Abstract
Dendritic cells derived from monocytes cultured in the presence of type I interferon were found to induce efficient T cell responses against tumor antigens in vitro. We vaccinated eight stage III or IV melanoma patients with dendritic cells generated with interferon-β and interleukin-3, activated by poly I: C, and pulsed with the tumor-specific antigen NA17.A2. This dendritic cell vaccine was well-tolerated with only minor and transient flu-like symptoms and inflammatory reactions at the injection sites. In most patients, isotopic imaging documented dendritic cells (DC) migration from the intradermal injection site to the draining lymph nodes. Finally, mixed lymphocyte-peptide culture under limiting dilution conditions followed by tetramer labeling indicated that three out of eight patients mounted a CD8 T cell response against the NA17.A2 antigenic peptide. We conclude that DC generated in type I-IFN represent an interesting alternative to DC generated in IL-4 and GM-CSF for cancer immunotherapy.
Keywords: Dendritic Cell, Melanoma Patient, Inguinal Region, Dendritic Cell Vaccine, Gamma Knife Surgery
Introduction
Most clinical trials of dendritic cell-based vaccines in cancer patients use dendritic cells (DC) derived either from blood monocytes cultured in the presence of GM-CSF and interleukin (IL)-4 [11, 27] or from CD34+ precursors cultured with GM-CSF, Flt3-L and TNF [1, 20]. Encouraging results have been obtained, and a lot of effort is now devoted to improve this type of immunotherapy and develop it on a larger scale [2, 6, 9]. Belardelli et al. [22, 26] described a population of DC derived from monocytes cultured in the presence of GM-CSF and IFNα (GM/IFN DC). These cells are strongly immunogenic, express high levels of HLA molecules, costimulatory molecules, and CCR7, a chemokine receptor favoring migration in secondary lymphoid organs. They spontaneously produce IL-15, which promotes Th-1 type responses and survival of T-lymphocytes. We observed that all these features were shared by DC generated in the presence of IL-3 and IFN-β (IL3/IFNβ DC) [4]. In addition, we established that both the GM/IFN and IL3/IFNβ DC secreted high amounts of IFN-α in response to Poly I:C; whereas, only low levels were secreted under the same condition by DC differentiated in IL-4 and GM-CSF [4]. Recently, GM/IFN and IL3/IFNβ DC were found capable of stimulating in vitro naïve CD8+ T cells recognizing tumor antigens such as the Melan-A/MART-1 peptide [18, 24, 28]. We report here the first results of a pilot clinical trial in which melanoma patients received IL3/IFNβ DC presenting the NA17.A2 antigen, a peptide encoded by an intron sequence of the gene coding for N-acetylglucosaminyltransferase V, and presented by HLA-A2 [12].
Materials and methods
Preparation of dendritic cell vaccine
Dendritic cells were generated according to GMP guidelines in a closed culture system using double-tray Cell Factory (Nunc, Roskilde, Denmark). Briefly, blood mononuclear cells isolated from cytapheresis products were washed in PBS supplemented with 5% human serum albumin (300 ml PBS + 75 ml HSA 20%), resuspended at 5×106 cells/ml in 320 ml of X-VIVO 20 medium (Cambrex Europe, Verviers, Belgium) supplemented with 1% autologous serum, and transferred into a double-tray cell-factory. Cells were then allowed to adhere for 2 h at 37°C in a 5% CO2 atmosphere. Non-adherent cells were discarded and adherent cells were washed and cultured in 320 ml of RPMI medium (Cambrex Europe) containing 100 U/ml of IFN-β (Avonex, Biogen, Cambridge, UK) and 50 U/ml of IL-3 (Cell Genix, Freiburg, Germany). After 48 h, IFN-β and IL-3 were added to the cultures, at the same concentrations. At day 5, cells were matured by overnight (18 h) incubation with 10 μg/ml of poly I:C (Sigma, Bornem, Belgium). Non-adherent cells were harvested at day 6 and pulsed for 2 h with NA17.A2 peptide (VLPDVFIRC, Clinalfa, Switzerland) (10 μg/ml). Since 60–65% of Belgian/French population are DPB1*0401 [10], DC were also pulsed with the MAGE-3.DP4 peptide (KKLLTQHFVQENYLEY, Clinalfa, Switzerland) (10 μg/ml) in order to potentially solicit help from CD4+ T cells [13].
Cytokine measurements
Commercially available Elisa Kits were used to determine the concentrations of IFN-α, (BioSource International, Fleurus, Belgium) and IL-12 p70 (Endogen, Woburn, MA, USA) in culture supernatants.
Patients and vaccination protocol
Eight HLA-A2-positive patients with Stage IIIb or IV melanoma expressing the NA17 tumor-associated antigen as determined by RT-PCR were vaccinated with peptide-pulsed IL3/IFNβ DC and tested for blood frequency of anti-NA17.A2 CD8 T cells. Patients were 18 to 75-year old, had a Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and a life expectancy of more than 4 months. Three vaccinations were given at 3-week intervals. Each vaccination consisted of 20×106 cells injected half subcutaneously and half intradermally, and 2.5×106 cells injected in an inguinal lymph node under ultrasound guidance. The Ethics Committee of the Erasme Hospital approved the study and all patients signed an informed consent. Adverse events were graded using the common terminology criteria for adverse events (CTCAE), Version 3.0 (Publish Date December 12, 2003).Clinical responses were measured according to RECIST criteria as a secondary endpoint of this study.
Imaging procedure for dendritic cell migration
Dendritic cells, prepared and matured as described above, then resuspended at a concentration of 107 DC/100 μl of RPMI medium [3], were incubated for 30 min at 20°C with 1.11 MBq/107 DC of Indium-111 oxine (Mallinckrodt Medical), then washed thrice with the medium. Patients received subcutaneous (SC) or intradermal (ID) injections of 5×106 labeled DC (in 0.3 ml of PBS) in the proximal inguinal region of each leg. A 60-min dynamic acquisition centered on the inguinal region was acquired immediately on a Sopha DSX gamma camera equipped with a parallel-hole medium-energy collimator. Ten-minute images were also acquired repeatedly during the first 45 h post-injection. Migration of DC-related tracer labeling was defined as the occurrence of at least one visually detectable focal uptake in the inguinal region. In one patient, a 3.7 MBq injection of Tc-99m nanocolloids (NanocisR, CIS bio International)—a radiopharmaceutical used for the sentinel node detection—was performed 19 h after the administration of DC, precisely in the area of labeled DC injection. Dynamic acquisition and 2-min static images centered on the inguinal region were acquired on the same gamma camera.
Detection of anti-NA17 T cells
Blood frequencies of anti-NA17.A2 CD8+ T cells were estimated using mixed lymphocyte-peptide cultures under limiting dilution conditions, followed by labeling with tetramers, as described [5, 14]. Briefly, PBMC collected and frozen 3 weeks after the third vaccination were thawed, resuspended at 107 cells/ml in Iscove’s medium supplemented with 1% human serum (HS) and 2 μM of peptide NA17.A2 (VLPDVFIRC), incubated over 1 h at room temperature, and washed. These peptide-pulsed cells were distributed at 2×105 cells/well in round-bottom microwells in Iscove’s medium with 10% HS, L-arginine (116 mg/L), L-asparagine (36 mg/L), L-glutamine (216 mg/L), IL-2 (20 U/ml) and IL-7 (10 ng/ml). On days 5 and 7, 50% of the medium was replaced by fresh medium containing IL-2 and IL-7. Peptide NA17.A2 was added on day 7 at a concentration of 2 μM. During the second week of stimulation, the microcultures were divided according to proliferation, in a medium containing IL-2 alone. At day 14, aliquots of the microcultures were labeled with anti-CD8 antibodies coupled to fluorescein, an HLA-A2 tetramer containing the NA17.A2 peptide and coupled to phycoerythrin, and a control HLA-A2 tetramer containing an EBV peptide (GLCTLVAML encoded by gene BMLF1) and coupled to allophycocyanin. The proportion of microcultures containing CD8+ cells specifically stained with the A2/NA17 tetramer was used to estimate a blood frequency of precursors of cells that can be stained by the tetramer (anti-NA17.A2 TETp frequency), taking into account the proportion of CD8+ cells in the PBMC.
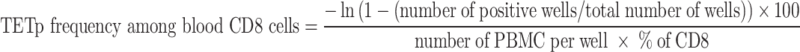 |
Results and discussion
Characteristics of the IL3/IFNβ dendritic cell vaccine
The resulting DC populations were more than 80% pure as determined by morphology. Contaminating cells were mostly B cells. Although far less potent than DC as antigen-presenting cells, these cells might contribute also to antigen presentation without interfering with the nature of the immune response. The major characteristics of the IL3/IFNβ DC vaccine preparations are given in Table 1. Flow cytometry analysis disclosed a mature phenotype including the expression of high levels of CD80, CD83 and CD86. These cells also produced high amounts of IL-12p70 and IFN-α. These IL3/IFNβ Dc were still able to produce these cytokines upon rechallenge with CD40L (data not shown).This is consistent with previous studies that report that poly(I:C) used for DC maturation preserves their ability to secrete bioactive IL-12 upon rechallenge notably with CD40L [17, 25].
Table 1.
Characteristics of IL3/IFNβ DC injected to patients
| DC enumeration | Immunophenotyping of DC (%) | Cytokine production in DC supernatant (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute number×106 | Purity of DC (%) | CD14 | CD1a | CD80 | CD83 | CD86 | IL12 p70 | IFNα | |
| Mean | 67 | 86 | 91 | 3 | 88 | 56 | 98 | 422 | 432 |
| SD | 37 | 8 | 6 | 1 | 14 | 22 | 8 | 484 | 311 |
Results are from 24 vaccine preparations: 3 vaccines per patient × 8 patients
SD standard deviation
Safety of the IL3/IFNβ dendritic cell vaccine and clinical outcomes
The IL3/IFNβ DC vaccine was well-tolerated with neither serious adverse event, nor grade 3 or 4 adverse reaction, in any of the eight patients. Seven patients developed a grade 1–2 local inflammatory reaction at the injection site (erythema with various degrees of skin infiltration) which faded away after 48 h. Transient grade 1–2 flu-like symptoms and fatigue developed in all patients with fever up to 40°C in one patient within 6 h after vaccine administration; in all cases, symptoms disappeared within 48 h. Neither vitiligo nor other signs of autoimmunity were observed.
Out of the four patients who had measurable disease at the onset of vaccination, three displayed tumor progression after vaccination. One patient remained stable for 9 months with the exception of one small brain metastasis 5 months after inclusion, treated with gamma knife surgery. Out of the four patients with no detectable tumor, one showed tumor progression while the others remained free of disease for >13, >16 and >18 months (table 2).
Table 2.
Characteristics of patients vaccinated
| Patient number | Age/sex | Stage | Previous treatments | Presence of metastases at inclusion | Clinical outcome |
|---|---|---|---|---|---|
| 1 | 36/F | IIIb | S, IFNα | No detectable | NED (>13 months) |
| 2 | 41/M | IIIb | S, RxTh | No detectable | PD |
| 3 | 60/M | IVM1c | S | No detectable | NED (>18 months) |
| 4 | 37/F | IVM1c | S, γK | No detectable | NED (>16 months) |
| 5 | 37/M | IVM1c | S, RxTh | Liver, subcutaneous | PD |
| 6 | 58/M | IVM1c | S | Lung, liver, subcutaneous | PD |
| 7 | 47/M | IVM1c | γK | Lung, subcutaneous | SDa (9 months) |
| 8 | 65/M | IVM1c | S, I, C | Liver | PD |
Abbreviations: NED no evidence of disease, ED evidence of disease= presence of metastases, F female, M male, S surgery; IFNα, interferon-α, RxTh radiotherapy, γK gamma-knife, C chemotherapy, I immunotherapy, PD progressive disease, SD stable disease
aExcept for one new 4 mm brain metastasis 5 months after inclusion, treated by gamma-knife
In vivo migration of IL3/IFNβ dendritic cells
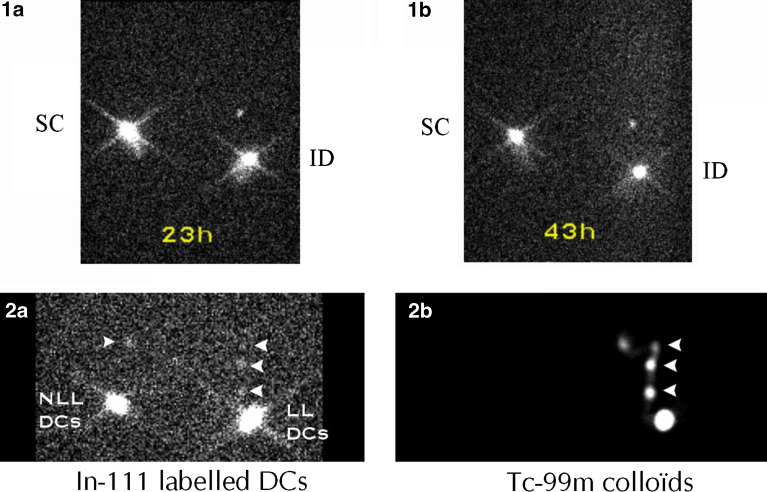
Evidence of IL3/IFNβ DC migration was found after ID, but not after SC injection (Fig. 1a, b). These results are consistent with those published by Morse et al. [19]. Their data indicated that DC trafficking is markedly dependent on their mode of delivery: SC administration seems to be ineffective in causing DC migration to regional lymphatics, IV administration results in DC migration to the spleen, whereas intradermal administration leads to regional transit in some patients.
Fig. 1.
Migratory capacities of IL3/IFNβ DC. 1a, b In-111 labeled autologous IL3/IFNβ DC were injected in the proximal inguinal region of each leg of a patient, either subcutaneously (SC) on one side, or intradermally (ID) on the opposite side. Using a Sopha DSX gamma camera, 1a and 1b images, acquired, 23 h and 43 h post-injection, respectively, showed migration only with ID injection. 2a An image obtained 15.5 h after ID administration of labeled DCs either loaded (LL) with NA17.A2 antigen on one side, or non loaded (NLL) on the opposite side, demonstrated tracer migration towards nodular inguinal sites (arrow heads). 2b The patient was secondarily injected with Tc-99m nanocolloïds on the site of LL DCs injection. The acquisition obtained 30 min later demonstrated that the migration detected on In-111 2a image corresponded to three draining lymph nodes in the inguinal region
Migration after ID injection was documented in six out of the eight patients studied. In patient 1, only a faint uptake in the inguinal region draining the vaccination site could be observed. In the other patients, tracer migration was more apparent as illustrated in Fig. 1. Focal uptake in the inguinal sites draining the sites of labeled DC injection represented 0.56±0.16% (mean ± SD) for loaded (LL) DC, and 0.78±0.23% (mean ± SD) for non-loaded (NLL) DC of the initially administered radioactivity. This is in accordance with previous results that observed that a significant percentage of both immature and mature IL-4/GM-CSF generated DC remained at the site of the injection [7]. However in contrast to them, the maximum intensity of migration estimated by visual analysis of radioactivity was around 24 h post-injection, but that could be explained by the use of a different type of DC.
Blood frequencies of anti-NA17.A2 T cells
Frequencies of precursors of CD8 T cells stained by a tetramer containing the NA17.A2 peptide were evaluated in the blood of the eight patients (Table 3). Pre-vaccination frequencies were evaluated for five patients. In four patients, they were below or about 10−6 of the blood CD8 T cells. We obtained similar frequencies in several other melanoma patients, not included in this study. In two individuals without cancer, we estimated frequencies at 0.1 and 0.5×10−6 of the CD8 cells. Therefore, we believe that these values correspond to the frequency of naïve anti-NA17.A2 T cells. This frequency is similar to that of the naïve CD8 cells against another tumor-specific antigen, peptide MAGE-3168-176presented by HLA-A1 molecules [16]. In patient 4, the pre-vaccination frequency was 4.5×10−6, and a frequency of 7×10−6 was observed in blood collected 9 months earlier. We conclude that this patient had a spontaneous anti-NA17.A2 T cell response prior to vaccination.
Table 3.
Migratory and immunostimulatory properties of DC IL3/IFNβ injected to patients
| Patient number | Status of disease at inclusion | DC migration | Anti-NA17.A2TETp frequency (×10−6 of the blood CD8 cells) | T cell response | |
|---|---|---|---|---|---|
| Before | After | ||||
| 1 | No detectable tumor | + | <0.8 | 9.4 | + |
| 2 | + | <0.7 | 5.7 | + | |
| 3 | − | NT | <0.5 | − | |
| 4 | + | 4.5 | 21 | − | |
| 5 | Presence of metastases | − | NT | <0.7 | − |
| 6 | + | NT | <0.2 | − | |
| 7 | + | 1.1 | 6.1 | + | |
| 8 | + | <0.4 | <0.3 | − | |
Abbreviations: NT not tested, TETp tetramer positive
After vaccination, 4 out of the 8 patients had anti-NA17.A2 TETp at frequencies below 10−6 of the CD8 cells, not higher than what we consider to be naïve frequency. Three patients had frequencies that were ≥5-fold times higher than before vaccination, indicating that they responded to the vaccine. In patient 4, the post-vaccination frequency was also 5-fold higher than before vaccination, but this may correspond to fluctuations of a pre-existing response. We conclude that three out of the eight vaccinated patients mounted an anti-NA17.A2 T cells response that can be detected with our methods. As illustrated in Table 3, all patients with a high anti-NA17 CTL frequency displayed migration of Indium-labeled DC, and had small or no tumor burden at the time of inclusion: three had no detectable metastasis, and one had small lung and subcutaneous metastasis.
Concluding remarks
The results of this pilot trial establish that vaccination of cancer patients with IL3/IFNβ DC represents a safe and valid tool to induce CD8+ T cell responses against tumor-antigens in melanoma patients. Together with previous reports demonstrating the efficiency of similar DC in the induction of anti-HIV responses in SCID mice reconstituted with human lymphocytes [15, 26], our data suggest that DC generated in type I IFN might represent an interesting alternative to DC generated in IL-4 and GM-CSF for the development of more efficient cellular vaccines. Indeed, we found that IL3/IFNβ DC produce higher levels of IFN-α [4] and IL-6 [8], the latter cytokine being able to circumvent suppressive signals elicited by regulatory T cells which jeopardize anti-tumor responses in cancer patients [21, 23]. Since simultaneous immune responses to multiple tumor antigens might be required for efficient therapeutic vaccination in cancer patients, we will soon initiate a randomized trial comparing multipeptide vaccines based on IL3/IFNβ or IL4/GM-CSF DC.
Acknowledgements
We thank Ms. C. Van Helleputte and Ms. E. Thille for their expert technical assistance, and Mrs. D. Duriau and A. Manunta for their help in data management. This work was supported by BruCells SA and the government of the Brussels Region.
References
- 1.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 2.Berzofsky JA, Terabe M, Oh S, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI200421926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blocklet D, Toungouz M, Kiss R, et al. 111In-oxine and 99mTc-HMPAO labelling of antigen-loaded dendritic cells: in vivo imaging and influence on motility and actin content. Eur J Nucl Med Mol Imaging. 2003;30:440–447. doi: 10.1007/s00259-002-1001-4. [DOI] [PubMed] [Google Scholar]
- 4.Buelens C, Bartholome EJ, Amraoui Z, et al. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993–998. doi: 10.1182/blood.V99.3.993. [DOI] [PubMed] [Google Scholar]
- 5.Coulie PG, Karanikas V, Colau D, et al. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA. 2001;98:10290–10295. doi: 10.1073/pnas.161260098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother. 2004;53:275–306. doi: 10.1007/s00262-003-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries IJ, Krooshoop DJ, Scharenborg NM, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 8.Detournay O, Mazouz N, Goldman M, Toungouz M. IL-6 produced by type I IFN DC controls IFN-gamma production by regulating the suppressive effect of CD4+ CD25+ regulatory T cells. Hum Immunol. 2005;66:460–468. doi: 10.1016/j.humimm.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 10.Gjertson DW, Terasaki PI. HLA (1998) American Society for Histocompatibility and Immunogenetics, pp 190–191
- 11.Godelaine D, Carrasco J, Lucas S, et al. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J Immunol. 2003;171:4893–4897. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]
- 12.Guilloux Y, Lucas S, Brichard VG, et al. A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanomas is encoded by an intron sequence of the N-acetylglucosaminyltransferase V gene. J Exp Med. 1996;183:1173–1183. doi: 10.1084/jem.183.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karanikas V, Lurquin C, Colau D, et al. Monoclonal anti-MAGE-3 CTL responses in melanoma patients displaying tumor regression after vaccination with a recombinant canarypox virus. J Immunol. 2003;171:4898–4904. doi: 10.4049/jimmunol.171.9.4898. [DOI] [PubMed] [Google Scholar]
- 15.Lapenta C, Santini SM, Logozzi M, et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198:361–367. doi: 10.1084/jem.20021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonchay C, van der BP, Connerotte T, et al. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci USA. 2004;101(suppl 2):14631–14638. doi: 10.1073/pnas.0405743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 18.Mazouz N, Detournay O, Buelens C et al (2005) Immunostimulatory properties of human dendritic cells generated using IFN-beta associated either with IL-3 or GM-CSF. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed]
- 19.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 20.Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardoll D, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887–892. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 22.Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–3029. doi: 10.1182/blood.V98.10.3022. [DOI] [PubMed] [Google Scholar]
- 23.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 24.Renneson J, Salio M, Mazouz N, Goldman M, Marchant A, Cerundolo V. Mature dendritic cells differentiated in the presence of interferon-beta and interleukin-3 prime functional antigen-specific CD8 T cells. Clin Exp Immunol. 2005;139:468–475. doi: 10.1111/j.1365-2249.2005.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouas R, Lewalle P, El Ouriaghli F, Nowak B, Duvillier H, Martiat P. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int Immunol. 2004;16:767–773. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- 26.Santini SM, Lapenta C, Logozzi M, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurner B, Haendle I, Roder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tosi D, Valenti R, Cova A, et al. Role of cross-talk between IFN-alpha-induced monocyte-derived dendritic cells and NK cells in priming CD8+ T cell responses against human tumor antigens. J Immunol. 2004;172:5363–5370. doi: 10.4049/jimmunol.172.9.5363. [DOI] [PubMed] [Google Scholar]