA-A2402 binding motif by scanning the HER-2 protein with a customized computer program which accounts for both primary and secondary HLA binding anchor residues contained within the HLA-A24 epitopes [16, 21]. In addition to the identified wild type peptides, sequences possessing suboptimal residues at anchor positions were modified to enhance the binding capacity for HLA-A*2402 molecules. Preferred anchor residues for A*2402 have been determined by Epimmune and others [21] to be tyrosine (Y) at position 2 and phenylalanine (F) at the carboxy terminus. In the present study, nineteen nonamers carrying the HLA-A24 binding motif were selected, including ten wild type peptides (IC50<1,000 nM) and nine substitution analog peptides (IC50<50 nM; Table 1). All of the substitution analog peptides showed a high binding affinity for HLA-A24 (IC50<50 nM), while wild type peptides showed a range of affinity from high to weakly intermediate.
Table 1.
Relative binding affinity of HER-2/neu-derived peptides to HLA-A24
| Peptide | Sequence | A24 binding2 IC50 |
|---|---|---|
| Wild type peptide | ||
| HER-2(8) | RWGLLLALL | 11.41 |
| HER-2(780) | PYVSRLLGI | 77.14 |
| HER-2(951) | VYMIMVKCW | 79.93 |
| HER-2(440) | AYSLTLQGL | 97.98 |
| HER-2(907) | SYGVTVWEL | 101.42 |
| HER-2(905) | VWSYGVTVW | 173.21 |
| HER-2(63) | TYLPTNASL | 323.50 |
| HER-2(968) | RFRELVSEF | 744.21 |
| HER-2(342) | CYGLGMEHL | 780.14 |
| HER-2(887) | KWMALESIL | 887.79 |
| Substitution analog | ||
| HER-2(8AA) | RYGLLLALF | 1.47 |
| HER-2(780A) | PYVSRLLGF | 9.23 |
| HER-2(905AA) | VYSYGVTVF | 17.89 |
| HER-2(887AA) | KYMALESIF | 19.97 |
| HER-2(951A) | VYMIMVKCF | 19.98 |
| HER-2(907A) | SYGVTVWEF | 27.82 |
| HER-2(968A) | RYRELVSEF | 37.21 |
| HER-2(414AA) | AYPDSLPDF | 42.97 |
| HER-2(63A) | TYLPTNASF | 46.02 |
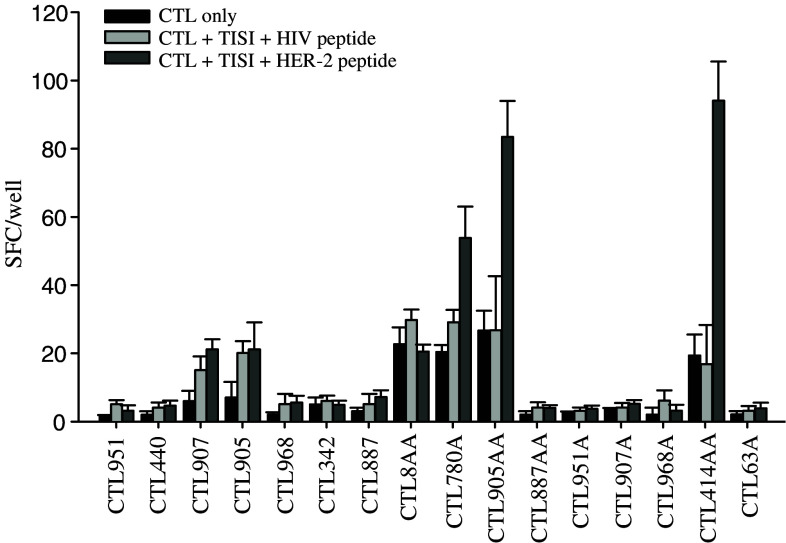
Of these peptides, we excluded the already known peptide epitopes HER-2(8), HER-2(780) and HER-2(63) [9, 24, 31], and generated 16 different peptide-specific CTL lines from five different HLA-A24(+) healthy donors by using mature DC cells pulsed with each of the remaining peptides. Then, the CTL lines were tested for their specificity against the cognate peptide used for each CTL induction, in an Elispot analysis. The reactivities of the peptide-induced CTL lines are shown in Fig. 1. In the present study, each peptide was considered positive if the spot forming cells (SFC) against TISI targets pulsed with cognate peptide was more than twofold SFC against CTL only in an Elispot analysis. Three CTL lines (CTL780A, CTL905AA and CTL414AA induced by the HER-2(780A), HER-2(905AA) and HER-2(414AA) epitopes, respectively) out of the 16 T cell lines significantly recognized TISI targets pulsed with each inducing cognate peptide.
Fig. 1.
The specificities of the HER-2 peptide-inducing CTL lines were evaluated with Elispot assay. Sixteen HER-2-derived peptide-specific CTL lines, designated by the inducing peptide epitope, were generated from five different HLA-A24(+) healthy donors by using mature DC cells pulsed with each peptide. Then, the CTL lines were tested for their specificity against cognate peptides, which were used for each CTL induction, in an Elispot assay described in Material and methods. Elispot assays were performed against all the CTL lines generated from five different healthy donors. Representative data from five independent experiments are shown. In the present study, each peptide was considered positive if the spot forming cells (SFC) of the CTL line against TISI targets pulsed with cognate peptide is more than the twofold SFC of CTL only. As a result, CTL780A, CTL905AA and CTL414AA significantly recognized TISI targets pulsed with each cognate peptide. Error bars indicate the standard error of the mean
HER-2 peptide-specific CTL lines can specifically recognize HLA-A24 tumor cell lines overexpressing HER-2
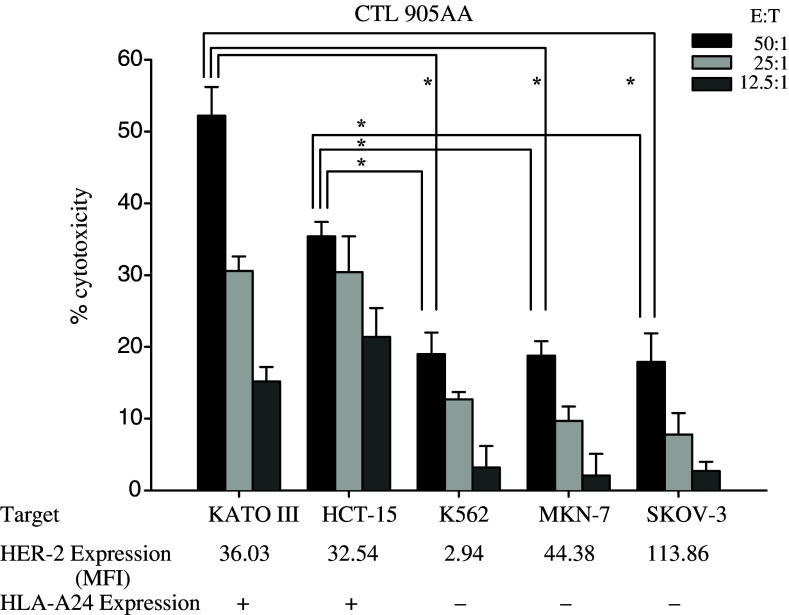
The CTL780A, CTL905AA and CTL414AA lines, wherein each CTL line was generated from five different healthy donors, were tested against HER-2-expressing tumor cell lines in an Elispot analysis. In the present study, the response of the CTL line against the tumor cell line was positive if the SFC against HLA-A24 positive, HER-2 positive tumor cell line was more than threefold SFC against HLA-A24 negative, HER-2 positive tumor cell line in an Elispot analysis. Out of the three CTL lines, only the CTL 905AA line recognized HLA-A24 positive tumor cell lines overexpressing HER-2 (PC-9 and HCT-15), but not a HLA-A24 negative HER-2 positive MKN-7 tumor cell line (Fig. 2). To further confirm the reactivity, CTL 905AA lines, which were generated from five different healthy donors, were tested against several targets in a cytotoxicity assay. The CTL 905AA line lysed HER-2(+), HLA-A24(+) HCT-15 and KATOIII cells, but not MKN-7, SKOV-3 or K562 cells (Fig. 3). These results indicated that the HER-2(905AA) peptide-induced CTLs recognized and lysed HER-2-expressing and HLA-A24(+) tumors.
Fig. 2.
Reactivities for the HER-2-expressing tumor by peptide-inducing CTL lines were evaluated with the Elispot assay. The CTL780A, CTL905AA and CTL414AA lines, which were generated from five different healthy donors, were tested against tumor cell lines in an Elispot analysis described in Material and methods. Representative data from five independent experiments are shown. In the present study, the response of the CTL line against the tumor cell line was positive if the SFC of the CTL line against a HLA-A24 positive HER-2 positive tumor cell line is more than threefold the SFC of a CTL line against a HLA-A24 negative HER-2 positive tumor cell line. Out of the three CTL lines, only the CTL 905AA line recognized HLA-A24 positive tumor cell lines overexpressing HER-2 (PC-9 and HCT-15), but not the HLA-A24 negative HER-2 positive MKN-7 tumor cell line. Error bars indicate the standard error of the mean
Fig. 3.
Cytotoxic assay by CTL905AA line. CTL905AA lines generated from five different healthy donors were tested against several targets using 4 h 51Cr-release assays at various effector/target ratios described in Material and methods. Representative data from five independent experiments are shown. CTL905AA line lysed HER-2(+), HLA-A24(+) HCT-15 and KATOIII, but not MKN-7, SKOV-3 or K562. HER-2 expression on the tumor cells was evaluated by flow cytometric analysis. Statistical analysis was performed with the Student’s t test. *P<0.05. MFI mean fluorescence intensity
CTL905AA clones recognize HLA-A24 tumor cell lines overexpressing HER-2 and TISI target cells pulsed with the HER-2(905) wild type peptide
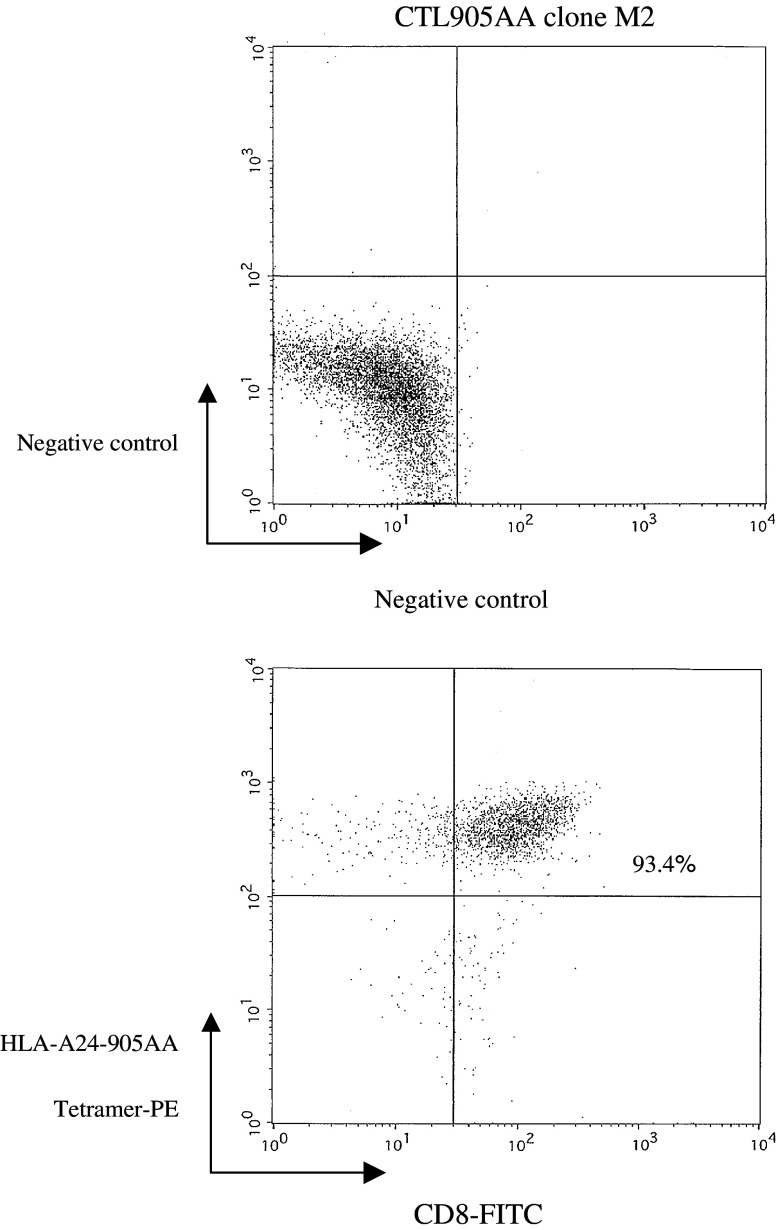
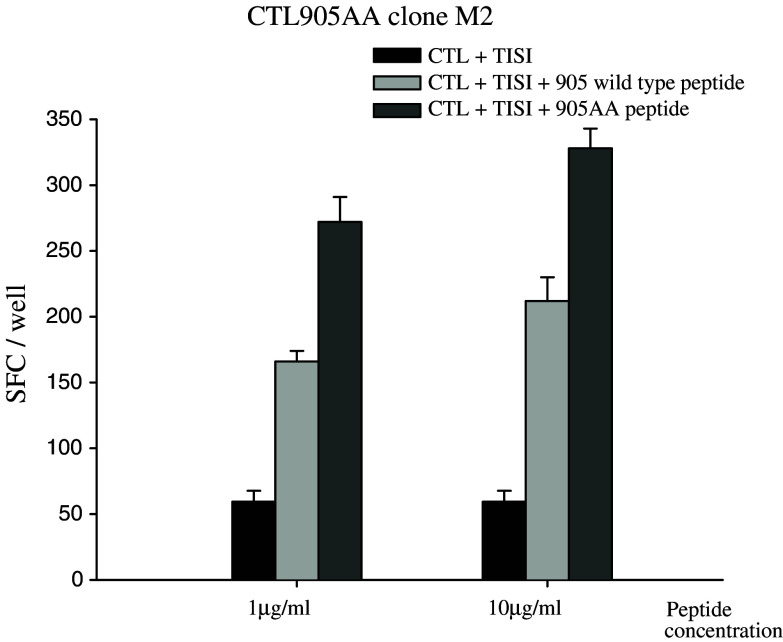
To further analyze the specificity of the HER-2(905AA) peptide, CTL clones were generated by limiting dilution methods from the CTL905AA line. Using the HER-2(905AA)–HLA-A24 tetramer, CTL 905AA clone, clone M2 was stained positive for both CD8 and the 905AA tetramer (Fig. 4), indicating that the T cell clone M2 was HER-2(905AA)-specific. In addition, clone M2 recognized the TISI cells pulsed with HER-2(905AA) peptide and also, to a lesser extent, TISI pulsed with the HER-2(905) wild type peptide (Fig. 5). These results revealed that the MHC anchor-substituted analog epitope HER-2(905AA) was more effective at breaking tolerance and inducing CTL which recognized the HER-2(905) wild type peptide, than the HER-2(905) wild type peptide which was less effective at inducing a primary in vitro CTL response (Fig. 1).
Fig. 4.
Tetramer assay for the CTL905AA clone M2. HER-2(905AA)-reactive CTL clones were generated by limiting dilution methods from the CTL905AA line. HER-2(905AA) tetramer analysis showed that CTL905AA clone M2 was stained positive for both CD8 and the HLA-A24-HER-2(905AA) tetramer (93.4%)
Fig. 5.
The reactivity of the CTL905AA clone M2 in Elispot analysis. The CTL905AA clone M2 recognized TISI pulsed with HER-2(905AA) peptide and also, to a lesser extent, TISI pulsed with the HER-2(905) wild type peptide
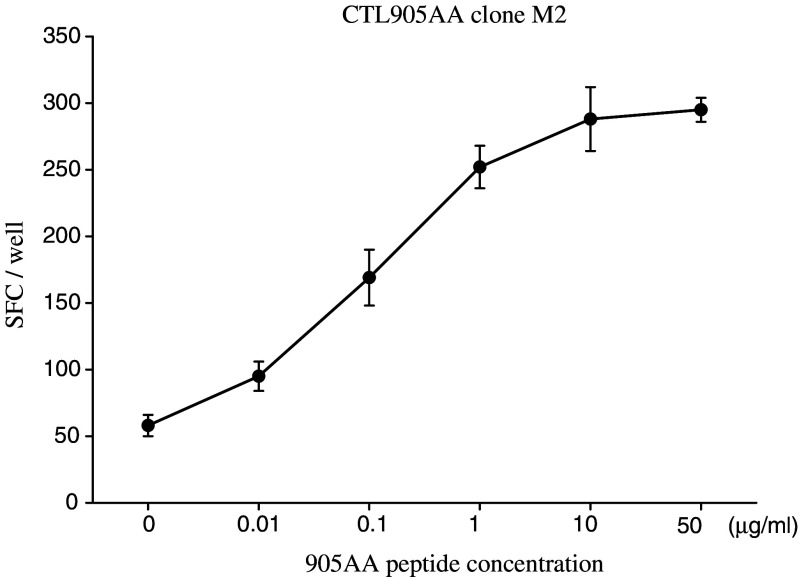
To further confirm the reactivity of the HER-2(905AA) peptide, various doses of the HER-2(905AA) peptides were tested for their capacity for sensitizing TISI by the clone M2. As expected, the reactivity of HER-2(905AA) peptide was dose-dependent (Fig. 6).
Fig. 6.
Dose-dependent reactivity of the CTL905AA clone M2. To further confirm the reactivity of the CTL905AA clone M2, various doses of HER-2(905AA) peptide were tested for their capacity for sensitizing TISI by the CTL905AA clone M2 in an Elispot analysis. The reactivity of clone M2 for HER-2(905AA) peptide was dose-dependent. Error bars indicate the standard error of the mean
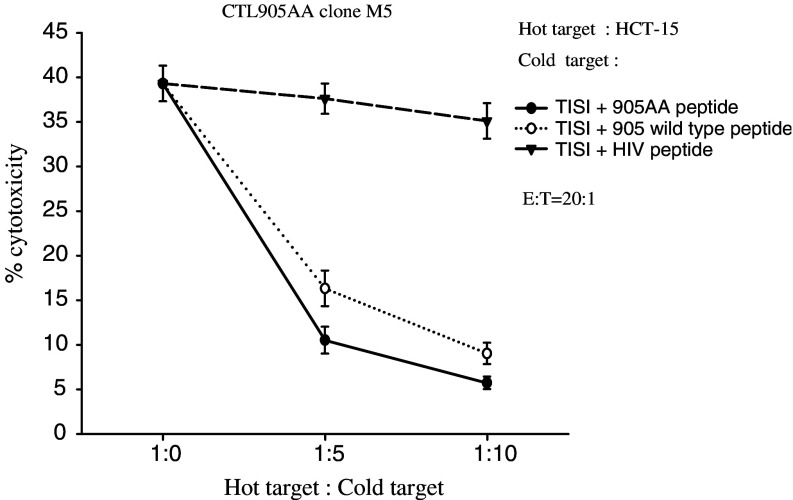
Moreover, another CTL clone derived from the CTL905AA line, M5, also demonstrated cytotoxicity against HER-2(+), HLA-A24(+) targets (HCT-15, MRKnu-1, KATOIII and PC-9) specifically (Fig. 7). When cold target inhibition assays were performed, a significant (84.3 or 75.2% inhibition at the 1:10 hot to cold ratio) inhibition of the killing for the HCT-15 was observed only with non-radiolabeled TISI loaded with HER-2(905AA) peptide or HER-2(905) wild type peptide but not with TISI loaded with an irrelevant control HIV peptide (Fig. 8). Collectively, these data indicated the HER-2(905AA) MHC anchor-substituted analog can efficiently induce HER-2-specific, HLA-A24 restricted CTLs.
Fig. 7.
The specificity of the CTL905AA clone M5 was evaluated with cytotoxic assay. The CTL905AA clone M5 was tested against several tumor cell lines using 4 h 51Cr-release assays at various effector/target ratios. The clone M5 lysed the HER-2(+) and HLA-A24(+) tumor cell lines HCT-15, MRKnu-1, KATOIII and PC-9, while M5 did not react with WiDr, SKOV-3, MKN-7, K562 or TISI cells. HER-2 expression on the tumor cells was evaluated by the flow cytometric analysis. MFI mean fluorescence intensity
Fig. 8.
Cold target inhibition assays with the CTL905AA clone M5. Cold target inhibition assays were performed using non-radiolabeled TISI cells loaded with the HER-2(905AA) peptide, the HER-2(905) wild type peptide or an irrelevant HIV peptide at various hot/cold target ratios. A significant (84.3 or 75.2% inhibition at the 1:10 hot to cold ratio) inhibition of the killing for the HER-2(+) and HLA-A24(+) HCT-15 mediated by clone M5 was observed when non-radiolabeled TISI cells loaded with HER-2(905AA) peptide or HER-2(905) peptide were added, but not when TISI cells were loaded with the control HIV peptide. E:T effector:hot target ratio
Discussion
In the present study, we have screened seven wild type peptides and nine HLA anchor-substituted analogs derived from HLA-A24 binding, HER-2-derived peptides as possible CTL epitopes. Then, we showed that the analog HER-2(905AA) can efficiently induce HER-2-specific, HLA-A24 restricted CTLs, which recognize and lyse tumor cells presenting the naturally processed wild type HER-2 epitope.
Deliberate substitutions of amino acids in peptide epitopes are generally thought to be effective in inducing peptide-specific CTLs by improving the binding affinity to HLA molecules. In previous studies, analogs substituted at MHC anchor residues have been tested in several tumor Ags, such as GP2, NY-ESO-1, gp100 as well as MART-1, and some of them successfully improved the immunogenicity of the CTL epitopes [3, 15, 25, 32, 35]. In the present study, to improve the immunogenicity of relatively low binding, HER-2-derived peptides, we generated anchor-substituted analogs (Table 1) and tested them for the immunogenicity. Although every substituted analog resulted in the enhancement of the binding affinity to HLA-A24 molecules, only analogs HER-2(780A), HER-2(905AA) and HER-2(414AA) were effective in inducing a peptide-specific CTL response (Fig. 1). Furthermore, out of three analogs, only the HER-2(905AA)-specific CTL resulted in the recognition and lysis of HLA-A24 tumor cell lines overexpressing HER-2 and the EBV-transformed cell line TISI pulsed with its wild type peptide. In addition, the cold target inhibition assay using the HER-2(905AA)-specific CTL clone further supported that a newly identified HER-2(905) peptide epitope is presented as the CTL epitope on HER-2 overexpressing tumor cell lines (Fig. 8).
In general, low binding affinity for the MHC class I molecule makes it difficult to induce peptide-specific CTL as epitope peptides that have low binding affinity may permit T cells to escape from negative selection; however, these epitope peptides and T cells may be useful for tumor-specific immunity. In the present study, the HER-2(905AA) analog peptide, but not the HER-2(905) wild type peptide, was effective in inducing a peptide-specific CTL response, and the HER-2(905AA)-specific CTL specifically lysed TISI target cells pulsed with HER-2(905AA) compared to TISI targets pulsed with HER-2(905). It is possible that increased immunogenicity with the HER-2(905AA) peptide analog may be derived from a combination of efficient binding to HLA-A24 molecules and better interaction with T cell receptors of specific CTLs. It has been shown that MHC anchor-substituted analogs derived from gp100 or NY-ESO-1 can induce CTL responses more efficiently than their corresponding wild type peptide epitopes [3, 25].
Recently, we and a few others have suggested that tumor-specific immunotherapy based on HER-2-derived peptides may be a useful and novel approach to the treatment of cancer patients with HER-2 overexpressing tumors. In fact, we have shown that DCs pulsed with HER-2-derived, HLA-A2 restricted peptides can induce specific T cell responses in patients with gastric cancer [20]. HLA-A24 is one of the most common alleles in Japanese people and is shared by more than 60% of the Japanese gastric cancer patients [7]. Thus, it would be desirable to identify additional HLA-A24 restricted immunodominant epitope peptides derived from HER-2, in order to broaden tumor-specific immunotherapy based on HER-2. The HER-2(905AA) peptide analog could be used as cancer vaccine to induce potent anti-tumor CTL responses. We believe that HER-2-specific, HLA-A24 restricted CTLs generated by HER-2(905AA) may react with HER-2 overexpressing tumor in vivo. In conclusion, the substitution analog peptide, HER-2(905AA), can efficiently induce HER-2-specific, HLA-A24 restricted CTLs.
Acknowledgments
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- 1.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celis E, Tsai V, Crimi C, DeMars R, Wentworth PA, Chesnut RW, Grey HM, Sette A, Serra HM. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci USA. 1994;91:2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JL, Dunbar PR, Gileadi U, Jager E, Gnjatic S, Nagata Y, Stockert E, Panicali DL, Chen YT, Knuth A, Old LJ, Cerundolo V. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 4.Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, Mattei S, De Plaen E, Lurquin C, Szikora JP, Renauld JC, Boon T. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 6.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 7.Date Y, Kimura A, Kato H, Sasazuki T. DNA typing of the HLA-A gene: population study and identification of four new alleles in Japanese. Tissue Antigens. 1996;47:93–101. doi: 10.1111/j.1399-0039.1996.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 8.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikuta Y, Okugawa T, Furugen R, Nagata Y, Takahashi Y, Wang L, Ikeda H, Watanabe M, Imai S, Shiku H. A HER2/NEU-derived peptide, a K(d)-restricted murine tumor rejection antigen, induces HER2-specific HLA-A2402-restricted CD8(+) cytotoxic T lymphocytes. Int J Cancer. 2000;87:553–558. doi: 10.1002/1097-0215(20000815)87:4<553::AID-IJC15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, Kobayashi M, Mai M, Suzuki T, Ooi A. Amplification of the c-erbB-2 (HER-2/neu) gene in gastric cancer cells. Detection by fluorescence in situ hybridization. Am J Pathol. 1997;151:761–768. [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 14.Kawashima I, Hudson SJ, Tsai V, Southwood S, Takesako K, Appella E, Sette A, Celis E. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum Immunol. 1998;59:1–14. doi: 10.1016/S0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 15.Keogh E, Fikes J, Southwood S, Celis E, Chesnut R, Sette A. Identification of new epitopes from four different tumor-associated antigens: recognition of naturally processed epitopes correlates with HLA-A*0201-binding affinity. J Immunol. 2001;167:787–796. doi: 10.4049/jimmunol.167.2.787. [DOI] [PubMed] [Google Scholar]
- 16.Kondo A, Sidney J, Southwood S, del Guercio MF, Appella E, Sakamoto H, Celis E, Grey HM, Chesnut RW, Kubo RT, Sette A. Prominent roles of secondary anchor residues in peptide binding to HLA-A24 human class I molecules. J Immunol. 1995;155:4307–4312. [PubMed] [Google Scholar]
- 17.Kono K, Halapi E, Hising C, Petersson M, Gerdin E, Vanky F, Kiessling R. Mechanisms of escape from CD8+ T-cell clones specific for the HER-2/neu proto-oncogene expressed in ovarian carcinomas: related and unrelated to decreased MHC class 1 expression. Int J Cancer. 1997;70:112–119. doi: 10.1002/(SICI)1097-0215(19970106)70:1<112::AID-IJC17>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Kono K, Rongcun Y, Charo J, Ichihara F, Celis E, Sette A, Appella E, Sekikawa T, Matsumoto Y, Kiessling R. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int J Cancer. 1998;78:202–208. doi: 10.1002/(SICI)1097-0215(19981005)78:2<202::AID-IJC14>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Kono K, Takahashi A, Amemiya H, Ichihara F, Sugai H, Iizuka H, Fujii H, Matsumoto Y. Frequencies of HER-2/neu overexpression relating to HLA haplotype in patients with gastric cancer. Int J Cancer. 2002;98:216–220. doi: 10.1002/ijc.10179. [DOI] [PubMed] [Google Scholar]
- 20.Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394–3400. [PubMed] [Google Scholar]
- 21.Kubo RT, Sette A, Grey HM, Appella E, Sakaguchi K, Zhu NZ, Arnott D, Sherman N, Shabanowitz J, Michel H, et al. Definition of specific peptide motifs for four major HLA-A alleles. J Immunol. 1994;152:3913–3924. [PubMed] [Google Scholar]
- 22.Linehan DC, Goedegebuure PS, Peoples GE, Rogers SO, Eberlein TJ. Tumor-specific and HLA-A2-restricted cytolysis by tumor-associated lymphocytes in human metastatic breast cancer. J Immunol. 1995;155:4486–4491. [PubMed] [Google Scholar]
- 23.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 24.Okugawa T, Ikuta Y, Takahashi Y, Obata H, Tanida K, Watanabe M, Imai S, Furugen R, Nagata Y, Toyoda N, Shiku H. A novel human HER2-derived peptide homologous to the mouse K(d)-restricted tumor rejection antigen can induce HLA-A24-restricted cytotoxic T lymphocytes in ovarian cancer patients and healthy individuals. Eur J Immunol. 2000;30:3338–3346. doi: 10.1002/1521-4141(200011)30:11<3338::AID-IMMU3338>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 26.Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiessling R. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol. 1999;163:1037–1044. [PubMed] [Google Scholar]
- 27.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 28.Ruppert J, Sidney J, Celis E, Kubo RT, Grey HM, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 29.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 30.Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer. 2002;98:833–837. doi: 10.1002/ijc.10257. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Tsunoda T, Nukaya I, Sette A, Matsuda K, Umano Y, Yamaue H, Takesako K, Tanimura H. Mapping the HLA-A24-restricted T-cell epitope peptide from a tumour-associated antigen HER2/neu: possible immunotherapy for colorectal carcinomas. Br J Cancer. 2001;84:94–99. doi: 10.1054/bjoc.2000.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka Y, Amos KD, Joo HG, Eberlein TJ, Goedegebuure PS. Modification of the HER2/NEU-derived tumor antigen GP2 improves induction of GP2-reactive cytotoxic T lymphocytes. Int J Cancer. 2001;94:540–544. doi: 10.1002/ijc.1508. [DOI] [PubMed] [Google Scholar]
- 33.Traversari C, van der Bruggen P, Luescher IF, Lurquin C, Chomez P, Van Pel A, De Plaen E, Amar-Costesec A, Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992;176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai V, Southwood S, Sidney J, Sakaguchi K, Kawakami Y, Appella E, Sette A, Celis E. Identification of subdominant CTL epitopes of the GP100 melanoma-associated tumor antigen by primary in vitro immunization with peptide-pulsed dendritic cells. J Immunol. 1997;158:1796–1802. [PubMed] [Google Scholar]
- 35.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]