Abstract
Cancer vaccines, while theoretically attractive, present difficult challenges that must be overcome to be effective. Cancer vaccines are often poorly immunogenic and may require augmentation of immunogenicity through the use of adjuvants and/or immune response modifiers. Toll-like receptor (TLR) ligands are a relatively new class of immune response modifiers that may have great potential in inducing and augmenting both cellular and humoral immunity to vaccines. TLR7 ligands produce strong cellular responses and specific IgG2a and IgG2b antibody responses to protein immunogens. This study shows that a new TLR7 ligand, 3M-019, in combination with liposomes produces very strong immune responses to a pure protein prototype vaccine in mice. Female C57BL/6 mice were immunized subcutaneously with ovalbumin (OVA, 0.1 mg/dose) weekly 4×. Some groups were immunized to OVA plus 3M-019 or to OVA plus 3M-019 encapsulated in liposomes. Both antibody and cellular immune responses against OVA were measured after either two or four immunizations. Anti-OVA IgG antibody responses were significantly increased after two immunizations and were substantially higher after four immunizations in mice immunized with OVA combined with 3M-019. Encapsulation in liposomes further augmented antibody responses. IgM responses, on the other hand, were lowered by 3M-019. OVA-specific IgG2a levels were increased 625-fold by 3M-019 in liposomes compared to OVA alone, while anti-OVA IgG2b levels were over 3,000 times higher. In both cases encapsulation of 3M-019 in liposomes was stronger than either liposomes alone or 3M-019 without liposomes. Cellular immune responses were likewise increased by 3M-019 but further enhanced when it was encapsulated in liposomes. The lack of toxicity also indicates that this combination may by safe, effective method to boost immune response to cancer vaccines.
Keywords: Vaccination, T cells, TLR, Adjuvant
Introduction
Toll-like receptors (TLRs) are critical elements in innate immune responses to pathogens [6, 22, 33]. The family of TLRs recognizes a broad spectrum of pathogen components including flagellum, lipopolysaccharide, RNA, and DNA. Activation of TLRs induces immediate production of cytokines and other molecules that may have direct effects leading to pathogen death. In addition, cytokines and immune response modifiers activate and direct subsequent induction of adaptive immune responses [25]. The involvement of TLR activation in subsequent adaptive immunity has sparked interest in the potential of TLR ligands to act as adjuvants for vaccines [8, 29, 36, 39, 40].
TLR-7 recognizes single-stranded RNA [14] leading to production of interferon, IL-6, IL-12, and other cytokines and chemokines. This pattern favors a TH1 response that, in turn, leads to antiviral cellular responses and preferential IgG2a antibody production [3, 4, 34]. Imidazoquinolines are a family of synthetic organic compounds that activate TLR-7 and induce the same immune response as RNA binding [14]. Imiquimod was the first imidazoquinoline developed and has been approved for topical treatment of genital warts [1, 15, 19] and the skin cancer conditions, superficial basal cell carcinoma and actinic keratosis. We have recently reported the adjuvant potential of topical imiquimod treatment [16]. Other imidazoquinolines including resiquimod [12, 13, 24, 37, 38] and S-27609 [9] have proven to be effective adjuvants.
In this study we have examined the adjuvant potential of a new imidazoquinoline agent (3M-019) both alone and encapsulated in liposomes. The very strong immune responses induced by this combination may have implications for vaccine production against viral infections and even cancer vaccines.
Materials and methods
Reagents
Imidazoquinolines 3M-019 and S-27609 were generous gifts provided by 3M Pharmaceuticals (St Paul, MN). Both of these derivatives of the basic imidazoquinoline structure differ in the substitutions on the side chains and bind to TLR7 in mice and TLR7 & 8 in humans. The exact structures are proprietary information held by the company. For use they were dissolved in 0.03 M citrate buffered saline (pH 6.0) at a concentration of 4.0 mg/ml. Ovalbumin (OVA) was obtained from Sigma Chemical Co (St. Louis, MO). Dimyristoylphosphatidylcholine (DMPC) was obtained from Avanti Polar Lipids (Alabaster AL).
Mice
All animal studies were approved by the Institutional Animal Care and Use Committee and conformed to the Principles of laboratory animal care. Female C57BL/6 mice (5–6 weeks old) were obtained from Taconic labs (Germantown, NY) and were maintained with standard mouse chow and conditions in the animal facility. Mice were acclimated for at least 1 week and then weighed prior to initiation of studies to assure that all mice weighed at least 15 g at the outset.
Cells
E.G7-OVA cells were originally obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in this laboratory for several years. Suspension cultures of these cells were grown in RPMI 1640 medium (Cellgro medium, Mediatech, Inc. Herndon, VA) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Norcross, GA). Antibiotics were obtained from Invitrogen (Carlsbad, CA) and used at the following concentrations: geniticin (G418, 0.4 mg/ml), streptomycin (100 μg/ml), and penicillin (100 U/ml).
Immunizations
Mice were immunized to a standard dose (100 μg) of OVA injected subcutaneously once a week for a total of four injections. All injections in the lower abdominal region switching sides each week and were given in a total volume of 0.1 ml of citrate-buffered saline. Both 3M-019 and OVA stocks were made up at 2× final concentration so that mixing equal volumes produced the desired concentrations. Both agents were mixed prior to liposome encapsulation. Different concentrations of imidazoquinolines were produced by dilution of the stock and were mixed with OVA and then encapsulated in liposomes as described previously [11, 16, 17]. Other groups of mice were immunized to OVA encapsulated in liposomes alone, to OVA + imidazoquinoline without liposomal encapsulation, or to lethally irradiated E.G7-OVA cells. The liposomes, consisting of the pure lipid dimyristoylphosphatidylcholine (DMPC, Avanti Polar Lipids, Alabaster AL), were formed by three cycles of freeze/thaw/sonicate as described [16] to yield samples containing 10 mg DMPC/dose. The E.G7-OVA cells were harvested from culture, washed 4 × with PBS, then exposed to a lethal dose of gamma radiation in a cell irradiator. Irradiated cells were collected by centrifugation, counted, resuspended in PBS at 5.0 × 107 cells/ml and injected in 0.1 ml (5 × 106 cells) S.C. in the lower abdominal region. As a negative control, a group of mice were immunized to 0.03 M citrate buffered saline (pH 6.0) diluted with an equal volume of PBS.
Assay of anti-OVA antibody responses
ELISA assay of antibody levels were performed as previously described [16, 17]. Mice were bled at three different time points under anesthesia from the retro-orbital sinus. Blood was collected immediately prior to the first immunization for baseline measurements and 1 week after the second and fourth immunizations. In most cases the sera at each time point were pooled by immunization group, and stored at −80°. The presence of anti-OVA IgG, IgM, and IgG subclass antibodies was measured by ELISA using Nunc maxisorb plates (Nalge Nunc International, Pittsburgh, PA). All wells were coated by overnight incubation of 1.0 μg of OVA in 0.1 ml in coating buffer (0.05 M carbonate buffer, pH 9.6). The antigen was removed and plates blocked with 5% milk/PBS by 2 h incubation at 37°C. Initial assay of IgG and IgM antibodies to OVA were performed with sera diluted 1:20 and 1:10 respectively in 5% milk/PBS. Overnight incubations of sera at 4°C were followed by extensive washes with 0.05% tween 20/PBS. Biotin-linked anti-mouse secondary goat antibodies to mouse IgG or IgM (ICN/Cappel, Aurora, OH) were diluted 1:500 in blocker and incubated for 1 h at 37°C. Plates were washed extensively and then incubated for 1 h at 37°C with avidin:peroxidase complex (extravidin, Sigma Chemical Co, St Louis, MO) diluted 1:1,000 in milk. After incubation the plates were washed 6× with tween-20 wash buffer. Peroxidase activity was determined by incubation with 0.10 ml of TMB substrate (KPL, Gaithersburg, MD). Reactions were stopped after 2 min by addition of 0.10 ml of 5% phosphoric acid and the OD read at 450 nm against blank wells that had no sera added.
The levels of anti-OVA IgG subclasses were determined by ELISA as above using a mouse IgG subtyping kit (Sigma, Chemical Co, St. Louis, MO) consisting of goat anti-mouse IgG subclass specific antibodies for the secondary antibody incubation. This was followed by 1 h incubation with biotin-conjugated rabbit anti-goat IgG (Sigma Chemical Co.), then avidin:peroxidase followed by substrate as above. IgG2a and IgG2b titers against OVA were quantified by end-point titer analysis as described [16, 39]. This analysis used specific affinity purified goat anti-mouse IgG2a and IgG2b antibodies (Immunology Consultants Laboratory, Inc. Newberg OR) that have less than 1% cross reactivity with other antibody classes or subclasses. Sera were initially diluted 1:100 in blocker and 1:5 serial dilutions made from there up to a final dilution over 7.8 × 106. The secondary and tertiary antibodies were used as described above. The reactions were stopped 10 min after addition of substrate. The endpoint titer was defined as the lowest dilution that gave an absorbance of >0.100 above background of sera from PBS-immunized mice.
Assay of cellular responses
Anti-OVA cellular immunity was determined by ELISPOT assay of interferon-γ release as previously described [11, 17]. Briefly, spleens were obtained from mice after sacrifice by CO2 narcosis, splenocytes were collected, and red blood cells lysed by hypotonic shock in ice-cold 0.87% ammonium chloride buffer. Remaining splenocytes were washed twice with culture medium and counted. Splenocytes were added to wells of ELISPOT plates (Multiscreen filtration plates, Millipore, Billerica, MA, previously coated with anti-mouse interferon-γ antibodies Biosource International, Camarillo, CA) in 0.10 ml of medium (RPMI 1640 supplemented with 10% FBS). 1:2 serial dilutions of 0.05 ml were made from these wells, then 2 × 104 irradiated E.G7-OVA target cells added in 0.05 ml of medium to give the proper E:T ratios of 100:1, 50:1, 25:1, and 12.5:1. After 48 h cells were lysed in 0.05% tween20/PBS. Biotinylated anti-mouse IFN (Biosource International) was added and incubated at 4°C overnight followed by extravidin alkaline phosphatase (Biosource International) diluted in 0.1% tween/PBS for 1 h at room temperature. After extensive washes with 0.05% tween/PBS the spots were visualized by addition of BCIP/NBT substrate (KPL) for 5 min.
To determine the contribution of OVA-specific CD8+ T cells to the overall cellular response, ELISPOT assays were performed in the presence of blocking antibodies specific to mouse CD8+ cells as described previously [11, 17]. H35 is a rat monoclonal antibody specific for the mouse CD8 molecule that was produced from H35 hybridomas grown in this laboratory. H35 was added (at 10 μg/well) to wells at each E:T ratio before the start of the 48 h incubation while other wells had no blocking antibody or antibody (PK136) against mouse NK cells as controls. Anti-OVA specific CD8+ T cell activity was determined from the difference in the number of spots in wells with and without blocking monoclonals.
Safety
The site of injection was examined 24 h after immunization for signs of inflammation or local reactions. Mice were also observed for piloerection, lethargy, or hyperactivity as measures of abnormal response to treatment. All mice were weighed weekly to detect systemic effects that could cause loss of weight.
Statistical analyses
Comparisons of all groups anti-OVA IgG, IgM, and IgG subclass antibody levels in individual mice were done by ANOVA analysis, comparison of all pairs using Tukey–Kramer HSD, and comparison of individual pairs using Student’s t test. Analysis of the spots developed by ELISPOT assay of cellular immunity was performed by multiple ANOVA analysis (Manova fit program) of duplicate wells at each E:T ratio.
Results
Antibody responses
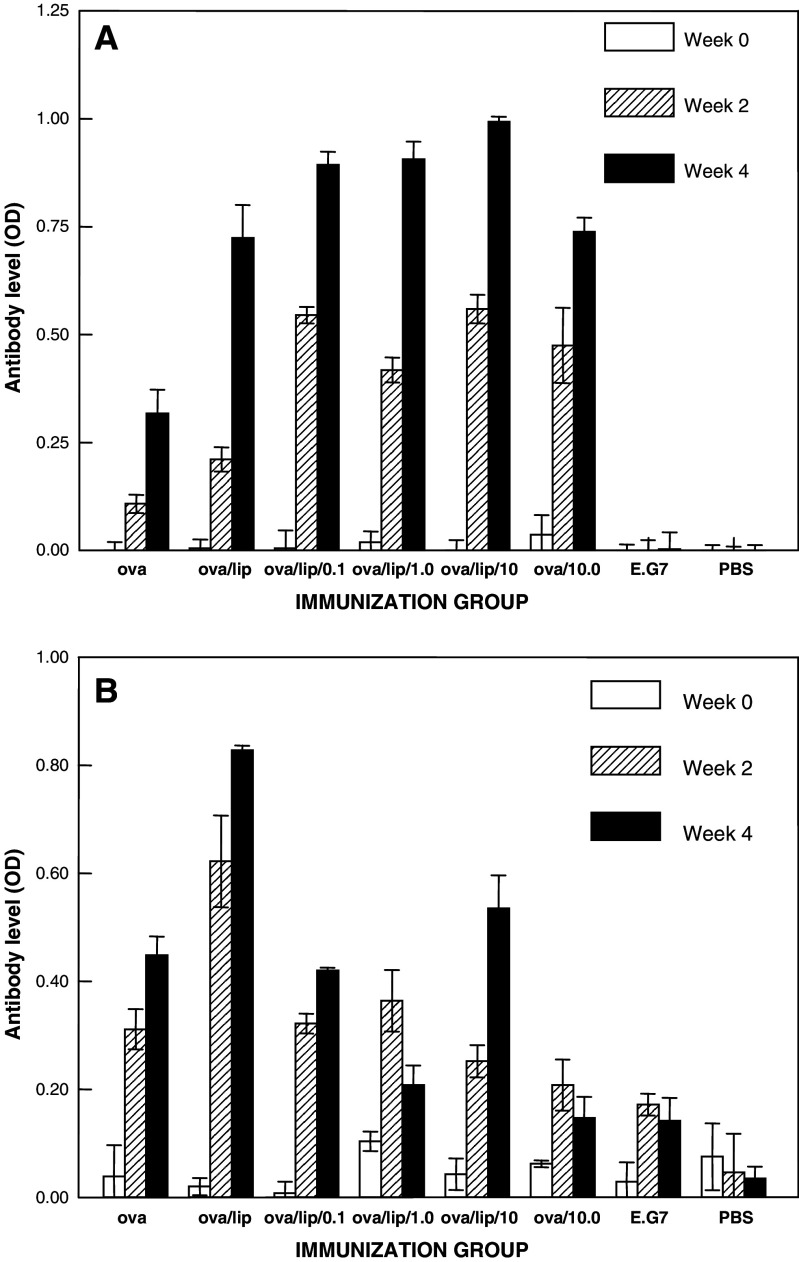
Specific anti-OVA IgG, IgM, and IgG subclass levels were measured in sera collected prior to immunization, at 1 week after the second immunization, and 1 week after the fourth immunization. The relative levels of anti-OVA IgG (panel A) and IgM (panel B) for these sera are shown in Fig. 1. Immunization to OVA alone led to low IgG antibody levels after two immunizations but significantly higher levels after the fourth immunization. When OVA was combined with 3M-019 (at a dose of 10 mg/kg), the anti-OVA IgG responses were significantly higher after both two and four immunizations, but especially evident after the second immunization. As we have previously reported [16, 17], encapsulation of OVA alone in liposomes caused similar increases in specific IgG responses at each post-immune time point as well. Encapsulation of OVA together with any dose of 3M-019 in liposomes appeared to increase somewhat further the anti-OVA IgG responses above those caused by liposome encapsulation of OVA alone. The augmentation was greater after two immunizations than that seen after four immunizations. IgG levels against OVA in mice immunized to the OVA-expressing E.G7-OVA cells were not significantly above the level of PBS-immunized mice or the plate background.
Fig. 1.
IgG and IgM antibody responses to OVA pre-immunization, after two immunizations, and after four immunizations. Mice were bled before immunization at the start (week 0) of each study, 1 week after the second immunization (week 2), and 1 week after the fourth immunization (week 4). Sera from mice in each group of a typical study were pooled and total IgG (panel A) and IgM (panel B) anti-OVA antibody levels determined by ELISA. Each bar represents the mean absorbance ± standard deviation of triplicate wells
The relative anti-OVA IgM antibody levels in the same sera produce a totally different picture than the IgG data as shown in Panel B (Fig. 1). The anti-OVA IgM levels were significant after two immunizations but further increased after four immunizations to OVA alone. Encapsulation of OVA alone into liposomes produced a similar augmentation of antibody levels to that seen for IgG. When OVA was combined with the highest dose of 3M-019 and injected without encapsulation a marked reduction in specific IgM response was detected, especially after four immunizations. Encapsulation of OVA and 3M-019 at all doses led to anti-OVA IgM responses intermediate between the high levels resulting from liposomes and the low levels seen when 3M-019 was combined with OVA but used without liposomes. The stimulatory effect of liposomes is counterbalanced by the reduction caused by imidazoquinolines. Anti-OVA IgM antibody levels in mice immunized to E.G7-OVA cells were not significantly above control levels in mice immunized to PBS.
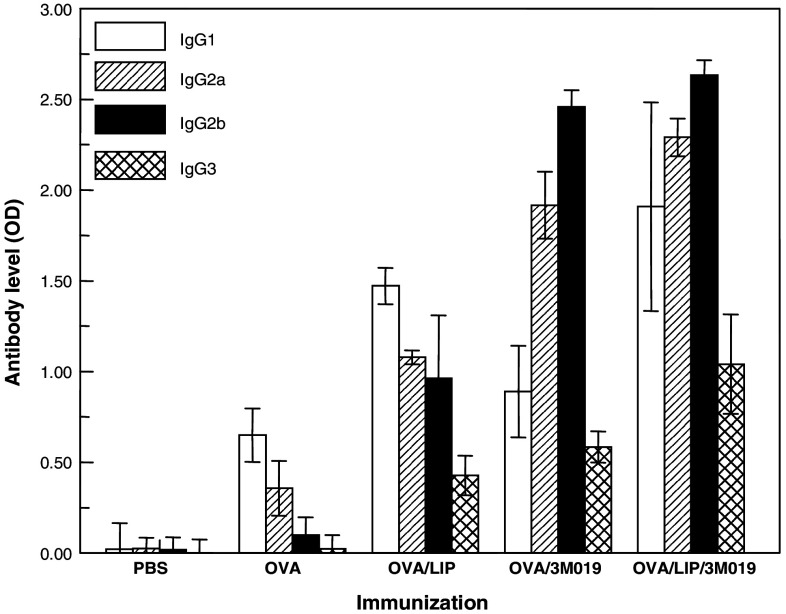
The increases in IgG levels with concomitant reduction in IgM responses indicate that 3M-019 produces an early class switch from IgM to IgG. The IgG subclass levels against OVA were analyzed (Fig. 2) to confirm this switch and to identify the IgG subclasses involved. In this study sera were collected from individual mice (eight mice group except OVA/lip which had six mice) after four immunizations. Immunization to OVA alone induced predominantly IgG1 with lower levels of IgG2a, IgG2b, and IgG3. Encapsulation of OVA in liposomes alone induced a similar pattern of subclass responses as OVA alone but with higher levels of each. When OVA was combined with 3M-019 and injected without liposomes, all subclass anti-OVA levels increased compared to OVA alone and the pattern shifted significantly to responses dominated by IgG2a and IgG2b. The combination of OVA plus 3M-019 encapsulated in liposomes induced a pattern of anti-OVA IgG subclass antibodies similar to that seen by this ligand and OVA without liposomes but with higher antibody levels.
Fig. 2.
Relative OVA specific IgG subclass responses to immunization. Individual sera collected from mice 1 week after the fourth immunization were analyzed by ELISA for specific IgG subclasses antibodies to OVA using a mouse subtyping kit. Each sample was diluted 1:20 in milk blocker, and then added to all the wells of a single row in a microtiter plate. Specific subclass secondary antibodies (goat) were then added to triplicate columns followed by biotinylated anti-goat (rabbit), and then extravidin:peroxidase. Reactions were stopped 2 min after the addition of substrate. Each bar represents the mean absorbance ± standard deviation of triplicate wells of sera collected from eight mice for all groups except OVA/Lip which consisted of six mice
The significance of these differences has been evaluated by ANOVA and individual pairs compared by Students t test. Liposomes increased all subtypes compared to OVA alone (P < 0.001) while 3M-019 increased IgG2a, IgG2b, and IgG3 (P < 0.001) but not IgG1 (P < .09). The encapsulation of OVA + 3M-019 in liposomes increased all subtypes compared to either OVA/3M-019 alone (P < 0.001) or OVA/Lip alone (P < 0.001 for IgG2a, IgG2b, and IgG3 and P < 0.004 for IgG1).
A similar pattern of IgG subclass responses to OVA were seen in sera collected after two immunizations (data not shown). Even after only two immunizations the subclass distribution favored IgG2a and IgG2b in all groups treated with 3M-019; however, liposomes appeared to only increase IgG1 without yet affecting IgG2b.
The IgG subclass data above suggest that the major adjuvant effect of 3M-019 on anti-OVA antibody responses is a class switch leading to augmentation of IgG2a and IgG2b levels. Although significant differences in antibody levels can be measured by ELISA as above, the magnitude of the differences and the actual antibody titers cannot. Endpoint titers were measured to quantify the 3M-019-induced enhancement of both of anti-OVA IgG2a and IgG2b subclasses. The endpoint titers for anti-OVA IgG2a and IgG2b were determined in sera collected after two immunizations (week 2) and after four immunizations (week 4). The adjuvant strength is indicated by the ratio of the endpoint titer to that measured in mice immunized to OVA alone. The results for IgG2a show that endpoint titers are relatively low after only two immunizations but are substantially increased after four immunizations. The highest titer after two immunizations was 500 found in the group immunized to the highest dose of 3M-019 encapsulated in liposomes. This was 25 times higher than the endpoint seen in mice immunized to OVA alone or OVA encapsulated in liposomes. The lower doses of 3M-019 in liposomes and the highest dose without liposomes led to a fivefold increase in the titers. All endpoints were increased after four immunizations. Encapsulation of the 10 mg/kg dose of 3M-019 in liposomes led to an IgG2a endpoint titer that was 625 times greater than that of OVA alone and 25 times greater than OVA encapsulated alone in liposomes. Liposome encapsulation of this dose was 125 times stronger than the same dose without liposomes. The results were the same even when the 3M-019 dose was reduced tenfold. When the dose of 3M-019 was reduced 100-fold to 0.10 mg/kg the adjuvant strength was lowered to that seen with liposomes alone.
Examination of the anti-OVA IgG2b results reveals an even more striking adjuvant capability of 3M-019. The endpoint titer in mice immunized to OVA alone was extremely low after two immunizations but increased to 1:500 after four immunizations. Encapsulation of 3M-019 showed dose-dependent increases in the endpoints after both two and four immunizations. The adjuvant strength of highest dose was over 3,000 after four immunizations. This was 625 stronger than liposomes and 25 times higher than the same dose used without liposomes. (See Table 1)
Table 1.
Determination of endpoint titers of anti-OVA IgG2a and IgG2b in immunized mice
| Immunization | 3M-019 (mg/kg) | IgG2a | Ratio to OVA | IgG2a | Ratio to OVA | IgG2b | Ratio to OVA | IgG2b | Ratio to OVA |
|---|---|---|---|---|---|---|---|---|---|
| Post 2 | Post 4 | Post 2 | Post 4 | ||||||
| OVA | – | 20 | 1 | 12,500 | 1 | 4 | 1 | 500 | 1 |
| OVA/LIP | – | 20 | 1 | 312,500 | 25 | 20 | 5 | 2,500 | 5 |
| OVA/LIP | 0.1 | 100 | 5 | 315,000 | 25 | 100 | 20 | 2,500 | 5 |
| OVA/LIP | 1 | 100 | 5 | 7,812,500 | 625 | 500 | 100 | 62,500 | 125 |
| OVA/LIP | 10 | 500 | 25 | 7,182,500 | 625 | 2,500 | 500 | 1,562,500 | 3,125 |
| OVA | 10 | 100 | 5 | 62,500 | 5 | 500 | 100 | 62,500 | 125 |
| PBS | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Pooled sera, after the second and fourth immunizations were analyzed for endpoint titers of anti-OVA IgG2a and IgG2b in separate ELISA plates. The initial dilution of each sera was 1:4 in the analyses of post 2 sera and 1:100 for post 4 sera. 1:5 serial dilutions were made from each starting sera. After overnight incubation with these sera, each plate was incubated with specific anti-mouse IgG2a or IgG2b secondary antibodies (from goat) followed by tertiary and peroxidase as described in Fig. 2. Reactions were stopped after 10 min incubation with substrate at room temperature
Cellular responses
In vitro assays were performed to measure total OVA-specific immune cells and CD8+ T cells present in the spleens of mice. Target cells were E.G7-OVA which constitutively present OVA derived peptides on the cell surface within the MHC I context [21].
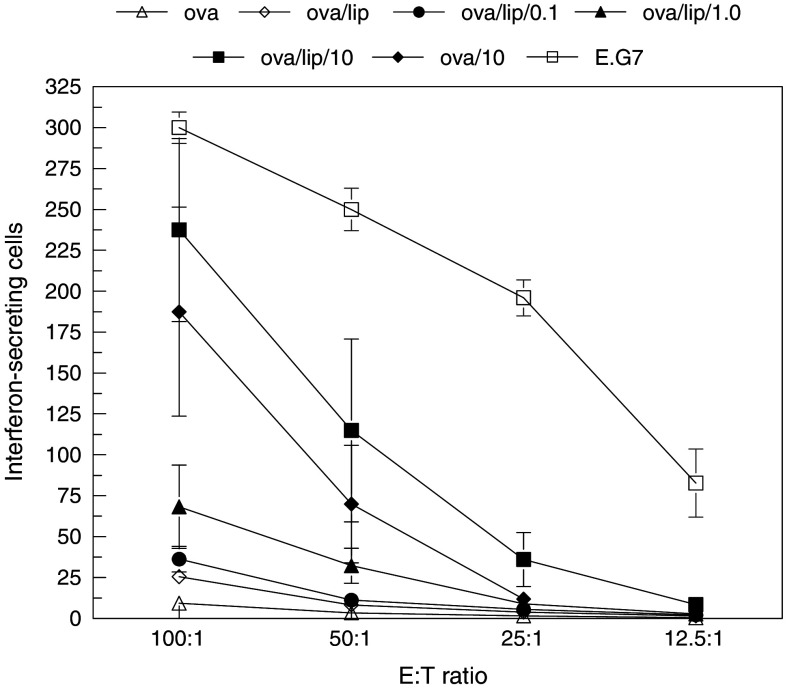
Mice were sacrificed 1 week after either the second or fourth immunization. Splenocytes were isolated and their ability to recognize target cells was determined by ELISPOT assay of interferon-γ production. The results in Fig. 3 show a dose-dependent increase in cellular response with the amount of 3M-019 encapsulated in liposomes. Immunization to OVA alone had no detectable anti-OVA cellular immune response above background seen in PBS-immunized mice. Liposome encapsulation of OVA had a very minimal affect on cellular immunity. The inclusion of 3M-019 at the middle and highest dose induced a significantly stronger cell response than OVA alone (P < 0.001 by MANOVA analysis). Cellular responses were also significantly (P < 0.001) higher than mice immunized to OVA encapsulated in liposomes. Mice immunized to the highest dose of 3M-019 mixed with OVA and injected without liposomes had a significant cellular response. For comparison, the cell response of mice immunized to E.G7-OVA cells was the strongest. Immunization to these cells could induce immune cells recognizing a far greater peptide repertoire than mice immunized to OVA.
Fig. 3.
Effect of immunization on cellular immune responses. The ability of splenocytes cells from immunized mice to recognize target OVA-expressing tumor cells was determined by interferon production detected by ELISPOT assay. Splenocytes were isolated from mice sacrificed 1 week after the fourth immunization and incubated in replicate wells with 1.5 × 104 target E.G7-OVA cells at the E:T ratios shown. The 3M-019 groups are indicated by the dose which is given in mg/kg mouse. Spots representing individual activated immune cells were counted and plotted. Each point represents the mean value ± the standard deviation of triplicate wells
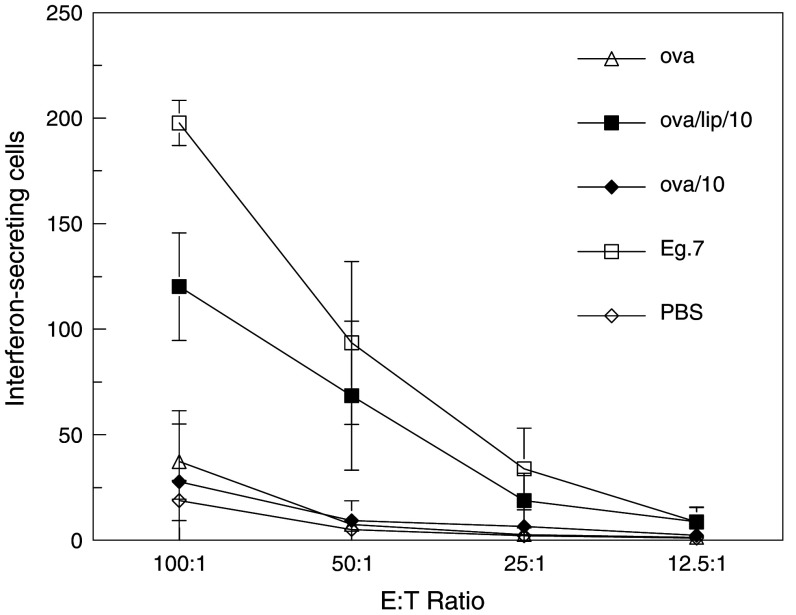
To further examine the effect of 3M-019 on induction of cellular immunity, the ELISPOT assay was performed with cells isolated from mice sacrificed after two immunizations (Fig. 4). The results of this study show that the highest dose of 3M-019 encapsulated in liposomes produced a strong cell response even after only two immunizations. This was greater than half the strength of the positive control group immunized to E.G7-OVA cells. The combination of 3M-019 and liposomes was significantly stronger (P < 0.05) than OVA alone or OVA plus the same dose of 3M-019 but without liposome encapsulation by MANOVA analysis.
Fig. 4.
Cellular immune responses after two immunizations. Triplicate mice from each group were sacrificed 1 week following the second immunization and ELISPOT assay performed to determine recognition of target cells by interferon secretion as described for Fig. 3. The highest dose (10 mg/kg) was used either with or without liposomes for the immunization. Each point represents the mean value of three separate mice, each run in duplicate wells at each E:T. Error bars represent the standard deviation of six wells
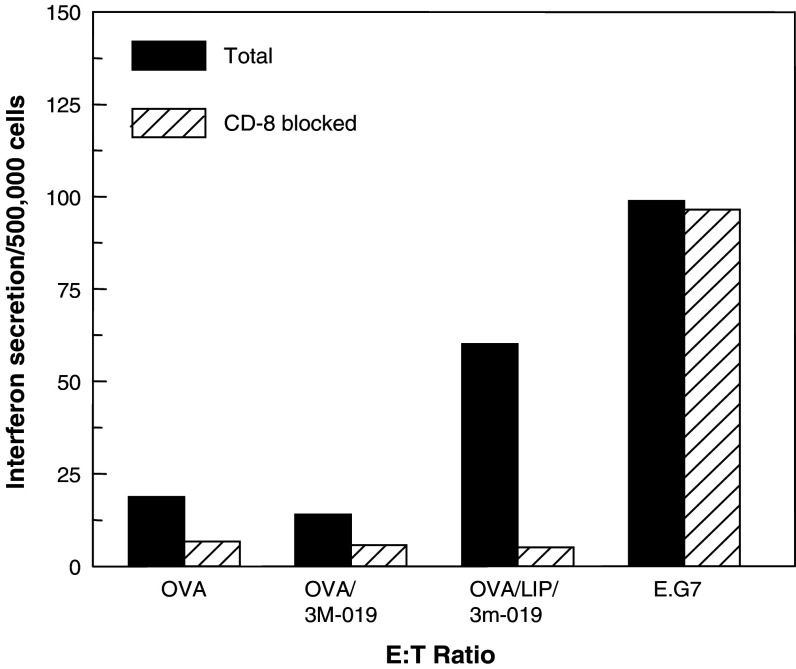
Analysis of the cells involved in the overall cellular immunity was achieved by the use of specific blocking antibodies. ELISPOT assays were run in the presence or absence of specific monoclonal antibodies to either CD8+ T cells or NK cells. Results from blocking CD8+ T cells are shown in Fig. 5. Blocking of CD8 response led to a substantial reduction in the number of interferon γ-secreting cells in mice immunized to OVA + 3M-019 in liposomes. The fact that almost 90% of the response was eliminated by this blocking indicated that a vast majority of the cells recognizing E.G7-OVA targets are OVA-specific cells. Elimination of CD8+ T cells reduced to a lesser extent the low response seen in mice immunized to OVA or OVA + 3M-019 without liposomes. The high cellular response seen in mice immunized to E.G7-OVA cells was not reduced at all by blocking anti-CD8 monoclonal antibodies indicating that the response was not due to CD8. Because these cells do not express B7 molecules, it is likely that immunization to these high numbers could lead to CD8+ T cell anergy rather than activation. Furthermore, the immunizing E.G7-OVA cells were irradiated, and thus may undergo apoptosis, form apoptic bodies, and be processed by macrophages rather than by dendritic cells. Processing of phagocytosed antigens generally favors a CD4 response. No blocking was seen when PK136 monoclonal antibody to mouse NK cells was used (data not shown).
Fig. 5.
Anti-OVA specific CD 8+ T cell responses. Effect of treatment on specific CD8 cellular response to target (E.G7-OVA) cells in the presence or absence of blocking anti mouse CD8 monoclonal antibodies. The dose of 3M-019 in this study was 10 mg/kg. The ELISPOT assay for interferon release was performed as described for Fig. 4 but in the presence and absence of 10 μg/well of anti-CD8 monoclonal antibody. The interferon-secreting cells/500,000 were calculated from an average of the 100:1 and 50:1 E:T ratios
Safety
The weight of each individual mouse, ear-punched for identification, was measured weekly and increased throughout the immunization period. ANOVA analysis of groups in every study indicates that no differences in mean weights among the groups occurred. Liposomes, with or without imidazoquinolines, caused a milky granuloma at the injection site but no inflammation was detected. All mice remained active and showed no signs of abnormal behavior. These observations suggest that imidazoquinolines alone or encapsulated in liposomes may be safely used as vaccine modulators at the doses described in this study.
Discussion
These studies demonstrate that liposome encapsulation of TLR7 ligands together with immunogen results in more powerful immunity that either TLR ligand or liposomes alone. Antibody responses are significantly boosted, but more importantly, strong cellular immune responses could be induced to a pure protein vaccine that, by itself, did not induce any cellular immune response. The immunization schedule and route of injections may not be optimal for induction of the strongest immunity, but this allows for the measurement of the strength of adjuvants in a relatively short time frame.
Imidazoquinolines are synthetic organic compounds that activate TLR7 in mice and TLR 7 & 8 in humans. Imidazoquinolines mimic the natural TLR receptor ligand which is a single-stranded RNA. The immune responses resulting from imidazoquinoline activation show strong anti-viral activity. Imiquimod, the first imidazoquinoline developed, has been approved by the FDA for topical human use for genital warts [1, 15, 19] and, more recently, has shown activity in the treatment of certain skin cancers [20, 23, 30, 32, 35, 41]. No significant systemic toxicity or side effects have been noted in human applications.
The ability of imidazoquinolines to activate potent immune responses has stimulated interest in the potential of these compounds as immune response modifiers of vaccines for cancer [8, 16, 29, 39]. Many studies have shown that imidazoquinolines augment specific CD4+ and CD8+ T cell responses against the viral infections including HIV and increased anti-viral protective immunity in mice [42]. The imidazoquinolines studied to date include imiquimod, resiquimod (R848), and S-27609. Several preclinical studies in mice have demonstrated their ability to potentiate immune responses to prototype vaccines such as OVA. We have recently demonstrated that topical treatment of mice with imiquimod was able to augment the immune responses to OVA that was injected S.C. [16].
In this study, a new imidazoquinoline derivative identified as 3M-019 was found to be a very powerful modulator of immune responses to OVA. We have further shown that encapsulation of 3M-019 into liposomes together with OVA produced even stronger immune responses. Levels of OVA-specific IgG2a and IgG2b were up to 625 and 3,125 times higher in mice immunized to liposome encapsulated imidazoquinoline plus OVA than the levels seen in mice immunized to OVA alone. The combination was significantly better than either OVA in liposomes alone or OVA plus 3M-019 without liposomes. Even more striking was the effect on anti-OVA cellular immune responses. OVA immunization is a very poor inducer of cellular immune responses at best. 3M-019 together with OVA produced a strong cellular immune response. This response could be further augmented if both were encapsulated in liposomes. Cellular immunity was substantially OVA-specific CD8+ T cells as the activity could be reduced by more than 90% if CD8+ T cells were blocked. Both antibody and cellular responses were augmented at an early time point, after only two immunizations, suggesting that imidazoquinoline/liposomes combinations may provide greater vaccine-induced immunity in shorter time frames.
The combination of 3M-019 and liposomes has produced the strongest immune responses that we have seen in our studies of several immune response modifiers. Immune responses were stronger than those induced by IL-2/liposomes [17] or topical imiquimod treatment [16]. Others have studied newer imidazoquinoline derivatives such as S-27609 [9]. We have also examined S-27609 alone and in liposomes (unpublished data) to compare its adjuvant strength to 3M-019. Both imidazoquinolines induced strong antibody and cellular immune responses at the highest dose (10 mg/kg) alone or encapsulated in liposomes with OVA. However, at doses of 1.0 or 0.1 mg/kg 3M-019 in liposomes with OVA produced stronger antibody and cellular responses than S-27609.
In summary, a new imidazoquinoline derivative combined with liposomes produces a tremendous amplification of immune responses to a pure protein prototype vaccine. All animals treated with the imidazoquinoline or combination of imidazoquinoline and liposomes appeared to be healthy, as they gained weight during the immunization period and displayed normal behavior. No detectable local effects of treatment were detected. Our results suggest that 3M-019 might also be safely used in humans as an adjuvant for vaccines. This could be especially important to vaccines that are weakly immunogenic. Cancer vaccines are a prime example where immune responses are often very poor [2, 5, 7, 10, 18, 26–28, 31].
Acknowledgment
We are most grateful to Dr. Richard Miller of 3M Pharmaceutical for providing imidazoquinolines for this study. This research was supported by grants CA096804 (DJ), The Skin Cancer Foundation, and the Gaisman Foundation.
Footnotes
This article was supported in part by grants CA096804 (DJ).
References
- 1.Arican O, Guneri F, Bilgic K, Karaoglu A. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627–631. doi: 10.1111/j.1346-8138.2004.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky JA, Oh S, Terabe M. Peptide vaccines against cancer. Cancer Treat Res. 2005;123:115–136. doi: 10.1007/0-387-27545-2_5. [DOI] [PubMed] [Google Scholar]
- 3.Bishop GA, Hsing Y, Hostager BS, Jalukar SV, Ramirez LM, Tomai MA. Molecular mechanisms of B lymphocyte activation by the immune response modifier R-848. J Immunol. 2000;165:5552–5557. doi: 10.4049/jimmunol.165.10.5552. [DOI] [PubMed] [Google Scholar]
- 4.Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA. The immune response modifier resiquimod mimics CD40-induced B cell activation. Cell Immunol. 2001;208:9–17. doi: 10.1006/cimm.2001.1769. [DOI] [PubMed] [Google Scholar]
- 5.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 6.Bowie AG, Haga IR. The role of toll-like receptors in the host response to viruses. Mol Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Bystryn JC, Reynolds SR. Melanoma vaccines: what we know so far. Oncology (Williston Park) 2005;19:97–108. [PubMed] [Google Scholar]
- 8.Craft N, Bruhn KW, Nguyen BD, Prins R, Lin JW, Liau LM, Miller JF. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol. 2005;175:1983–1990. doi: 10.4049/jimmunol.175.3.1983. [DOI] [PubMed] [Google Scholar]
- 9.Doxsee CL, Riter TR, Reiter MJ, Gibson SJ, Vasilakos JP, Kedl RM. The immune response modifier and Toll-like receptor 7 agonist S-27609 selectively induces IL-12 and TNF-alpha production in CD11c + CD11b + CD8- dendritic cells. J Immunol. 2003;171:1156–1163. doi: 10.4049/jimmunol.171.3.1156. [DOI] [PubMed] [Google Scholar]
- 10.Ferrone S. Immunotherapy dispenses with tumor antigens. Nat Biotechnol. 2004;22:1096–1098. doi: 10.1038/nbt0904-1096. [DOI] [PubMed] [Google Scholar]
- 11.Gershman N, D J, Bystryn J-C. Potentiation of B16 melanoma vaccine immunogenicity by IL-2 liposomes. Vaccine Res. 1994;3:83–92. [Google Scholar]
- 12.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/S0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 13.Harrison CJ, Jenski L, Voychehovski T, Bernstein DI. Modification of immunological responses and clinical disease during topical R-837 treatment of genital HSV-2 infection. Antiviral Res. 1988;10:209–223. doi: 10.1016/0166-3542(88)90032-0. [DOI] [PubMed] [Google Scholar]
- 14.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 15.Hengge UR, Ruzicka T. Topical immunomodulation in dermatology: potential of toll-like receptor agonists. Dermatol Surg. 2004;30:1101–1112. doi: 10.1111/j.1524-4725.2004.30335.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnston D, Bystryn JC. Topical imiquimod is a potent adjuvant to a weakly-immunogenic protein prototype vaccine. Vaccine. 2006;24:1958–1965. doi: 10.1016/j.vaccine.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 17.Johnston D, Reynolds SR, Bystryn JC. Interleukin-2/liposomes potentiate immune responses to a soluble protein cancer vaccine in mice. Cancer Immunol Immunother. 2006;55:412–419. doi: 10.1007/s00262-005-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kast WM, Levitsky H, Marincola FM. Synopsis of the 6th Walker’s Cay colloquium on cancer vaccines and immunotherapy. J Transl Med. 2004;2:20. doi: 10.1186/1479-5876-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin P, Torres G, Tyring SK. Changing paradigms in dermatology: antivirals in dermatology. Clin Dermatol. 2003;21:426–446. doi: 10.1016/j.clindermatol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int J Dermatol. 2005;44:14–19. doi: 10.1111/j.1365-4632.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/S0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 22.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oster-Schmidt C. Two cases of squamous cell carcinoma treated with topical imiquimod 5% J Eur Acad Dermatol Venereol. 2004;18:93–95. doi: 10.1111/j.1468-3083.2004.00852.x. [DOI] [PubMed] [Google Scholar]
- 24.Otero M, Calarota SA, Felber B, Laddy D, Pavlakis G, Boyer JD, Weiner DB. Resiquimod is a modest adjuvant for HIV-1 gag-based genetic immunization in a mouse model. Vaccine. 2004;22:1782–1790. doi: 10.1016/j.vaccine.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Re F, Strominger JL. Heterogeneity of TLR-induced responses in dendritic cells: from innate to adaptive immunity. Immunobiology. 2004;209:191–198. doi: 10.1016/j.imbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds SR, Zeleniuch A-Jacquotte, Shapiro RL, Roses DF, Harris MN, Johnston D, Bystryn JC. Vaccine-induced CD8+ T-cell responses to MAGE-3 correlate with clinical outcome in patients with melanoma. Clin Cancer Res. 2003;9:657–662. [PubMed] [Google Scholar]
- 27.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santini SM, Belardelli F. Advances in the use of dendritic cells and new adjuvants for the development of therapeutic vaccines. Stem Cells. 2003;21:495–505. doi: 10.1634/stemcells.21-4-495. [DOI] [PubMed] [Google Scholar]
- 29.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, Chen Q, Parente P, Jefford M, Masterman KA, Caron D, Chen W, Maraskovsky E, Cebon J. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 30.Sidbury R, Neuschler N, Neuschler E, Sun P, Wang XQ, Miller R, Tomai M, Puscasiu E, Gugneja S, Paller AS. Topically applied imiquimod inhibits vascular tumor growth in vivo. J Invest Dermatol. 2003;121:1205–1209. doi: 10.1046/j.1523-1747.2003.12521.x. [DOI] [PubMed] [Google Scholar]
- 31.Slingluff CL, Jr, Engelhard VH, Ferrone S. Peptide and dendritic cell vaccines. Clin Cancer Res. 2006;12:2342s–2345s. doi: 10.1158/1078-0432.CCR-05-2541. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan TP, Dearaujo T, Vincek V, Berman B. Evaluation of superficial basal cell carcinomas after treatment with imiquimod 5% cream or vehicle for apoptosis and lymphocyte phenotyping. Dermatol Surg. 2003;29:1181–1186. doi: 10.1111/j.1524-4725.2003.29399.x. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 34.Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell Immunol. 2000;203:55–65. doi: 10.1006/cimm.2000.1673. [DOI] [PubMed] [Google Scholar]
- 35.Urosevic M, Dummer R. Role of imiquimod in skin cancer treatment. Am J Clin Dermatol. 2004;5:453–458. doi: 10.2165/00128071-200405060-00010. [DOI] [PubMed] [Google Scholar]
- 36.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Vasilakos JP, Smith RM, Gibson SJ, Lindh JM, Pederson LK, Reiter MJ, Smith MH, Tomai MA. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 2000;204:64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- 38.Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, Reiter MJ, Vasilakos JP, Tomai MA. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 1999;191:10–19. doi: 10.1006/cimm.1998.1406. [DOI] [PubMed] [Google Scholar]
- 39.Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848) Vaccine. 2005;23:5263–5270. doi: 10.1016/j.vaccine.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitelli JA. Use of imiquimod for treating skin cancer. J Am Acad Dermatol. 2005;52:177. doi: 10.1016/j.jaad.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Zuber AK, Brave A, Engstrom G, Zuber B, Ljungberg K, Fredriksson M, Benthin R, Isaguliants MG, Sandstrom E, Hinkula J, Wahren B. Topical delivery of imiquimod to a mouse model as a novel adjuvant for human immunodeficiency virus (HIV) DNA. Vaccine. 2004;22:1791–1798. doi: 10.1016/j.vaccine.2003.10.051. [DOI] [PubMed] [Google Scholar]