Abstract
Tumors contain many antigens that may be recognized by the immune system. It is not known whether these antigens, and the epitopes within these antigens, can all be recognized by the anti-tumor immune response or if such responses are restricted to a few dominant epitopes. Effector function of endogenous cytotoxic T lymphocytes (CTL) generated during tumor progression has previously been assessed by indirect, ex vivo assays, which often focused on a single antigen. Therefore, we evaluated the endogenous in vivo CTL response to multiple neo tumor antigens using murine Lewis lung carcinoma tumor cells transfected with ovalbumin or a polyepitope construct. Both express multiple MHC class I-restricted epitopes. Ovalbumin contains a known hierarchy of epitopes for given MHC molecules, whilst the polyepitope expresses a number of dominant epitopes. We show that as tumors progress, potent effector CTL are generated in vivo that are restricted to dominant epitopes; we did not see the responses to subdominant or cryptic epitopes. Our data show that the CTL recognizing tumor antigens vary in their lytic capacity, as the CTL responding to two of the four epitopes were particularly potent killers. The presence of these effector CTLs did not prevent tumor growth. However, intra-tumoral IL-2 treatment altered the potency, but not the hierarchy, of these CTL such that they mediated tumor regression. These results have implications for immunotherapy protocols.
Keywords: Tumor Immunity, T cells, Cytotoxic, Antigens/peptides/epitopes
Introduction
Anti-cancer immunotherapy strategies are based upon the underlying hypothesis that following immune recognition of tumor antigens, CD8+ cytotoxic T lymphocytes (CTL) will destroy solid tumors. However, results of clinical trials targeting specific tumor antigens have generally been disappointing. We speculated that this is because the tumor-bearing host already has an immunological relationship with a progressing tumor. This relationship is likely to be complex and includes the generation of CTL responses to multiple, class I-restricted epitopes co-expressed by the tumor. These epitopes can originate from a single antigenic molecule (intra-molecular epitopes) or from unrelated co-expressed molecules (inter-molecular epitopes) expressed by a tumor. Immune responses to multiple, co-expressed, tumor epitopes is an understudied area despite studies showing that a wide range of carcinomas express many antigens [1–8]. Therefore, we studied the relationship between the immune system and multiple antigens co-expressed by a progressing tumor and the consequent CTL response.
Tumor antigens and immunodominance
When epitopes originate from a single (intra-molecular) antigen the immune system often focuses on one dominant epitope [9]. In some instances weaker responses are seen towards other epitopes, termed subdominant (reviewed in [10]). Responses to subdominant epitopes have been shown to be biologically significant in viral infections [11, 12] and in autoimmune models [13, 14]. Both situations are relevant to tumor immunology as tumors can express foreign antigens (including those with viral origins) and self-antigens. Furthermore, CTL to subdominant peptides have been detected during tumor growth [15, 16] and, when these peptides are used as immunogens, they can confer protection [17, 18]. Importantly, some immunotherapeutic regimens have revealed CTL responses to weaker tumor antigens (via a process described as epitope spreading) during tumor regression [18–25]. In eptiope spreading, epitopes distinct from and non-cross-reactive with an inducing epitope become targets of an evolving immune response. The additional epitopes may be of intra-molecular or inter-molecular origins. Thus, optimal immunotherapeutic regimens might initiate a cascade of events that lead to T-cell cross priming against a range of tumor-associated epitopes. However, studies looking at hierarchical CTL responses to tumor antigens have generally been limited to in vitro systems [26] and no studies, until now, had assessed in vivo tumor antigen presentation and its relationship to the generation and functional activity of CTL specific for subdominant or cryptic peptides. We believe that it is essential to understand how the immune system responds to multiple tumor-derived epitopes expressed by a growing solid tumor prior to commencing any immunological-based therapy. Therefore, this study examined the in vivo response of host CTL to multiple, dominant and subdominant antigens co-expressed by a developing tumor.
Pre-existing anti-tumor CTLs
Immune responses to immunotherapeutic regimens may be confounded by a pre-existing anti-tumor CTL response. It has been proposed that T cells responding to dominant epitopes maintain their status by ‘immunodomination’; i.e., CD8+ T cells specific for immunodominant antigens suppress the immunogenicity of subdominant antigens [10]. Interestingly, ‘superdominance’ has also been reported where the CTL response focused on only two of five pooled immunodominant inter-molecular epitopes [27]. Hence, when multiple antigens are co-expressed by a tumor, the presence of one or more immunodominant, or even superdominant, epitope/s may influence the immune response to ‘weaker’ epitopes during tumor progression, or when immunotherapies targeting tumor antigens are used.
We have recently shown that the tumor-associated antigens are continuously presented in sentinel lymph nodes as tumors progress, and that even when a potent, endogenous, CTL response is generated to the immunodominant epitope of a single tumor antigen, the effect on tumor growth is minimal [28, 29]. We have also shown that delivering IL-2 directly into a tumor enhances this CD8+ T cell response to levels that mediate tumor regression under certain conditions; however, we only looked at responses to a single MHC class I-restricted tumor epitope [30]. This paper extends these studies, and examines CTL responses to subdominant epitopes expressed within the same tumor antigen, as well as to several co-expressed, ‘dominant’, tumor-associated epitopes.
We show that the endogenous anti-tumor CTL response that evolves during tumor progression recognizes many tumor antigens; however, this response focuses upon immunodominant epitopes and may have features of immunodomination or even superdominance. After intra-tumoral (i.t.) IL-2 therapy this response is enhanced to levels that mediate tumor regression. However, the hierarchy remains unaltered and there is no evidence of epitope spreading.
Material and methods
Mice
Female C57BL/6 (H-2b) mice were obtained from the Animal Resources Centre (Murdoch, WA, Australia) and maintained under standard housing conditions in the QEII Medical Centre animal holding facility. The OT-1 (H-2b) TCR transgenic mouse line, expressing a TCR recognizing the dominant H-2b restricted OVA epitope (SIINFEKL) [31] was kindly supplied by Dr. F. Carbone and Dr. W. Heath (University of Melbourne, Australia and Walter Eliza Hall Institute, Australia, respectively).
Murine tumor cell lines
The murine LL (LL2 or CRL-1642) and thymoma cell lines, EL4 (TIB-39) and EG7 (CRL-2113), used in this study were all obtained from American Type Culture Centre (ATCC, Manassas, VA, USA). LL, EL4 and EG7 are H-2b restricted tumors derived from C57BL/6 mice. EG7 yields secreted OVA and has been described previously [32].
Cell culture and maintenance
All cell lines were maintained in a complete media (CM) which consisted of RPMI 1640 supplemented with 10% FCS (both supplied by Gibco, Invitrogen, Grand Island, NY, USA), 60 mg/l penicillin (CSL, Melbourne, Australia), 48 μg/ml gentamicin (Pharmacia, Bentley, Australia), 5×10−5 M 2-ME (Merck, West Point, PA, USA) and 20 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES; Sigma-Aldrich, St. Louis, MO, USA) pH 7.2. Transfected cell lines were maintained in a CM supplemented with 400 μg/l of the neomycin analog G418 (Geneticin, Gibco, In vitrogen). Cells were cultured at 37°C in a 5% CO2 humidified atmosphere.
Plasmids and transfections
The DNA encoding the secreted form of OVA (sOVA) placed under the control of the CMV promoter in the mammalian expression plasmid pCI (kindly donated by Dr. Andrew Lew, Walter and Eliza Hall Institute, Australia) has been described previously [33]. The polyepitope DNA encoding ten minimal MHC class I epitopes was inserted in the PEFBos plasmid under the control of the human EF-1α chromosomal promoter gene [34].
Lewis lung carcinoma transfectants were prepared by co-transfecting the LL parental cell line with cDNA encoding for sOVA [31] or the polyepitope and the neomycin selection marker, using FuGENETM 6 Transfection Reagent (Boehringer Manheim). Polyepitope and sOVA expressing lines growing in CM were cloned by limiting the dilution three and four times respectively. Clones were screened by an OVA specific ELISA or an in vitro CTL 51Cr release assay.
In vivo tumor growth and immunological protection experiments
Mice were injected subcutaneously (s.c.) at day 0 with 5×105 viable cells per mouse in 100 μl PBS, and tumor development monitored using microcallipers. The immunogenicity of the selected tumor cell lines was determined by s.c. injecting mice with 106 irradiated (20,000 rad total dose) tumor cells in 100 μl PBS. Fourteen to twenty-four days later, a secondary s.c. challenge with viable tumor cells (5×105 cells per mouse in 100 μl PBS) was given. Mice were regularly checked and sacrificed when the tumor dimension reached 100 mm2 as per University of Western Australia (UWA) ethics approval.
Peptides
The dominant peptide OVA257–264 (SIINFEKL), subdominant peptides OVA55–62 (KVVRFDKL), and OVA176–183 (NAIVFKGL) and the cryptic peptide OVA11–18 (CFDVFKEL), as well as two of the polyepitope peptides Sendai virus nucleoprotein324–332 (FAPGNYPAL) and Adenovirus 5 E1A234–243 (SGPSNTPPEI), were manufactured by the Centre for Cell and Molecular Biology (University of Western Australia, Perth) at a purity of >89%. The Influenza nucleoprotein366–374 (ASNENMDAM) was manufactured by Auspep (Parkerville Victoria) at a purity of >95%.
In vitro CTL assays
Effector cells were prepared from the spleens of either OT-I TCR mice (used to screen polyepitope transfected clones), or pooled experimental groups of C57BL/6 non-transgenic mice. The effector cells were expanded in vitro in a 1:1 ratio with splenic cells from naive mice as a source of normal APC that were pulsed for 90 min with 5 μg/ml of peptide and irradiated at 2,100 rad. Excess peptide was removed by washing, before APCs were added to the effector cells and incubated at 37°C for 5 days in CM.
Target cells included our sOVA or polyepitope transfected LL tumor clones, EG7, or EL4 cells pulsed with 10 μg/ml of peptide, were labeled with 100 μCi of 51Cr (Amersham Health PLC, North Ryde, NSW, Australia) for 90 min and washed before use. Effector cells were added to corresponding targets at varying effector to target cell ratios and incubated at 37°C for 4 h. Supernatants were collected after centrifugation and 51Cr release determined (Packard Topcount, Zurich, Switzerland). The mean of duplicate samples was calculated and the percentage of specific 51Cr release was determined as follows:
 |
Experimental 51Cr release represented counts from target cells mixed with effector cells, control 51Cr release represented counts from targets incubated with medium alone (spontaneous release), and maximum 51Cr release represents counts from targets exposed to 5% triton X-100. Note that the results from the in vitro CTL assay were analyzed with two different methods; i.e., comparing % lysis of targets and lytic units to achieve 20% lysis/106 effector cells. Both approaches showed a similar outcome (data not shown).
OVA levels determined by ELISA
Microtiter plates were coated overnight with 2 μg/ml anti-OVA antibody (Cappel-Organon Technica, Durham, NC, USA) at 4°C, blocked with PBS + 1% BSA (Sigma-Aldrich) and washed with PBS + 0.05% Tween 20 (Sigma-Aldrich). Samples were serially diluted in PBS + 0.05% Tween 20 and 1% BSA. OVA (chicken OVA fraction V; Sigma-Aldrich) was used as a standard curve. The ELISA plate was incubated overnight at 4°C, followed by sequential incubations at 37°C for 1 h each in rabbit anti-OVA (2 μg/ml; Harlan Sera-Lab, Loughborough, England), anti-rabbit horseradish peroxidase (HRP) (DAKO, Glostrup, Denmark), and the HRP substrate 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (Sigma-Aldrich), washing thoroughly between incubations with PBS + 0.05% Tween20. Quantification of OVA was determined by a Spectromax 250 spectrophotometer, using Softmax Pro, version 2.2.1, software (both Molecular Devices, Sunnyvale, CA, USA).
In vivo analysis of tumor antigen cross-presentation
5,6-Carboxy-succinimidyl-fluorescein-ester (CFSE; Molecular Probes, Eugene, OR, USA) labeling was performed as previously described [35]. Briefly, lymph node (LN) cells from TCR transgenic OT-1 mice were resuspended in RPMI at 2×107cells/ml and incubated with 2.5 μM CFSE (Stock solution 5 mM in DMSO) for 10 min at room temperature. Cells were washed through an FCS underlay twice and PBS alone twice, and 107 cells injected i.v. into each recipient mouse. CFSE-labeled cells were recovered from secondary lymphoid organs 3 days post-adoptive transfer and CD8+ cells analyzed by FACS analysis.
In vivo analysis of CTL function
In this study we used a modified version of a ‘2 peak’ (or 2 populations) in vivo CTL assay [36] with which we have extensive experience [28–30, 37]. Here, we used our previously described ‘3 peak’ in vivo CTL assay [37]. Briefly, erythrocytes from C57BL/6 pooled spleen and LN cell suspensions were lysed, the cells washed and then divided into three populations. One population was pulsed with 10−6 M of one of the peptides which we are interested in and a second population pulsed with 10−6 M of another peptide (as stated in the results section) for 90 min at 37°C, then washed in PBS and labeled with high (5 μM) or low (0.05 μM) CFSE concentrations, respectively. Control uncoated target cells (the third population) were labeled with an intermediate concentration of CFSE (0.5 μM). For i.v. injection 1×107 cells of each population was mixed in 200 μl PBS per recipient mouse. Specific in vivo cytotoxicity was determined by collecting the DLN, non-DLN and spleen from recipient mice 16–18 h post-i.v. injection and differentially labeled fluorescent target cell populations detected by flow cytometry. The ratio between the percentages of uncoated versus peptide-coated targets (CFSEInt/CFSEhigh) was calculated to obtain a numerical value of cytotoxicity. Further controls included naïve mice and parental tumor treated recipient mice. To normalize data allowing inter-experimental comparisons, ratios were calculated between the percentages of peptide-coated targets in control mice versus tumor-bearing mice.
FACS analysis
Tissues were prepared as single cell suspensions in PBS supplemented with 2% FCS for CFSE analysis. PE-conjugated mAb anti-CD8 (clone 53-6.7 PharMingen, San Diego, CA, USA) was used for two-color analysis. Analysis of 2000 (or more) CFSE-labeled control targets or CD8+ cells was performed on a FACScan (Becton Dickinson, Mountain View, CA, USA) using Cell Quest software.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was prepared from tumor cells using Ultraspec according to the manufacturer’s directions (Fisher Biotec, Australia). Preparation of cDNA by reverse transcription (RT) of 2 μg of RNA was performed using the Qaigen Omniscript RT kit (Qaigen Pty Ltd, Australia), and Oligo(dt)15 primer (Promega, Madison, WI, USA). For sOVA, a 174 bp amplicon was generated using the nucleotide primers (5′–3′) AAG GAT GAA GAC ACA CAA GCAA and CAG GCA ACA GCA CCA ACA. For the polyepitope a 228 bp amplicon was generated using the nucleotide primers (5′ – 3′) GGA TCC CCA CCA TGT CTA GA and GGC GCT TGG GAT GTA GCT CA. The cDNA was co-amplified with GAPDH using nucleotide primers (5′–3′) CGG AAG GGG CGG AGA TGA TGA and GAA GGT CGG TGT GAA CGG ATT to yield a 362 bp amplicon. PCR was performed on cDNA samples using the Quantitch SYBR® Green PCR Master Mix and run on a Real Time PCR Biorad Icycler IQ instrument for 40×30 s cycles at each of 95, 60 and 72°C and 95, 52 and 72°C for OVA and polyepitope respectively. Samples were visualized on a 3% agarose gel (Ameresco, Solon, OH, USA) and stained with ethidium bromide (Sigma, St Louis, MO, USA).
Intra-tumoral injection of IL-2
Recombinant human IL-2 (Cetus Corporation, Emeryville, CA, USA) was diluted in PBS to the required concentration and 100 μl injected via a 26 g needle directly into tumors. This was performed three times per week.
Statistical analysis
Statistically significant differences were evaluated by the Student’s t test using GraphPad PRISM (San Diego, CA, USA) software.
Results
Transfected clones expressing a neo-tumor antigen grow in syngeneic mice
Expression of cDNA coding for either sOVA or the polyepitope in transfected LL clones was confirmed by RT-PCR (Fig. 1a). Transfected LL clones were screened for their susceptibility to lysis by OVA-specific CTL using an in vitro CTL assay (data not shown). This assay confirmed that SIINFEKL (the dominant class I-restricted epitope of OVA) is transported to the cell surface in MHC class I molecules to levels that render the tumor cells recognizable by the CTL. Effector cells for this assay were in vitro activated SIINFEKL-specific CD8+ CTLs prepared from OT-1 mice. The polyepitope and sOVA LL clones most efficiently lysed by these CTL (shown in Fig. 1b) were also assessed for their capacity to grow in normal C57BL/6 mice following s.c. injection of tumor cells (Fig. 1c, d). Tumor incidence at day 22 for LL and LLsOVA was 100%, whilst LLpoly tumors progressed in an average of 85% of mice at the same time point (Fig. 1d). Both LLsOVA and LLpoly grew at a slightly slower rate than the parental LL cell line (Fig. 1c). To confirm that the LLsOVA cell line secreted OVA, supernatants were removed from LLsOVA cells and OVA (detected by ELISA) was determined to be 0.28 pg/cell/24 h. These cell lines were used for the experiments described below.
Fig. 1.
Characterization of polyepitope and secreted OVA LL transfectants. The parental Lewis lung (LL) cell line was transfected with cDNA coding for secreted OVA (LLsOVA), or the polyepitope (LLpoly), and the neomycin selection marker. a Specific PCR product could be demonstrated for both cell lines using OVA and polyepitope-specific primers, GAPDH primers were included as controls. b The resulting clones were screened using primed OVA-specific effector T cells from OT-1 mice in a 51Cr release assay; the LLsOVA, and LLpoly transfectants, as well as the SIINFEKL-pulsed LL controls, were readily lysed. c, d 5×105 viable LL, LLpoly or LLsOVA tumor cells were s.c inoculated into naïve C57BL/6 and their tumor growth rates compared. c The growth kinetics of LLpoly and LLsOVA were slower than the parental line LL. d All LL and LLsOVA inoculated mice developed tumors. 15% of LLpoly injected mice did not develop tumors
Antigen presentation of the neo tumor antigen (SIINFEKL) occurs early in tumor development
Since both the LLsOVA and LLpoly tumor models express SIINFEKL as a tumor antigen, the first series of experiments assessed whether or not SIINFEKL is presented to the host immune system during tumor progression. Proliferation of adoptively transferred, CFSE-labeled, OT-1 lymphocytes (indicating antigen presentation) was detected 8–9 days after tumor cell inoculation in the DLN of mice bearing the transfected tumors, and not in mice given the parental tumor (shown in Fig. 2 a, b).
Fig. 2.
Tumor antigen is presented in tumor-draining lymph nodes. CFSE-labeled, SIINFEKL-specific T cells from OT-1 mice were adoptively transferred at days 5 or 6 into mice inoculated s.c on day 0 with Lewis lung, LLsOVA or LLpoly tumor cells. a CFSE+CD8+ cells were reisolated from the DLN 3 days post-transfer for analysis. Representative FACs profiles from individual mice are shown (n=6 mice/time point) b The mean (±SE) percentage of CFSE+CD8+ cells proliferating in the DLN is indicated on the histogram: there was no statistical difference between LLsOVA and LLpoly tumor-bearing mice
This indicates that SIINFEKL is presented in the early stages of tumor development, even when the LLsOVA or LLpoly tumors are not palpable. These results confirm that both transfected tumor cell lines express SIINFEKL as a tumor antigen, and that this epitope is readily transported to the DLN, and presented to naïve T cells within the first 2 weeks of tumor growth.
The next series of experiments investigated whether or not an in vivo effector CTL response was generated to the multiple neo tumor epitopes, including SIINFEKL, expressed by LLsOVA and LLpoly tumor cells.
Tumor antigen-specific CTL activity is focused on the dominant epitope in vivo
The OVA protein consists of a number of epitopes that bind to MHC class I (Kb) molecules; SIINFEKL is recognized as dominant, whilst KVVRFDKL and NAIVFKGL have been described as subdominant [38]. Peptides that are not generally seen by the immune system are described as cryptic; such as CFDVFKEL in OVA [26]. Nonetheless, CFDVFKEL does bind to Kb molecules and has the potential to be recognized by the immune system [26]. We used the LLsOVA model, to assess whether or not endogenous CTL responses were generated in vivo to these epitopes using CFSE labeled peptide-pulsed targets that were adoptively transferred into the host 16 h prior to the collection for analysis. The value of this assay is that it provides an in vivo readout of CTL activity (shown as percent of peptide-pulsed targets lysed). CTL activity directed against SIINFEKL could be detected 7 days after tumor cell injection in all lymphoid compartments investigated; shown in Fig. 3a, b.
Fig. 3.
Effector CTLs recognizes the dominant and not the subdominant epitopes in vivo. Targets cells pulsed separately with the dominant OVA epitope (SIINFEKL; SIIN), or the two subdominant OVA epitopes (KVVRFDKL and NAIVFKGL; KVV and NAIV respectively) were differentially labeled with CFSE. Control uncoated target cells were labeled with an intermediate concentration of CFSE (shown as asterisk). 107 cells of each population were pooled before i.v. injection into recipient mice that had been injected s.c. with 5×105 LLsOVA tumor cells 8 days earlier. Note that separate groups of mice received either three-pooled targets (a three-peak assay) consisting of SIIN, uncoated and NAIV targets, or two-pooled targets (a two-peak assay) consisting of uncoated and KVV targets. Specific in vivo cytotoxicity was determined by collecting DLN, contralateral LN and spleens from recipient mice 16 h after i.v. injection, for analysis. a Representative FACS profiles of target cells from naïve and tumor-bearing individual mice 16 h after i.v. injection are shown. b In vivo lysis is restricted to the dominant epitope (SIINFEKL). Pooled data, shown as mean (± SE), is from two experiments with three mice/peptide
There appeared to be an occasional weak response to the subdominant peptides, NAIVFKGL and KVVRFDKL, however the responses were not significantly above background levels (Fig. 3c, d). Note that we have observed a weak, but reproducible, response to the KVVRFDKL peptide in mice given 200 μg of OVA in incomplete freunds adjuvant (IFA) [37] confirming that this assay is sensitive enough to detect responses to subdominant epitopes. The cryptic peptide was not included in the in vivo studies.
Multiple tumor antigens can be recognized simultaneously by the immune system
A notable difference between the sOVA and polyepitope models is the relationship between the epitopes that are co-expressed. In the sOVA model there is a clear hierarchy of dominant, subdominant and cryptic epitopes for intra-molecular OVA-derived peptides. However, each of the epitopes in the polyepitope model is considered dominant in its original context, i.e., as a viral antigen (SGPSNTPPEI, FAPGNYPAL and ASNENMDAM) and a protein antigen (SIINFEKL). Interestingly, responses to these epitopes show a clear hierarchy in polyepitope vaccination experiments [39, 40]. In our polyepitope tumor model, endogenous CTL activity was clearly directed against three of the four polyepitopes recognized by either the Kb or Db C57BL/6 MHC class I alleles. In vivo CTL activity was systemic at day 8 of tumor growth (Fig. 4a, b). The highest level of CTL activity was seen against SIINFEKL-specific targets (87.8±6.2%) followed by FAPGNYPAL (75.5±4.1%) and SGPSNTPPEI coated targets (52±6.4%). The response to ASNENMDAM was not significantly above background levels seen in the parental LL tumor-bearing mice. Thus SIINFEKL and SGPSNTPPEI appear to the dominant Kb and Db epitopes respectively. These data confirm that the immune system recognizes multiple, co-expressed tumor antigens and suggests that a hierarchy may be established when these epitopes are presented as tumor antigens.
Fig. 4.
In vivo CTL activity is directed against three of the polyepitopes expressed by Llpoly. Targets cells were pulsed separately with each polyepitope peptide and differentially labeled with CFSE. ASENMDAM, FAPGNYPAL, SGPSNTPPEI and SIINFEKL are referred to as ASN, FAP, SGP and SIIN respectively. The two control uncoated target cell populations were labeled with an intermediate concentration of CFSE (shown as asterisk). 107 cells of each population were pooled as two groups with two peptides/group (i.e., a three-peak assay) before i.v. injection into recipient mice that had been injected s.c. with 5×105 LLpoly tumor cells 8 days earlier. Specific in vivo cytotoxicity was determined 16 h after i.v. injection by flow cytometry. a FACS histograms are the DLN from representative individual tumor-bearing animals. b Pooled data, shown as mean (± SE) is from two experiments each with three mice/target peptide group
A similar CTL response pattern is seen in mice that did not develop tumors
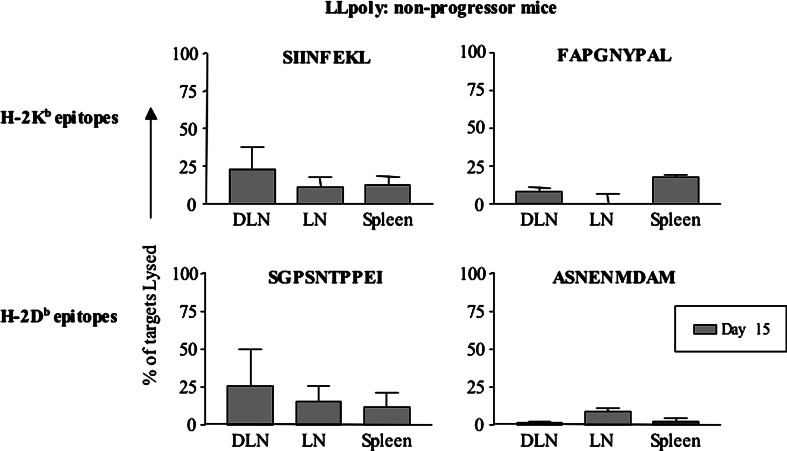
As previously mentioned, tumor incidence in the LLpoly model is 85%. We considered that tumor free mice might have developed a potent anti-tumor immune response with significantly higher CTL activity than that seen in the progressors. We were surprised to find that the mice which did not develop tumors (non-progressors) at day 15 (note that earlier time points could not be assessed as it was not clear which animals would develop tumors) showed weak systemic killing of SIINFEKL and SGPSNTPPEI peptide-coated targets, in association with low levels of killing of FAPGNYPAL coated targets and barely detectable killing of ASNENMDAM coated targets (Fig. 5). Interestingly, the hierarchy was the same with SIINFEKL being the dominant Kb epitope and SGPSNTPPEI being the dominant Db epitope. These data imply that the LLpoly model generates an immune response that is on the threshold of offering protection but only achieves this infrequently.
Fig. 5.
An equivalent in vivo CTL response is seen in mice that did not develop LLpoly tumors. Mice that had not developed tumor by day 14 were included in the in vivo CTL assay and showed a similar response to mice with tumor at the same time point (n=2 for SIINFEKL and SGPSNTPPEI, and n=3 for FAPGNYPAL and ASNENMDAM)
Precursor CTL are restricted to the dominant epitope of an intra-molecular tumor antigen
The in vivo CTL assay results demonstrate that functional, tumor-specific, effector CTLs can be detected in tumor-bearing mice. A second approach used to investigate CTL populations is the 51Cr release in vitro CTL assay. In this assay splenocytes from tumor-bearing mice are restimulated in culture with APC pulsed with the relevant epitope for 5 days revealing precursor CTL populations, as well as the recall response. Seven days after LLsOVA tumor cell inoculation CTLs to SIINFEKL were readily detected (Fig. 6a). A very weak response, although not statistically significant, was observed to the subdominant epitope KVVRFDKL when used as a target and restimulation peptide at day 7 (Fig. 6b). No response was seen for the cryptic peptide (Fig. 6c).
Fig. 6.
Precursor CTLs is limited to the dominant peptide in LLsOVA tumor-bearing mice. Splenocytes taken from mice 7 days after LLsOVA s.c. injection were restimulated in vitro with SIINFEKL (SIIN), KVVRFDKL (KVV) or CFDVFKEL (CFD) peptide. Five days later, a standard 51Cr release in vitro CTL assay was performed using EL4 target cells pulsed with the same peptides. EG7 was used as a positive target control; data not shown. Negative controls included LL tumor-bearing mice. a–c Data (shown as mean ± SE) is from two experiments (10 and 6 mice/experiment) at an effector to target ratio of 25:1. Restimulation with SIIN generated CTL that lysed SIIN-coated targets, whilst restimulation with KVV or CFD did not generate KVV- or CFD-specific CTL respectively (shown in bold). Italics show ‘bystander’ CTL responses when peptides other than the restimulation peptide are used as targets. Statistically significant differences between LL and LLsOVA tumor-bearing mice are indicated by an asterisk (P=0.002)
We assessed whether the splenoctyes from LLsOVA-bearing mice that had been restimulated with SIINFEKL generated a ‘bystander effect’ to the weaker epitopes, i.e., when KVVRFDKL and CFDVFKEL were target peptides (indicated in italics in Fig.6a). As expected, we saw a response only to SIINFEKL and not to the subdominant or cryptic epitopes. Similarly, restimulation with the subdominant or cryptic peptides did not enhance a CTL response to the other peptides (Fig. 6b, c). These results confirm that CTL are only generated against SIINFEKL when OVA is a tumor antigen.
Precursor CTL responses are generated to all dominant tumor antigens
Splenocytes from LLpoly-bearing mice restimulated with each peptide readily lyse targets labeled with the same peptide (including ASNENMDAM; Fig. 7). The level of CTL activity for each peptide varied from 40 to 20% killing. This result supports those seen with the in vivo CTL studies (refer to Fig. 4), with the exception of the ASNENMDAM peptide. Importantly, this assay shows that ASNENMDAM is indeed processed and presented in vivo to levels that generate a precursor CTL population, however, these CTLs are not functional in vivo after an encounter with their cognate antigen.
Fig. 7.
Potent precursor CTLs are detected to all epitopes in LLpoly tumor-bearing mice. Splenocytes from LLpoly-bearing mice were in vitro restimulated with SIINFEKL (SIIN), FAPGNYPAL (FAP), SGPSNTPPEI (SGP) or ASNENMDAM (ASN) peptides and a 51Cr release in vitro CTL assay performed using EL4 target cells pulsed with the same peptides. Controls included mice given LL. Pooled data from two experiments at a 25:1 effector to target ratio is shown as mean ± SE. Significant differences (P<0.05 indicated by an asterisk) were seen between LL versus LLpoly tumor-bearing mice for the restimulation to target pairs, SIIN–SIIN (a), FAP–FAP (b), SGP–SGP (c) and ASN–ASN (d) all highlighted in bold. Italics show ‘bystander’ CTL responses when peptides other than the restimulation peptide are used as targets
Interestingly, a ‘bystander effect’ was seen when FAPGNYPAL and ASNENMDAM were used as restimulation peptides; i.e., detectable responses were seen to the other epitopes (indicated in italics in Fig. 7). Note that these were the two ‘weaker’ in vivo CTL responders. This did not occur when SGPSNTPPEI and SIINFEKL were the restimulation peptides, as the in vitro response remained focused on these two epitopes.
CTL responses to multiple tumor antigens can be enhanced to levels that mediate protection and offer curative therapy
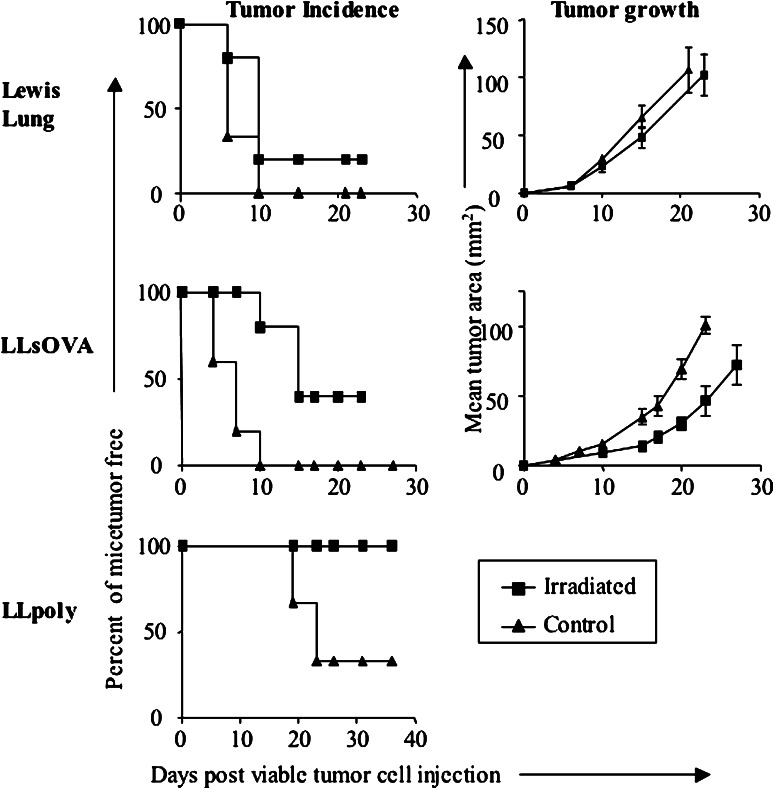
The data described above suggests that the presence of tumor-specific CTL may not prevent tumors from progressing. To confirm this, mice were vaccinated with irradiated tumor cells and challenged 14–24 days later with viable tumor cells. Figure 8 shows that 80% of mice vaccinated with LL do not generate a protective response, however, 37.5% of mice given LLsOVA were protected from LLsOVA. In contrast, mice vaccinated with LLpoly offered complete protection against subsequent challenge with LLpoly. These data show that generating a CTL response to multiple dominant tumor antigens can reliably offer protection.
Fig. 8.
Vaccination with irradiated tumor cells offers protection to mice if the tumor cell expresses multiple dominant antigens. Mice were given 106 irradiated (20,000 rad) tumor cells s.c. in PBS and rechallenged with 5×105 viable tumor cells 12–24 days later (LL=12 days, LLsOVA=18 days, LLpoly=24 days). Tumor incidence and mean tumor area were measured. Graphs represent mean tumor area in tumor-bearing mice
Thus far, we have shown that when tumor cells co-express multiple dominant tumor antigens, a tumor is less likely to develop and, when used as a vaccine the resulting T cells response can be highly protective. The next series of experiments assessed the potential of i.t. IL-2 as an immune-enhancing agent used to treat established tumors. We have successfully used this regimen to cure established tumors [30]. Intra-tumoral IL-2 did not reduce tumor growth or offer a significant increase in survival in LL-bearing mice (Fig. 9 a, d). In contrast, there was a survival advantage for LLsOVA and LLpoly tumor-bearing mice (Fig. 9 e, f) in which significantly reduced tumor growth was seen, particularly in LLpoly-bearing mice (Fig. 9 b, c), following several rounds of IL-2 therapy.
Fig. 9.
Intra-tumoral IL-2 enhances CTL responses to dominant but not subdominant epitopes. Mice given 5×105 tumor cells s.c. received 20 μg of IL-2 or PBS intra-tumorally from day 7 or 9 of tumor growth (when tumors were palpable); tumor growth (a–c) and survival were monitored (e–g ). In vivo CTL responses in LLsOVA and LLpoly tumor-bearing mice were analyzed 16 h after the adoptive transfer of CFSE-labeled, peptide-coated target lymphocytes (g, i). In vitro CTL responses were also assessed in LLsOVA (h)
An examination of IL-2-treated versus PBS-treated mice showed that in vivo CTL responses to the dominant, and not subdominant epitopes were enhanced in LLsOVA-bearing animals (Fig. 9g). These results were confirmed using the in vitro CTL assay (Fig. 9h); hence we did not see intra-molecular epitope spreading in mice with regressing tumors. Similarly, the CTL response to the three dominating epitopes was increased after IL-2 treatment in LLpoly-bearing mice (SIIN and SGP P<0.05, FAP P=0.074; Fig. 9i) to levels that retarded tumor growth and lead to resolution of the tumor in 80% of mice (Fig. 9f). Interestingly, in both models whilst the CTL response was enhanced, the hierarchy remained unchanged.
Discussion
It is becoming increasingly clear that the immune system is engaged in an intimate relationship with a progressing tumor. Whilst, it is generally accepted that CTL responses are pivotal in destroying tumor cells; studies looking at anti-tumor CTL have often focused on responses to a single antigen despite clear evidence that tumors co-express many antigens at any one time. There is very little information about in vivo CTL responses to co-expressed, multiple tumor antigens; this is true for co-expressed immunodominant antigens, as well as for co-expressed intra-molecular epitopes with differing affinities for MHC class I molecules (i.e., weaker antigens that are described as subdominant or cryptic). Therefore, our current study examines the nature of immune responses to multiple tumor antigens and indicates that if a tumor expresses one or more dominant antigens a functional CTL response will be generated. However, whilst this response may be able to retard tumor growth it will ultimately not prevent the tumor from progressing.
Two models were used: the first secretes a neo tumor antigen, i.e., intact ovalbumin (LLsOVA). Importantly, many tumor antigens are secreted (e.g., prostate-specific antigen in prostrate cancer [41] and alpha feto-protein in hepatic carcinomas [42]) so this model is relevant to the clinical tumor immunology. The second model expresses a string of multiple dominant epitopes (a polyepitope; LLpoly) and may represent tumor antigens of viral origins (such as the E6 and E7 antigens from papilloma virus in cervical cancer [43, 44], which are known to be immunogenic. The common epitope in both models is SIINFEKL as it is the dominant epitope in the LLsOVA model and a co-dominant epitope in the LLpoly model. Hence, where relevant, we used SIINFEKL as our ‘readout’ epitope.
Both LLsOVA and LLpoly tumor cell lines expressed SIINFEKL to levels that enabled recognition and lysis by antigen-specific effector CTLs, and grew slightly slower than the parental line (15% of LLpoly-bearing mice did not develop tumor) suggesting that an immune response to the neo tumor antigens had been generated. We showed that SIINFEKL was presented to T cells in vivo during tumor growth; this is consistent with our earlier studies [28, 30, 37, 45]. Tumor antigen presentation occurred early in tumor progression (before the tumors were palpable). We then used an in vivo CTL assay that directly measures the capacity of endogenous CTLs to lyse antigen-bearing targets in vivo. This overcomes problems encountered in most other studies which relied on assays that indicate potential effector function, and not the authentic in vivo effector (lytic) capacity of CTL (e.g., tetramer staining, the ELISPOT assay and intracellular cytokine staining). In vitro CTL assays were carried out as a comparison.
In the LLsOVA model CTLs readily lysed SIINFEKL-coated target cells early in tumor development. No response was seen towards either of the subdominant epitopes. These results show that the hierarchy of dominance (described when OVA is used as a native protein) is maintained when OVA is seen as a tumor antigen; i.e., the tumor did not appear to significantly alter responses to OVA [26, 37, 38].
In vivo CTL analysis of the endogenous effector CTL in LLpoly mice shows that at day 7 there is a high level of killing to three of the four epitopes in the polyepitope. This result clearly demonstrates that if several dominant epitopes are expressed by a tumor, then the host immune system can recognize and respond to each of the epitopes. The strongest responses were seen to SIINFEKL and FAPGNYPAL. The lack of a response to ASNENMDAM suggests that in the polyepitope model ASNENMDAM may be a subdominant epitope, as has been shown by others using the polytope in a different (non-tumor) context [40]. This contrasts to our in vitro analysis which showed effector CTL activity against all the four peptides [46]; confirming that all the epitopes were processed and presented in vivo by APC to naive T cells. This hierarchical response was also seen in experiments using polyepitope expressing Vaccinia virus or Kunjin virus vaccines [47–49] and may represent superdominance [27]. If so, superdominance appears to occur regardless of the context the epitopes are seen in, and presentation as a tumor antigen does not alter this hierarchy. However, it is possible that preferential antigen processing (biased towards SIINFEKL and away from ASNENMDAM) may also explain these CTL responses (investigating this issue is well beyond the scope of this project). Nonetheless, the original aim of this study was to determine whether or not the immune system recognized co-expressed multiple tumor antigens and these data clearly show that it does.
However, our studies also show that this anti-tumor immune response does not (generally) prevent tumor growth. This may be also true for humans as tumor-specific T cells can be found in blood, and in the tumor itself [50, 51]. There is evidence that this immune response may restrain tumors until an event occurs that allows the tumor to progress. Tumors can spontaneously regress only to emerge in different locations later [52]. This may be true for many cancers as autopsies of individuals who died of trauma often reveal microscopic colonies of cancer cells, referred to as in situ tumors [53]. Indeed, virtually all autopsied individuals aged 50–70 have in situ carcinomas in their thyroid gland, whereas only 0.1% of individuals in this age group are diagnosed with thyroid cancer. However, it is acknowledged that our murine tumor models have been deliberately (artificially) selected because of the antigens they express and may not reflect the human situation. Nonetheless, in defense of our model, early anti-tumor immune events cannot be measured in patients until reliable early diagnoses can be conducted; herein we started to monitor the anti-tumor response before tumors were palpable.
Interestingly, in the odd instance when LLpoly tumors did not grow, the in vivo CTL response was not significantly higher than that seen in mice with progressing tumors. This response was measured 2 weeks after tumor cell inoculation and without the additional tumor antigen levels offered by a developing tumor. Hence, it is possible that the early CTL response may have been significantly more potent in the non-progressors. Nonetheless, the data do imply that CTL recognition of tumors that express multiple dominant antigens can occasionally prevent tumor growth and this is supported by the vaccination experiments, which generated complete protection against LLpoly tumors. Thus, if sufficient CTL are generated to multiple tumor antigens they can impede tumor development.
Immunotherapy with cytokines continues to represent an attractive approach to achieve tumor regression by enhancing CTL activity and/or prolonging CTL survival, and we have previously shown that i.t. IL-2 can induce tumor regression [30]. In this study, i.t. IL-2 enhanced CTL responses to the dominant epitope of OVA (without altering responses to the subdominant epitope) offering reduced tumor growth rates in LLsOVA-bearing mice. However, i.t. IL-2 was more effective in retarding tumor growth in LLpoly-bearing mice; i.e., tumors that express several dominant epitopes. Together, these data suggest that pre-existing precursor CTL populations will influence the anti-tumor immune response after challenge with an immunotherapeutic strategy.
It has been suggested that cancer patients may benefit from therapies that induce the phenomenon of epitope spreading [18, 20, 22, 54, 55]. In these studies the event triggering epitope spreading is often a tumor antigen-expressing vaccine and not a cytokine [22–24, 55–59]. We saw no evidence of intra-molecular epitope spreading in tumors regressing after IL-2 immunotherapy. Hence, use of cytokines may retain the preset tumor immune response, whereas tumor antigen-based vaccines may induce the spreading of CTL responses to previously silent tumor antigens.
In summary, we have shown that CTL are generated to multiple neo tumor antigens co-expressed by a progressing tumor, and that these CTL are functional in vivo. However, they are generally unable to eradicate the tumor. Interestingly, the CTL response to tumor antigens is restricted by the dominant nature of tumor antigens. Should several dominant epitopes co-exist on a tumor, the host can generate a response to most of them. Cytokine-based immunotherapies can enhance responses back to dominant epitope/s, and do not reveal responses to weaker antigens. Nonetheless, this response can be enough to retard tumor growth. Immunotherapies targeting tumors that express several neo antigens are particularly effective. Hence, these data should assist in the choice of the most useful immunotherapy for a particular cancer. Immunogenic tumors are highly likely to respond favorably to an adjuvant-like therapy whilst non-immunogenic tumors are likely to require a multi-modality assault that will (at least) include a vaccine that specifically targets the tumor plus an adjuvant.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia (NH&MRC), the State Government Insurance Commission (SGIC) and the RAINE Medical Research Foundation.
Abbreviations
- CM
Complete media
- DLN
Draining lymph node
- LL
Lewis lung carcinoma
- LN
Lymph node
- TIL
Tumor-infiltrating lymphocyte
- sOVA
Secretory ovalbumin
References
- 1.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21(6):873. doi: 10.1081/CNV-120025091. [DOI] [PubMed] [Google Scholar]
- 2.Stoler DL, Chen N, Basik M, Kahlenberg MS, Rodriguez-Bigas MA, Petrelli NJ, Anderson GR. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc Natl Acad Sci USA. 1999;96(26):15121. doi: 10.1073/pnas.96.26.15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mashino K, Sadanaga N, Tanaka F, Yamaguchi H, Nagashima H, Inoue H, Sugimachi K, Mori M. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85(5):713. doi: 10.1054/bjoc.2001.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandeneynde BJ, Vanderbruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9(5):684. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 5.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50(1):3. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioannides CG, Freedman RS, Platsoucas CD, Rashed S, Kim YP. Cytotoxic T cell clones isolated from ovarian tumor-infiltrating lymphocytes recognize multiple antigenic epitopes on autologous tumor cells. J Immunol. 1991;146(5):1700. [PubMed] [Google Scholar]
- 7.Eifuku R, Yoshino II, Imahayashi S, Fujie H, Takenoyama M, Yoshimatsu T, Hanagiri T, So T, Ichiyoshi Y, Nomoto K, Yasumoto K. Induction of tumor-specific cytotoxic T lymphocytes from regional lymph node lymphocytes of human breast cancer. Breast Cancer. 1998;5(4):367. doi: 10.1007/BF02967433. [DOI] [PubMed] [Google Scholar]
- 8.Van den Eynde B, Hainaut P, Herin M, Knuth A, Lemoine C, Weynants P, van der Bruggen P, Fauchet R, Boon T. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int J Cancer. 1989;44(4):634. doi: 10.1002/ijc.2910440413. [DOI] [PubMed] [Google Scholar]
- 9.Johnston JV, Malacko AR, Mizuno MT, McGowan P, Hellstrom I, Hellstrom KE, Marquardt H, Chen L. B7-CD28 costimulation unveils the hierarchy of tumor epitopes recognized by major histocompatibility complex class I-restricted CD8+ cytolytic T lymphocytes. J Exp Med. 1996;183(3):791. doi: 10.1084/jem.183.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Anton LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in class I- restricted T cell responses to viruses. Immunity. 2000;12(1):83. doi: 10.1016/S1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 12.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8(6):683. doi: 10.1016/S1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14(5):203. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 14.Voskuhl RR, Farris RW, II, Nagasato K, McFarland HF, Dalcq MD. Epitope spreading occurs in active but not passive EAE induced by myelin basic protein. J Neuroimmunol. 1996;70(2):103. doi: 10.1016/S0165-5728(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 15.Newmaster RS, Mylin LM, Fu TM, Tevethia SS. Role of a subdominant H-2Kd-restricted SV40 tumor antigen cytotoxic T lymphocyte epitope in tumor rejection. Virology. 1998;244(2):427. doi: 10.1006/viro.1998.9148. [DOI] [PubMed] [Google Scholar]
- 16.Pilon SA, Kelly C, Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol. 2003;170(3):1202. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- 17.Feltkamp MC, Vreugdenhil GR, Vierboom MP, Ras E, van der Burg SH, ter Schegget J, Melief CJ, Kast WM. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur J Immunol. 1995;25(9):2638. doi: 10.1002/eji.1830250935. [DOI] [PubMed] [Google Scholar]
- 18.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20(11):2624. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 19.Ranieri E, Kierstead LS, Zarour H, Kirkwood JM, Lotze MT, Whiteside T, Storkus WJ. Dendritic cell/peptide cancer vaccines: clinical responsiveness and epitope spreading. Immunol Invest. 2000;29(2):121. doi: 10.3109/08820130009062294. [DOI] [PubMed] [Google Scholar]
- 20.el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, Feldman M, Eisenbach L. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29(10):3295. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Anderson BW, Kudelka AP, Honda T, Pollack MS, Gershenson DM, Gillogly MA, Murray JL, Ioannides CG. Induction of determinant spreading and of Th1 responses by in vitro stimulation with HER-2 peptides. Cancer Immunol Immunother. 2000;49(9):459. doi: 10.1007/s002620000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatsumi T, Gambotto A, Robbins PD, Storkus WJ. Interleukin 18 gene transfer expands the repertoire of antitumor Th1-type immunity elicited by dendritic cell-based vaccines in association with enhanced therapeutic efficacy. Cancer Res. 2002;62(20):5853. [PubMed] [Google Scholar]
- 23.Markiewicz MA, Fallarino F, Ashikari A, Gajewski TF. Epitope spreading upon P815 tumor rejection triggered by vaccination with the single class I MHC-restricted peptide P1A. Int Immunol. 2001;13(5):625. doi: 10.1093/intimm/13.5.625. [DOI] [PubMed] [Google Scholar]
- 24.Liau LM, Jensen ER, Kremen TJ, Odesa SK, Sykes SN, Soung MC, Miller JF, Bronstein JM. Tumor immunity within the central nervous system stimulated by recombinant Listeria monocytogenes vaccination. Cancer Res. 2002;62(8):2287. [PubMed] [Google Scholar]
- 25.Lally KM, Mocellin S, Ohnmacht GA, Nielsen MB, Bettinotti M, Panelli MC, Monsurro V, Marincola FM. Unmasking cryptic epitopes after loss of immunodominant tumor antigen expression through epitope spreading. Int J Cancer. 2001;93(6):841. doi: 10.1002/ijc.1420. [DOI] [PubMed] [Google Scholar]
- 26.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993;150(4):1212. [PubMed] [Google Scholar]
- 27.Sandberg JK, Grufman P, Wolpert EZ, Franksson L, Chambers BJ, Karre K. Superdominance among immunodominant H-2Kb-restricted epitopes and reversal by dendritic cell-mediated antigen delivery. J Immunol. 1998;160(7):3163. [PubMed] [Google Scholar]
- 28.Nelson DJ, Mukherjee S, Bundell C, Fisher S, van Hagen D, Robinson B. Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J Immunol. 2001;166(9):5557. doi: 10.4049/jimmunol.166.9.5557. [DOI] [PubMed] [Google Scholar]
- 29.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59(5):1071. [PubMed] [Google Scholar]
- 30.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171(10):5051. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 31.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 32.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54(6):777. doi: 10.1016/S0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 33.Boyle JS, Koniaras C, Lew AM. Influence of cellular location of expressed antigen on the efficacy of DNA vaccination: cytotoxic T lymphocyte and antibody responses are suboptimal when antigen is cytoplasmic after intramuscular DNA immunization. Int Immunol. 1997;9(12):1897. doi: 10.1093/intimm/9.12.1897. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura A, Morita M, Nishimura Y, Sugino Y. A rapid and highly efficient method for preparation of competent Escherichia coli cells. Nucleic Acids Res. 1990;18(20):6169. doi: 10.1093/nar/18.20.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171(1):131. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 36.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory ctl: correlation of effector function with phenotype and cell division. J Immunol. 1998;161(10):5338. [PubMed] [Google Scholar]
- 37.Nelson D, Bundell C, Robinson B. In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J Immunol. 2000;165(11):6123. doi: 10.4049/jimmunol.165.11.6123. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Khilko S, Fecondo J, Margulies DH, McCluskey J. Determinant selection of major histocompatibility complex class I-restricted antigenic peptides is explained by class I-peptide affinity and is strongly influenced by nondominant anchor residues. J Exp Med. 1994;180(4):1471. doi: 10.1084/jem.180.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le TT, Drane D, Malliaros J, Cox JC, Rothel L, Pearse M, Woodberry T, Gardner J, Suhrbier A. Cytotoxic T cell polyepitope vaccines delivered by ISCOMs. Vaccine. 2001;19(32):4669. doi: 10.1016/S0264-410X(01)00243-2. [DOI] [PubMed] [Google Scholar]
- 40.Schirmbeck R, Fissolo N, Chaplin P, Reimann J. Enhanced priming of multispecific, murine CD8+ T cell responses by DNA vaccines expressing stress protein-binding polytope peptides. J Immunol. 2003;171(3):1240. doi: 10.4049/jimmunol.171.3.1240. [DOI] [PubMed] [Google Scholar]
- 41.Gurova KV, Roklin OW, Krivokrysenko VI, Chumakov PM, Cohen MB, Feinstein E, Gudkov AV. Expression of prostate specific antigen (PSA) is negatively regulated by p53. Oncogene. 2002;21(1):153. doi: 10.1038/sj.onc.1205001. [DOI] [PubMed] [Google Scholar]
- 42.Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of Human T-cell Responses to an HLA-A2.1-restricted peptide epitope derived from alpha-Fetoprotein. Cancer Res. 1999;59(13):3134. [PubMed] [Google Scholar]
- 43.Chen L, Mizuno M, Singhal M, Hu S, Galloway D, Hellstrom I, Hellstrom K. Induction of cytotoxic T lymphocytes specific for a syngeneic tumor expressing the E6 oncoprotein of human papillomavirus type 16. J Immunol. 1992;148(8):2617. [PubMed] [Google Scholar]
- 44.Thornburg C, Boczkowski D, Gilboa E, Nair SK. Induction of cytotoxic T lymphocytes with dendritic cells transfected with human papillomavirus E6 and E7 RNA: implications for cervical cancer immunotherapy. J Immunother. 2000;23(4):412. doi: 10.1097/00002371-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, Robinson BW, Scott B. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162(10):5838. [PubMed] [Google Scholar]
- 46.Thomson SA, Elliott SL, Sherritt MA, Sproat KW, Coupar BE, Scalzo AA, Forbes CA, Ladhams AM, Mo XY, Tripp RA, Doherty PC, Moss DJ, Suhrbier A. Recombinant polyepitope vaccines for the delivery of multiple CD8 cytotoxic T cell epitopes. J Immunol. 1996;157(2):822. [PubMed] [Google Scholar]
- 47.Mateo L, Gardner J, Chen Q, Schmidt C, Down M, Elliott SL, Pye SJ, Firat H, Lemonnier FA, Cebon J, Suhrbier A. An HLA-A2 polyepitope vaccine for melanoma immunotherapy. J Immunol. 1999;163(7):4058. [PubMed] [Google Scholar]
- 48.Thomson SA, Sherritt MA, Medveczky J, Elliott SL, Moss DJ, Fernando GJ, Brown LE, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160(4):1717. [PubMed] [Google Scholar]
- 49.Anraku I, Harvey TJ, Linedale R, Gardner J, Harrich D, Suhrbier A, Khromykh AA. Kunjin virus replicon vaccine vectors induce protective CD8+ T-cell immunity. J Virol. 2002;76(8):3791. doi: 10.1128/JVI.76.8.3791-3799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawakami Y, Dang N, Wang X, Tupesis J, Robbins PF, Wang RF, Wunderlich JR, Yannelli JR, Rosenberg SA. Recognition of shared melanoma antigens in association with major HLA-A alleles by tumor infiltrating T lymphocytes from 123 patients with melanoma. J Immunother. 2000;23(1):17. doi: 10.1097/00002371-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Evans E, Man S, Evans A, Borysiewicz L. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Res. 1997;57(14):2943. [PubMed] [Google Scholar]
- 52.Robinson BW, Robinson C, Lake RA. Localised spontaneous regression in mesothelioma—possible immunological mechanism. Lung Cancer. 2001;32(2):197. doi: 10.1016/S0169-5002(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 53.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427(6977):787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 54.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber J, Sondak VK, Scotland R, Phillip R, Wang F, Rubio V, Stuge TB, Groshen SG, Gee C, Jeffery GG, Sian S, Lee PP. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected Stage II melanoma. Cancer. 2003;97(1):186. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 56.Chiong B, Wong R, Lee P, Delto J, Scotland R, Lau R, Weber J. Characterization of long-term effector-memory T-cell responses in patients with resected high-risk melanoma receiving a melanoma peptide vaccine. J Immunother. 2004;27(5):368. doi: 10.1097/00002371-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 57.el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, Feldman M, Eisenbach L. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29(10):3295. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Lehmann F, Marchand M, Hainaut P, Pouillart P, Sastre X, Ikeda H, Boon T, Coulie PG. Differences in the antigens recognized by cytolytic T cells on two successive metastases of a melanoma patient are consistent with immune selection. Eur J Immunol. 1995;25(2):340. doi: 10.1002/eji.1830250206. [DOI] [PubMed] [Google Scholar]
- 59.Godelaine D, Carrasco J, Lucas S, Karanikas V, Schuler-Thurner B, Coulie PG, Schuler G, Boon T, Van Pel A. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J Immunol. 2003;171(9):4893. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]