Abstract
The adoptive transfer of in vitro-induced and expanded tumor-specific cytotoxic T lymphocytes (CTL) presents a promising immunotherapeutic approach for the treatment of cancer. The in vitro induction of tumor-reactive CTL requires repeated stimulation of CTL precursors with dendritic cells (DC). To circumvent problems like scarcity of blood DC precursors and donor variability, it would be attractive to use DC from a non-autologous, unlimited source. DCs derived from the human acute myeloid leukemia (AML) cell line MUTZ-3 are attractive candidates since these DCs closely resemble monocyte-derived DC (MoDC) in terms of phenotype and T cell stimulatory capacity. Here we demonstrate that functional CTL clones could be generated against multiple tumor-associated antigens, i.e., human telomerase reverse transcriptase (hTERT), ErbB3-binding protein-1 (Ebp1), carcinoembryonic antigen (CEA) and Her-2/neu, by stimulating CD8β+ CTL precursors with peptide-loaded allogeneic, HLA-A2-matched MUTZ-3-derived DC. A consistent induction capacity, as determined by MHC tetramer-binding, was found in multiple donors and comparable to autologous peptide-loaded MoDC. Functional characterization at the clonal level revealed the priming of CTL that recognized endogenously processed epitopes on tumor cell lines in an HLA-A2-restricted fashion. Our data indicate that MUTZ-3-derived DC can be used as stimulator cells for in vitro priming and expansion of functional TAA-specific effector CTL. MUTZ-3-derived DCs thus represent a ready and standardized source of allogeneic DC to generate CTL for therapeutic adoptive transfer strategies.
Keywords: Human dendritic cell line, Tumor-associated antigens, Immunization, Immunotherapy, Adoptive T cell transfer
Introduction
The identification and characterization of tumor-associated antigens (TAA) and TAA-derived peptides recognized by cytotoxic T lymphocyte (CTL) have opened new possibilities for immunotherapeutic approaches to treat human cancers. Different strategies can be used, including vaccination with antigenic peptides [26], autologous tumor cells [37], autologous dendritic cells (DC) [21] or the adoptive transfer of in vitro-generated and expanded tumor-specific CTL [7, 16, 25, 41]. The latter approach seems very promising since adoptive therapy strategies are able to circumvent tolerogenic mechanisms that influence the magnitude and avidity of the anti-tumor response by enabling the selection and activation of highly reactive anti-tumor T cell populations ex vivo. Recent clinical successes have been achieved with adoptive transfer of tumor-reactive CTL in combination with nonmyeloablative chemotherapy in metastatic melanoma patients [7].
DCs, which are professional antigen-presenting cells (APCs), have been shown to be very potent in inducing specific CTL both in vitro and in vivo [9, 15, 23, 32, 33]. DCs loaded with antigenic preparations like synthetic peptides, recombinant proteins or tumor lysates are able to successfully induce specific CTL in vitro from low-frequency healthy donor-derived CTL precursors [9, 23, 30, 33]. To induce robust antigen-specific CTL responses in vitro, CTL precursors need to be stimulated with antigen-loaded DC repeatedly. Due to scarcity of blood DC precursors and donor variability in terms of DC differentiation, generating and expanding tumor-specific CTL remains difficult and labor-intensive. To circumvent these problems, it would be preferable to use DC cultured from an unlimited and readily available source.
We recently showed that DC could be cultured from the MUTZ-3 cell line, a CD34+ human acute myeloid leukemia cell line [19]. Upon stimulation with GM-CSF, IL-4 and TNF-α, MUTZ-3 precursor cells acquire a DC morphology and phenotype consistent with conventional myeloid DC, expressing high levels of MHC, co-stimulatory and adhesion molecules. Furthermore, they are capable of antigen processing and presentation via MHC class I and II, resulting in stimulation of specific CD8+ and CD4+ T cells. Given the fact that the MUTZ-3 cell line expresses the highly prevalent HLA class I antigens HLA-A2, HLA-A3, HLA-B44 and HLA-B56 covering 70% of the Caucasian population, DC generated from this cell line might serve as universal stimulators for the generation of effector CTL in vitro.
In vitro priming of T cells for the generation of a tumor-specific response is dependent on the characterization of epitopes that are able to elicit an efficient immune response. In this study, we selected immunogenic HLA-A2-binding epitopes derived from the classical colorectal carcinoma antigen, CEA [34], the pan-carcinoma antigen, hTERT [38], the adenocarcinoma antigen Her-2/neu and the newly identified colorectal carcinoma antigen, Ebp1, which was recently identified by us as an immunogenic protein, capable of eliciting CD8-mediated responses both in vivo and in vitro (Santegoets et al. submitted). Generating tumor-reactive CTL for these adenocarcinoma-associated antigens is technically challenging since these TAAs are also self-proteins, with lower precursor frequencies and preferential outgrowth of low avidity CTL.
Here, we examined the capacity of MUTZ-3-derived DC (hereafter referred to as MUTZ-3 DC) to induce functional tumor-specific CTL from HLA-A2-matched CD8β+ CTL precursors and compared it to the priming capacity of commonly used autologous MoDC. We were able to demonstrate that functional hTERT-, Ebp1-, CEA- and Her-2/neu-specific CTL clones could be generated by stimulating CD8β+ CTL precursors with peptide-loaded allogeneic, HLA-A2-matched MUTZ-3 DC and that the efficiency of this induction was comparable to autologous peptide-loaded MoDC. Our data indicate that MUTZ-3 DCs represent a ready and standardized source of allogeneic DC that can be used as HLA-matched stimulator cells for in vitro priming and expansion of tumor-specific CTL for therapeutic adoptive transfer strategies.
Materials and methods
Cell lines
The CD34+ human acute myeloid leukemia cell line MUTZ-3 (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany) was cultured in MEM-α medium containing ribonucleosides and deoxyribonucleosides (Life Technologies, Paisley, UK) supplemented with 20% Fetal calf serum (Perbio, Helsingborg, Sweden), 100 I.E./ml sodium penicillin (Yamanouchi Pharma, Leiderdorp, The Netherlands), 100 μg/ml streptomycin sulfate (Radiumfarma-Fisiopharma, Naples, Italy), 2.0 mM L-glutamine (Invitrogen, Breda, The Netherlands), 0.01 mM 2-mercapoethanol (Merck, Darmstadt, Germany) and 10% 5637-conditioned medium (CM) [13, 19].
The EBV-transformed B cell line JY and the TAP-deficient cell line T2 (both HLA-A2+) were cultured in IMDM (BioWhittaker, Verviers, Belgium) supplemented with 10% fetal calf serum, 100 I.E./ml sodium penicillin, 100 μg/ml streptomycin sulphate, 2.0 mM L-glutamine and 0.01 mM 2-mercapoethanol (complete medium). The breast cancer cell lines MCF-7, MDA-MB231 (HLA-A2+), colon carcinoma cell lines SW620, SW403 (HLA-A2+), HT-29 (HLA-A2−), gastric carcinoma cell line KATO-3 (HLA-A2+), prostate cancer cell line PC-3 (HLA-A2−) (all from ATCC, Manassas, VA, USA) and melanoma cell line melAKR (HLA-A2+; Netherlands Cancer Institute, Amsterdam, The Netherlands) were all cultured in DMEM (BioWhittaker) complete medium.
Synthetic peptides
The HLA-A2-restricted peptides hTERT540 (ILAKFLHWL) and hTERT988Y (YLQVNSLQTV), CEA571 (YLSGANLNL), Ebp159 (HVDGFIANV) and Her-2/neu 369 (KIFGSLAFL) were synthesized by solid-phase strategies on an automated multiple peptide synthesizer (Syro II, MultiSyntech, Witten, Germany) using Fmoc-chemistry. Peptides were analyzed by reversed-phase high-performance liquid chromatography (HPLC), dissolved in DMSO (Merck) and stored at −20°C.
In vitro generation of monocyte-derived and MUTZ-3-derived DC
MoDCs were generated as described [2]. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood of normal human volunteers by density centrifugation over Lymphoprep (Nycomed AS, Oslo, Norway). PBMCs were allowed for 1–2 h to adhere to the bottom of plastic culture flasks (Nunc, Intermed, Denmark) at 37°C. Non-adherent cells were removed and the adherent cells were cultured for 5–7 days in IMDM complete medium supplemented with 100 ng/ml GM-CSF (Schering-Plough, Madison, NJ, USA) and 1,000 U/ml IL-4 (Strathmann Biotec, Hamburg, Germany).
MUTZ-3 DCs were generated as described [19]. Briefly, MUTZ-3 progenitors were cultured in 12 well tissue culture plates at a concentration of 1×105/ml in MEM-α medium without 5637-CM in the presence of 100 ng/ml GM-CSF, 1,000 U/ml IL-4 and 2.5 ng/ml TNF-α (Strathmann Biotec) for 7 days. Every 3 days new cytokines were added. At day 7, maturation of both MoDC and MUTZ-3 DC was induced by adding MCM mimic, a cytokine cocktail consisting of 50 ng/ml TNF-α, 100 ng/ml IL-6, 25 ng/ml IL-1β (Strathmann Biotec) and 1 μg/ml PGE2 (Sigma).
Antibodies, tetramers and flow cytometry
PE- or FITC-labeled Abs directed against human CD34 (Strathmann Biotec), CD40, CD83, CD8β (Immunotech, Marseille, France), CD1a, CD8α, CD14, CD27, CD28, CD45RO, CD45RA, CD80, CD86, CCR7, DC-SIGN, TCRαβ, TCRγδ (all from BD Biosciences, Mountain view, CA, USA) and APC-labeled-anti-ΔNGFR (Chromoprobe, Aptos, CA, USA) were used for flow cytometric analysis. PE- and/or APC-labeled HLA-A2 tetramers (Tm) presenting the hTERT988Y, hTERT540, Ebp159, Her-2/neu 369, and CEA571 epitopes were prepared as described previously [11]. Antibody and/or tetramer staining was performed in PBS supplemented with 0.1% BSA and 0.02% natrium-azide for 30 min at 4°C and 15 min at 37°C, respectively. Stained cells were analyzed on a FACScalibur (BD Biosciences) using Cell Quest software. To exclude dead cells in flow cytometric tetramer analysis, 0.5 μg/ml propidium iodide (ICN Biomedicals, Zoetermeer, The Netherlands) was used. mAb- and tetramer-guided flow sorting was performed on a FACStarPlus (BD Biosciences) using CellQuest software.
Primary CTL induction in vitro
Antigen-specific CTLs were generated as described [30]. Briefly, CD8β+ CTL precursors were isolated from PBMC of HLA-A2+ healthy donors and a prostate cancer patient by positive selection on an automated magnetic cell sorting (MACS) device (autoMACS; Miltenyi Biotec, Bergisch Gladbach, Germany). For this purpose, total PBMCs were stained with unlabeled anti-CD8β mAb (Immunotech) and microbead-conjugated anti-mouse IgG Abs (Miltenyi Biotec). Mature MoDC and MUTZ-3 DCs, prepared as described above, were loaded with 25 μg/ml peptide in the presence of 3 μg/ml β2-microglobulin (Sigma-Aldrich, St. Louise, MO, USA) for 2–4 h at room temperature and irradiated (40 Gy). 1×105 peptide-loaded DCs were cultured for 10 days with 1×106 CD8β+ CTL precursors and 1×106 irradiated (80 Gy) CD8β− autologous PBMC in Yssel’s medium [43] supplemented with 1% hAB serum (ICN Biochemicals), 10 ng/ml IL-6 and 10 ng/ml IL-12 in a 24 well tissue-culture plate. At day 1, 10 ng/ml IL-10 (R&D Systems) was added. From day 10, CTL cultures were stimulated every week for 5 weeks with 1×105 fresh peptide-loaded DCs in the presence of 5 ng/ml IL-7 (Strathmann Biotec). Two days after each restimulation, 10 U/ml IL-2 (Strathmann Biotec) was added. One day prior to each restimulation, a sample was taken and analyzed by flow cytometry using both PE- and APC-labeled tetramers presenting the relevant epitope. Tetramer-positive CTL were isolated by CD8+/tetramer+ flow sorting and subsequently cloned by limiting dilution [43]. For this purpose, CTLs were weekly stimulated with irradiated feeder-mix consisting of allogeneic PBMC and JY cells in Yssel’s medium supplemented with 100 ng/ml phytohemagglutin (PHA; Murex Biotech, Dartford, UK) and 20 U/ml IL-2. To allow extensive characterization of the generated CEA571-specific CTL clones, the clones were rescued from replicative senescence by introducing human telomerase reverse transcriptase (hTERT) as described elsewhere [12]. Briefly CEA571-specific CTLs, stimulated for 48 h with feeder-mix as described above, were transduced with retrovirus encoding LZRS-hTERT-IRES-ΔNGFR, in fibronectin-coated plates (Retronectin, Takara, Japan) in the presence of 100 U/ml IL-2. During transduction, the plates were centrifuged at 2,000×g for 90 min at 23°C and subsequently incubated at 37°C. After 4,5 h, cells were washed and cultured overnight in Yssel’s medium containing 20 U/ml IL-2. Next day, retroviral transduction was repeated. After 48 h, transduction efficiency was checked by flow cytometric analysis of nerve growth factor receptor (ΔNGFR)-marker gene expression. Subsequently, ΔNGFR+ CTLs were positively selected by MACS and cloned by limiting dilution as described.
Intracellular IFN-γ detection
To determine the capacity of CTL clones to produce IFN-γ upon recognition of a specific target, intracellular IFN-γ staining was performed. Target cells used included HLA-A2+/CEA+, HLA-A2+/Ebp1+, HLA-A2−/CEA+ or HLA-A2−/Ebp1+ tumor cell lines and JY cells or T2 cells pulsed with either relevant or irrelevant peptide. CTLs were cultured with target cells at an effector:target cell (E:T) ratio of 2:1 in 96-well round-bottom plate. One hour after the start of stimulation, 0.5 μl of GolgiPlug (BD Biosciences) was added to each well. After 6 h, cells were harvested, washed, stained with APC-labeled tetramer and PE-labeled anti-CD8 mAb. After fixation with 4% paraformaldehyde (Merck) and permeabilization with 1×BD Perm/wash solution (BD Biosciences), cells were labeled with FITC-conjugated anti-IFN-γ Ab (BD Biosciences). Stained cells were analyzed on a FACScalibur.
Chromium release assay
Cytotoxic activity of CTL clones was determined by standard chromium release assay as described [30].
CD107a membrane expression
Cytotoxic potential was determined by a flow cytometric degranulation assay as described [3, 27]. In this assay, the potential for granule-dependent perforin/granzyme-mediated target cell killing was determined. As a marker for degranulation, the cumulative exposure of granular membrane protein CD107a (also known as lysosomal-associated membrane protein-1 (LAMP-1)) on the cell surface of a responding antigen-specific T cell was measured by flow cytometry. For this purpose, CEA571- or Her-2/neu 369-specific CTLs were stimulated with various target cells for 5 h at 37°C in a 1:1 ratio in the presence of anti-CD107a-PE (BD Biosciences) and 4 μM monensin (Sigma). Following the stimulation, cells were washed, stained with APC-labeled tetramer and FITC-labeled anti-CD8 mAb, respectively, and analyzed on a FACScalibur.
Adenoviral infection
Replication-deficient recombinant type-5 adenoviruses (with deletion of the E1 region), encoding green fluorescent protein (GFP) and CEA were used (kindly provided by Dr. Nikolay Korokhov, Vectorlogics Inc., Birmingham, AL, USA). MelAKR cells were incubated with the Ad5GFP-CEA at a multiplicity of infection (MOIvp) of 1,000, in 100 μl of serum-free DMEM medium, at 37°C for 1 h, after which the cells were washed and cultured overnight in complete DMEM medium with 10% FCS.
Results
Characterization of mature MoDC and MUTZ-3 DC
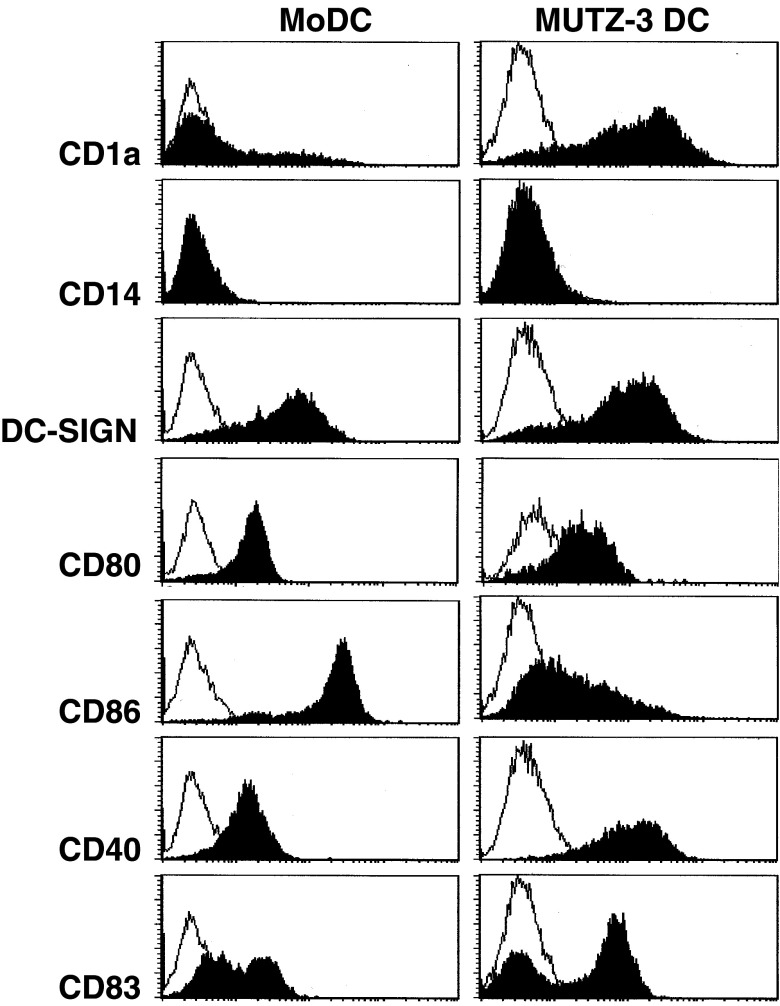
Like DCs cultured from monocytes (MoDC) or CD34+ bone marrow-derived precursors (CD34-derived DC), DCs cultured from the human cytokine-dependent myeloid cell line MUTZ-3 (MUTZ-3 DC) exhibit true DC morphology and characteristics. After differentiation in the presence of GM-CSF, IL-4 and TNF-α and maturation with a cytokine cocktail containing TNF-α, IL-6, IL-1β and PGE2, MUTZ-3 DC expressed intermediate to high levels of the co-stimulatory molecules CD80, CD86 and CD40, and of DC-specific molecules CD1a, DC-SIGN and CD83 (Fig. 1). Thus, MUTZ-3 DCs phenotypically resemble MoDC (see Fig. 1) and CD34-derived DC, as previously described [19].
Fig. 1.
Phenotypic characterization of mature MoDC and MUTZ-3-derived DC. DCs were stained with FITC and/or PE-conjugated antibodies against CD1a, CD14, CD86, CD40, DC-SIGN and CD83 and analyzed by flow cytometry. Open histograms isotype-matched controls; closed histograms the marker as indicated to the left
Induction of hTERT988Y-specific CD8+ T cells in vitro using peptide-pulsed autologous MoDC or allogeneic HLA-A2-matched MUTZ-3 DC
DCs have the unique ability to induce and activate tumor-specific CTLs both in vivo and in vitro. In order to determine whether MUTZ-3 DC can be used to generate primary tumor-specific CTL in vitro and whether their priming efficiency is comparable to that of MoDC, both autologous MoDC and allogeneic MUTZ-3 DC were used as stimulator cells in an in vitro CTL induction protocol. To this end, CD8β+ CTL precursors isolated from PBMC of HLA-A2+ healthy donors were stimulated weekly with either peptide-pulsed autologous MoDC or allogeneic but HLA-A2-matched MUTZ-3 DC in 12 parallel cultures at 1×106 CD8β+ CTL precursors per culture. The epitopes selected were the previously described HLA-A2-restricted hTERT-derived peptides, hTERT540 and/or the P1Y heteroclitic variant of hTERT988 (hTERT988Y), in which aspartic acid (D) at position 988 is substituted for tyrosine (Y) for increased binding affinity for HLA-A2 [29, 38]. From the second round of stimulation onwards, T cell cultures were monitored for the expansion of hTERT-specific CD8+ T cells through HLA-A2-tetramer binding (see Fig. 2). Table 1 shows the capacity of both MoDC and MUTZ-3 DC to induce hTERT-specific CD8+ T cells, as tested in two individual HLA-A2+ healthy donors by tetramer binding. Comparable T cell priming efficiencies were observed against the hTERT988Y epitope with allogeneic HLA-A2-matched MUTZ-3 DC and fully HLA-matched autologous MoDC. Of note, both MoDC and MUTZ-3 DC were equally ineffective in generating hTERT540-specific CD8+ T cells (Table 1). The highest achieved percentage of Tm-hTERT+988Y CD8+ T cells using MoDC and MUTZ-3 DC was comparable, varying between 0.15 and 3.5% for the different inductions, and was reached at various stimulation rounds (see Table 1). No difference was observed in the number of in vitro stimulations (IVS) needed to detect the first hTERT988Y-specific CD8+ T cells by tetramer analysis; both MoDC- and MUTZ-3 DC-induced hTERT988Y-specific CD8+ T cells could already be detected as early as round two or three of stimulation (Table 1). In conclusion, equivalent T cell priming efficiencies (i.e., induction rates) were observed for autologous MoDC and allogeneic HLA-A2-matched MUTZ-3 DC.
Fig. 2.
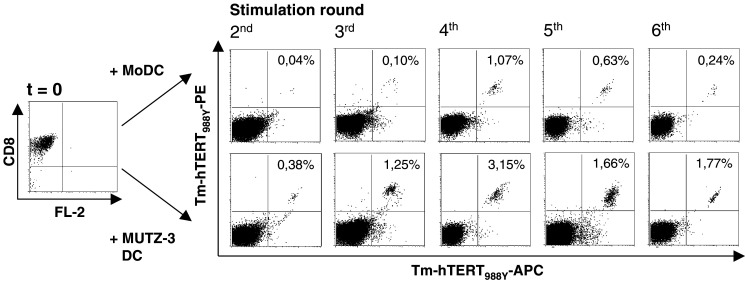
Flow cytometric HLA-A2+ tetramer (Tm)-binding analysis of CD8β+ CTL precursors stimulated with mature hTERT988Y-loaded MoDC or MUTZ-3 DC. From day 0 (t=0) magnetic bead-isolated CD8+ CTL precursors were stimulated repeatedly with either peptide-pulsed autologous MoDC or HLA-A2-matched MUTZ-3 DC. Flow cytometric PE-/APC-labeled tetramer analysis on CD8β+ CTL precursors was performed on day 7 after second, third, fourth, fifth and sixth stimulation cycles. Staining with both PE- and APC-labeled Tm was performed to exclude false positive staining by single Tm. Results for live, propidium iodide-negative cells are shown. Percentages Tm-hTERT988Y-PE+/Tm-hTERT988Y-APC+ CD8+ T cells are shown in the upper right quadrants
Table 1.
MoDC- versus MUTZ-3 DC-mediated induction of hTERT-specific CD8+ T cells
| Donor | Antigen | Epitope | MoDC | MUTZ-3 DC | ||||
|---|---|---|---|---|---|---|---|---|
| Induction efficiencya | Earliest Tm+ frequency (%) (number of IVS )b | Max. Tm+ T cell frequency (%) (number of IVS)c | Induction efficiencya | Earliest Tm+ frequency (%) (number of IVS)b | Max. Tm+ T cell frequency (%) (number of IVS)c | |||
| 1 | hTERT | 988Y | 2/12 | 0.13 (3) | 0.80 (4) | 2/12 | 0.26 (2) | 0.40 (3) |
| 1.30 (3) | 3.50 (4) | 0.10 (3) | 2.10 (6) | |||||
| 540 | 0/12 | NA | NA | 0/12 | NA | NA | ||
| 2 | hTERT | 988Y | 5/12 | 0.17 (2) | 0.25 (4) | 3/12 | 0.22 (3) | 0.72 (4) |
| 0.29 (2) | 0.29 (2) | 0.10 (2) | 1.50 (5) | |||||
| 0.10 (3) | 0.15 (4) | 0.38 (2) | 3.15 (4) | |||||
| 0.18 (1) | 0.48 (2) | |||||||
| 0.10 (3) | 1.07 (4) | |||||||
aDetermined by flow cytometric HLA-A2+ tetramer analysis and expressed as proportion of positively responding bulk cultures. For each peptide, 12 parallel CD8β+ T cell cultures were started. Cultures were scored positive when percentage of tetramer-(Tm) positive cells >0.1% of live cells
bEarliest frequencies listed are first observed tetramer-positive cells for each individual culture detected after indicated number of in vitro stimulations (IVS) in parentheses
cMaximum frequencies listed are highest achieved percentage of tetramer-positive cells for each individual culture detected after indicated number of in vitro stimulations (IVS) in parentheses
NA not applicable
Induction of antigen-specific, HLA-A2-restricted CD8+ T cells directed against different tumor-associated epitopes by allogeneic MUTZ-3 DC in vitro
To further establish the MUTZ-3 DCs’ utility as universal HLA-A2+ stimulator cells for in vitro priming and expansion of CTL against different TAA across a range of allogeneic backgrounds, we included the immunodominant HLA-A2-restricted CEA-derived peptide CEA571, also known as CAP-1 [34] and the recently identified, HLA-A2-restricted Ebp1-derived peptide Ebp159 (Santegoets et al. submitted). hTERT540-specific CD8+ T cells could be generated in 1/12 individual cultures from one donor tested, while hTERT988Y-specific CD8+ T cells could be generated in 6/36 individual cultures from two out of three donors tested. Furthermore, detectable Ebp159-specific CD8+ T cells were generated in 9/24 individual cultures from two donors (2/2 donors positive), while CEA571-specific CD8+ T cells could be induced in 6/48 individual cultures from four different donors (4/4 donors positive) (Table 2). Frequency of MUTZ-3 DC-induced tetramer-positive T cells was shown to be variable, ranging between 0.15 and 4.12%. These findings are summarized in Table 2. Importantly, tumor peptide-reactive CD8+ T cells could be primed by MUTZ-3 DC in all donors tested. These data demonstrate that MUTZ-3 DC-mediated and HLA-A2-restricted T cell priming is reproducible for different peptides in multiple donors against a variety of allogeneic MHC backgrounds.
Table 2.
MUTZ-3 DC-mediated induction of CEA-, hTERT- and Ebp1-specific CD8+ T cells
| Donor | Antigen | MUTZ-3 DC | |
|---|---|---|---|
| Induction efficiencya | Max. Tm+ T cell frequency (%) (number of IVS)b | ||
| 3 | hTERT988Y | 2/12 | 0.41 (4) |
| 2.89 (5) | |||
| hTERT540 | 1/12 | 0.25 (5) | |
| 4 | hTERT988Y | 4/12 | 0.24 (3) |
| 0.56 (3) | |||
| 0.44 (3) | |||
| 4.12 (5) | |||
| CEA571 | 1/12 | 0.15 (7) | |
| Ebp159 | 5/12 | 1.82 (3) | |
| 0.54 (3) | |||
| 0.79 (6) | |||
| 0.61 (3) | |||
| 0.12 (3) | |||
| 5 | hTERT988Y | 0/12 | NA |
| CEA571 | 1/12 | 0.78 (7) | |
| 6 | CEA571 | 2/12 | 0.55 (2) |
| 0.57 (5) | |||
| Ebp159 | 4/12 | 0.28 (5) | |
| 0.89 (5) | |||
| 1.49 (5) | |||
| 2.66 (5) | |||
| 7 | CEA571 | 2/12 | 0.26 (7) |
| 1.36 (7) | |||
aDetermined by flow cytometric HLA-A2+ tetramer analysis and expressed as proportion of positively responding bulk cultures. For each peptide, 12 parallel CD8β+ T cell cultures were started. Cultures were scored positive when percentage of tetramer-positive cells >0.1% of live cells
bMaximum frequencies listed are highest achieved percentage of tetramer-positive cells for each individual culture detected after indicated number of in vitro stimulations (IVS) in parentheses
NA not applicable
Isolation, expansion and characterization of functional CD8+ T clones
To further characterize the MUTZ-3 DC-generated CD8+ T cells in terms of phenotype and cytotoxic potential, MUTZ-3 DC-induced CEA571- and Ebp159-specific CD8+ T cells were isolated and cloned. Tetramer+CD8+ T cells in the priming cultures tended to reach a maximum percentage after varying numbers of restimulation cycles, after which their numbers waned (see Fig. 2). This overgrowth by non-specific clones most likely signals a decreased proliferative potential of the antigen-specific effector T cells. To optimize the chances of successful cloning and expansion, specific tetramer+ T cells were therefore isolated before this point was reached. CEA571- and Ebp159-specific CD8+ T cells were isolated by CD8/tetramer-guided flow sorting from individual cultures containing 0.17% and 0.44% Tm+ T cells, respectively (not shown). After cloning by limiting dilution and further expansion, CEA571- and Ebp159-specific T cell cultures of up to 95 and 70% pure, respectively, could be isolated and expanded (Figs. 3a, 4a).
Fig. 3.
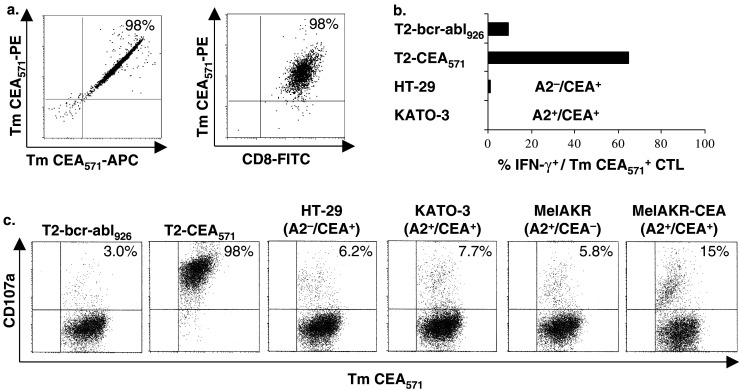
Flow cytometric tetramer-binding analysis and functional analysis of CEA571-specific CTL clones. CEA571-specific CD8+ T cells were generated by repeated stimulation of CD8β+ CTL precursors with peptide-pulsed MUTZ-3 DC. a Flow cytometric PE-/APC-labeled tetramer (Tm)-binding analysis and PE-labeled Tm/FITC-CD8 staining of CEA571-specific CD8+ T cells after Tm CEA571/CD8-guided FACSsort and limiting dilution cloning. Lytic activity of CEA571-specific CD8+ T cell clones were analyzed by b intracellular IFN-γ staining and c flow cytometric degranulation assay. Data shown are representative of two separately tested clones. Target cells used were HLA-A2+ JY or T2 cells loaded with the CEA571 peptide or control HLA-A2-restricted peptides Flu-M158 or Bcr-abl926, CEA expressing cell lines KATO-3 and the HT-29, wild-type CEA−/HLA-A2+ MelAKR and AdGFP-CEA-transduced MelAKR. Percentage IFN-γ+ cells and CD107a+ cells shown were gated for live tetramer-CEA+571/CD8+ T cells
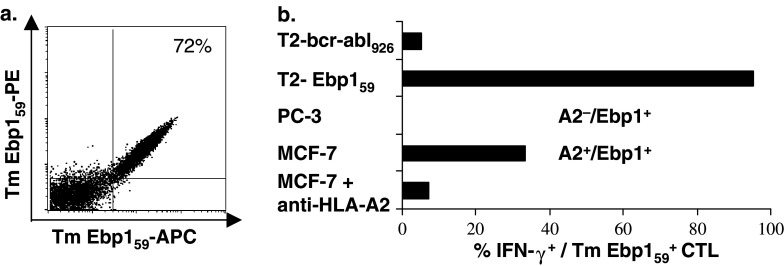
Fig. 4.
Flow cytometric tetramer-binding analysis and functional analysis of an Ebp159-specific CTL clone. Ebp159-specific CD8+ T cells were generated by repeated stimulation of CD8β+ CTL precursors with peptide-pulsed MUTZ-3 DC. a Flow cytometric PE-/APC-labeled tetramer (Tm)-binding analysis of an Ebp159-specific CD8+ T cell line after Tm Ebp159/CD8-guided FACSsort and limiting dilution cloning. b Functional activity of the Ebp159-specific CD8+ T cell line was determined by intracellular IFN-γ staining. Target cells used were HLA-A2+ T2 cells loaded with the Ebp159 peptide or control HLA-A2-restricted peptide bcr-abl926, Ebp1+/HLA-A2− cell line PC-3 and Ebp1+/HLA-A2+ cell line MCF-7, with or without anti-HLA-A2 neutralizing antibody BB7.2. Percentage IFN-γ+ cells shown was gated for live tetramer-Ebp1+59/CD8+ T cells
After isolation and expansion, CEA571- and Ebp159-specific T cell clones were used for phenotypic and functional analysis. Flow cytometric analysis revealed that the generated CD8+ T cells could be defined as effector memory T cells, according to the CD8+CD27−CD28−CD45RA+ CD45RO+/−CCR7−TCRαβ+ phenotype (Table 3) [10, 28].
Table 3.
Phenotype of CEA571-specific CD8+ T cells
| Antigen | Expression* | |
|---|---|---|
| Clone 5#1 | Clone 6#1 | |
| CD8α | 99.1 | 98.4 |
| CD8β | 99.4 | 97.9 |
| CD27 | 0 | 0 |
| CD28 | 1.0 | 0.7 |
| CD45RO | 13.2 | 43.8 |
| CD45RA | 97 | 96.4 |
| TCRαβa | 61.6 | 57.9 |
| TCRγδ | 0 | 0 |
| CCR7 | 0 | 0 |
*Indicated as percentage positive cells, determined by flow cytometry
aTCR expression varies with time after stimulation and increases to 100% ≥7 days after stimulation
Functional activity of the generated T cell clones was determined by IFN-γ production assay, CD107a flow cytometric degranulation assay and standard chromium release assay. IFN-γ production assays showed that the obtained CEA571-specific CD8+ T cell clones were able to recognize the exogenously loaded CEA571 epitope, but not its endogenously expressed counterpart in the HLA-A2+/CEA+ gastric carcinoma cell line KATO-3 (Fig. 3b), nor in the breast or coloncarcinoma lines MCF-7, SW-620 or SW-403 (data not shown). Similarly, in a chromium release assay, CEA571-specific CD8+ T cells were able to specifically lyse peptide-loaded JY target cells, but not the HLA-A2+/CEA+ KATO-3 cells unless exogenously loaded with CEA571 peptide, excluding intrinsic apoptosis resistance or defective HLA expression as possible causes of the observed lack of CTL-induced lysis (data not shown). Functional avidity analysis, based on cytolysis of JY targets loaded with titered peptide, revealed the CEA571-specific CD8+ T cell clones to exhibit low avidity antigen recognition (half maximum lysis at 500 nM, data not shown). We also assessed tumor cell recognition in the high-sensitivity CD107a degranulation assay [3, 27]. Again, only exogenously loaded and not endogenously expressed CEA571 epitope on target cells was recognized by the CEA571-specific CTL clones, unless over-expression of CEA was induced by high-efficiency adenoviral transduction (Fig. 3c). Transduction efficiency of the HLA-A2+/CEA− melanoma cell line MelAKR was 100% as assessed by GFP transgene expression upon infection with an adenovirus encoding both CEA and GFP (data not shown). These data indicate that despite low avidity antigen recognition, CEA571-specific CTL generated by MUTZ-3 DC are able to detect endogenously processed and presented CEA571 peptide.
Functionality of the Ebp159-specific CD8+ T cell line was determined by IFN-γ production. As shown in Fig. 4b, the Ebp159-specific CTL line was able to recognize both exogenously loaded target cells and HLA-A2+ tumor cells expressing endogenous Ebp1. The observation that the recognition of HLA-A2+/Ebp1+ tumor cells could be blocked almost completely by incubating the target cells with an anti-HLA-A2 antibody, confirmed that the recognition of these tumor cells is HLA-A2 restricted and not mediated by NK cells.
To demonstrate the feasibility of generating high avidity cytolytic T cell clones from cancer patients, we stimulated CD8β+ CTL precursors obtained from a prostate cancer patient with MUTZ-3 DC loaded with low peptide concentrations (1 μg/ml for the first stimulation and 10 ng/ml thereafter). In doing so, we were able to generate high avidity Her-2/neu 369-specific CTL (up to 95% pure, Fig. 5a), with half maximal CD107a translocation at 1 pM peptide as analyzed by avidity analysis on HLA-A2+ JY targets cells loaded with tenfold dilutions of the Her-2/neu 369 peptide (data not shown). This CTL clone was indeed able to specifically recognize and lyse the HLA-A2+/Her-2/neu + tumor cell line SW620 (Fig. 5b). Importantly, the recognition of this tumor cell line was as effective as the recognition of the exogenously loaded JY target cells. In conclusion, employing allogeneic HLA-A2-matched MUTZ-3 DC, tumor peptide-specific CTL clones with endogenously expressed epitope and tumor recognition capabilities could be generated.
Fig. 5.
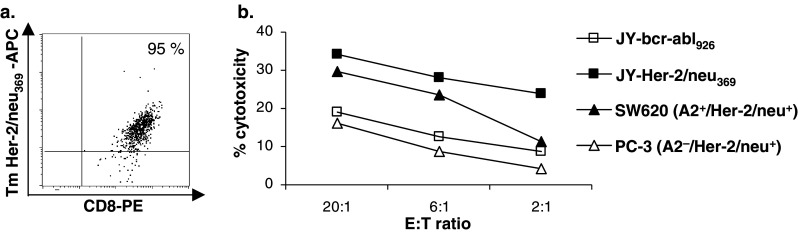
Flow cytometric tetramer-binding analysis and functional analysis of a prostate cancer patient-derived Her-2/neu 369-specific CTL clone. Her-2/neu 369-specific CD8+ T cells were generated by repeated stimulation of CD8β+ CTL precursors with peptide-pulsed MUTZ-3 DC. a Flow cytometric APC-labeled Tm/PE-labeled CD8 staining of an Her-2/neu 369-specific CD8+ T cell line after Tm Her-2/neu 369/CD8-guided FACSsort and limiting dilution cloning. Results for live, propidium iodide-negative cells are shown. Percentage Tm Her-2/neu 369-APC+/CD8-PE+ T cells is shown in the upper right quadrants. b Functional activity of the Her-2/neu 369-specific CD8+ T cell clone was determined by chromium release assay. Target cells used were HLA-A2+ JY cells loaded with the Her-2/neu 369 peptide or control HLA-A2-restricted peptide bcr-abl926, the Her-2/neu +/HLA-A2− cell line PC-3 and the Her-2/neu +/HLA-A2+ cell line SW620
Discussion
The adoptive transfer of in vitro-induced and expanded tumor-specific CTL presents a promising immunotherapeutic approach for the treatment of cancer. Recently, objective clinical responses were observed in refractory metastatic melanoma patients receiving in vitro-generated and expanded tumor-reactive T cells and IL-2 infusions, either with or without immunodepleting chemotherapy [7, 41]. Tumor-infiltrating lymphocytes (TILs) are considered to be an appropriate source of tumor-reactive T cells, as they recognize a variety of TAA. However, since TILs are often not available due to lack of accessible tumors, it would be useful to generate tumor-reactive CTL from peripheral blood-derived CTL precursors. Due to low precursor frequencies in the blood, repeated IVS with APC is needed to generate sufficient numbers of tumor-reactive CTL. DCs are preferably used for these stimulations since they have been shown to be very potent inducers of specific CTL both in vitro and in vivo [9, 23, 33]. In this study, we report on the applicability of MUTZ-3 DC as stimulator cells for the generation and expansion of TAA-specific effector CTL for adoptive transfer in a clinical setting.
It is well known that the mature MoDCs are capable of inducing functional tumor-specific CTL in vitro [9, 30, 33]. Here we show that allogeneic HLA-A2-matched MUTZ-3 DC can similarly induce tumor antigen-specific CD8+ T cells. Importantly, T cell priming efficiencies were shown to be equivalent in terms of induction rate for allogeneic MUTZ-3 DC and autologous MoDC. From previous studies in our lab employing the same priming method with autologous MoDC [30, 31], we know that tumor antigen-specific CD8+ T cells with similar functional avidity (i.e., low to intermediate) were induced as described here for MUTZ-3 DC. Our observation that both MoDC and MUTZ-3 DC induce tumor antigen-specific CD8+ T cells with comparable efficiency and avidity indicates that there is no interference of allogeneic CTL responses against mismatched HLA antigens with the CTL response against the selected antigen. This is in line with findings from other groups, observing no or low-level allogeneic CTL response when priming with either allogeneic melanoma cell lines or peptide-pulsed allogeneic DC [5, 24]. Overall, these results indicate that allogeneic MUTZ-3 DC can be used to induce TAA-specific CD8+ T cells from low frequency CTL precursors directed against various TAAs. Importantly, these tumor-specific CD8+ T cells were generated in all donors tested, independent of HLA background.
A requirement for achieving effective adoptive T cell therapy is the ability to generate tumor-reactive CTLs capable of recognizing tumor cells expressing the antigen of interest. Functional characterization of MUTZ-3 DC-induced and expanded Ebp159- and CEA571-specific CD8+ T cells revealed that the Ebp159-specific CD8+ T cells were able to recognize both exogenously loaded target cells and HLA-A2+ tumor cells expressing endogenous Ebp1, whereas the primed and expanded CEA571-specific CD8+ T cells were only able to recognize target cells exogenously loaded with CEA571 peptide. The fact that we generated CEA571-specific CD8+ T cells that are of low avidity may account for the observation that endogenously processed and presented CEA571 epitope could only be detected on tumor cells transduced with adenoviral vectors to induce high levels of the CEA protein, but not on wild-type tumor cells expressing physiological levels of CEA.
The selection of low avidity CEA571-specific CD8+ T cells in the current study can be explained by the possible absence and/or unresponsiveness of high and intermediate avidity CEA-specific CTL. This is most likely due to negative selection in the thymus and/or the induction of peripheral tolerance [14, 36]. Furthermore, as reported previously [1, 4, 39] and currently appreciated as a well-known phenomenon, the repetitive stimulation of antigen-specific CTL with APC expressing high densities of peptide/MHC complexes favors low avidity CTL outgrowth. Consequently, the induction of low avidity CEA571-specific CD8+ T cells in this study is most likely due to the selected antigen and the priming method rather than to the use of MUTZ-3 DC. Indeed, we were able to select tumor-reactive CTL directed against Her-2/neu and Ebp1, another self-protein which is known to be involved in multiple signal transduction pathways and cellular proliferation and differentiation processes [40, 42, Santegoets et al. submitted].
In addition, from previous studies it has become clear that generating tumor-reactive CEA571-specific CTL from normal individuals remains difficult. Most CEA571-specific CTL reported originate from CEA vaccinated cancer patients, in which CEA-specific T cell responses may have been potentiated and possible tolerance may have been broken [34, 35, 45]. Moreover, most of those tumor-reactive CEA571-specific CTL reported previously were not CD8+ CTL clones as we describe here, but CD8 and CD4 containing bulk T cell lines. Nevertheless, it has been shown possible to generate CEA571-specific tumor-reactive CTL in vitro from normal individuals [17].
Although it is well appreciated that high avidity CTLs show superior anti-tumor activity in vivo [1, 6, 44], it has been demonstrated that low avidity CTLs can also mediate tumor rejection in vivo [18, 20]. For future clinical application, it would be preferable to induce tumor-reactive CTL with a broad range of avidities. We are therefore currently optimizing our CTL induction protocol in order to generate higher avidity tumor-specific CTL displaying anti-tumor activity at lower TAA-expression levels. Employing MUTZ-3 DC loaded with lower concentrations of Her-2/neu-derived HLA-A2-binding peptides, we are indeed able to generate higher avidity effector CTL from cancer patient-derived CTL precursors with the ability to recognize and kill tumor cells. In addition, for future clinical application we are developing methods to generate MUTZ-3 DC under serum-free GMP conditions [8, 22].
In summary, our data clearly show that MUTZ-3 DC can be used to induce tumor-reactive CTL clones in vitro. MUTZ-3 DCs thus represent a ready and standardized source of allogeneic HLA-matched DC capable of generating and expanding functional TAA-specific effector CTL for therapeutic adoptive transfer strategies. Moreover, the capacity of MUTZ-3 DC to induce antigen-specific CTL against a variety of HLA backgrounds also suggests its possible utility as DC vaccine in in vivo cancer immunotherapy.
Acknowledgments
The authors wish to thank NEMOD Biotherapeutics for their financial support and the Maurits & Anna de Kock Foundation for financial support in the purchase of an HPLC.
Abbreviations
- APC
Antigen-presenting cell
- CEA
Carcinoembryonic antigen
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cell
- GFP
Green fluorescent protein
- hTERT
Human telomerase reverse transcriptase
- MoDC
Monocyte-derived dendritic cell
- ΔNGFR
Truncated form of nerve growth factor receptor
- PBMC
Peripheral blood mononuclear cell
- PHA
Phytohemagglutin
- TIL
Tumor-infiltrating lymphocyte
- Tm
Tetramer
References
- 1.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender A, Sapp M, Schuler G, et al. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 3.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 4.Carbone FR, Moore MW, Sheil JM, et al. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988;167:1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley NJ, Slingluff CL, Jr, Darrow TL, et al. Generation of human autologous melanoma-specific cytotoxic T-cells using HLA-A2-matched allogeneic melanomas. Cancer Res. 1990;50:492–498. [PubMed] [Google Scholar]
- 6.Dudley ME, Nishimura MI, Holt AK, et al. Antitumor immunization with a minimal peptide epitope (G9-209-2M) leads to a functionally heterogeneous CTL response. J Immunother. 1999;22:288–298. doi: 10.1097/00002371-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figdor CG, de Vries IJ, Lesterhuis WJ, et al. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 9.Fonteneau JF, Larsson M, Somersan S, et al. Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J Immunol Methods. 2001;258:111–126. doi: 10.1016/S0022-1759(01)00477-X. [DOI] [PubMed] [Google Scholar]
- 10.Hamann D, Roos MT, van Lier RA. Faces and phases of human CD8 T-cell development. Immunol Today. 1999;20:177–180. doi: 10.1016/S0167-5699(99)01444-9. [DOI] [PubMed] [Google Scholar]
- 11.Heemskerk MH, Hooijberg E, Ruizendaal JJ, et al. Enrichment of an antigen-specific T cell response by retrovirally transduced human dendritic cells. Cell Immunol. 1999;195:10–17. doi: 10.1006/cimm.1999.1520. [DOI] [PubMed] [Google Scholar]
- 12.Hooijberg E, Ruizendaal JJ, Snijders PJ, et al. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–4245. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 13.Hu ZB, Ma W, Zaborski M, et al. Establishment and characterization of two novel cytokine-responsive acute myeloid and monocytic leukemia cell lines, MUTZ-2 and MUTZ-3. Leukemia. 1996;10:1025–1040. [PubMed] [Google Scholar]
- 14.Jameson SC, Hogquist Bevan KA MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 15.Jochmus I, Osen W, Altmann A, et al. Specificity of human cytotoxic T lymphocytes induced by a human papillomavirus type 16 E7-derived peptide. J Gen Virol. 1997;78(Pt 7):1689–1695. doi: 10.1099/0022-1317-78-7-1689. [DOI] [PubMed] [Google Scholar]
- 16.Kast WM, Offringa R, Peters PJ, et al. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989;59:603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima I, Hudson SJ, Tsai V, et al. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum Immunol. 1998;59:1–14. doi: 10.1016/S0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 18.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34:752–761. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 19.Masterson AJ, Sombroek CC, De Gruijl TD, et al. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 2002;100:701–703. doi: 10.1182/blood.V100.2.701. [DOI] [PubMed] [Google Scholar]
- 20.Morgan DJ, Kreuwel HT, Fleck S, et al. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 21.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 22.Nestle FO, Banchereau J, Hart D. Dendritic cells: on the move from bench to bedside. Nat Med. 2001;7:761–765. doi: 10.1038/89863. [DOI] [PubMed] [Google Scholar]
- 23.Oelke M, Moehrle U, Chen JL, et al. Generation and purification of CD8+ melan-A-specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin Cancer Res. 2000;6:1997–2005. [PubMed] [Google Scholar]
- 24.Peiper M, Goedegebuure PS, Alldinger I, et al. Comparison of various sources of antigen-presenting cells for the generation of GP2-tumor peptide specific cytotoxic T-lymphocytes. Anticancer Res. 2002;22:3357–3363. [PubMed] [Google Scholar]
- 25.Prevost-Blondel A, Zimmermann C, Stemmer C, et al. Tumor-infiltrating lymphocytes exhibiting high ex vivo cytolytic activity fail to prevent murine melanoma tumor growth in vivo. J Immunol. 1998;161:2187–2194. [PubMed] [Google Scholar]
- 26.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 29.Scardino A, Gross DA, Alves P, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 30.Schreurs MW, Scholten KB, Kueter EW, et al. In vitro generation and life span extension of human papillomavirus type 16-specific, healthy donor-derived CTL clones. J Immunol. 2003;171:2912–2921. doi: 10.4049/jimmunol.171.6.2912. [DOI] [PubMed] [Google Scholar]
- 31.Schreurs MW, Kueter EW, Scholten KB, et al. Identification of a potential human telomerase reverse transcriptase-derived, HLA-A1-restricted cytotoxic T-lymphocyte epitope. Cancer Immunol Immunother. 2005;54:703–712. doi: 10.1007/s00262-004-0611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurner B, Haendle I, Roder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai V, Kawashima I, Keogh E, et al. In vitro immunization and expansion of antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy using peptide-pulsed dendritic cells. Crit Rev Immunol. 1998;18:65–75. doi: 10.1615/critrevimmunol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 34.Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 35.Tsang KY, Zhu M, Even J, et al. The infection of human dendritic cells with recombinant avipox vectors expressing a costimulatory molecule transgene (CD80) to enhance the activation of antigen-specific cytolytic T cells. Cancer Res. 2001;61:7568–7576. [PubMed] [Google Scholar]
- 36.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 37.Vermorken JB, Claessen AM, van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 38.Vonderheide RH, Hahn WC, Schultze JL, et al. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 39.Wentworth PA, Celis E, Crimi C, et al. In vitro induction of primary, antigen-specific CTL from human peripheral blood mononuclear cells stimulated with synthetic peptides. Mol Immunol. 1995;32:603–612. doi: 10.1016/0161-5890(95)00037-F. [DOI] [PubMed] [Google Scholar]
- 40.Xia X, Lessor TJ, Zhang Y, et al. Analysis of the expression pattern of Ebp1, an ErbB-3-binding protein. Biochem Biophys Res Commun. 2001;289:240–244. doi: 10.1006/bbrc.2001.5942. [DOI] [PubMed] [Google Scholar]
- 41.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo JY, Wang XW, Rishi AK, et al. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br J Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yssel H, de Vries JE, Koken M, et al. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 44.Zeh HJ, Perry-Lalley D, III, Dudley ME, et al. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 45.Zhu MZ, Marshall J, Cole D, et al. Specific cytolytic T-cell responses to human CEA from patients immunized with recombinant avipox-CEA vaccine. Clin Cancer Res. 2000;6:24–33. [PubMed] [Google Scholar]