Abstract
Background and aim
A new marker, CD208, was recently explored as a mature interdigitating dendritic cell (DC), and the correlation between the infiltration of CD208-positive cells and clinical factors has been reported in various types of cancers. In this study, we tried to clarify the clinical implication of CD208-positive cell infiltration in gastric cancer immunohistochemically.
Patients and methods
A total of 128 gastric cancer patients who underwent a curative operation were enrolled. DCs in tumor nests were identified with two DC markers, CD208 and S-100 protein (S100), by immunohistochemistry. The correlation between clinicopathological features and the CD208- or S100-positive cell infiltration degree was analyzed.
Results
Infiltration of S100-positive cells did not correlate with the degree of CD208-positive cell infiltration. Patients with high CD208-positive cell infiltration in the peritumor had a poorer surgical outcome than those with low CD208 infiltration (p < 0.05). Multivariate analysis revealed that CD208-positive cell infiltration was not an independent prognostic factor.
Conclusion
We showed that intratumoral CD208-positive cells, as mature DCs, had an inverse correlation to patients’ postoperative outcome in gastric cancer, unlike a conventional DC marker. Evaluation of CD208-positive cell infiltration with S100-positive cell infiltration in gastric cancer is useful to predict antitumor immunological conditions in gastric cancer.
Keywords: Gastric cancer, Dendritic cells, CD208, S100 protein, Survival
Introduction
Gastric cancer is one of the most common gastrointestinal malignancies in Asian countries, including Japan [1, 2]. When patients undergo a curative operation for gastric cancer, a favorable prognosis is expected; however, some patients show an unexpected postoperative course despite a curative operation for early stage cancer [3]. The postoperative outcome of gastric cancer mainly depends on TNM factors, namely, the depth of tumor invasion and the presence or absence of lymph node metastasis and distant metastasis. In addition to these critical clinicopathological factors, the prognosis of gastric carcinoma is influenced by several biological variables, including immunological parameters [4, 5]. Among several immunological factors, we previously reported the significant clinical impact of intratumoral dendritic cell (DC) infiltration in esophageal [6] and gastric [7] cancer. According to previous reports, DC infiltration correlated with local immune interaction and patient prognosis in breast [8], pancreas [9], colon [10], and gall bladder cancer [11]. Functionally, DC is thought to work as an antigen-presenting cell, presenting a tumor-specific antigen to helper T cells and stimulating tumor-specific cytotoxic T lymphocytes. DCs play a significant role in the induction of an immune response and an imbalance in the proportion of macrophages, and DCs within the tumor could affect the immune response to cancer; however, the role of the T cell-mediated immune response in controlling the cancer progression of tumor-infiltrating lymphocytes remains poorly documented.
Recently, various types of DCs have been classified using special immunological markers. CD208 is a member of the lysosome-associated membrane glycoprotein family, and is specifically expressed by human DCs on activation, being used as a marker of mature DCs [12, 13]. A recent study identified intratumoral CD208-positive cell infiltration in various types of cancers, such as colon [14], breast [15], and other tumors, and the authors discussed the clinical implication of CD208-positive cell infiltration beyond conventional intratumoral DC infiltration; however, there has been no information on gastric cancer. In this study, we tried to clarify the clinical impact of CD208-positive cell infiltration in comparison with the conventional DC marker, S100 protein-positive cell infiltration, in gastric cancer.
Patients and methods
One hundred twenty-eight consecutive gastric cancer patients who received R0 resection at Kagoshima University Hospital between 1996 and 2001 were enrolled in this study. The patient group was composed of 92 men and 36 women, ranging in age from 31 to 82 years (mean 61 years). No patients received preoperative chemotherapy. All patients underwent R0 resection with more than D1 lymph node dissection (Table 1). Clinical factors were assessed by the Japanese Classification of Gastric Carcinoma [16].
Table 1.
Patient’s information
| Gender | |
| Male | 92 |
| Female | 36 |
| Age (years) | |
| Mean (range) | 61 (31–82) |
| Operation | |
| Total gastrectomy | 62 |
| Distal | 52 |
| Proximal | 14 |
| Stage | |
| I | 60 |
| II | 21 |
| III | 25 |
| IV | 22 |
| Histology | |
| Differentiated | 69 |
| Undifferentiated | 59 |
Immunohistochemistry
Intratumoral CD208-positive cells and S100-positive cells were assessed according to previous reports [13, 14] and visualized by avidin–biotin complex immunohistochemistry. Paraffin-embedded sections (4 μm), including tumor nests, were obtained from the 128 gastric cancer patients, which were deparaffinized and soaked in phosphate-buffered saline (PBS) prior to immunohistochemical analysis. Sections were treated with 3% H2O2 for 30 min to block endogenous tissue peroxidase, followed by treatment with bovine serum for 30 min to reduce nonspecific binding. CD208 monoclonal antibody (DC-LAMP, France) and S100-protein (Ancell DAKO) polyclonal antibody were retrospectively diluted at 1:100 and 1:200, respectively, and incubated with the sections for 120 min at room temperature. Sections were rinsed in PBS and visualized using standard techniques for labeled avidin–biotin immuno-peroxidase staining. Lymphocytes of normal spleen sections were used as positive controls for DC infiltration. The positivity of CD208 and S100 protein was observed at not only the cell membrane but also in the cytoplasm of infiltrating tumor cells.
Evaluation of intratumoral CD208-positive or S100-positive cells’ infiltration in gastric cancer
The degree of CD208- and S100-positive cells was evaluated according to previous reports [13, 14]. The numbers of CD208- and S100-positive cells were calculated in 25 representative high-power fields (×400) in not only each tumor nest but also at the invasive front of the tumor. All immunostained slides were evaluated by two independent observers (SI and SN), who were unaware of the clinical data or disease outcome. The average number of CD208- or S100-positive cells was measured and calculated. A group with high and low DC infiltration was distinguished based on the 75th percentile of the average DC infiltration scores of all patients divided into high and low infiltration groups according to previous reports. Namely, patients with more than 1.0/HPF-positive cells were regarded as the CD208-positive group, and patients with more than 40 S100 protein-positive cells/HPF were regarded as the S100-positive group. Clinicopathological factors were described according to the general rules of gastric cancer in Japan [16].
Statistical analysis
Statistical analysis of clinical features was performed by the χ 2-test, and the degree of infiltration between the two groups was compared by Pearson’s correlation coefficient. Survival curves were produced using the Kaplan–Meier method, and statistical significance was calculated using the generalized Wilcoxson method. Multivariate analysis was performed to determine prognostic factors. A p value of <0.05 was considered significant.
Results
Distribution of CD208-positive and S100-positive cells in gastric cancer
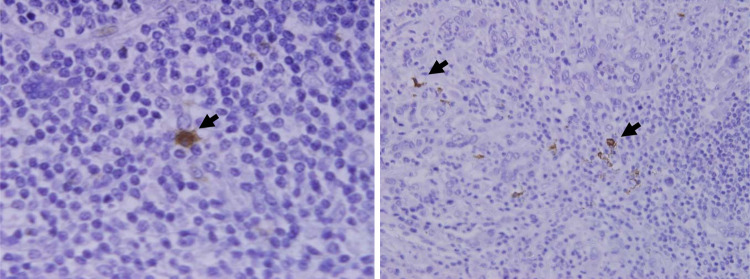
CD208-positive cells were identified as an irregular-shaped cell in cancerous stroma and lymph follicle. Under a high-power view, CD208 positive cells had some dendrites (Fig. 1). Intratumoral CD208-positive cells were predominantly observed in stroma and sparsely found in epithelium. CD208-positive cells were also distributed in lymph follicles in the invasive margin or peritumoral space (Fig. 2). S100-positive cells were mainly found in tumor stroma. Nerve tissue in the gastric wall also showed positivity of S100-protein. Infiltrating S100-positive cells were identified as a brownish cell having dendrites by high-power fields (Fig. 3).
Fig. 1.
High-power view of CD208-positive cells’ infiltration into tumor nest. Under a high-power view, CD208 positive cells had some dendrites (arrow head)
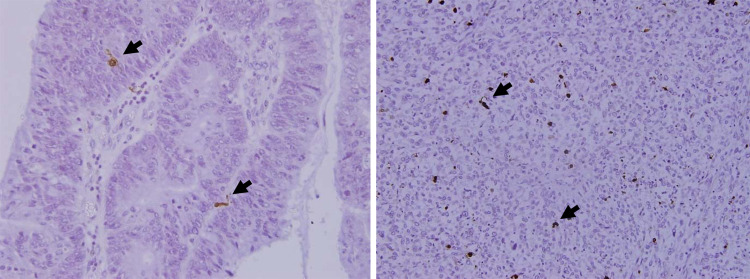
Fig. 2.
Infiltration of CD208-positive cell into tumor nest. CD208-positive cells were found in both peritumoral and intratumoral space sparsely
Fig. 3.
Infiltration of S100 protein-positive cell into tumor nest. S100 positive cells were found as a brawn with a dendrite in peritumoral space (arrow head)
Infiltration degree of CD208-positive and S100-positive cells and its correlation
Among these 128 gastric cancer patients, intratumoral CD208-positive cells infiltrated the intratumoral space from 0 to 14.5 (average 2.9) and the peritumoral space from 0 to 19 (average 3.6). The infiltration degree in two tumoral space was similar. In contrast, S100-positive cell infiltration ranged from 0 to 182 (average 19). According to the previous evaluation, the degree of CD208-positive cell infiltration in intratumoral and peritumoral areas and S100 protein-positive cells infiltration were divided into high and low. The number of patients with high and low CD208-positive cell infiltration was 36 and 92 in the intratumoral area, respectively, and 63 and 65 in the peritumoral area, respectively. The number of patients with high and low S100-positive cell infiltration was 51 and 77, respectively (Table 2).
Table 2.
Details of CD208- or S100-positive cells’ infiltration in gastric cancer
| Average | Infiltrating degree | ||
|---|---|---|---|
| High | Low | ||
| CD208-positive cell | |||
| Intratumoral | 2.9 (0–14.5) | 36 | 92 |
| Peritumoral | 3.6 (0–19.0) | 63 | 65 |
| S100 protein-positive cell | |||
| Intratumoral | 19 (0–182) | 51 | 77 |
Correlation between CD208-positive and S100-positive cells
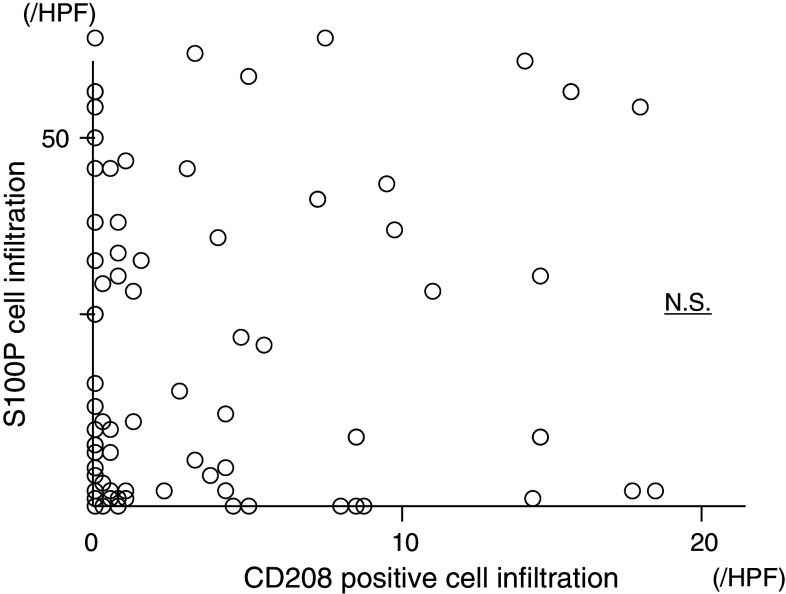
CD208- and S100-positive cells’ infiltration in 128 gastric cancer patients is plotted in Fig. 4. The correlation between CD208- and S100 protein-positive cells was analyzed by Pearson’s correlation. No significant correlation was found between the CD208- and S100-positive cells’ infiltration.
Fig. 4.
Correlation between S100 protein- and CD208-positive cells’ infiltration. Infiltration degree of S100- and CD208-positive cells was plotted. There was no significant correlation between two DCs’ infiltration
Clinicopathological features according to CD208-positive and S100-positive cells’ infiltration
According to the infiltration degree of CD208-positive cells, clinicopathological features were assessed between high and low CD208-positive cells groups. The degree of CD208-positive cell infiltration in intratumoral and peritumoral areas did not correlate with the depth of invasion and lymph node metastases. Only tumor histology significantly correlated with CD208 cell infiltration in intratumoral and peritumoral areas (p < 0.05) (Table 3). S100-positive cell infiltration significantly and positively correlated with the depth of invasion and nodal involvement, respectively (p < 0.05) (Table 4).
Table 3.
Correlation between CD208-positive cell infiltration and clinical factors
| Clinical factors | CD208 infiltration degree | p value | |
|---|---|---|---|
| High n = 36 |
Low n = 92 |
||
| Intratumoral infiltration | |||
| Gender | |||
| Male | 23 | 69 | N.S. |
| Female | 13 | 23 | |
| Tumor depth | |||
| T1 | 12 | 31 | N.S. |
| T2 | 11 | 34 | |
| T3 | 13 | 27 | |
| Nodal involvement | |||
| Yes | 17 | 52 | N.S. |
| No | 19 | 40 | |
| Lymphatic invasion | |||
| Yes | 25 | 61 | N.S. |
| No | 11 | 31 | |
| Venous invasion | |||
| Yes | 18 | 46 | N.S. |
| No | 18 | 46 | |
| Histology | |||
| Differentiated | 11 | 48 | <0.05 |
| Undifferentiated | 25 | 44 | |
| Clinical factors | High n = 63 |
Low n = 65 |
p value |
|---|---|---|---|
| Peritumoral infiltration | |||
| Gender | |||
| Male | 47 | 45 | N.S. |
| Female | 16 | 20 | |
| Tumor depth | |||
| T1 | 21 | 22 | |
| T2 | 22 | 23 | N.S. |
| T3- | 20 | 20 | |
| Nodal involvement | |||
| Yes | 36 | 33 | N.S. |
| No | 27 | 32 | |
| Lymphatic invasion | |||
| Yes | 44 | 42 | N.S. |
| No | 19 | 23 | |
| Venous invasion | |||
| Yes | 31 | 33 | N.S. |
| No | 32 | 32 | |
| Histology | |||
| Differentiated | 31 | 28 | <0.05 |
| Undifferentiated | 32 | 37 | |
Table 4.
Correlation between S100-positive cell infiltration and clinical factors
| Clinical factors | S100-positive cells’ infiltration | p value | |
|---|---|---|---|
| High n = 77 |
Low n = 51 |
||
| Gender | |||
| Male | 32 | 66 | N.S. |
| Female | 19 | 17 | |
| Depth | |||
| T1 | 20 | 26 | <0.05 |
| T2 | 16 | 29 | |
| T3 | 15 | 22 | |
| Nodal metastasis | |||
| Yes | 26 | 36 | <0.05 |
| No | 25 | 41 | |
| Lymphatic invasion | |||
| Yes | 38 | 50 | N.S. |
| No | 13 | 27 | |
| Venous invasion | |||
| Yes | 27 | 36 | N.S. |
| No | 24 | 41 | |
| Histology | |||
| Differentiated | 26 | 32 | N.S. |
| Undifferentiated | 25 | 45 | |
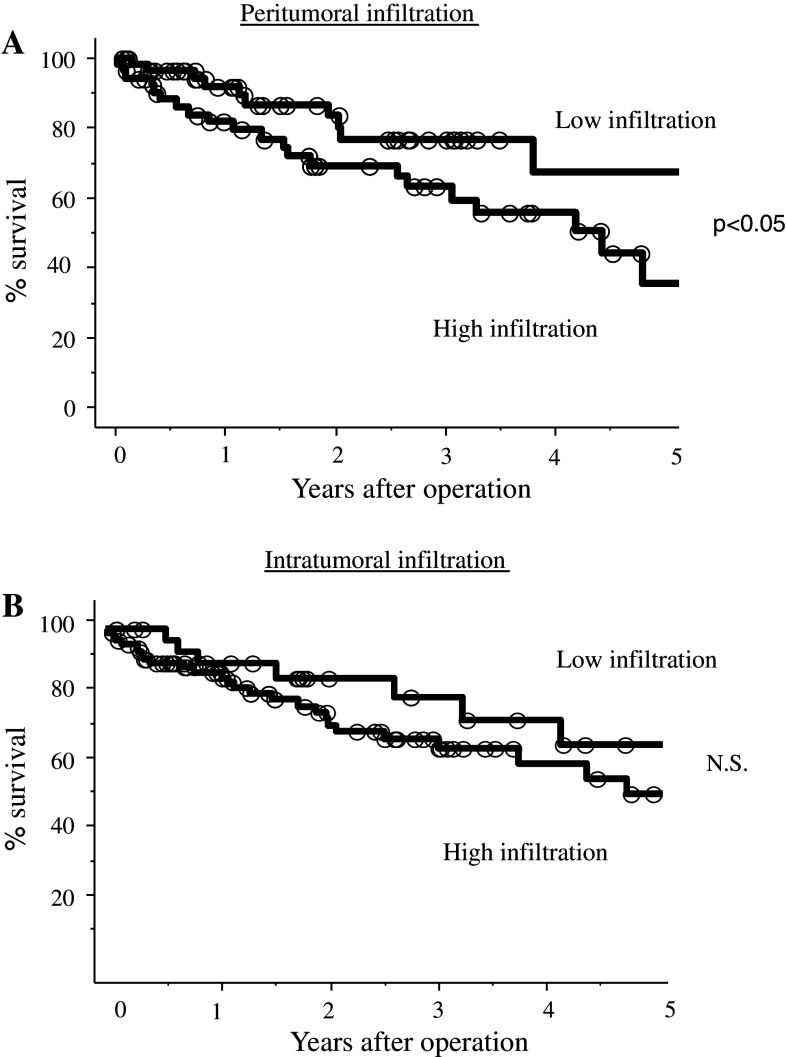
The CD208 high peritumoral infiltration group had a significantly poorer outcome than the CD208 low infiltration group (p < 0.05) (Fig. 5a); however, there was no significant difference in intratumoral CD208-positive cell infiltration (Fig. 5b).
Fig. 5.
Survival curves of gastric cancer patients according to intratumoral CD208-positive cell infiltration: a Peritumoral infiltration and b Intratumoral infiltration. High peritumoral CD208-positive cells infiltration group had significantly better outcome than those with low infiltration (p < 0.05). However, there was no significant difference between two CD208 groups with respect to intratumoral infiltration
Five clinical factors—age, gender, depth of invasion, nodal involvement, and CD208 positivity—were significant prognostic factors by univariate analysis used for multivariate analysis; however, CD208 was not an independent prognostic factor.
Discussion
Galon et al. documented the clinical significance of the Th1 adaptive immunocyte infiltration degree, such as CD3-positive or CD45RO-positive cells in the tumor nest in colon cancer, which is a stronger prognostic factor than the TNM stage [17]. DC infiltration is also regarded as a group of Th1 adaptive immunocytes, and we can therefore reconsider the significance of intratumoral DC infiltration with respect to Th1 adaptive immunocytes.
We analyzed intratumoral CD208-positive cell infiltration in gastric cancer for the first time, and found that the distribution and percentage of CD208-positive cells was similar to previous reports on colon cancer [14]. CD208-positive cells were mainly present in peritumoral and cancer stromal areas. We analyzed the correlation between the positivity of CD208 cells and the clinicopathological features, divided into two areas, and a significance difference in patient survival was found only in peritumoral area, which agreed with a previous report [14].
In this study, we used two types of DC markers. We showed that a new DC marker, CD208-positive cells, had different clinical traits from the previous marker, S100 protein. DC infiltration into a tumor nest is considered to reflect the helper T cell-related immunological reaction; therefore, it is reasonable that the conventional DC marker, S100 protein-positive cell infiltration, positively correlated with better survival, and the same results were found as in other types of tumors.
To the contrary, intratumoral CD208-positive cell infiltration in melanoma is not in accordance with our results. Ladányi et al. demonstrated that CD208-positive cells were associated with activated CD25+ or OX40+ lymphocytes and the degree of CD208 cell infiltration was an independent prognostic factor in melanoma [18]. Elliott et al. showed accumulation of CD208-positive cell in sentinel lymph node was related with prevention of lymph node micro-metastasis in melanoma [19]. However, as for other types of cancers, clinical implication of intratumoral CD208 cell infiltration generally agreed with this study. Treilleux et al. demonstrated a positive correlation between intratumoral CD208 cell infiltration and tumor aggressiveness and adverse outcome in breast cancer [15]. Moreover, Sandel et al. demonstrated a negative prognostic impact of CD208 cell infiltration in colon cancer [14]. Most part of histology of gastric cancer was adenocarcinoma-like colon cancer, which may lead to similar results of intratumoral CD208-positive cell infiltration in colon cancer.
This study indicated that the degree of mature markers of tumor-infiltrating dendritic cells negatively correlated with the surgical outcome in gastric cancer patients who had received curative operation. This negative correlation of CD208-positive cells with clinical prognosis is speculative. Sandel et al. [14] speculated that this negative correlation between mature DCs and the surgical outcome is derived from immunosuppressive cytokines which hinder mature DCs on their way to migrate to secondary lymphoid tissue after antigen capture. van Seters et al. [20] analyzed the distribution pattern of several immunocompetent cells in vulvar neoplasia and demonstrated that the reduction of immature DCs and the influx of mature DCs into tumor nests occurred in an immunosuppressed condition in human papilloma virus infection. Yuan et al. immunohistochemically analyzed several types of DCs in colon cancer and showed that DC types infiltrating tumor nests changed from immature to mature according to the adenoma carcinoma sequence [21]. These speculations may support the inverse significant prognostic factor of CD208-positive cell infiltration.
In conclusion, CD208-positive cells as a marker of mature DCs in gastric cancer showed a different infiltrating degree, pattern, and clinical trait to S100-positive cells as a conventional DC marker. Our study showed that functional subsets of tumor-infiltrating DCs affected local tumor cell–immune cell interactions and correlated with the clinical prognosis of gastric cancer patients in different ways. Immunocyte expression profiling analysis using various DCs seems to be useful as a valuable prognostic tool in the treatment of gastric cancer.
References
- 1.Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H. Surgical treatment for gastric cancer: the Japanese approach. Semin Oncol. 1996;23:360–368. [PubMed] [Google Scholar]
- 2.Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Modern surgery for gastric cancer—Japanese perspective. Scand J Surg. 2006;95:232–235. doi: 10.1177/145749690609500404. [DOI] [PubMed] [Google Scholar]
- 3.Orita H, Matsusaka T, Wakasugi K, Kume K, Fujinaga Y, Fuchigami T, Iwashita A. Clinicopathologic evaluation of recurrence in early gastric cancer. Surg Today. 1992;22:19–23. doi: 10.1007/BF00326120. [DOI] [PubMed] [Google Scholar]
- 4.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Iwashige H, Aridome K, Hokita S, Aikou T. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87–91. doi: 10.1016/S0304-3835(01)00503-1. [DOI] [PubMed] [Google Scholar]
- 5.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, Miyazono F, Hokita S, Aikou T. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23(5A):4079–4083. [PubMed] [Google Scholar]
- 6.Ishigami S, Natsugoe S, Matsumoto M, Okumura H, Sakita H, Nakashima S, Takao S, Aikou T. Clinical implications of intratumoral dendritic cell infiltration in esophageal squamous cell carcinoma. Oncol Rep. 2003;10:1237–1240. [PubMed] [Google Scholar]
- 7.Ishigami S, Aikou T, Natsugoe S, Hokita S, Iwashige H, Tokushige M, Sonoda S. Prognostic value of HLA-DR expression and dendritic cell infiltration in gastric cancer. Oncology. 1998;55:65–69. doi: 10.1159/000011837. [DOI] [PubMed] [Google Scholar]
- 8.Hillenbrand EE, Neville AM, Coventry BJ. Immunohistochemical localization of CD1a-positive putative dendritic cells in human breast tumours. Br J Cancer. 1999;79(5–6):940–944. doi: 10.1038/sj.bjc.6690150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallal RM, Christakos P, Lee K, Egawa S, Son YI, Lotze MT. Paucity of dendritic cells in pancreatic cancer. Surgery. 2002;131:135–138. doi: 10.1067/msy.2002.119937. [DOI] [PubMed] [Google Scholar]
- 10.Ambe K, Mori M, Enjoji M. S-100 protein-positive dendritic cells in colorectal adenocarcinomas. Distribution and relation to the clinical prognosis. Cancer. 1999;63:496–503. doi: 10.1002/1097-0142(19890201)63:3<496::AID-CNCR2820630318>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Nakakubo Y, Miyamoto M, Cho Y, Hida Y, Oshikiri T, Suzuoki M, Hiraoka K, Itoh T, Kondo S, Katoh H. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer. 2003;89:1736–1742. doi: 10.1038/sj.bjc.6601331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Aït-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/S1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 13.Salaun B, de Saint-Vis B, Clair-Moninot V, Pin JJ, Barthélemy-Dubois C, Kissenpfennig A, Peronne C, Bates E, Mattei MG, Lebecque S. Cloning and characterization of the mouse homologue of the human dendritic cell maturation marker CD208/DC-LAMP. Eur J Immunol. 2003;33:2619–2629. doi: 10.1002/eji.200324175. [DOI] [PubMed] [Google Scholar]
- 14.Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn CM, Ensink NG, Tollenaar RA, van de Velde CJ, Kuppen PJ. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res. 2005;11:2576–2582. doi: 10.1158/1078-0432.CCR-04-1448. [DOI] [PubMed] [Google Scholar]
- 15.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 16.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma, 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/PL00011681. [DOI] [PubMed] [Google Scholar]
- 17.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 18.Ladányi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Tímár J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott B, Scolyer RA, Suciu S, Lebecque S, Rimoldi D, Gugerli O, Musat E, Sharma RN, Lienard D, Keilholz U, Testori A, Eggermont A, MacKie R, Robert C, Cook M, Thompson JF, Angevin E, Spatz A. A long-term protective effect of mature DC-LAMP+ dendritic cell accumulation in sentinel lymph nodes containing micrometastatic melanoma. European Organization for Research and Treatment of Cancer Melanoma Group. Clin Cancer Res. 2007;13:3825–3830. doi: 10.1158/1078-0432.CCR-07-0358. [DOI] [PubMed] [Google Scholar]
- 20.van Seters M, Beckmann I, Heijmans-Antonissen C, van Beurden M, Ewing PC, Zijlstra FJ, Helmerhorst TJ, KleinJan A. Disturbed patterns of immunocompetent cells in usual-type vulvar intraepithelial neoplasia. Cancer Res. 2008;68:6617–6622. doi: 10.1158/0008-5472.CAN-08-0327. [DOI] [PubMed] [Google Scholar]
- 21.Yuan A, Steigen SE, Goll R, Vonen B, Husbekk A, Cui G, Florholmen J. Dendritic cell infiltration pattern along the colorectal adenoma-carcinoma sequence. APMIS. 2008;116:445–456. doi: 10.1111/j.1600-0463.2008.00879.x. [DOI] [PubMed] [Google Scholar]