Abstract
By the use of a neural network capable of performing quantitative predictions of peptides binding to HLA-A*0201 molecules, we identified a number of nonamer peptides derived from the catalytic subunit of telomerase, human telomerase reverse transcriptase (hTERT). Five nonimmunogenic peptides with measured binding affinities for HLA-A*0201 ranging from 155 to 1,298 nM were modified at the P1, P2 and P9 positions, respectively, to achieve stronger HLA-A*0201 binding. One peptide, mp30–38 (mp30), with an L to V substitution at position 9 was subsequently found to be immunogenic in mp30 immunized HLA-A*0201/H2Kb or HHD transgenic mice. The T cell reactivity obtained was directed against both the mp30 and against the unmodified p30. Anti-mp30 specific T cells generated in HLA-A*0201 transgenic mice were dependent on TCR-CD8/MHC-I α3 binding and therefore not capable of recognizing mp30-pulsed human HLA-A*0201+ cells or murine HLA-A*0201 transfectants. In order to show reactivity against naturally processed peptide in human tumor cells, an hTERT positive HLA-A*0201 negative colon carcinoma cell line (CCL220) was transfected with an HLA-A*0201/H2Kb cDNA construct and used as target in ELISPOT and cytotoxicity assays. The data show that T cells from mp30 immunized HHD transgenic mice react specifically against the CCL220 transfectant indicating that p30 is naturally processed. In conclusion, we have identified a new CTL HLA-A*0201 restricted hTERT epitope, which is now, included in an ongoing phase 2 vaccine trial of patients with disseminated cancer.
Keywords: Peptides, Telomerase, Epitope prediction
Introduction
Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase and its expression is rate-limiting for telomerase activity, which maintains the protective structure, the telomeres, at the ends of eukaryotic chromosomes [11]. In the majority of human somatic cells, the expression of hTERT is suppressed and the repetitive sequences in the ends of chromosomes are shortened at cell divisions, ultimately resulting in apoptosis [10]. Telomerase expression has been directly linked to tumor development, and inhibition of telomerase in human tumour cells may therefore arrest growth [9, 12]. Thus, the vast majority of human cancers expresses hTERT and this molecule might therefore qualify as an universal tumor-antigen [7], and as a target for an effective cancer vaccine therapy.
Previously, MHC class I binding peptides derived from hTERT have been shown to elicit CD8+ T cell responses in vivo capable of recognizing hTERT expressing tumour cells [3, 13, 15, 16, 19, 25–28].
Like most tumour-associated antigens (TAA), hTERT is a self-antigen and central tolerance towards hTERT-derived peptides with high affinity for the MHC molecules is therefore to be expected. However, hTERT-derived peptides with intermediate binding affinities for MHC class I molecules might be insufficiently presented to trigger naive CTLs, but presented at levels capable of stimulating already activated CTLs. In the present work we have used a neural network capable of performing quantitative predictions of peptides binding to HLA-A*0201 molecules, and identified a number of nonamer hTERT-derived peptides with intermediate binding affinities. These peptides were then modified in order to improve their binding affinity for HLA-A*0201 above the threshold necessary for activation of naive T cells. This strategy has been used previously by us and others in studies including p53- and hTERT-derived peptides with low or intermediate affinity for the HLA-A*0201 molecule [8, 13, 18, 19, 24]. However, the position of the residues to be modified and substituted differed between the studies. In the present study modifications were performed in the two primary anchor position residues (P2, P9).
Materials and methods
HLA-A*0201 transgenic mice
C57-A2Kb transgenic mice, which express a chimeric heavy chain of the MHC-I molecule (HLA-A*0201 α1 and α2 and H-2Kb α3, transmembrane and intracytoplasmic domains), were kindly provided by Dr. N. Holmes, University of Cambridge, Cambridge, UK. The mice were backcrossed with C57Bl/6Bom (Bomholtgård, Ry, Denmark). HHD transgenic mice, which express the chimeric MHC-I heavy chain (HLA-A*0201 α1 and α2 and H-2Db α3, transmembrane and intracytoplasmic domains) with human β2-microglobulin (β2 m) covalently linked to the NH2 terminus of the heavy chain by a 15-amino acid long peptide were kindly provided by F. A. Lemonnier. HHD mice are H-2Db−/− and mouse beta2 m−/− double knockout mice.
Predictions of hTERT peptide binding to HLA-A*0201
The complete hTERT protein sequence was obtained from NP_937983 and virtually digested into all possible nonamer peptides. Artificial neural networks (ANN) were used to predict some of the most important events of antigen processing and presentation: proteasome digestion (NetChop 1.0) and HLA class I binding (NetMHC 1.0, current versions are available at the immunological section of http://www.cbs.dtu.dk/services/). Briefly, ANN are trained, using an existing set of data representing the event in question, to capture the general features of that event thereby enabling the ANN to predict the outcome for any nonamer [5, 14].
HLA-A*0201-binding assays
Peptides were synthesized using standard FMOC chemistry at Schafer-N (http://www.schafer-n.com). Purity was ascertained by reverse-phase HPLC and identity was confirmed by mass spectroscopy analysis. Peptide-binding affinities to purified HLA-A*0201 molecules were measured using an in vitro biochemical binding assay [6]. Briefly, the concentration of test peptide needed to effect 50% inhibition (IC50) of the binding of a radio labelled tracer peptide was measured by spun column gel filtration.
Stable transfection of CCL220 with HLA-A*0201/H2-Kb cDNA
Human colon carcinoma cells (CCL-220, HLA-A*0201−) were transfected by Lipofectamine 2000 (Invitrogen Corporation, CA, USA) with a plasmid vector encoding the chimeric heavy chain of the MHC class I molecule (A2Kb) and the neomycin resistance gene. Briefly, 7.5 μl Lipofectamine 2000 was diluted in 50 μl RPMI-1640 (serum, and antibiotics free) and incubated for 5 min at room temperature (RT) before being mixed with 50 μl RPMI-1640 containing 3 μg cDNA. After incubating for 20 min at RT, 100 μl of the cDNA–Lipofectamine 2000 complexes was added directly to 0.5 × 106 cells in 400 μl RPMI-1640. Cells were incubated at 37°C in a 5% CO2 incubator for 6 h, then additional 500 μl RPMI-1640 containing 20% FCS was added. The next day, transfected cells were passaged with complete culture medium supplemented with G418 (Sigma, St Louis, USA) at 800 μg/ml. The transfected cells were verified for HLA-A*0201 expression with flow cytometry by using the PA2.1 (anti-HLA-A*0201) unlabelled monoclonal antibody (HB-117, ATCC, Rockville, MD, USA) and subsequently FITC-conjugated rabbit anti-mouse immunoglobulins (Dako, Copenhagen, Denmark). Approximately 85% of the CCL220 cells were stably HLA-A*0201 positive in this culture system.
Cell lines and anti-CD8 antibody
CCL220, CCL220-A2Kb, EL4-A2Kb and EL4-A2 (a kind gift from Dr. Linda A. Sherman) were cultured in complete culture medium RPMI-1640 (Gibco-BRL, Rockville, MD, USA) supplemented with 10% fetal calf serum (FCS), penicillin and streptomycin, and 2 × 10−5 M mercaptoethanol. CCL220-A2Kb, EL4-A2 and EL4-A2Kb were also cultured in the presence of G418 (800 μg/ml). Ascites generated by hybridoma YTS 169.4 (a kind gift from Dr. J. Reimann) containing 1 mg anti-CD8 mAb/ml were used.
Generation of mature dendritic cells from bone marrow cells
Bone marrow cells from transgenic mice were kept over night in culture medium (RPMI 1640 with 10% FCS, penicillin, streptamycin, 2-mercaptoethanol) and the non-adhering cells were isolated by plastic adherence and cultured with GM-CSF, 10 ng/ml, and IL-4, 20 ng/ml (PreproTech Inc., Rocky Hill, NJ, USA). On day 3, fresh culture medium with GM-CSF and IL-4 were supplied. On day 6, the cells were harvested and cultured over night with GM-CSF, IL-4 and LPS, 1 μg/ml. On day 7, the mature dendritic cells were harvested and used for vaccination.
Generation of CTLs in transgenic mice
HHD transgenic mice, 8–12 weeks old, were injected subcutaneously in the flank with 3 × 106 syngenic mature dendritic cells pulsed with the relevant A2-binding hTERT-peptide and a T-helper peptide PADRE (Pan DR reactive epitope), which binds multiple HLA-DR alleles and H-2 I-Ab/d or I-Eb/d antigens [2]. On day 11 after vaccination, the mice were sacrificed and the splenocytes were recovered and re-stimulated with the vaccination-peptide. Briefly, splenocytes (100 × 106 ml) were loaded with the A2-binding vaccination-peptide, 100 μg/ml, for 2 h at 37°C and 5% CO2. On day 1 after re-stimulation, IL-2 (Roche, Indianapolis, USA), 50 IU/ml, was added. After 5 days, the CTLs were harvested and depleted for NK and B cells using anti CD49bDX5 antibodies and anti CD19 antibodies (MACS, Mylteneyi Biotec, Gladbach, Germany), respectively. Cells were depleted on a column, MACS LS, according to manufacturer’s protocol. The non-depleted cells were then used directly for 51Cr-release assay or kept for another 4 days under resting conditions with IL-2, and then used for ELISPOT assay. C57-A2Kb transgenic mice were immunized and splenocytes restimulated as above and unfractionated splenocytes were used directly for the ELISPOT assay.
ELISPOT assay
The generated CTLs were assayed in 20-h ELISPOT cultures (96-well nitrocellulose microtitre plates, Millipore, Billerica, MA, USA) for IFN-γ production. Briefly microtitre plates were coated overnight with 50 μl per well of anti-IFN-γ monoclonal antibody (BD Pharmingen, San Diego, CA, USA) at 10 μg/ml in cabonate buffer, pH 9.6. Effector:stimulator cell ratio, 3:1, was added in at least triplicates with titration of cells ranging from 300,000:100,000 down to 18,750:6,250. The plates were incubated in 37°C, 5% CO2 for 20 h. The plates were processed using biotinylated anti-IFNγ (5 μg/ml, biotin rat antimouse IFN-γ BD Pharmingen, San Diego, CA, USA), streptavidin-conjugated horseradish peroxidase (1:1,000, DAKOcytomation, Glostrup, Denmark) and Alkaline Phosphate Conjugate Substrate Kit (BIORAD, Hercules, CA, USA). Spots were quantified using the ELISPOT reader, ELR02 (Autoimmun Diagnostika GmbH, Strassberg, Germany). Peptide pulsed (40 μg/ml for 2 h, 37°C, 5% CO2) or unpulsed EL4-A2 or EL4-A2Kb transfectants, CCL220-A2Kb transfectants or wildtype CCL220 cells were used as stimulator cells in the assay.
In the case of human blood cells, ELISPOT analysis was performed after one week in vitro culture of separated mononuclear cells with peptide (5 μg) and IL-2 (40 IU/ml). We cultured 3 × 105 to 3 × 104 cells per well, depending on the availability of patient material, together with hTERTmp30 peptide (5 μg/ml) and TAP deficient HLA-A*0201+ 104 T2 cells. After an overnight incubation, IFN-γ spots were developed. The number of spots was determined by a digitalized ELISPOT counter (Immunospot, CTL Inc., CA, USA).
Cytotoxic assay
Target cells, CCL220 and CCL220-A2Kb, were either left unpulsed or pulsed with the vaccination-peptide or the wildtype peptide in a final concentration of 40 μg/ml for 2 h at 37°C in culture medium. Target cells were labelled (30 μCi Na512CrO4 per 1 × 106 cells), then washed four times and resuspended to 3 × 104 ml. Target cells (100 μl) were then mixed with effector cells at effector:target ratios (E:T) of 100:1, 50:1, 25:1 and 12.5:1. After incubation for 4 h at 37°C in 5% CO2, 51Cr-release was measured in 50 μl supernatant using Lumaplates (Perkin-Elmer, Boston, USA) and a microplate scintillation counter. Spontaneous and total 51Cr-releases were measured by adding culture medium or a detergent (Triton X-100) 2%, respectively. The percentage of specific lysis was calculated as 100 × (experimental release − spontaneous release)/(total release − spontaneous release).
Western blot
For detection of hTERT in human CCL220 tumor cells, cell lysates of 5 × 106 cells were subjected to electrophoresis on 10% SDS polyacrylamide gels. The separated proteins were electrotransferred to nitrocellulose membranes. The membranes were treated with hTERT-specific rabbit polyclonal antibody H-231 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by swine anti-rabbit IgG horseradish peroxidase (DAKOcytomation, Glostrup, Denmak). The HeLa nuclear extract (sc-2120, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a positive control for hTERT expression. The ECL Plus kit (Amersham Biosciences, Piscataway, NJ, USA) was used to detect antigen/antibody complexes and chemiluminescent signals were detected using X-ray film. The western blot positive HeLa extract control showed one band at 74–76 kDa and the same band was found in the cell-lysate of CCL220.
Clinical vaccination trial
Two HLA-A2 positive patients with progressive metastatic renal cell carcinoma were enrolled in a clinical phase I/II study testing the efficacy and toxicity of dendritic cell (DC) based vaccination (ClinicalTrials.gov Identifier: NCT00197860). Briefly, autologous DCs were pulsed with HLA-A2 matched hTERT mp30 peptide. On the day of vaccination, 1 × 107 peptide pulsed DCs were injected. Vaccination no. 1–4 were given weekly and no. 5–10 were given biweekly. A leukapheresis sample collected prior to treatment (week-2) and blood samples drawn at successive time points during treatment were analyzed (manuscript in preparation). Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation (Lymphoprep, Nycomed Diagnostika, Oslo, Norway) and cryo-preserved.
Results
Selection of new HLA-A*0201-binding hTERT-derived epitopes
Four peptides were chosen with predicted HLA-A*02 binding affinities from 15 to 382 nM and with at least one suboptimal anchor residue for HLA-A*0201 binding that theoretically, by substitution of an optimal amino acid, might improve the binding (Table 1).
Table 1.
HLA-A*0201 binding affinity (IC50 in nM) of six wild type and five amino acid modified peptides of hTERT
| Position | Wild type peptide | Modified peptide | ||||
|---|---|---|---|---|---|---|
| Sequence | pIC50 | mIC50 | Modification | Sequence | mIC50 | |
| p30–38 | RLGPQGWRL | 15 | 620 | p 38 L–V | RLGPQGWRV | 423 |
| p122–30 | YLPNTVTDA | 382 | 155 | p130 A–V | YLPNTVTDV | 160 |
| p359–67 | RLVETIFLG | 144 | 900 | ND | – | – |
| p540–48a | ILAKFLHWL | 38 | 340 | p548 L–V | ILAKFLHWV | 525 |
| p572–80b | RLFFYRKSV | ND | 1,298 | p572 R–Y | YLFFYRKSV | 206 |
| p1017–25 | LQLPFHQQV | 172 | 576 | p1018 Q–L | LLLPFHQQV | 340 |
Table 1 also includes the peptides, p540–548 [15, 27, 28] and p572–580 [13, 19], since the immunogenicity of p540–548 and a p572–580 analogue peptide have been demonstrated previously. Table 1 shows also the biochemically measured peptide binding affinities for HLA-A*0201. In order to improve the HLA-A*0201 binding affinity of these peptides, they were subjected to aminoacid substitutions as indicated in the table. However, after modification only minor changes in the affinity were observed: the affinities of three peptides were increased, in one peptide, there was hardly any change and in another, the affinity decreased after modification. The discrepancies between the predicted and the measured binding affinities are within the ranges normally observed in this system [5].
Immunogenicity of analogue hTERT peptides and cross-reactivity with the wild type epitope
Immunogenicity was assessed by immunizing C57-A2Kb transgenic mice. Each peptide was pulsed on dendritic cells and subsequently injected into transgenic mice and IFN-γ ELISPOT was used as readout for immunogenicity. Except for the known immunogenic peptide p540–548 [15, 27, 28], none of the hTERT-derived wild type peptides in Table 1 were capable of eliciting IFN-γ spots (data not shown).
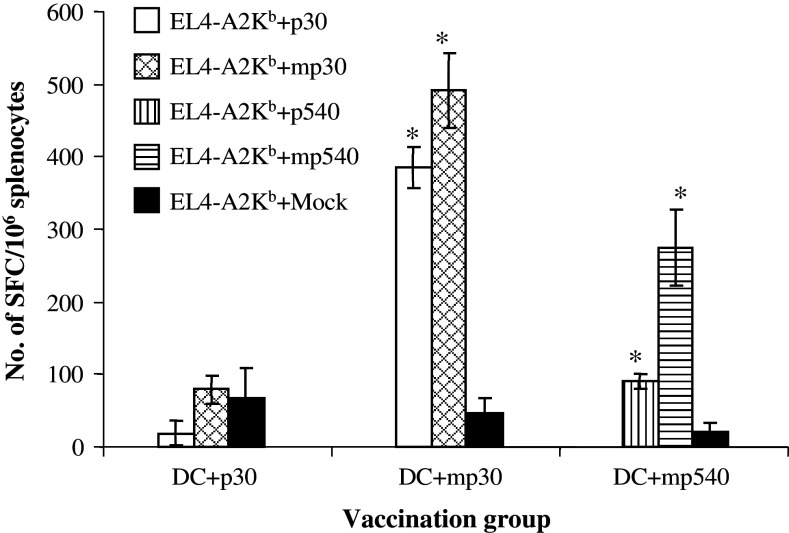
Next, the aminoacid modified peptides depicted in Table 1 were tested for immunogenicity by injection of peptide pulsed dendritic cells into individual groups of three transgenic mice. As shown in Fig. 1, the mp30–38 peptide (mp30) was found to be immunogenic in vivo. The data also show that T cells against mp30 crossreact with wildtype p30. Like the wildtype counterpart, p540–548 [15, 27, 28], the analogue mp540–548 epitope was shown to be immunogenic, and the peptide specific T cells crossreacted with wildtype p540–548. None of the other modified peptides were found to be immunogenic, although, their binding affinity for HLA-A*0201 were measured to be comparable to the intermediate binding affinity of mp30.
Fig. 1.
Immunogenicity and cross reactivity in the efferent arm of the immune response. Groups of C57-A2Kb transgenic mice (n = 3) were vaccinated with 3 × 106 syngenic DCs pulsed with p30, mp30 or mp540 (10 μg/ml), and PADRE (5 μg/ml). Ten days after immunization, the mice were sacrificed and the pooled splenocytes (from 3 mice) were pulsed with the peptide used for immunization (100 μg/ml) and expanded in vitro for 5 days, on day 5, the splenocytes were tested in ELISPOT wells for IFN-γ production after exposure to EL4-A2Kb cells pulsed with wildtype peptide, modified peptide and Mock (control), respectively, *P < 0.001 (t test)
CD8 dependence of T cells with specificity for mp30–38
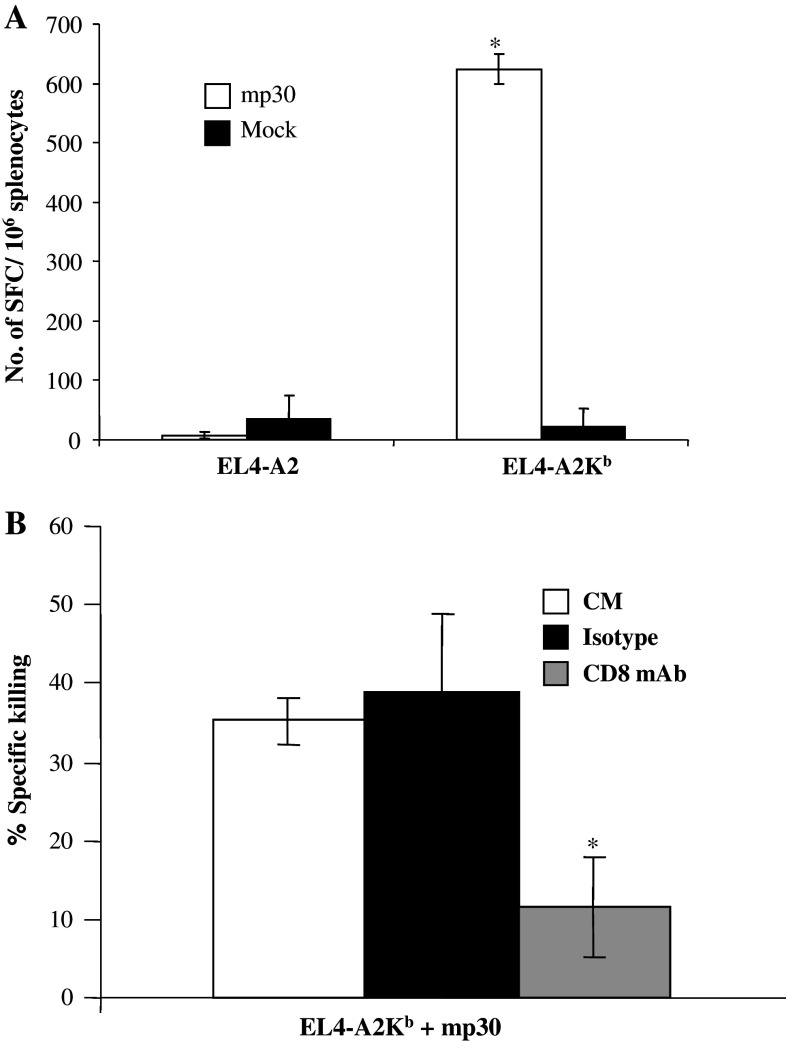
The function of anti-mp30 specific T cells generated in transgenic mice was found to be dependent on their CD8 mediated interaction with MHC-I α3 domain on target cells (Fig. 2a). Thus, if peptide-pulsed mouse EL4 cells transfected with HLA-A*0201 were used as stimulator cells in an ELISPOT assay, there was no response. In contrast, peptide-pulsed target EL4 cells transfected with the chimeric A2Kb cDNA construct provided the necessary contact between TCR/CD8 and the peptide-MHC-I complex to induce IFN-γ production by mp30 specific T cells. In addition, when 5 μg anti-CD8 antibody was added to the cytotoxicity assay, the level of CTL mediated killing dropped by 66% (Fig. 2b).
Fig. 2.
CD8-dependence of the generated CTLs. C57-A2Kb transgenic mice (3 mice) were immunized with 3 × 106 syngenic DCs pulsed with mp30 (10 μg/ml) and PADRE (5 μg/ml). Ten days after immunization, the mice were sacrificed and the pooled splenocytes (from 3 mice) were pulsed with mp30 (100 μg/ml) and expanded in vitro for 5 days. Day 5, splenocytes (300,000/well) were tested in ELISPOT wells for IFN-γ production when stimulated with either EL4-A2 cells (100,000) pulsed with mp30 or an HLA-A*0201-binding irrelevant peptide (Mock) or EL4-A2Kb cells (100,000) pulsed with mp30 or an HLA-A*0201-binding irrelevant peptide (a). In some cases, splenocytes were tested in the 4 h cytotoxicity assay (b). Target cells were mp30-pulsed EL4-A2Kb and incubated with effector cells at ratio of 1:50 in the presence of anti-CD8 antibody or isotype control. *P < 0.001 (t test)
Target cells are more efficiently sensitized for lysis by modified than by non-modified peptide
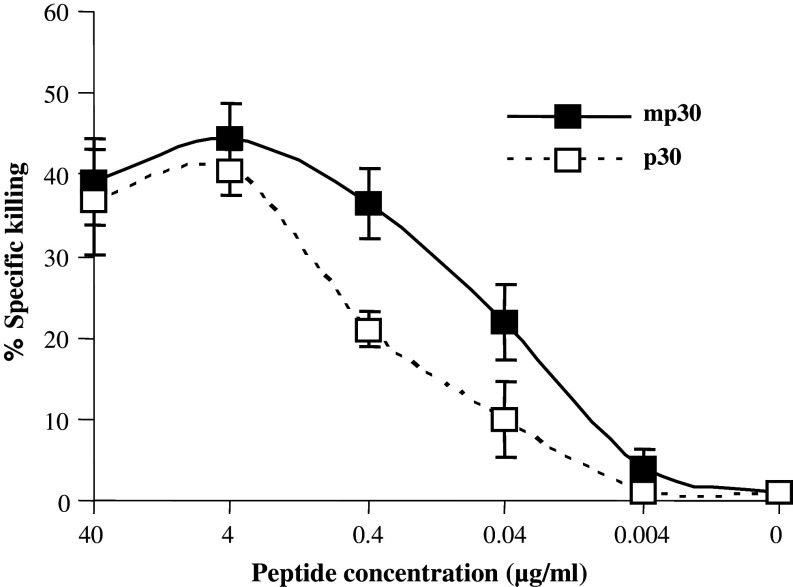
Spleen cell-derived mp30 specific CTLs from immunized mice were studied for their capacity to lyse target cells pulsed with a dose titration of mp30–38 versus p30–38 peptide. As shown in Fig. 3 mp30–38 peptide sensitized target cells show half-maximal lysis at approximately tenfold lower peptide concentrations than p30–38 peptide.
Fig. 3.
Target cells are more efficiently sensitized for lysis by modified than by non-modified peptide. Spleen cells derived from the HHD transgenic mice immunized with mp30 pulsed DCs were studied for their capacity to lyse EL4-A2Kb cells pulsed with a dose titration of mp30–38 versus p30-38 peptide. The effector cells and target cells pulsed with different concentration of peptides were cultured at ratio of 50:1 for 4 h. Filled squares are EL4-A2Kb pulsed with mp30, open squares are EL4-A2Kb pulsed with p30. Error bars represent standard deviations
Natural processing and presentation of the p30
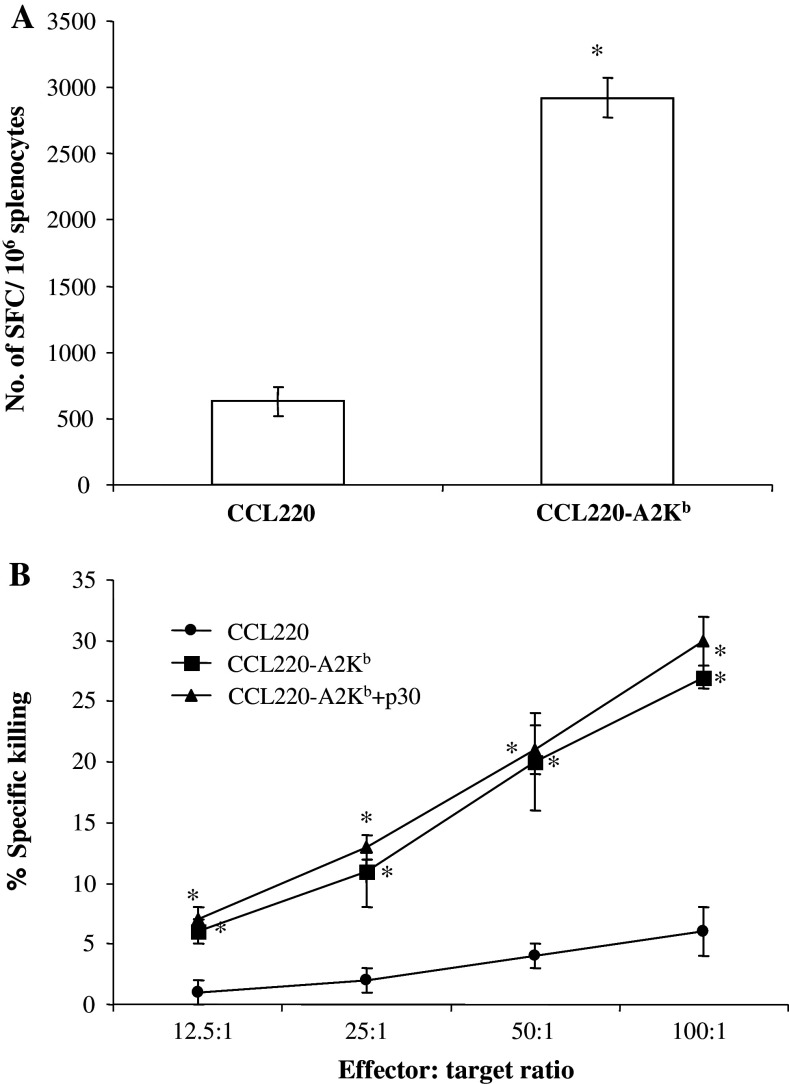
CCL220 cells express telomerase as judged from western blot experiments (data not shown). HLA-A*0201 negative CCL220 cells were transfected with the chimeric HLA-A*0201/Kb cDNA construct. Transfected and nontransfected cells were used as targets for CTL immunity generated in HHD transgenic mice immunized with dendritic cells pulsed with mp30. Data in Fig. 4 show that only transfected CCL220 cells are recognized by anti-mp30–38 T cells both in the ELISPOT assay (Fig. 4a) and in the cytotoxicity assay (Fig. 4b). Further, the data in Fig. 4b illustrate that pulsing of the transfected target cells with wildtype p30 does not significantly increase their sensitivity to killing, suggesting that the p30 epitope from the point of view of immunogenicity is optimally processed and presented by the CCL220 cells.
Fig. 4.
T cells specific for mp30 epitope release IFN-γ after exposure to CCL220-A2Kb transfectants and kill CCL220-A2Kb transfectants. HHD transgenic mice (n = 4) were vaccinated with 3 × 106 syngenic DCs pulsed with mp30–38 (10 μg/ml) and PADRE (5 μg/ml). Eleven days after immunization, the mice were sacrificed and the pooled splenocytes (from 4 mice) were pulsed with the vaccination peptide (100 μg/ml) and expanded in vitro for 5 days. On day 5, the splenocytes were harvested and left under resting conditions (IL-2 was added) for another 4 days. After resting, the splenocytes were tested in ELISPOT wells for IFN-γ production in response to CCL220-A2Kb and CCL220, respectively (a). For the cytotoxicity assay (b), the splenocytes were tested on day 5. Target cells were CCL220-A2Kb, p30 pulsed CCL220-A2Kb and CCL-220, respectively. *P < 0.001 (t test)
Response against mp30–38 in vaccinated patients
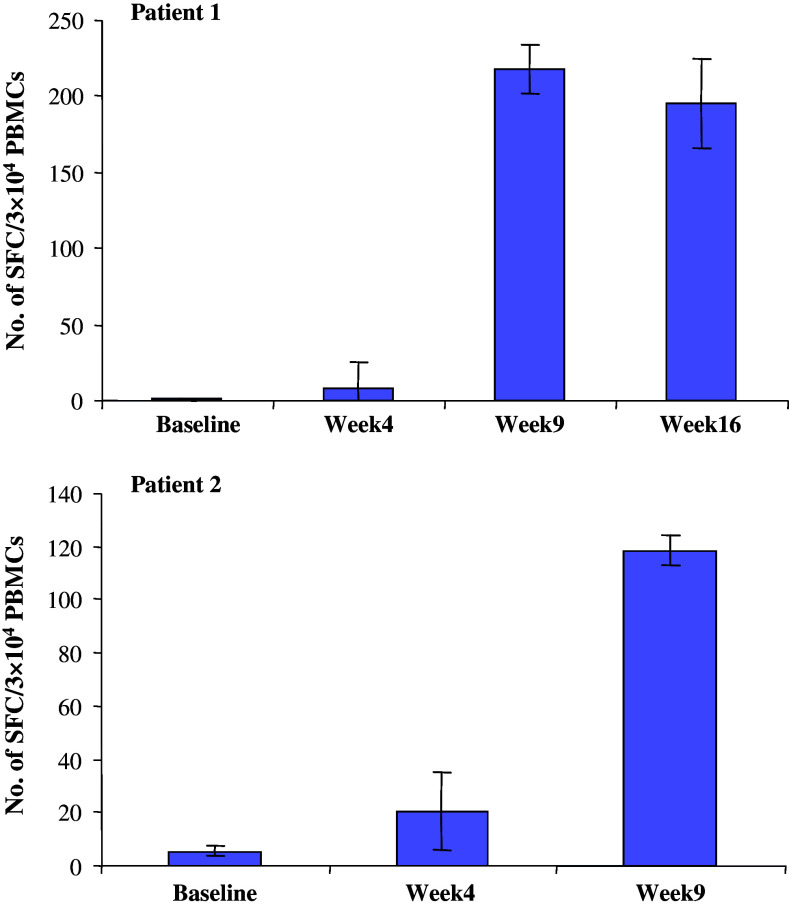
Two patients with metastatic renal carcinoma were enrolled into a vaccination program (see “Materials and methods”). PBMC were obtained prior to and at various intervals after initiation of the mp30/DC vaccine injections. The ELISPOT data in Fig. 5 demonstrate that none of the patients had detectable levels of reactivity prior to vaccination, but both patients showed a vaccine-dose-related increase in anti-mp30 reactivity with a maximum after six vaccinations.
Fig. 5.
Changes in the frequency of hTERTmp30 specific T cells in 7 days cultures of PBMCs from two renal cell carcinoma patients vaccinated for 6 (Patient 2) or 10 (Patient 1) times (week 1–16) with mp30 loaded autologous dendritic cells. Vaccination-induced T cell responses against mp30 were measured by ELISPOT analysis and data show the mean number of peptide specific IFN-γ spots in response to mp30 by 3 × 104 in vitro stimulated PBMCs. Non-specific IFN-γ spots are subtracted. Bars, range of duplicates
Discussion
In the present study, we focused on hTERT peptides with a relatively low affinity for the HLA-A*0201 molecule. Consequently, T cell immunity against these peptides could be expected not to be intrathymically deleted but preserved and available for tumour rejection responses. Aminoacid substitutions were performed primarily at one of the peptide anchor positions in order to improve binding to HLA-A*0201 and immunogenicity of the modified peptides. Only one of these modified peptides (mp30–38), with a selected residue modification in the P9 anchor position, was found to have an improved binding affinity for the HLA-A*0201 molecule and by immunization capable of inducing peptide specific T cells. Thus, dendritic cells pulsed with mp30 peptide and injected into HLA-A*0201 transgenic mice induced a specific HLA-A*0201 restricted and CD8 dependent response. We also demonstrated that the generated CTLs cross reacted with the p30 wild type peptide counterpart and showed that mp30–38 peptide sensitized target cells show half-maximal lysis at approximately tenfold lower peptide concentrations than p30–38 peptide. Thus it appears that the modified p30–38 peptide is improved not only as an immunogen, but also as an antigen.
In order to include the mp30 peptide epitope, in a vaccine for immunotherapy, it is necessary to prove that the wild type p30–38 is processed and presented at the surface of human telomerase expressing HLA-A*0201 positive tumor cells. For this purpose, we used a HLA-A*0201 negative, but HLA-A*0201/Kb transfected, telomerase positive colon carcinoma cell line, CCL-220, as target cells for mp30–38 immune murine T cells, and we showed reactivity in this system against the naturally processed peptide p30–38. The non-transfected, HLA-A*0201 negative cell line was used as a convenient genetically identical background control. Thus, we propose that CTL precursors for p30–38 peptide can be triggered in cancer patients by a vaccine including the mp30 peptide. In support of this notion, an immune responses against mp30 was recorded in PBMC of two HLA-A*0201+ renal cell carcinoma patients after vaccination with autologous dendritic cells pulsed with the mp30 peptide (Fig. 5), strongly supporting that the human TCR repertoire is capable of recognizing and responding to this peptide in vivo.
Previously, the immunogenic HLA-A*0201-restricted hTERT epitope, p540–48, included as an immunogenic control in the present work, has been extensively studied and used in vaccines for cancer patients [4, 15, 23, 26–28]. This epitope might be poorly processed as judged from our own predictions, since the C-flush value [14] is as low as 0.04 (data not included). The C-flush value is the probability of processing of an epitope (0 ≤ C-flush value ≤ 1). In comparison, the predicted C-flush value of the p30 peptide is 1. The fact that three to four in vitro restimulations are needed to induce p540-specific CTLs from CD8-enriched PBMCs in healthy as well as in cancer patients [28] raises some doubt about the efficiency of presentation of the p540 epitope. In addition, Ayyoub et al. [4] incubated a long hTERT precursor peptide (amino acids 534–554) with proteasomes and analyzed the products by mass spectrometry and cytotoxic assays. It showed that p540 is inefficiently processed. Finally, CTLs from HLA-A*0201+ melanoma patients only show cytotoxicity against A2+ target cells in the presence of exogeneously added p540–48 peptide but no direct cytotoxicity against HLA-A*0201+ hTERT+ melanoma or colon carcinoma cells [4]. The clinical outcome from a phase I trial [26] in which patients were vaccinated with DCs pulsed with p540 is hard to judge because of the small number of patients included and the absence of a patient control group. The results from other vaccination studies with p540 are not encouraging [17].
A number of other immunogenic HLA-A*0201-restricted epitopes have been identified using HLA-A*0201 transgenic mice as well as by in vitro immunization of donor PBMCs. Minev et al. [15] used transgenic mice and generated a peptide specific response against p865. They also showed that patient CTLs specific for p865 lysed a variety of telomerase expressing, HLA-A*0201 positive cell lines of breast, colon, lung and melanoma origin. Another two A2-restricted hTERT-derived epitopes, p572 [13, 19] and p988 [19], were identified coincidentally. In contrast to p540 and p865, p572 and p988 show relative low binding affinity for HLA-A*0201 and therefore (as for p30) likely spared from deletion due to self-tolerance. Consequently, analogue peptides of p572 and p988 (the affinity for HLA-A*0201 was increased by aminoacid substitution in P1) were identified to be immunogenic in HLA-A*0201 transgenic mice, and the wildtype peptide counterparts were shown to be endogenously processed and presented by HLA-A*0201 positive tumour cells and recognized by CTLs raised by the analogue peptide. In the present study, a substitution in the anchor P9 in the p30 epitope was introduced, since the binding to HLA-A*0201 is affected positively by this change without interfering with TCR binding. This is not the case as with substitution in P1 in which position there is a risk for TCR binding interference.
In addition to the above mentioned A2-restricted epitopes, one HLA-A3-restricted [25], two HLA-A24-restricted [3], three HLA-B0702-restricted [1] and finally, one HLA-A1 restriced [20] hTERT-derived epitope have been identified to meet the needs of candidate epitopes for a cancer vaccine.
From an immunotherapeutic point of view, it has been speculated that an effective tumor vaccine may require the induction of both CTL as well as T-helper cell responses against hTERT [29]. Two hTERT MHC class II-restricted epitopes have been identified capable of inducing CD4+ T cell reponses in the context of several commonly found HLA-DR alleles [21, 22].
The result of the present study may extend the number of hTERT epitopes that can potentially be targeted by CTLs in an hTERT-based vaccine. A vaccine that include both common MHC class II and class I allele-restricted hTERT epitopes may have greater success than vaccines comprising only one or a few allele-restricted epitopes.
Acknowledgments
This work was supported by the Danish Cancer Society, Aase og Ejnar Danielsens Fond, Dagmar Marshalls Fond, Else og Mogens Wedell-Wedellsborg Fond.
References
- 1.Adotévi O, Mollier K, Neuveut C, Cardinaud S, Boulanger E, Mignen B, et al. Immunogenic HLA-B*0702-restricted epitopes derived from human telomerase reverse transcriptase that elicit antitumor cytotoxic T-cell responses. Clin Cancer Res. 2006;12:3158–3167. doi: 10.1158/1078-0432.CCR-05-2647. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/S1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 3.Arai J, Yasukawa M, Ohminami H, Kakimoto M, Hasegawa A, Fujita S. Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood. 2001;97:2903–2907. doi: 10.1182/blood.V97.9.2903. [DOI] [PubMed] [Google Scholar]
- 4.Ayyoub M, Migliaccio M, Guillaume P, Lienard D, Cerottini JC, Romero P, et al. Lack of tumor recognition by hTERT peptide 540–548-specific CD8+ T cells from melanoma patients reveals ineffeicient antigen processing. Eur J Immunol. 2001;31:2642–2651. doi: 10.1002/1521-4141(200109)31:9<2642::AID-IMMU2642>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Buus S, Lauemoller SL, Worning P, Kesmir C, Frimurer T, Corbet S, et al. Sensitive quantitative predictions of peptide-MHC binding by a ‘query by committee’ artificial neural network approach. Tissue Antigens. 2003;62:378–384. doi: 10.1034/j.1399-0039.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 6.Buus S, Stryhn A, Winther K, Kirkby N, Pedersen LO. Receptor–ligand interactions measured by an improved spun column chromatography technique. A high efficiency and high throughput size separation method. Biochim Biophys Acta. 1995;1243:453–460. doi: 10.1016/0304-4165(94)00172-t. [DOI] [PubMed] [Google Scholar]
- 7.Greener M. Telomerase: the search for a universal cancer vaccine. Mol Med Today. 2000;6:257. doi: 10.1016/S1357-4310(00)01731-7. [DOI] [PubMed] [Google Scholar]
- 8.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–433. doi: 10.1172/JCI200419418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 10.Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 11.Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, et al. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert B, Pitts AE, Baker SI, Hamilton SE, Wrigth WE, Shay JW, et al. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández J, Garcia-Pons F, Lone YC, Firat H, Schmidt JD, Langlade-Demoyen P, et al. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc Natl Acad Sci USA. 2002;99:12275–12280. doi: 10.1073/pnas.182418399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesmir C, Nussbaum AK, Schild H, Detours V, Brunak S. Prediction of proteasome cleavage motifs by neural networks. Protein Eng. 2002;15:287–296. doi: 10.1093/protein/15.4.287. [DOI] [PubMed] [Google Scholar]
- 15.Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci USA. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 17.Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. Immunization of patients with the hTERT: 540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenourly expressing telomerease. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen TR, Buus S, Brunak S, Nissen MH, Sherman LA, Claesson MH. Identification and design of p53-derived HLA-A*0201-binding peptides with increased CTL immunogenicity. Scand J Immunol. 2001;53:1–9. doi: 10.1046/j.1365-3083.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 19.Scardino A, Gross DA, Alves P, Schultze JL, Graff-Dubois S, Faure O, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 20.Schreurs MWJ, Kueter EWM, Scholten KBJ, Kramer D, Meijer CJLM, Hooijberg E. Identification of a potential human telomerase reverse transcriptase-derived, HLA-A1-restricted cytotoxic T-lymphocyte epitope. Cancer Immunol Immunother. 2005;54:703–712. doi: 10.1007/s00262-004-0611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroers R, Huang XF, Hammer J, Zhang J, Chen SY. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
- 22.Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, et al. Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res. 2003;9:4743–4755. [PubMed] [Google Scholar]
- 23.Speiser DE, Cerottini JC, Romero P. Can hTERT peptide (540–548)-specific CD8 T cells recognize and kill tumor cells? Cancer Immun. 2002;2:14–19. [PubMed] [Google Scholar]
- 24.Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, et al. A general strategy to enhance immunogenicity of low-affinity HLA-A*0201.1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol. 2000;30:3411–3421. doi: 10.1002/1521-4141(2000012)30:12<3411::AID-IMMU3411>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 25.Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res. 2001;7:3343–3348. [PubMed] [Google Scholar]
- 26.Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.CCR-0620-3. [DOI] [PubMed] [Google Scholar]
- 27.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 28.Vonderheide RH, Schultze JL, Anderson KS, Maecker B, Butler MO, Xia Z, et al. Equivalent induction of telomerase-specific cytotoxic T lymphocytes from tumor-bearing patients and healthy individuals. Cancer Res. 2001;61:8366–8370. [PubMed] [Google Scholar]
- 29.Wei WZ, Ratner S, Shibuya T, Yoo G, Jani A. Foreign antigenic peptides delivered to the tumor as targets of cytotoxic T cell. J Immunol Methods. 2001;258:141–150. doi: 10.1016/S0022-1759(01)00484-7. [DOI] [PubMed] [Google Scholar]