Abstract
Introduction
We and others previously observed immunosurveillance against transplantable tumors in mice, and enhancement thereof by blockade of negative regulation by T reg cells or the NKT-IL-13-myeloid cell-TGF-β regulatory circuit. However, it was unknown whether natural immunosurveillance inhibits growth of completely spontaneous autochthonous tumors, and whether it can be improved by inhibition of negative regulation.
Materials and methods
To examine the existence of T cell-mediated immunosurveillance against spontaneous tumors, BALB-neuT mice were treated with anti-CD4 and/or anti-CD8. A role for IL-13 in the suppression of immunosurveillance was investigated by treating mice with IL-13 inhibitor.
Results
We show that even spontaneous autochthonous breast carcinomas arising in Her-2/neu transgenic mice appear more quickly when the mice are depleted of T cells, evidence for T-cell mediated immunosurveillance slowing tumor growth. This immunosurveillance could be further enhanced by blockade of IL-13 (but not IL-4) which slowed the appearance of these autologous tumors compared to control antibody-treated mice.
Conclusion
Thus, even completely spontaneous, autochthonous breast cancers can be controlled in part by natural immunosurveillance, and blockade of negative regulation can improve this control.
Keywords: IL-13, Breast cancer, Immunosurveillance
Introduction
Tumor immunity and natural immunosurveillance have been most often studied with transplantable animal tumors, but the role of natural immunosurveillance, defined as spontaneous immunologic rejection or retardation of tumor growth without any immune manipulation, in completely autochthonous, spontaneous tumors, is not yet well understood. Although it is still in debate whether or not tumor immunosurveillance exists against spontaneous tumors [23, 24], more evidence suggesting a role of immunosurveillance to some degree in preventing development and growth of tumors has been accumulated. Several molecules such as IFN-α/β, IFN-γ, STAT1, and perforin have been suggested to be involved in tumor immunosurveillance against spontaneous tumors [10, 25, 27]. However, little is known about suppressive mechanisms blocking tumor immunosurveillance to allow spontaneous tumors to grow [2].
Here, we investigated whether natural anti-tumor immunosurveillance existed in unmanipulated BALB-neuT mice, which develop neoplastic mammary lesions starting from 4 weeks of age [3], and palpable spontaneous autochthonous mammary tumors from 14 to 15 weeks. We also asked whether any such immunosurveillance could be magnified by blocking IL-13 in vivo, as we previously found that IL-13 made by NKT cells was a mediator inhibiting tumor immunosurveillance in several transplanted mouse tumor models [28–30]. As immunosurveillance is not always apparent even for tumors induced with carcinogens or other mutagens, that might alter self, it was not expected that any immunosurveillance would be detected against completely spontaneous autochthonous tumors arising from early overexpression of an oncogene to which the mouse was at least partially tolerant. Surprisingly, our findings imply the existence of natural T cell immunosurveillance even against an autochthonous tumor, but also the existence of a negative immunoregulatory mechanism involving IL-13 that limits the effectiveness of this immunosurveillance [22, 28–31].
Materials and methods
Mice and tumor assessment
Virgin female BALB-neuT mice, BALB/c mice transgenic for the rat transforming Her-2/neu oncogene expressed under the control of mouse mammary tumor virus promoter [3], develop an independent mammary carcinoma in each of the ten mammary glands starting at 4 weeks of age [3]. Tumors become palpable and individually identifiable asynchronously from the 15th to the 25th week of age, and were inspected once or twice a week. Tumor multiplicity was calculated as the cumulative number of incident tumors/total number of mice. Female BALB/c mice were purchased from Animal Production Colonies, Frederick Cancer Research and Development Center, NIH (FCRDC, Frederick, MD). All mice were maintained in a pathogen-free animal facility. Animal experiments were conducted in accordance with protocols approved by the National Cancer Institute (NCI) Animal Care and Use Committee.
Antibodies and in vivo depletion of T cells
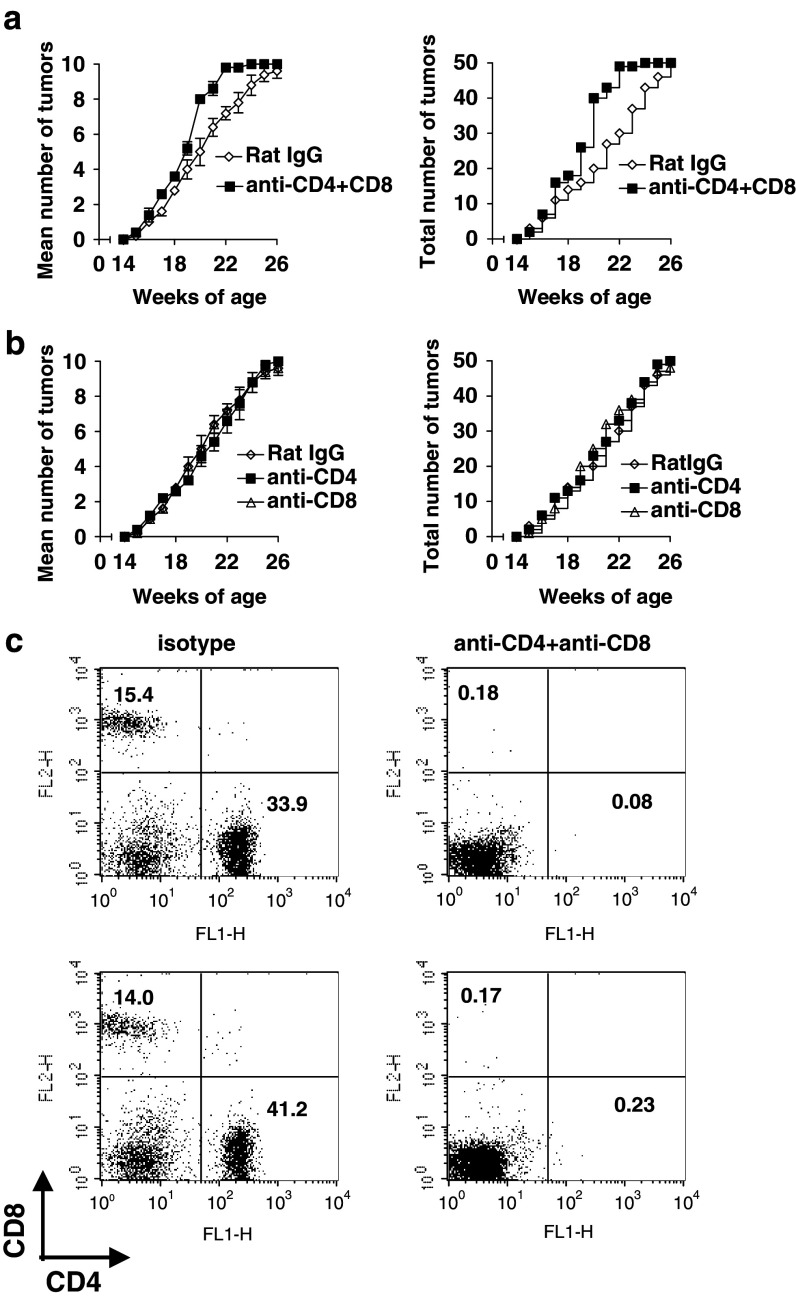
CD4+ and CD8+ T cells were depleted by i.p. injection of 500 μg of rat anti-CD4 (clone GK1.5) or anti-CD8 (clone 2.43) antibody, respectively, from the Frederick Cancer Research and Development Center. As a control, rat IgG was used (ICN, Costa Mesa, CA). Starting at 8 weeks of age, the antibodies were injected on day 0, 1, 2, 5, 9, then once per week until 25 weeks of age. T cell depletion (>99% of total T cells) was verified by FACS analysis of PBMCs 1 day after the 5 initial treatments using FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 antibodies (BD PharMingen, San Diego, CA) (For representative examples, see Fig. 1c). Control experiments confirmed that the antibody used for depletion did not simply block binding of the fluorescent antibody.
Fig. 1.
Acceleration of tumor growth in BALB-neuT mice by depletion of both CD4+ T cells and CD8+ T cells. a BALB-neuT mice were treated with anti-CD4 antibody (GK1.5) and anti-CD8 (2.43) antibody (0.5 mg/injection at day 0, 1, 2, 5, 9) starting from the age of 8 weeks, then weekly until 25 weeks (anti-CD4 and anti-CD8 group vs rat IgG group, P < 0.001 by log-rank test on the entire curves). b BALB-neuT mice were treated with anti-CD4 antibody (GK1.5) or anti-CD8 (2.43) antibody. Control mice were treated with rat Ig. No significant difference was found between groups in b. Five mice were used for each group. Left panels mean number of tumors per mouse; right panels cumulative tumor incidence for the entire group (independent tumors in 50 mammary glands). Similar results were obtained in repeated experiments and a representative is shown. c PBMC from two mice (upper and lower panels, right) depleted by 5 injections of anti-CD4 and anti-CD8 as described above were obtained one day after the fifth dose of antibody and stained for with FITC-anti-CD4 and PE-anti-CD8 as described in “Materials and methods” section, or with isotype control antibodies (not shown). For comparison, at left are shown similar staining plots of PBMC obtained from mice treated in vivo with control rat IgG. Depleting antibodies were found not to compete with staining antibodies
IL-13 inhibitor and inhibition of IL-13 and IL-4 activity
IL-13 inhibitor, a fusion protein of murine IL-13 receptor-α2 and human IgG1 Fc(sIL-13Rα2-Fc), was made as described [8] and provided with its control isotype-matched human IgG from the External Research Department, Wyeth Research, Cambridge, MA. BALB-neuT mice, 4–5 or 8–10 weeks old, were treated with IL-13 inhibitor or control IgG (0.2 mg/injection) every other day for the first 2 weeks, then twice per week until 21 weeks of age. For the inhibition of IL-4 activity, BALB-neuT mice (4–5 weeks old) were injected i.p. with rat anti-IL-4 antibody (0.5 mg/injection) according to the same schedule as for IL-13 inhibition. Anti-IL-4 (clone 11B11) antibody was obtained from the FCRDC, as a control antibody for the anti-IL-4, rat IgG was purchased from ICN.
Statistical analysis
Cumulative tumor multiplicity was compared by a log-rank test, using JMP software (SAS Institute, Cary, NC). The tumors in each of the 10 mammary glands arise independently, and their rate of appearance is similar in all mice in a group, so the cumulative incidence of all tumors was compared among groups using the log-rank test.
Results
Acceleration of tumor growth in BALB-neuT mice by depletion of both CD4+ T cells and CD8+ T cells
To address whether T-cell mediated natural immunosurveillance may be influencing the rate of spontaneous autochthonous mammary carcinoma tumor appearance in BALB-neuT female mice, we depleted mice of CD4+ and/or CD8+ T cells from the age of 8–25 weeks. Depletion of T cells was confirmed by FACS analysis as less than 1% of pretreatment levels of CD4+ cells and about 1% of pretreatment levels of CD8+ cells in PBMCs (Fig. 1c). Effects on tumor growth were evaluated by counting palpable mammary tumors. Although the age of first tumor appearance did not differ between the groups, tumor development was accelerated by 2–3 weeks between the age of 18 and 22 weeks in BALB/c-neuT mice depleted of both CD4+ and CD8+ T cells compared to the control groups (P < 0.001, comparing the cumulative incidence of tumors in 50 mammary glands in each group, right panel) (Fig. 1a). Thus, this degree of T cell depletion was sufficient to reveal a T-cell-mediated delay in tumor growth that was lost on depletion of T cells. It is possible that T-cell depletion starting even earlier might reveal even greater acceleration of tumor growth, as the abnormality in mammary gland development starts as early as 3 weeks of age [6]. Nevertheless, the treatment starting from 8 weeks of age was sufficient to reveal evidence for immunosurveillance. The delay was not a non-specific effect of antibody administration, as the effect was observed relative to control, isotype-matched antibody-treated animals. (Although we cannot rule out an anti-antibody response to the injected depleting antibodies, this does not appear to have been sufficient to prevent T cell depletion as measured by FACS and by the biological effect. Further, the depleted mice would be less likely to make antibodies against the depleting antibodies since such antibody production would likely require CD4+ T cell help.) However, there was no significant difference between the control group and the groups depleted of either CD4+ T or CD8+ T cells alone (Fig. 1b). These results imply that immunosurveillance against spontaneous tumor development exists in vivo even without immunization and is mediated by both CD4+ and CD8+ T cells. Although the spontaneous anti-tumor immune response retarded tumor growth by 2–3 weeks, tumor development was inevitable as the oncogene continues to engender new tumors. Therefore, this natural immunosurveillance may be suppressed in the developing tumor environment. There are reports that anti-tumor immunity is negatively regulated by Th2-type cytokines such as IL-4, IL-13 [1, 5, 19, 29, 31] or by TGF-β [15, 16, 30], but very few studies exist on natural immunosurveillance against spontaneous autochthonous tumors.
Retardation of tumor growth in BALB-neuT mice by inhibition of IL-13
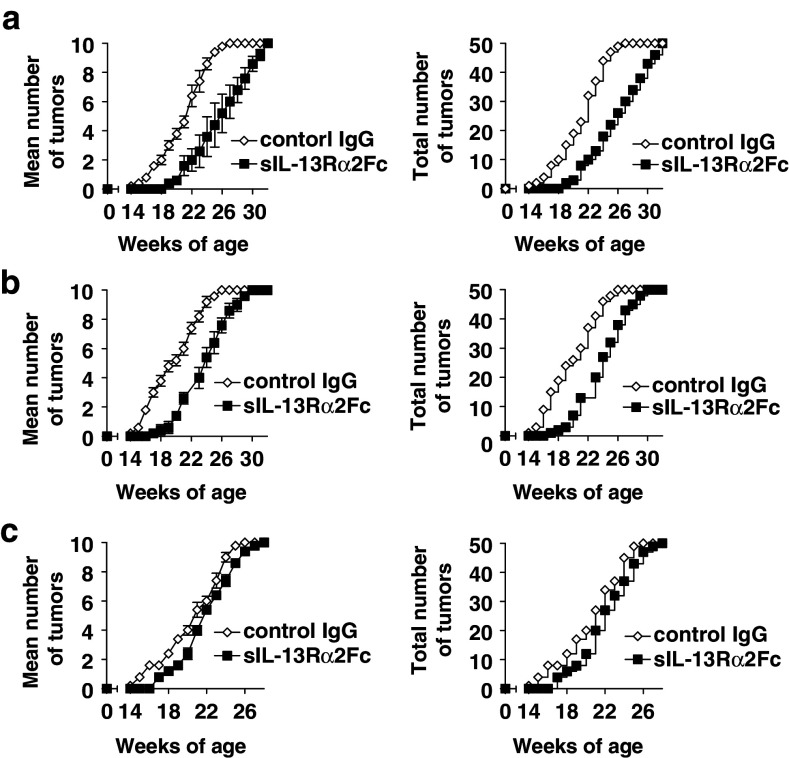
To confirm whether this acceleration of tumor growth by T cell depletion reflected a limited degree of T-cell mediated immunosurveillance that was reigned in by immunoregulatory mechanisms, we asked whether such putative immunosurveillance could be amplified by diminishing such negative regulation. As we had previously observed an inhibition of such a negative immunoregulatory mechanism dampening tumor immunosurveillance in two transplanted, non-autologous tumors by blockade of IL-13 but not IL-4 [22, 29], we addressed whether natural immunosurveillance could be recovered by inhibition of IL-13 using a soluble IL-13 inhibitor, sIL-13Rα2-Fc. BALB-neuT mice were treated with IL-13 inhibitor or control Ig, from the age of 4–5 weeks every other day (0.2 mg/injection, i.p.) for the first 2 weeks, then twice a week until the age of 21 weeks (Fig. 2a, b). Tumor incidences were compared between groups by the average number of palpable tumors per mouse (n = 5 mice per group) (left panels), and the total cumulative tumor incidence within the group (right panels). Tumor growth in 50% of mammary glands, was found around the age of 20–21 weeks in control Ig-treated mice. However, a similar level of tumor development was not reached until 4–5 weeks later in IL-13 inhibitor-treated mice (P < 0.001 for both A and B) (Fig. 2a, b). This retardation of tumor growth was dependent on the starting time point of IL-13 inhibitor treatment, as it was not apparent when treatment started at 8–10 weeks (Fig. 2c). Lack of efficacy of delayed treatment may reflect the fact that BALB-neuT mice highly express Her-2 protein on the terminal ductular-lobular units of the mammary glands by the 3rd week of age [3]. Thus, to unmask natural immunosurveillance from the suppressive effects of IL-13, it was critical to inhibit IL-13 activity from the very beginning of tumor development in vivo.
Fig. 2.
Retardation of tumor growth in BALB-neuT mice by in vivo inhibition of IL-13 activity. BALB-neuT mice (5/group) were treated with IL-13 inhibitor, sIL-13Rα2-Fc, (0.2 mg/injection) every other day for the first 2 weeks starting from the age of 4–5 weeks (two independent experiments in panels a and b) or from the age of 8–10 weeks (panel c), then twice weekly until 21 weeks. The control group was treated with control isotype-matched human IgG. Left panels mean tumor incidence/mouse; right panels cumulative tumor incidence/group (independent tumors in 50 mammary glands per group)
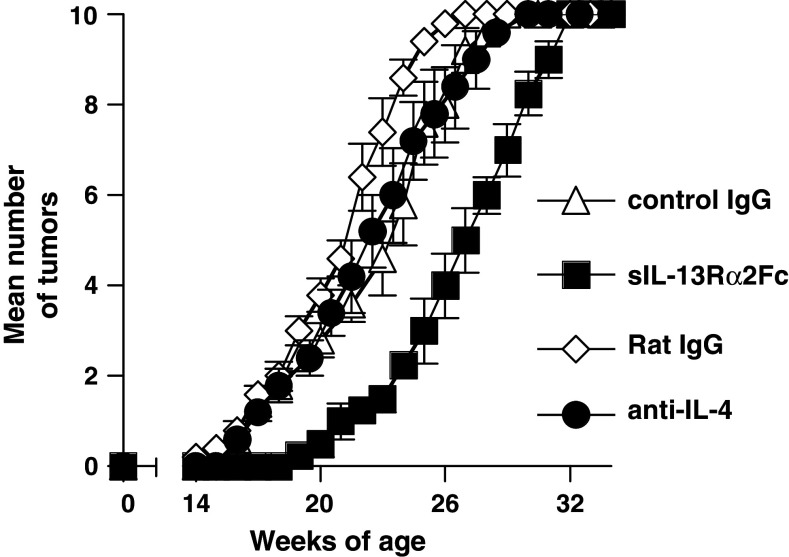
Consistent with our previous findings in other tumor models [22, 29], blockade of IL-4, also from age 4–5 weeks, did not delay tumor growth, in contrast to the effect of IL-13 blockade (Fig. 3). This finding is consistent with the idea that the regulatory mechanism in the Her-2 transgenic mice is similar to that we had identified previously in adoptively transferred tumor models [22, 29].
Fig. 3.
Blockade of in vivo IL-4 activity was not sufficient to delay tumor growth in BALB-neuT mice. BALB-neuT mice (5/group) were treated with anti-IL-4 antibody (i.p. 0.5 mg/injection), sIL-13Rα2-Fc (0.2 mg/injection), or their control antibodies as in Fig. 2. Rat Ig (0.5 mg/injection) was used as the control antibody for the anti-IL-4 and human isotype matched IgG1 for the IL-13 inhibitor
Discussion
The role of immunosurveillance in inhibiting tumor growth has long been debated. Evidence for immunosurveillance exists from experiments of Schreiber and coworkers [9–12, 25] in aging mice and in carcinogen-induced tumors. Also, studies using carcinogen in Jα18 KO and CD1 KO mice indicated that NKT cells can enhance tumor immunity against carcinogen-induced spontaneous tumors [26]. However, it is still uncertain how broadly immunosurveillance plays a role in controlling growth of autochthonous tumors [33]. We found that in several mouse tumor models, in which the CD25+ T regulatory cells do not appear to play a role [32], tumor immunosurveillance is suppressed by NKT cells that produce IL-13 [22, 28–31]. However, these studies were all carried out using transplantable tumors in which tumor antigens have been identified. Here, we asked whether even in the case of completely spontaneous autochthonous tumors arising in oncogene-transgenic animals, evidence for tumor immunosurveillance could be obtained. If T cells mediate such immunosurveillance, we reasoned that its presence might be revealed by depleting T cells in vivo and observing an effect on tumor growth. Acceleration of tumor appearance in the transgenic animals depleted of T cells, but not in controls treated with isotype-matched antibodies, indicated that such immunosurveillance existed but was grossly inadequate, as even in a T-cell replete animal, tumors appeared inexorably in all mammary glands. Indeed, it has been notoriously difficult to control completely this oncogene-mediated mammary carcinogenesis even with potent vaccines [7, 20]. Having thus found evidence for the existence of spontaneous T-cell-mediated immunosurveillance, we asked whether the reason this was not more effective was in part due to negative regulation of the immunity, and whether abrogation of a negative regulatory pathway would unmask natural immunosurveillance against authochthonous tumors.
We previously observed that IL-13, but not IL-4, was a critical molecule mediating the negative regulatory effect of NKT cells early in the immune response [22, 29, 30]. IL-13 production occurs early in the response of NKT cells. Therefore, we examined whether blockade of IL-13 would improve the limited spontaneous immunosurveillance observed in T-cell-replete untreated mice. Indeed, we found that blockade of IL-13 but not IL-4 further delayed tumor growth, consistent with this mechanism. However, it is possible that IL-13 comes from multiple sources, and production of CD1d knockout BALB-neuT transgenic mice would be required to confirm the origin of the cytokine as NKT cells. Nevertheless, these results are sufficient to suggest that even in a completely spontaneous autochthonous tumor, where no immune response would be expected, there is a naturally occurring T-cell response against the tumor that is minimally effective because it is kept in check by strong regulatory mechanisms, potentially of multiple types.
There is evidence for a role of different subsets of NKT cells in regulating immune responses. Type I NKT cells, that respond to α-GalCer, play a role in suppressing Th1-mediated autoimmune disease [34]. Also, type II NKT cells, without the canonical Vα14 receptor, are sufficient to suppress immunosurveillance when type I NKT cells are absent [32]. These are not activated by α-GalCer. There is evidence in humans that these type II NKT cells make IL-13 [13]. Recent evidence suggests that there are also different subsets of type I NKT cells expressing greater or lesser amounts of Th2 cytokines in mice [5]. In BALB-neu T mice, the role of type I NKT cells activated by α-GalCer is primarily protective. Their repeated stimulation with α-GalCer elicits a significant immunosurveillance against the onset of autochthonous carcinomas in BALB-neu T mice [17]. It has also been used in human clinical trials, although early phase trials have shown immunological effects but have not yet demonstrated a clear impact on tumor growth [4, 14, 18, 21]. The ability of IL-13 blockade to enhance spontaneous immunosurveillance suggests that type II NKT cells naturally primed by tumor growth may possibly contribute to the suppression of immunosurveillance even in the case of spontaneous, autochthonous tumors. We do not know the exact site where these NKT cells mediate their effect, but because the IL-13 must induce myeloid cells to make TGF-β to suppress [30], and because the TGF-β produced by the tumor itself does not seem to matter in this mechanism, we think that the most likely place is in the tumor draining lymph node where tumor immunity is being generated. We must also consider the possibility that some of the effect of IL-13 is through alternative mechanisms, such as influencing the production of cytokines that have proangiogenic effects supporting tumor growth.
By anti-CD4 and anti-CD8 antibody treatment, we showed that depletion of both CD4+ and CD8+ T cells accelerated tumor growth. Since this treatment cannot deplete CD4CD8 double negative population of T cells, it is possible that CD4CD8 double negative T cells are enriched in vivo in the treated mice. Recently it was reported that liver-derived CD4CD8 double negative NKT cells meditative protective tumor immunity [5]. However, in the mice treated with both anti-CD4 and anti-CD8 in this report did not show better protection against tumor. Further, many of suppressor T cells reported are either CD4 or CD8 positive. Therefore, the result suggested that there is an active T cell-mediated natural immunosurveillance in BALB-neu T mice although it is not strong enough to prevent tumor growth.
Overall, we conclude that T-cell-mediated tumor immunosurveillance occurs even against a completely spontaneous autochthonous tumor, and delays tumor growth, even though it does not fully prevent it. Blocking immunoregulation mediated by IL-13 further improves this immunosurveillance. Additional strategies to optimize immunosurveillance could potentially prophylactically reduce the incidence of some cancers or delay their progression.
Acknowledgments
We thank Dr. Scott Abrams and Dr. Crystal Mackall for critical reading of the manuscript and helpful suggestions. This work was in part supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The authors declare that there is no financial conflict of interests.
Footnotes
This work was in part supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Agarwal A, Rani M, Saha GK, Valarmathi TM, Bahadur S, Mohanti BK, Das SN. Disregulated expression of the Th2 cytokine gene in patients with intraoral squamous cell carcinoma. Immunol Invest. 2003;32:17. doi: 10.1081/IMM-120019205. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, Wei WZ, Cavallo F. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 3.Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, Bouchard P, Wolf S, Modesti A, Musiani P, Lollini PL, Colombo MP, Forni G. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of {alpha}-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Carlo E, Diodoro MG, Boggio K, Modesti A, Modesti M, Nanni P, Forni G, Musiani P. Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab Invest. 1999;79:1261. [PubMed] [Google Scholar]
- 7.Di Carlo E, Rovero S, Boggio K, Quaglino E, Amici A, Smorlesi A, Forni G, Musiani P. Inhibition of mammary carcinogenesis by systemic IL12 or p185neu DNA vaccination in Her-2/neu transgenic BALB/c mice. Clin Cancer Res. 2001;7(Suppl 3):830s. [PubMed] [Google Scholar]
- 8.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O’Hara RM, Jr., Beier DR, Turner KJ, Wood CR, Collins M. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317. [PubMed] [Google Scholar]
- 9.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 10.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 11.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 13.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702. [PubMed] [Google Scholar]
- 15.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 16.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha-galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci USA. 2003;100:9464. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi M, Kobayashi H, Pollard RB, Suzuki F. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J Immunol. 1998;160:5869. [PubMed] [Google Scholar]
- 20.Nanni P, Landuzzi L, Nicoletti G, De Giovanni C, Rossi I, Croci S, Astolfi A, Iezzi M, Di Carlo E, Musiani P, Forni G, Lollini PL. Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent. J Immunol. 2004;173:2288. doi: 10.4049/jimmunol.173.4.2288. [DOI] [PubMed] [Google Scholar]
- 21.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of V alpha24+ V beta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 22.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2004;114:80. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 23.Qin Z, Blankenstein T. A cancer immunosurveillance controversy. Nat Immunol. 2004;5:3. doi: 10.1038/ni0104-3. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber RD, Old LJ, Hayday AC, Smyth MJ. Response to ‘A cancer immunosurveillance controversy’. Nat Immunol. 2004;5:4. doi: 10.1038/ni0104-4. [DOI] [PubMed] [Google Scholar]
- 25.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 26.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192. doi: 10.1182/blood.V97.1.192. [DOI] [PubMed] [Google Scholar]
- 28.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 30.Terabe M, Matsui S, Park J-M, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-b production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in negative regulation of anti-tumor immunity. Cancer Immunol Immunother. 2003;53:79. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Va14Ja18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 34.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]