Abstract
The aim of the present phase I/II study was to evaluate the safety, immune responses and clinical activity of a vaccine based on autologous dendritic cells (DC) loaded with an allogeneic tumor cell lysate in advanced melanoma patients. DC derived from monocytes were generated in serum-free medium containing GM-CSF and IL-13 according to Good Manufacturing Practices. Fifteen patients with metastatic melanoma (stage III or IV) received four subcutaneous, intradermal, and intranodal vaccinations of both DC loaded with tumor cell lysate and DC loaded with hepatitis B surface protein (HBs) and/or tetanus toxoid (TT). No grade 3 or 4 adverse events related to the vaccination were observed. Enhanced immunity to the allogeneic tumor cell lysate and to TAA-derived peptides were documented, as well as immune responses to HBs/TT antigens. Four out of nine patients who received the full treatment survived for more than 20 months. Two patients showed signs of clinical response and received 3 additional doses of vaccine: one patient showed regression of in-transit metastases leading to complete remission. Eighteen months later, the patient was still free of disease. The second patient experienced stabilization of lung metastases for approximately 10 months. Overall, our results show that vaccination with DC loaded with an allogeneic melanoma cell lysate was feasible in large-scale and well-tolerated in this group of advanced melanoma patients. Immune responses to tumor-related antigens documented in some treated patients support further investigations to optimize the vaccine formulation.
Keywords: Allogeneic melanoma lysate, Dendritic cells, Immunotherapy, T-cell responses, Human clinical trial
Introduction
Dendritic cells (DC) are considered promising natural adjuvants for therapeutic cancer vaccines because of their unique potential to both prime and boost cellular immune responses. The methods to generate DC ex vivo developed during the last decade have opened the possibility to bypass antigen presentation deficiencies previously described in cancer patients [1–4]. Results from phase I/II trials have demonstrated that treatment with DC-based vaccines is biologically active resulting in the induction of vaccine-specific immunological responses in different type of cancers [5]. It is now recognized that vaccination with tumor antigens can result in objective clinical responses and regression of metastases in a selected populations of patients [5–7], although only a few studies have established a correlation between immune and clinical responses [8–10]. Nevertheless, detection of mutations in tumor antigens following vaccination [11] strengthens the belief that the immune system is able to interact with tumors in vivo.
Immune responses have been observed in DC-vaccine clinical trials using diverse sources of tumor antigen, including synthetic peptides, recombinant proteins, APC-tumor cell hybrids, DC pulsed with autologous or allogeneic tumor cell lysates, and mRNA-transfected DC [6]. To develop a vaccine that could benefit to a large population of patients, we investigated the use of allogeneic tumor cell lysates that contain a variety of defined and undefined tumor-associated antigens (TAA). When loaded on DC, tumor cell lysates are presented by both MHC class I and class II pathways [12–14]. Therefore, tumor-lysate loaded DC should recruit a broad repertoire of T cells, comprising both CD8 and CD4 T cells, more likely to circumvent tumor escape and heterogeneity. Preclinical models and clinical studies showed indeed that immunization with DC loaded with tumor-derived lysates can lead to the induction of specific anti-tumor T cell responses and to objective clinical responses [14–17]. Production of lysates from autologous tumors has proven to be very challenging and poorly controlled. We therefore prepared a master cell bank from an established tumor cell line that expresses high levels of tumor specific antigens and used this cell line as a source of tumor lysate. We selected the M17 tumor cell line that expresses high levels of tumor-specific antigens of the MAGE, GAGE, BAGE families but also melanoma-specific antigens. We have previously described a method that allows the generation of large quantities of autologous monocyte-derived DC yielding multiple doses of vaccine from a single apheresis product [18]. In the present study, we report the safety, immune responses and clinical activity following treatment with autologous monocyte-derived DC loaded with a tumor cell line lysate in patients with advanced melanoma.
Materials and methods
Study design and eligibility criteria
The study was designed as a phase I/II study to evaluate toxicity, anti-tumor activity, and the immune responses to vaccination. This work was sponsored by IDM and funded in part by a European grant (Contract BIO4-97-2216 “Cellular Vaccines”) and by Sanofi-Aventis. All patients had histologically confirmed stage III or IV melanoma according to the 1992 AJCC staging system. Eligibility criteria included: age between 18 and 75 years old, ECOG performance status 0 or 1, leucocyte count > 3,000/mm3, neutrophils >1,500/mm3, platelets >100,000/mm3, serum bilirubin <2.0 mg/dl, serum creatinine <2.0 mg/dl, SGOT / SGPT < 3 × upper limit of normal, viral screening for HIV, HBV, and HCV must be negative. Exclusion criteria were cerebral metastases, positive pregnancy test, autoimmune disease, or other medical conditions that would contraindicate study participation. Previous treatment with chemotherapy, cytokines, or active immunotherapy was permitted; however, any concomitant anticancer therapy or systemic corticosteroids were not allowed by the protocol. The trial was conducted in accordance with the Declaration of Helsinki and with the European Guidelines on Good Clinical Practice. Written informed consent was obtained from each patient before inclusion in the protocol. Patients were enrolled from November 2000 to June 2001 in two Belgium centers: Erasme Hospital and Jules Bordet Institute, Brussels, Belgium. The protocol and informed consent were approved by the local Ethics committee of Erasme Hospital.
Evaluation of patients and treatment schedule
The baseline tumor evaluation was performed within 35 days prior to the first vaccination. Clinical evaluation included a complete medical history, physical examination, chest X-ray, ECG, tumor staging and antigen expression, tumor burden and documentation of sites of disease, HLA class I typing, blood chemistry, hematology, urine analysis, autoantibody testing, and S-100 β serum levels. Physical examination and autoantibody testing were also performed at the time of first vaccination. Eligible patients underwent an apheresis to generate DC. Patients received 3 vaccinations at 3-week intervals, i.e., days 0, 22, and 43 followed by a fourth vaccination 6 weeks later (day 85). Each injection series consisted of four intradermal and four subcutaneous injections at 4 different sites, and one intranodal injection with ultrasound guidance. All injections were performed in lymph node bearing areas. Patients’ vital signs and skin reactions were monitored for 2 h after vaccination. Intradermal injections would be stopped if cutaneous necrosis grade 3 were induced following vaccination.
Evaluation criteria and statistical analysis
All adverse events were classified according to the NCI Common Toxicity Criteria. Clinical responses were based on WHO criteria (World Health Organisation handbook for reporting results of cancer treatment. WHO, 1979, Geneva). Complete response (CR) was defined as complete disappearance of all clinically detectable disease for at least 4 weeks. Partial response (PR) was defined as at least a 50% decrease in all measurable lesions without an increase in size of any target lesions or the appearance of new lesions. Stable disease (SD) was defined as absence of significant change for 4 weeks or an increase of less than 25% or a decrease of less than 50% in tumor size, and no new lesions. Progressive disease was defined as 25% or more increase in the sum of the products of the measurable lesions or appearance of new lesions. Baseline measurements of target lesions were made within 35 days prior to the first vaccination. Patients were evaluated for clinical response on days 64 and 113. The evaluable population was defined as all eligible patients who received at least four vaccinations.
Preparation of tumor cell lysate
Tumor cell lysate was prepared from the M17 cell line derived from a melanoma patient (INSERM U211, Nantes, France). A master cell bank of this cell line was produced after extensive safety testing for viruses, bacteria and mycoplasma (Genopoietic, Miribel, France).
The cell line is HLA-A2+ and expresses by real-time PCR high levels of MAGE-A1, MAGE-A2, MAGE-A3, BAGE, GAGE-1, GAGE-3, Na17A (GnTV), Tyrosinase, TRP-2, AIM2, PRAME, WT1; intermediate levels of MAGE-A10, LAGE-1, gp100; and low levels of Melan-A/MART-1 and TRP-1 (M-T Duffour and P. Doceur, unpublished data). Lysate of this tumor cell line was prepared by Genopoietic as follows: tumor cells were washed and resuspended in PBS at 25×106 cells/ml. Tumor cells underwent four freeze (−80°C) and thaw (room temperature) cycles. Larger particles were removed by sedimentation. Supernatants contained 3.5 mg/ml of total protein. The tumor cell lysate was aliquoted, frozen, and stored at −80°C until use. The final lysate product was negative for adventitious viruses, bacteria, and mycoplasma.
DC preparation, characterization and injection
Dendritic cells were generated under Good Manufacturing Practices as described previously [18, 19]. Briefly, mononuclear cells from patients were isolated from peripheral blood by apheresis on day-15. DC were generated in nonadherent EVA bags (Stedim, Aubagne, France) during a 7-day culture in IDM VacCell medium (Invitrogen, Paris, France) containing GM-CSF (500 U/ml, Leucomax, Shering-Plough) and IL-13 (50 ng/ml, Sanofi-Aventis, Labège, France). DC were further enriched by elutriation. For each patient, an average of 1.36×109 DC being 87% pure with a viability of 97% were generated from initial apheresis. Quality controls (viability, cell count, purity) were performed on all DC preparations, at all critical steps of the differentiation process. Sterility testing was performed on starting apheresis and on final formulated product samples during DC differentiation according to European Pharmacopoeia and before injection of thawed DC batches by gram staining. DC preparations were characterized by flow cytometry using a FACScalibur (Becton Dickinson, San Jose, CA, USA). The following mAb were used: CD11c (BU15), CD80 (MAB104), HLA-DR (B8.12.2), CD83 (HB15a) or isotype controls, all from Beckman Coulter Immunotech (Marseille, France). DC were then loaded overnight with either M17 lysate (140 μg/ml) or HBs (Berna Biotech, Berna, Switzerland) and TT (GSK-Bio, Belgium) proteins (10 μg/ml). After loading, DC were washed and frozen in 4% HSA, 10% DMSO at 40 × 106 DC per vial. Cells were frozen using an isopropanol freezing container (Nalgene, Rochester, NY, USA) which was kept at −80°C for 24 h. Cryovials were then stored in liquid nitrogen. Remaining purified unloaded-DC were kept for immunological tests. DC-vaccine preparations were thawed prior to patient injection. Cells were thawed in a 37°C water bath, washed once in 4% HSA and further resuspended at 20×106 DC/ml in 4% HSA. Thawed-loaded DC were on average 83% viable ranging from 78 to 87% between patients’ batches. DC loaded either with the lysate or HBs/TT were mixed at this point. For intranodal injection, 2×106 lysate-loaded DC and 2×106 HBs/TT-loaded DC were injected on a non-invaded lymph node localized by echography. For subcutaneous and intradermal injections, 5×106 lysate-loaded DC and 5×106 HBs/TT-loaded DC were injected on each of four sites, chosen in the vicinity of noninvaded lymph nodes.
Samples for immunological tests
For assessment of immune responses, partial aphereses were performed on patients on days 0, 22, 43, 85, and 113. Peripheral blood mononuclear cells (PBMC) were isolated by ficoll gradient centrifugation (lymphoprep, Axis Shield, Oslo, Norway). One part of the cells was used fresh and the remaining cells were frozen in fetal calf serum (Gibco-BRL, Grand Island, NY, USA) with 10% DMSO (Sigma Aldrich, St Louis, MO, USA) and stored in liquid nitrogen until use.
Proliferation assay
Peripheral blood mononuclear cells (1×105 cells/well) were incubated in triplicates in 96-well round bottom tissue culture plates with 10 μg/ml of TT or HBs proteins in IDM VacCell medium supplemented with 5% human AB serum (BioWhittaker Europe, Belgium). PHA-L (1 μg/ml, Sigma Aldrich) was used as a positive control. On day 5, 1 μCi/well of 3H-thymidine (Amersham, Orsay, France) was added and plates were harvested 16 h later. Pre- and postimmunization samples were tested in the same experiments for each patient. Coefficient of variations between replicates were <25%. Results are expressed as stimulation index (SI = cpm with antigen/cpm without antigen). SI was calculated when cpm in the triplicates with antigen were significantly different from cpm in the triplicates without antigen (Student’s t test, one-tailed, P<0.05). When no significant proliferation was detected, SI was given the value 1. Postvaccination samples were considered positive when SI postvaccination was ≥2-fold prevaccination samples.
IFN-γ ELISpot
Peripheral blood mononuclear cells (4 × 105/well) were stimulated overnight with autologous DC (2×104 DC/well) loaded with M17 tumor cell lysate (140 μg/ml) or cocultured with TT (10 μg/ml) or HBs (10 μg/ml) proteins. PBMC from HLA-A2 patients were also stimulated with DC and peptides derived from TAA (10 μg/ml). The following peptides were used: Melan-A/MART-126-35(27L):ELAGIGILTV (Cybergene AB, Huddinge, Sweden), NA17A:VLPDVFIRC; MAGE-3271-279 :FLWGPRALV or a cocktail of four different gp100-derived peptides : gp100209-217:ITDQVPFSV; gp100 154-162 :KTWGQYWQV;gp100280-288 :YLEPGPVTA; gp100476-485 :VLYRYGSFSV, from Neosystem (Strasbourg, France). Soluble anti-CD3 mAb (HIT3a, Pharmingen, France) added at 50 ng/ml was used as a positive control. ELISpot was performed as previously described [20]. Briefly, cells were incubated in Multiscreen nitrocellulose 96-well plates (Millipore, Bedford, MA, USA) precoated with 10 μg/ml IFN-γ mAb (1-D1K, Mabtech, Stockholm, Sweden). After an overnight culture, plates were washed, incubated with biotinylated anti-IFN-γ mAb (2 μg/ml; 7-B6-1; Mabtech) and IFN-γ spot forming cells (SFC) were revealed using a Vectastain Elite Kit (AbCys, Paris, France), followed by aminoethyl carbazol at 1 mg/ml in 50 mM acetate buffer with 0.015% H2O2 (all from Sigma). Counting of SFC was performed using a computer-assisted microscope (Carl Zeiss, Le Pecq, France). Secretion of IFN-γ was considered positive when SFC in the triplicates with antigen were significantly different from the SFC in the triplicates without antigen (Student’s t test, one-tailed, P<0.05) and SFC in wells with antigen were ≥7 after subtraction of the SFC from wells without antigen.
Histology
Two slides of 5-μm thick sections were obtained from biopsy specimens all fixed in 10% phosphate-buffered formalin. The sections were deparaffinized and then stained by haematoxylin (Gille 2, Merck, France) and eosin (Eosin B, Merck).
Results
Patients’ characteristics
The clinical characteristics of the patients are listed in Table 1. Fifteen patients were included in this study. Ten patients had AJCC stage IV melanoma and five had stage III melanoma. S-100β level was elevated in a total of seven patients (six with Stage IV and one with Stage III melanoma). All had previously been treated with at least surgery and ten patients had received systemic treatment with chemotherapy and/or biological therapy. Sites of metastasis included lungs, skin, lymph nodes, and liver.
Table 1.
Patients’ characteristics and disease status
| Patient ID | Age | Sex | PFS 0–4 | Prior treatment | Stage | Site of metastases | No. of Vaccination | Survivald (months) | Clinical evolution post-DC vaccine |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 73 | M | 1 | S, BC | IV | Lung, liver, axillary lymph node | 4 | 7 | Radiotherapy |
| 02 | 58 | M | 1 | S, ILP | III | In-transit mets | 7a | >25 | In CR 2 months post fourth vaccination; CR for 18 months |
| 03 | 49 | F | 0 | S, I | IV | Liver, lung, spleen | 4 | 13b | Chemotherapy |
| 04 | 62 | M | 0 | S, I | III | Cervical nodes | 4 | >23 | Surgery 15 days after fourth vaccination; disease-free for 19 months |
| 05 | 66 | F | 1 | S, I, DC vacc. | III | Leg | 3 | 19b | Chemotherapy and radiotherapy |
| 06 | 67 | M | 0 | S, C | IV | Lung, scalp, right temporal | 1 | <1 (0.7)b | |
| 07 | 42 | M | 0 | S | IV | Lung | 7a | >22 | SD for 10 months post fourth vaccination chemo and radiotherapy after relapse at another site |
| 08 | 62 | F | 1 | S, I, C, peptide vacc. | IV | Inguinal node, lung | 3 | 4b | Radiotherapy |
| 09 | 55 | F | 0 | S | III | In-transit mets | 4 | 5b | No additional treatment |
| 10 | 44 | F | 0 | S | III | In-transit mets | 4 | >23 | Chemotherapy followed by radiotherapy |
| 11 | 50 | M | 1 | S, I, C | IV | Cutaneous mets | 2 | 1.6b | |
| 12 | 59 | M | 1 | S, I, C, BC, DC vacc. | IV | Liver, lung, spleen | 4 | 16b | Chemotherapy |
| 13 | 62 | F | 0 | S, C | IV | Soft tissue, inguinal node, retroperitoneal nodes, bilateral lung mets. | 3 | 6c | Radiotherapy and chemotherapy |
| 14 | 75 | F | 0 | S | IV | Lung | 4 | 12b | Chemo- and radiotherapy |
| 15 | 47 | M | 0 | S, ILP, I, C | IV | Lung, left temporal | 3 | 5b | Radiosurgery |
PFS performance status ECOG, S surgery, I immunotherapy (IFN-alpha), C chemotherapy, BC biochemotherapy, ILP isolated limb perfusion, DC dendritic cell
aAdditional vaccinations were given at months 8, 10, and 12
bDeceased
cLost to follow-up
dTime from the first day of therapy (day 0) to the date of death/last visit
DC-Vaccine and toxicity
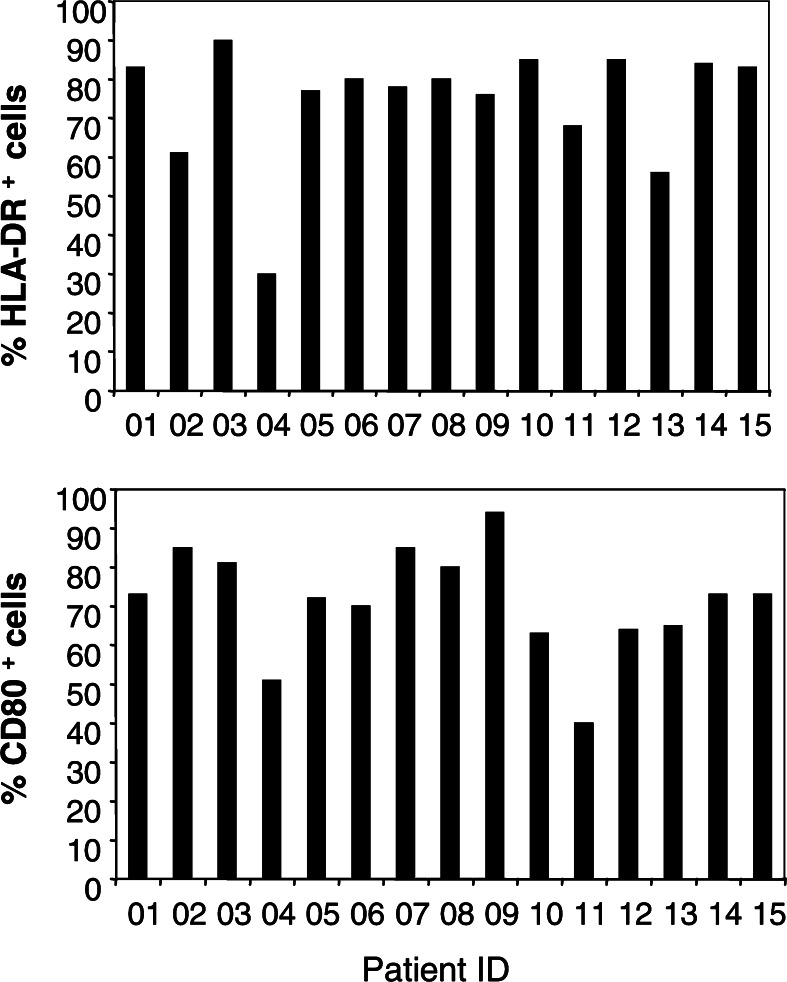
The DC vaccine was prepared from autologous monocytes cultured in GM-CSF and IL-13. Low variability between patients’ DC-preparations was observed as shown in Fig. 1: the majority of DC express HLA-DR and CD80 molecules (on average 74% and 71%, respectively). DC were not matured and did not express CD83 except in three patients (03, 06, and 07), where CD83 was spontaneously expressed by about 66% of the DC population. DC were loaded with tumor cell lysate and with HBs and/or TT antigens. HBs protein was considered as a good antigen to detect induction of primary immune responses since most patients included in the study were naïve for this antigen at baseline. TT protein was chosen to evaluate the ability of the vaccine to recall memory-immune responses. All patients received at least one vaccination. Nine patients completed four vaccinations and were considered evaluable for clinical activity (Table 1). Seven patients discontinued the study due to early progressive disease (patients 05, 06, 08, 10, 11, 13, and 15). The treatment was generally well-tolerated. Twelve patients experienced at least one adverse event (AE); most events were mild or moderate and no grade 3 or 4 events related to treatment were reported. No AE led to the discontinuation of patient from the study. Two serious adverse events were reported: one patient (02) was hospitalized for fever due to cholecystic lithiasis, and one patient (06) died during the study due to disease progression. Neither of these serious AE were related to treatment. The most frequent events reported were pain (40% of reported events), local injection site reaction (33%), anorexia (20%) and asthenia (20%). There were no manifestations of autoimmunity.
Fig. 1.
Expression of HLA-DR and CD80 by DC prepared from melanoma patients. Expression of HLA-DR and CD80 molecules in DC cell suspensions are shown for each patient preparation. The percentage of positive cells is given after gating on large (FSC/SSC) alive cells. Coefficient of variations between DC preparations were 20 and 18% for HLA-DR and CD80 molecules, respectively
Clinical activity in treated patients
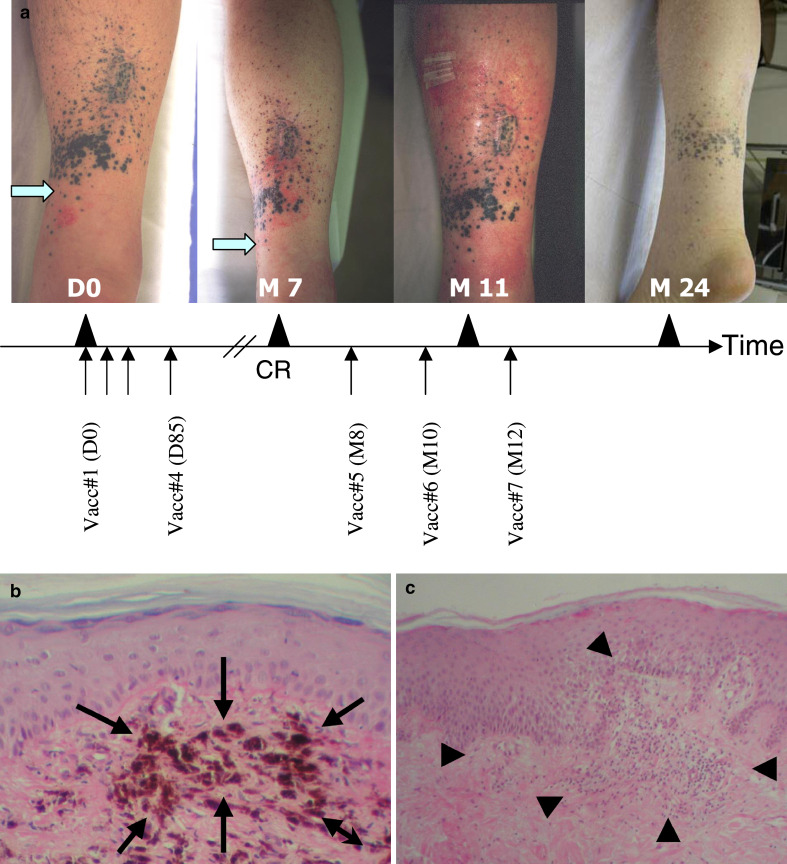
No patient achieved a complete or partial response by the time of the last study visit (day 113). Among the nine evaluable patients (i.e., who received the four doses of vaccine), six patients had still progressive disease during follow-up, one patient (04) had no evidence of disease following surgery after the fourth vaccination, one patient had disease stabilization (07), and one patient (02) showed a complete regression of his melanoma two months after the fourth vaccination (Fig. 2a). In patient 02, the in-transit metastases had flattened up and the biopsy revealed no viable melanoma cells. Histological studies of lesion biopsies showed the presence of melanophages (Fig. 2b). In addition, this patient presented an eczema at the inclusion which became a severe skin inflammatory reaction colocalized to the cutaneous metastases site at this time (Fig. 2a). He received three additional vaccinations at months 8, 10, and 12. After the 6th vaccination, an exacerbation of the skin inflammatory reaction was observed (Fig. 2a) and new biopsies were performed. A T-cell infiltrate was noted (Fig. 2c). The patient remained in complete response until the last available follow-up visit at month 24. Upon completion of the fourth vaccination, patient 07 was documented with stable disease and therefore received three additional vaccinations. He remained stable for 10 months but finally presented new metastasis at another site (vertebral) and received radiotherapy and chemotherapy.
Fig. 2.
Clinical evolution of patient 02 with a complete response after treatment with lysate-loaded DC. a Pictures of patient 02 were taken before treatment (day 0) with lysate-loaded DC and at different time points posttreatment (months 7, 11, and 24). The patient received two cycles of vaccinations (days 0, 22, 57, 85, and months 8, 10, and 12). The site of eczema presented by this patient at the inclusion is indicated with an arrow. The correspondent site is indicated in the picture taken after vaccination (month 7), showing the modification of the skin reaction. Complete response was diagnosed at this point by biopsy and histopathological analysis. The picture taken at month 11 shows the exacerbation of the eczema reaction following the sixth immunization. The last picture was taken 12 months after beginning of the treatment with lysate-loaded DC. b Histological examination (original magnification ×100) of the biopsy of a cutaneous metastasis in this patient 02 two months after the fourth vaccination showed the presence of melanophages (arrows) which were interpreted as signs of tumor regression. c The cutaneous biopsy performed after the sixth vaccination demonstrated a lymphocytic infiltrate and spongiosis (original magnification ×40, arrowheads) confirming the delayed immune response (eczema)
Immune responses
Immune responses to the vaccine were monitored in all 15 but one patient (06) who went out of study after the first vaccination. All patients were immunized with lysate-loaded DC and HBs- and/or TT-loaded DC. For HBs and TT antigens, 11 patients were vaccinated with both TT and HBs; four patients received either TT (patients 01 and 02) or HBs-loaded DC (patients 05 and 06), according to protocol specifications.
At baseline, reactivity to TT protein was detected in 10 out of the 13 patients vaccinated with TT-loaded DC (Fig. 3a). TT reactivity was increased (i.e., ≥2-fold over baseline) at different time points after vaccination in four patients (02, 03, 12, 13). Three other patients (01, 09, and 15) had an increased post-treatment at only one occasion. HBs reactivity was evaluated in the 12 patients vaccinated with HBs-loaded DC. HBs-specific proliferation was absent in most patients at baseline (Fig. 3b) and was clearly increased after vaccination in two patients (07 and 15). Two other patients (09 and 12) showed an increase only at one occasion posttreatment. In general, we observed that the proliferative response to HBs protein was often of much lower amplitude than the TT-specific responses. Immune responses are summarized in the Table 2.
Fig. 3.
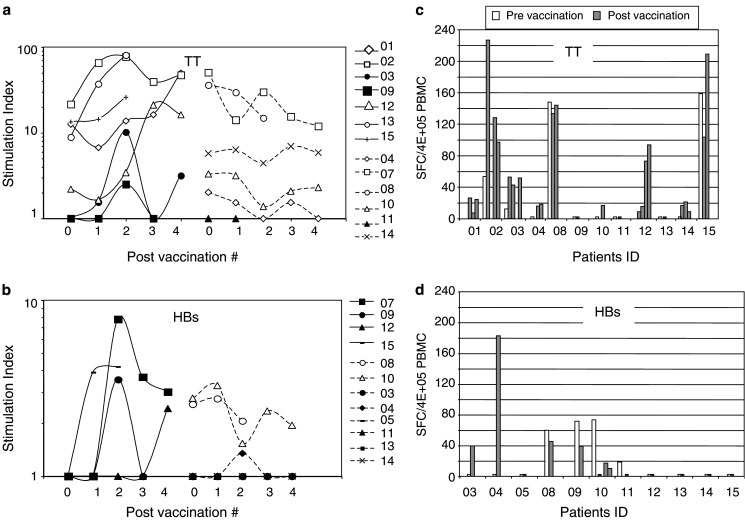
Immune responses to HBs- and TT-proteins in vaccinated patients. a, b PBMC were tested in proliferation assay for reactivity against (a) TT- or (b) HBs-proteins at baseline and after 1, 2, 3, or 4 vaccinations. Results are expressed as stimulation index (SI = cpm with antigen/cpm without antigen). Results from patients with pre-existing reactivity (open symbols) and those without reactivity at baseline (filled symbols) are shown. Patients with increased reactivity during treatment are presented on the left side of graphs (a) and (b). c, d Fresh PBMC were cultured with autologous DC and TT (c) or HBs (d) proteins and IFN-γ production was measured by ELISpot. Results are expressed as IFN-γ spot forming cell (SFC) among 4×105 PBMC. Patient 07 could not be evaluated by ELISpot because of a high reactivity against autologous DC at baseline
Table 2.
Summary of Immune Responses measured in patients treated with lysate-loaded DC
| Patient ID | Increased response to HBs/TTa | Increased response to lysate-DC | HLA-A2 | Increased response to HLA-A2 binding peptides |
|---|---|---|---|---|
| 01 | + | + | A2− | NA |
| 02 | + | − | A2− | NA |
| 03 | + | − | A2− | NA |
| 04 | + | − | A2− | NA |
| 05 | − | + | A2+ | − |
| 06 | ND | ND | A2+ | ND |
| 07 | + | ND | A2+ | ND |
| 08 | − | + | A2+ | − |
| 09 | + | ND | A2− | NA |
| 10 | + | − | A2− | NA |
| 11 | − | − | A2− | NA |
| 12 | + | − | A2+ | − |
| 13 | + | − | A2− | NA |
| 14 | + | + | A2+ | + |
| 15 | + | − | A2+ | + |
aReactivity increased in proliferation assay or IFN-γ ELIspot
ND not determined, NA not applicable (HLA-A2− patients)
IFN-γ ELISpot assay showed that TT reactivity detected by proliferation assay was in general associated with a type 1 immune response (Fig. 3c). Three patients (04, 10, and 14) have increased IFN-γ responses to TT after vaccination that were not detected by proliferation assay. HBs-specific proliferative and IFN-γ responses were usually not correlated (Fig. 3d). Of note, IFN-γ response to HBs protein was clearly induced after vaccination in patients 03 and 04 who showed no or very weak proliferative responses. We also measured anti-HBs Ab titers in vaccinated patients. Four patients (04, 07, 09, and 12) were negative at baseline but positive after the second or third vaccination (data not shown). This observation strengthens the reactivity to HBs detected in these patients by proliferation or ELISpot assays. The other patients did not have anti-HBs Ab except two patients (10 and 14) who were already positive at baseline.
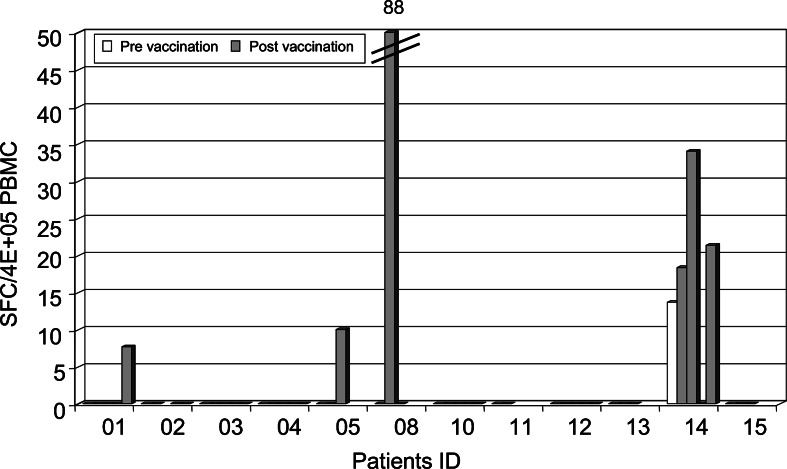
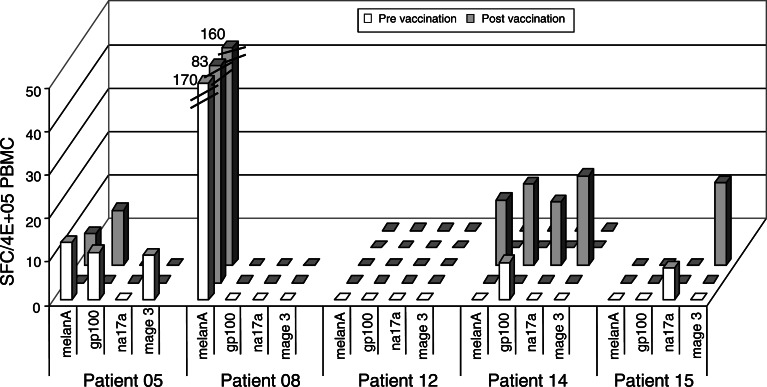
T cell responses against lysate or peptide-loaded DC were monitored ex vivo by IFN-γ ELISpot to determine the frequencies of circulating specific T cells induced (in particular, lysate-specific ones) and to better reflect the functional status of the T cell populations in vivo. Patient 07 could not be evaluated because of spontaneous IFN-γ production when DC were added to PBMC at baseline. As shown in Fig. 4, reactivity to lysate-loaded DC was enhanced in four patients (01, 05, 08, and 14). The frequency of IFN-γ-producing cells was relatively low in general except in patient 08 (about 2×10−4 IFN-γ+ cells among PBMC). Reactivity to the lysate was also detected at baseline and posttreatment in patient 09 but we could not compare the intensity of the responses because less DC were available for posttreatment samples. Seven patients (05, 06, 07, 08, 12, 14, and 15) were HLA-A2. We investigated whether T cell responses specific for defined MHC class I TAA-derived epitopes could be detected after vaccination. In four out of five HLA-A2+ patients evaluated, we detected the presence of Melan-A/MART-1-, gp100-, Na17A/GnTV- or MAGE-3-specific CD8+ T cells by IFN-γ ELISpot (Fig. 5). These antigens are expressed by M17 cell line used to prepare the vaccine. In patients 05, 08, and 15, we have detected IFN-γ-producing cells at baseline to peptides derived from MAGE3, MelanA/MART-1 and Na17A/GnTV antigens. Appearance or enhancement of IFN-γ reactivity to peptides after vaccination was observed in patients 14 and 15. We did not find any correlation with the tumor antigens expressed by the tumor biopsies collected before DC treatment (data not shown). A high frequency of Melan-A/MART-1-specific CD8+ T cells was detected for the patient 08. This response was not modified by the treatment despite induction of a specific response to the lysate (Figs. 4, 5). In fact, three patients (patients 05, 08, and 14) responded to both tumor lysate and TAA-derived peptides; however, responses against peptides or tumor lysate were not always detected at the same time points. In total, 4 out of 12 patients showed increased reactivity to the lysate after vaccination and 2 out of 5 HLA-A2+ patients showed enhanced reactivity against TAA-derived HLA-A2-restricted peptides (Table 2).
Fig. 4.
Immune responses to lysate-loaded DC detected by IFN-γ ELISpot. Fresh PBMC were cultured overnight with autologous unloaded DC or DC loaded with M17 lysate. Results are expressed as IFN-γ spot forming cells after subtraction of the background among 4×105 PBMC collected before (white bars) and during treatment (gray bars)
Fig. 5.
Immune responses to TAA derived peptides detected by IFN-γ ELISpot in HLA-A2+ patients. Fresh PBMC were cultured overnight with autologous DC and TAA-derived peptides (10 μg/ml). Results are expressed as IFN-γ spot forming cells among 4×105 PBMC collected before (white bars) and during treatment (gray bars)
Discussion
In the present study, 15 patients were treated with 9 patients receiving four doses of vaccine. Of these, two patients received three additional vaccinations after having shown signs of clinical response or disease stabilization. Our results indicate that the vaccine was well-tolerated. We were able to document immune responses against tumor associated- and control-antigens after vaccination in some patients, providing encouraging results for further development of this vaccine.
The use of tumor cell lysate allows for vaccination of melanoma patients regardless of their HLA haplotype. Access to autologous tumors and yield of autologous cell-lysate are usually limiting factors to produce enough vaccines in a reproducible and quality-controlled manner. Therefore, we investigated the use of allogeneic tumor cell lysate derived from an established melanoma cell line. In particular, the cell line expresses Mage-3, gp100, Melan-A/MART-1 and Na17A/GnTV antigens against which we detected immune responses in some patients. We used a process shown to generate large numbers of monocyte-derived DC in serum-free conditions suitable for cellular therapy in humans [18]. The vaccine was produced successfully for all patients included in the study.
Dendritic cells were administered by multiple routes as we believe there is no consensus on the best route of immunization for patients with tumors at different locations in the body. Immune responses were monitored by proliferation assay and IFN-γ ELISpot. Immune responses against vaccine-control antigens were enhanced in 11 patients which increased at more than one time point posttreatment in six patients (02, 03, 07, 12, 13, and 15). Previous studies using non-matured DC reported similar results [21–23]. The use of non-matured DC instead of matured DC can be limiting to induce persistent proliferative responses [23]. We cannot exclude that loading of TT and HBs on the same DC favors T cell competition for recognition of APC and limits priming or boosting effect of the vaccine. We show that some of these responses were associated with the presence of IFN-γ-producing cells indicating that the vaccine is able to stimulate type 1 immune responses. Immune responses against the allogeneic tumor-cell lysate loaded on DC were enhanced after treatment in four patients. Other studies using allogeneic or autologous tumor-based vaccines have reported induction of T-cell responses [15, 17, 22, 24–32]. The use of autologous DC allows for presentation of allogeneic material by the DC-self MHC molecules limiting direct recognition of allogeneic determinants by T cells in vivo. In addition, targeting tumor material into ex vivo generated-DC should circumvent dysfunctions of the APC observed in tumor-bearing patients [33]. In 2/5 HLA-A2+ patients, we show enhanced reactivity to TAA-derived peptides presented by self MHC. These results support the idea that tumor lysate-loaded DC can cross-present TAA epitopes [17, 28, 32, 34]. On the other hand, it is very difficult to strictly demonstrate that these peptide responses are directly related to lysate presentation by DC in vivo rather than to epitope spreading. The observation that reactivity to the lysate but not to the TAA-derived peptides was enhanced in patients 05 and 08 after treatment suggest that other non-identified epitopes present in the lysate may be involved. In particular, recognition of allogeneic determinants with a potential bias in the HLA haplotype of the responding patients [35] should be further investigated.
Although the study was not designed to demonstrate clinical efficacy of the vaccine, we could follow one patient (patient 02) who experienced a complete response, with a severe eczema in the vicinity of the metastasis sites. The inflammatory skin reaction observed in this patient may have favored tumor regression and whether recognition of vaccine-antigens is involved is not clear. Tumor-lysate specific response could not be evidenced in this patient. Another patient (patient 07) with stabilization of lung metastasis could not be evaluated for lysate or peptide reactivity. In parallel, the five patients with lysate or peptides responses progressed rapidly before receiving the four vaccinations. Thus, we could not observe any relation between clinical activity and systemic immune responses in the treated patients.
Our results indicate that administration of allogeneic tumor lysate-loaded, non-matured DC is a feasible approach to induce tumor responses. Should we use matured-DC for further optimization of the vaccine? Indeed, DC treated with maturation agents have been shown to better sensitize T cells [19, 23, 36]. However, recent discussions in the literature raised the question whether DC should be matured ex vivo or injected non-matured [37, 38]. Sallusto and colleagues pointed out that fully matured DC impact on T cell polarization [39] and Albert et al. [40] showed that matured DC can also induce tolerance in vivo. In parallel, some clinical studies have shown induction of immune responses after injection of non-matured DC [41–43] and the study by Barrat-Boyes et al. [44] indicates that non-matured DC can undergo spontaneous maturation in vivo. Prince et al. [45] have recently shown that GM-CSF/IL-13 generated DC can migrate in vivo when injected as non-matured DC. Finally, we are currently comparing 6 h matured DC with non-matured DC in another phase I/II clinical trial. Initial results suggest that there is no difference in systemic immune responses rates. We believe other ways of maturing DC, like in situ maturation, should be explored to improve therapeutic effects of current vaccines.
Acknowledgements
we would like to thank GSK-Bio for the tetanus Toxoid and Berna Biotech for the HBs protein. We also thank all the patients who participated in the study. This work was funded in part by a European grant (Contract BIO4-97-2216 “Cellular Vaccines”) and by Sanofi-Aventis.
Footnotes
Margarita Salcedo and Nadège Bercovici both contributed equally to this work
References
- 1.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 2.Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134:270–278. doi: 10.1046/j.1365-2249.2003.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, Mazzella F, Antonia SJ, Mellor AL, Munn DH. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest. 2003;83:1457–1466. doi: 10.1097/01.LAB.0000090158.68852.D1. [DOI] [PubMed] [Google Scholar]
- 5.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother. 2004;53:275–306. doi: 10.1007/s00262-003-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svane IM, Soot ML, Buus S, Johnsen HE. Clinical application of dendritic cells in cancer vaccination therapy. Apmis. 2003;111:818–834. doi: 10.1034/j.1600-0463.2003.11107813.x. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 9.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonchay C, van der Bruggen P, Connerotte T, Hanagiri T, Coulie P, Colau D, Lucas S, Van Pel A, Thielemans K, van Baren N, Boon T. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14631–14638. doi: 10.1073/pnas.0405743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riker A, Cormier J, Panelli M, Kammula U, Wang E, Abati A, Fetsch P, Lee KH, Steinberg S, Rosenberg S, Marincola F. Immune selection after antigen-specific immunotherapy of melanoma. Surgery. 1999;126:112–120. [PubMed] [Google Scholar]
- 12.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 14.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mule JJ. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 16.Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, Nestle FO. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102:2338–2344. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 17.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 18.Goxe B, Latour N, Chokri M, Abastado JP, Salcedo M. Simplified method to generate large quantities of dendritic cells suitable for clinical applications. Immunol Invest. 2000;29:319–336. doi: 10.3109/08820130009060870. [DOI] [PubMed] [Google Scholar]
- 19.Boccaccio C, Jacod S, Kaiser A, Boyer A, Abastado JP, Nardin A. Identification of a clinical-grade maturation factor for dendritic cells. J Immunother. 2002;25:88–96. doi: 10.1097/00002371-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Bercovici N, Givan AL, Waugh MG, Fisher JL, Vernel-Pauillac F, Ernstoff MS, Abastado JP, Wallace PK. Multiparameter precursor analysis of T-cell responses to antigen. J Immunol Methods. 2003;276:5–17. doi: 10.1016/S0022-1759(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 21.de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 22.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 23.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty NG, Sporn JR, Tortora AF, Kurtzman SH, Yamase H, Ergin MT, Mukherji B. Immunization with a tumor-cell-lysate-loaded autologous-antigen-presenting-cell-based vaccine in melanoma. Cancer Immunol Immunother. 1998;47:58–64. doi: 10.1007/s002620050504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, Hodi FS, Liebster L, Lam P, Mentzer S, Singer S, Tanabe KK, Cosimi AB, Duda R, Sober A, Bhan A, Daley J, Neuberg D, Parry G, Rokovich J, Richards L, Drayer J, Berns A, Clift S, Cohen LK, Mulligan RC, Dranoff G. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell MS, Kan-Mitchell J, Kempf RA, Harel W, Shau HY, Lind S. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1988;48:5883–5893. [PubMed] [Google Scholar]
- 27.Soiffer R, Hodi FS, Haluska F, Jung K, Gillessen S, Singer S, Tanabe K, Duda R, Mentzer S, Jaklitsch M, Bueno R, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Mihm M, Dranoff G. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Griffioen M, Borghi M, Schrier PI, Osanto S, Schadendorf D. Analysis of T-cell responses in metastatic melanoma patients vaccinated with dendritic cells pulsed with tumor lysates. Cancer Immunol Immunother. 2004;53:715–722. doi: 10.1007/s00262-004-0514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernando JJ, Park TW, Kubler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen-pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother. 2002;51:45–52. doi: 10.1007/s00262-001-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell MS, Kan-Mitchell J, Morrow PR, Darrah D, Jones VE, Mescher MF. Phase I trial of large multivalent immunogen derived from melanoma lysates in patients with disseminated melanoma. Clin Cancer Res. 2004;10:76–83. doi: 10.1158/1078-0432.CCR-0689-3. [DOI] [PubMed] [Google Scholar]
- 31.Vilella R, Benitez D, Mila J, Lozano M, Vilana R, Pomes J, Tomas X, Costa J, Vilalta A, Malvehy J, Puig S, Mellado B, Marti R, Castel T. Pilot study of treatment of biochemotherapy-refractory stage IV melanoma patients with autologous dendritic cells pulsed with a heterologous melanoma cell line lysate. Cancer Immunol Immunother. 2004;53:651–658. doi: 10.1007/s00262-003-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 33.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 34.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell MS, Harel W, Groshen S. Association of HLA phenotype with response to active specific immunotherapy of melanoma. J Clin Oncol. 1992;10:1158–1164. doi: 10.1200/JCO.1992.10.7.1158. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser A, Bercovici N, Abastado JP, Nardin A. Naive CD8+ T cell recruitment and proliferation are dependent on stage of dendritic cell maturation. Eur J Immunol. 2003;33:162–171. doi: 10.1002/immu.200390019. [DOI] [PubMed] [Google Scholar]
- 37.Adema GJ, de Vries IJ, Punt CJ, Figdor CG. Migration of dendritic cell based cancer vaccines: in vivo veritas. Curr Opin Immunol. 2005;17:170–174. doi: 10.1016/j.coi.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 40.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 41.Ribas A, Glaspy JA, Lee Y, Dissette VB, Seja E, Vu HT, Tchekmedyian NS, Oseguera D, Comin-Anduix B, Wargo JA, Amarnani SN, McBride WH, Economou JS, Butterfield LH. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27:354–367. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Smithers M, O’Connell K, MacFadyen S, Chambers M, Greenwood K, Boyce A, Abdul-Jabbar I, Barker K, Grimmett K, Walpole E, Thomas R. Clinical response after intradermal immature dendritic cell vaccination in metastatic melanoma is associated with immune response to particulate antigen. Cancer Immunol Immunother. 2003;52:41–52. doi: 10.1007/s00262-002-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 44.Barratt-Boyes SM, Zimmer MI, Harshyne LA, Meyer EM, Watkins SC, Capuano S, 3rd, Murphey-Corb M, Falo LD, Jr, Donnenberg AD. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol. 2000;164:2487–2495. doi: 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- 45.Mileshkin LR, Wall DM, Loveland BE, Thompson M, Coverdale J, Wong J, Xing PX, Taylor RR, Hicks RJ, Prince HM (2004) A Study of in vivo tracking of MUC-1 pulsed dendritic cells in patients with multiple myeloma. In: 19th Annual SBT meeting 2004, San-Francisco, CA