Abstract
Various clinical and experimental observations detected an immunological host defense in cutaneous melanoma. In order to investigate the prognostic value of leukocyte effector mechanisms, we examined the presence of different subsets of leukocytes in tumor samples of 58 patients diagnosed with primary cutaneous melanoma. The presence of T lymphocytes, cytotoxic T lymphocytes, B lymphocytes, CD16+ cells and macrophages was correlated to Breslow depth. A significantly higher amount of several subsets of leukocytes was found in samples with a more progressed tumor stage and survival analysis demonstrated that a higher amount of T lymphocytes and CD16+ cells was associated with a short survival. The amount of FOXP3+ regulatory T lymphocytes did not correlate with survival, nevertheless, it correlated with the amount of total infiltrate. In contrast, analysis of the expression of CD69, a marker for activated lymphocytes, demonstrated that patients with a higher amount of CD69+ lymphocytes had a better survival. In addition, a new parameter for aggressiveness of melanoma, tumor cell plasticity [i.e., the presence of periodic acid Schiff’s (PAS) reagent positive loops], also predicted short survival and a trend of a higher amount of tumor infiltrating leukocytes in tumors with PAS positive loops was observed. These findings demonstrate that leukocyte infiltration and the presence of PAS loops is a sign of tumor aggressiveness and may have prognostic value.
Keywords: Tumor infiltrating leukocytes, Prognosis, Immunosuppression, Activation status of lymphocytes, Angiogenesis, Tumor cell plasticity, Cutaneous melanoma
Introduction
Due to dedifferentiation and uncontrolled proliferation, cancerous cells express antigens that can be recognized by the immune system. Leukocytes are activated, expand clonally and migrate to the tumor [1]. The infiltration of these leukocytes into tumor tissues is controlled by the local microenvironment and precedes the lytic cascade in which leukocytes attack tumor cells [2]. Several studies report the amount of tumor infiltrating leukocytes as a prognostic factor in, e.g., prostatic adenocarcinoma [3], breast carcinoma [4], cervix squamous cell carcinoma [5], colorectal carcinoma [6], oesophageal carcinoma [7, 8], ovarian cancer [9, 10], head and neck cancer [11], non-small cell lung cancer [12]. However, most studies were only investigating the role of T lymphocytes in relation to survival. There is a complex relationship between tumor infiltrating leukocytes and the patient outcome due to heterogeneity of infiltration in different patients and different regions of the tumor, the variety of subtypes of leukocytes that are present in the tumor and the differences in localization of the leukocytes within the tumor and the surrounding tissue [2].
Even though the presence of a heavy leukocyte infiltrate is a frequent characteristic in melanoma, this fails to control tumor growth [13]. The prognostic value of tumor infiltrating leukocytes in human melanoma has been contradictory. Clark et al. proposed a definition of tumor infiltrating leukocytes categorizing them by intensity in brisk, non-brisk and absent. They saw a favorable outcome in patients with a brisk intensity of tumor infiltrating leukocytes [14]. These findings were confirmed by Clemente et al. [15]. However, in the large patient studies of Thorn and Barnhill, a more favorable outcome in patients with a higher intensity of leukocyte infiltration was not observed [16, 17]. The Clark-method appears to be subject to a large interobserver variability and does not distinguish between the several subsets of tumor infiltrating leukocytes. Furthermore, discrimination between intratumoral and peritumoral infiltration may be necessary, as it is a prerequisite of an attacking lymphocyte to infiltrate inside the tumor tissue [2].
In the current study we investigated a variety of subsets of leukocytes in intratumoral and peritumoral areas, and their prognostic value in patients diagnosed with primary cutaneous melanoma. In addition, we analyzed the activation status of the lymphocytes and the presence of immunosuppressive lymphocytes or T regulatory cells. Furthermore, we were interested in the relationship between leukocyte infiltration and angiogenesis. Since tumor cell plasticity is contributing to the circulatory system of tumors [18], we also investigated this parameter in relationship to leukocyte infiltration.
Materials and methods
Patients
We collected tumor tissues of 58 patients [69% (40/58) females, 31% (18/58) males], diagnosed with primary cutaneous melanoma between 1985 and 1995, from the archival tissue bank of the Department of Pathology, Maastricht. A clinical follow-up of 10 years was obtained. The diagnosis of melanoma was reconfirmed by a pathologist (D.C., V.W.). Of these patients, 64% (37/58) were diagnosed with superficial spreading melanoma. The other 36% (21/58) of the patients were diagnosed with a nodular type of melanoma. Radial growth phase was observed in 10% (6/58) of the patients, tumor with both radial and vertical growth phase were observed in 54% (31/58) of the patients and 36% (21/58) of the tumor samples presented only vertical growth phase. Mean age at diagnosis was 52.69 years (range 22–88 years). The presence of ulceration was found in 20.6% (12/58) of the patients. In this group of primary tumors, 37.9% (22/58) of the tumor tissues were located on extremities, 29.3% (17/58) on the trunk and 10.3% (6/58) on the head and neck. For 22.4% of the patients (13/58) there was no data in the archive on the location of the primary tumor. Lymph node metastasis was detected in 20.7% (12/58) and distant metastasis in 34.5% (20/58) of the patients, 31% (18/58) of the patients died from melanoma. Mean overall survival of the total patient group was 7.7 years (range 5 months to 10 years). Regression was observed in 31% (18/58) of the patients. Patient population was categorized in four groups based on Breslow thickness, according to the guidelines of the Dutch Society of Melanomas: ≤0.75 mm (24.1%, 14/58), group 1; 0.76–1.5 mm (39.7%, 23/58), group 2; 1.51–3 mm (20.7%, 12/58), group 3 and >3 mm (15.5%, 9/58), group 4. This revealed a strong correlation to survival (log rank, P < 0.0001). The presence of distant metastasis, positive lymph nodes, ulceration and the histological subtype (being superficial spreading melanoma versus nodular melanoma) demonstrated a significant negative correlation (P < 0.003) with survival, as we have previously described [19]. To compare our observations with previous reports on infiltration, tumor samples were classified according to the classification of Clark et al. [14]. 41.4% (24/58) of the tumor samples were classified as brisk, 37.9% (22/58) were analyzed as non-brisk and 20.7% (12/58) were observed with an absent infiltrate, according to this classification.
Immunohistochemistry
Paraffin sections (6 μm thickness) were deparaffinized in xylene and ethanol, incubated in 0.3% H2O2 in methanol to quench endogenous peroxidase activity for 20′, after which antigen retrieval was carried out by heating the sections in a Tris–EDTA buffer (10 mM Tris–1 mM EDTA, pH 8) for 15 min in a microwave oven for most of the antibodies except for the CD45 and CD20 antibodies. The staining with the CD68 antibody required an incubation of 30′ with 0.1% pepsin in 1 N HCl. The antigen retrieval step for the FOXP3 antibody was performed with a citric buffer (10 mM citric acid, pH 6) while the slides for the CD69 antibody were heated with an EDTA buffer (1 mM EDTA, pH 8). Subsequently, the slides were incubated in a 5% BSA/PBS solution for 30′. Sections were incubated for 1 h with the primary antibody for CD45, CD3 (Dakocytomation, Glostrup, Denmark), CD8 (Novacastra, Newcastle Upon Tyne, UK), CD68, CD20 (Dakocytomation, Glostrup, Denmark) and CD16 (Neomarkers, Fremont, CA, USA), FOXP3 (eBioscience, San Diego, CA, USA), CD69 (Novacastra, New Castle Upon Tyne, UK), followed by a secondary biotin-labelled rabbit anti-mouse IgG (Dakocytomation, Glostrup Denmark) for 30′ and avidin-biotin complex HRP (Dakocytomation) for 30′. The secondary antibody for FOXP3 was a biotin-labelled donkey anti-rat, since the primary antibody was of rat origin (Jackson Immunoresearch, West Grove, PA, USA). Diaminobenzidine (DAB) as a brown chromogen (Sigma, St Loius, MO, USA) but for most leukocyte infiltration DAB combined with 0.03% NiCl2 as a black chromogen was used to distinguish leukocytes in pigmented tumors. Because of the high deposition of melanin within the macrophages, CD68 staining was visualized with a red chromogen (avidin–biotin complex with alkaline phosphatase, Dakocytomation; alkaline phosphatase substrate kit II, Vector Laboratories, Burlingame, CA, USA) and the sections were afterwards treated with imsolmount (Klinipath, Duiven, the Netherlands) to prevent alkaline phosphatase bleaching. Finally, the slides were mounted with entellan (Merck, Darmstadt, Germany).
The same series of slides was stained with periodic acid Schiff’s (PAS). After deparafinization and rehydration slides were incubated in 1% periodic acid (VWR Prolab, Fontenay/Bois, France) for 5 min and subsequently, for 5 min, in PAS reagent (Sigma diagnostics, St Louis, MO, USA), dehydrated and mounted with entellan.
As we previously reported, a double staining with Ki67 and CD31/CD34 was performed on this same series of tumor samples [19]. In short, sections were incubated with a rabbit–anti human polyclonal Ki67 (Neomarkers, Fremont, CA, USA), followed by a polyclonal biotin-labelled swine anti-rabbit IgG (Dakocytomation, Glostrup, Denmark). This staining was developed with an avidin–biotin complex HRP (Dakocytomation) followed by DAB with 0.03% NiCl2 (black chromogen). A second staining was performed with a mixture of CD31 (Dakocytomation) and CD34 (Monosan, Uden, the Netherlands). This was followed by a biotin-labelled goat anti-mouse IgG (Dakocytomation) and an avidin–biotin complex AP (Dakocytomation). Finally, the slides were developed with alkaline phosphatase substrate kit III (Vector Laboratories, Burlingame, CA, USA), treated with imsolmount (Klinipath, Duiven, the Netherlands) and mounted with entellan (Merck, Darmstadt, Germany).
Analysis
The amount of each subset of tumor infiltrating leukocytes was quantified by three independent observers (F.H., C.B., A.W.) in four high power fields at 400 times magnification. Both intratumoral and peritumoral leukocytes were quantified, each in four randomly chosen regions that where representative for the whole tumor. The amount of FOXP3+ cells was quantified in eight high power fields at 400 times magnification both intratumoral and peritumoral. Since the amount of CD69+ cells was so low, we screened the whole tumor, both intratumorally and peritumorally, at 400 times magnification. With the CD16 staining we quantified only granulocytes and NK cells, while macrophages were quantified with a CD68 staining. The numbers are presented as the amount of tumor infiltrating leukocytes/mm2. Intratumoral leukocytes where leukocytes that were nested inside the tumor tissue in contact with tumor cells, while peritumoral leukocytes were located outside the border of the tumor.
The presence of PAS positive loops was scored as present or absent. We scored each tumor for the presence of parallel cross linked patterns or back to back loops [20].
Regression was evaluated to be present when there was a replacement of tumor tissue with fibrosis, degenerated melanoma cells, leukocytic proliferation and telangiectasia formation.
A radial growth phase was evaluated when the tumor had a tendency to grow horizontally within the epidermal and superficial dermal layers. A vertical growth phase was defined as a pattern of growth in which tumor cells spread vertically from the epidermis into the dermis.
As we previously described, we evaluated a parameter for active neovascularization or angiogenesis [19]. Therefore, the amount of proliferating endothelial cells, being Ki67 positive endothelial cells, was observed in four intratumoral high power fields (200×) and is shown as the number of proliferating EC/vessel. Proliferating Ki67 positive tumor cells were quantified in the same fields and presented as the amount of Ki67 positive tumor cells/mm2.
Statistical analysis
Statistical analyses were performed with SPSS-10 software (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov was used for normality-testing of the data. Student’s T-test, Mann–Witney and Kruskal–Wallis were applied to detect significant differences between groups. With the Spearman test, the correlation between the different data groups was examined. The equality of the survival distributions was analyzed using the Log Rank test. Results were considered to be statistically significant when P < 0.05.
Results
Leukocyte infiltration is positively correlated with tumor stage
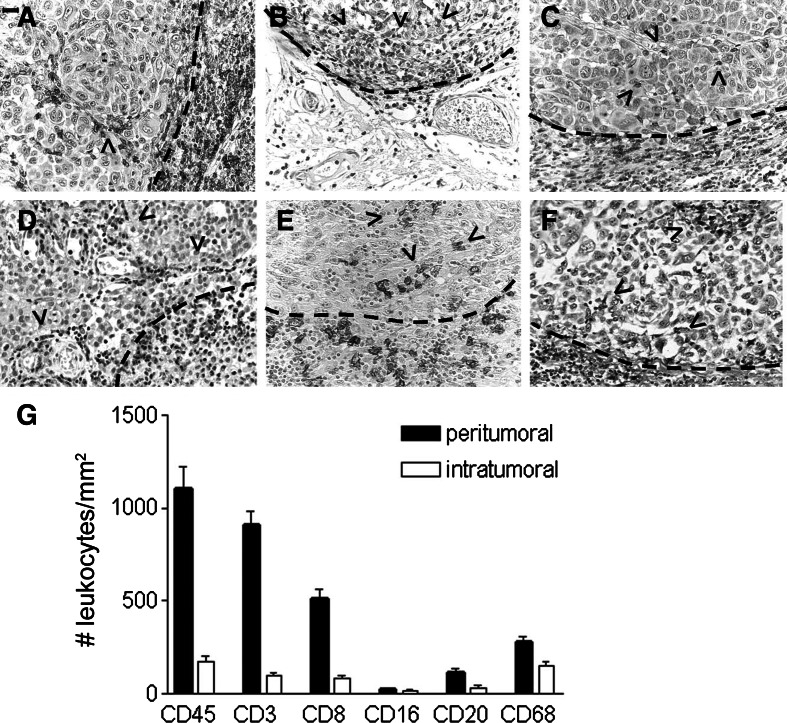
To study leukocyte infiltration in human cutaneous melanoma, we investigated the total amount of leukocytes (CD45+), as well as the subsets of T lymphocytes (CD3+), cytotoxic T lymphocytes (CD8+), B cells (CD20), CD16+ cells and macrophages (CD68+), in both peritumoral and intratumoral areas (Fig. 1a–f). As shown in Fig. 1g most leukocytes are present in peritumoral areas. Furthermore, we found that in peritumoral areas the majority of leukocytes are T lymphocytes, while within the tumor the leukocyte population consists of mostly macrophages. Another finding was that the number of CD16+ cells was very low as compared to the other leukocyte subsets. The composition of the leukocyte infiltrate was comparable for all patients (not shown).
Fig. 1.
Peritumoral and intratumoral leukocytes in cutaneous melanoma. Examples of immunostainings of peri-and intratumoral leukocytes. a All leukocytes (CD45+), b T lymphocytes(CD3+), c cytotoxic T lymphocytes(CD8+), d CD16+ cells, e B lymphocytes (CD20+), and f macrophages (CD68+). The dotted lines indicate the border between the tumor and the surrounding tissue. Arrow heads indicate intratumoral leukocytes. Scale bar, 20 μm. g The quantification of different subsets of leukocytes in the melanomas. The amount of peritumoral leukocytes are shown in black bars, while the amount of intratumoral leukocytes are demonstrated in white bars. Error bars show standard error of the mean
We found a significant correlation between the “brisk” classification (dividing tumor samples in three groups: absent, non-brisk and brisk) and the amount of peritumoral CD45+ (P = 0.001), CD3+ (P = 0.001), CD8+ (P = 0.001), CD20+ (P = 0.001), CD68+ (P = 0.001) and the amount of intratumoral CD45+ (P = 0.001), CD3+ (P = 0.001), CD8+ (P = 0.001), CD16+ (P = 0.027), CD20+ (P = 0.001).
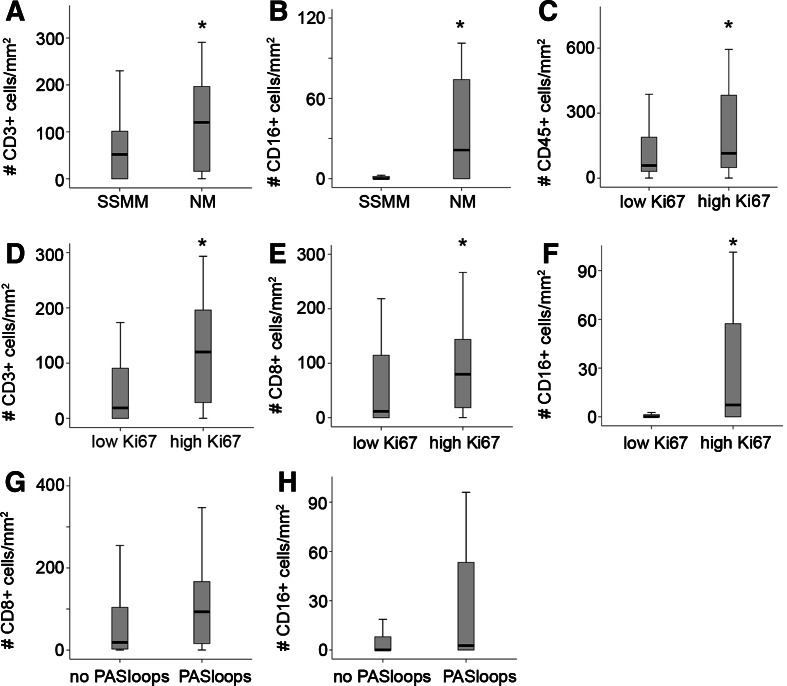
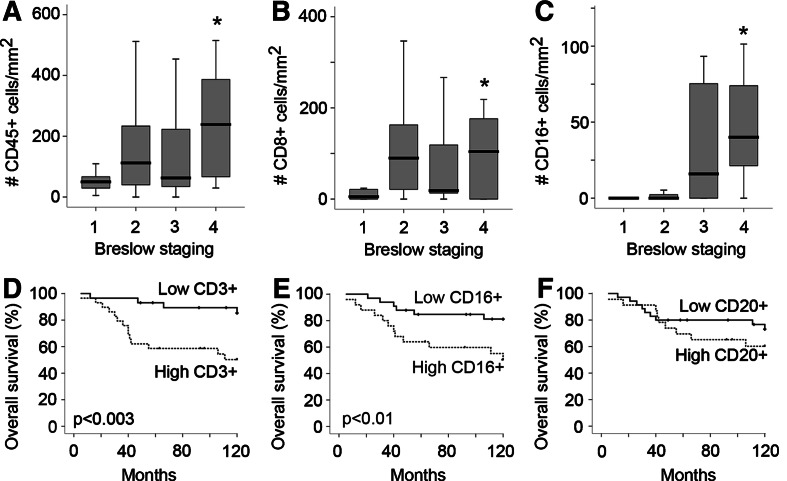
Leukocyte infiltration was positively correlated to aggressiveness of the tumors as determined by three different ways. (1) The group of nodular melanomas, known to have a poor prognosis (also in this study, log rank P = 0.039), were characterized by a significantly higher amount of intratumoral CD3+ (P = 0.036) and CD16+ cells (P = 0.001), and peritumoral CD45+ (P = 0.034), CD16+ (P = 0.001) and CD20+ (P = 0.044), as compared to superficial spreading melanoma (Fig. 2a, b). (2) There was a significant higher amount infiltration by different subsets of leukocytes in tumors with a higher amount of proliferating tumor cells [peritumoral CD3+ (P = 0.004), CD8+ (P = 0.005), CD16+ (P = 0.002) cells and intratumoral CD45+ (P = 0.038), CD3+ (P = 0.001), CD8+ (P = 0.037), CD16+ (P = 0.002)] (Fig. 2c–f). (3) We identified a higher amount of intratumoral leukocyte infiltration (for CD45+, CD8+ and CD16+ cells, respectively P = 0.042, P = 0.032, P = 0.0001, Fig. 3a–c) in patients with a Breslow depth >3 mm. A similar trend was observed for peritumoral leukocyte subsets.
Fig. 2.
Leukocyte infiltration in relation to parameters of progression and aggressiveness in cutaneous melanoma. The amount of several subsets of intratumoral leukocytes divided in two groups according to the histological subtype (a, b), the amount of proliferating tumor cells (c–f) and the presence of PAS loops (g, h). Significant difference between the groups (P < 0.05) is indicated by an asterisk. Black lines in the box plots present the median, upper and lower quartiles and the error bars show minimum and maximum data values
Fig. 3.
Staging and survival in relation to leukocyte infiltration. a–c The amount of leukocytes of the different subsets are stratified by Breslow staging (group 1, ≤0.75 mm; group 2, 0.76–1.5 mm; group 3, 1.51–3 mm and group 4, >3 mm). Significant difference between the four groups (P < 0.05) is indicated by an asterisk. Black lines in the box plots present the median and the error bars show quartiles. Kaplan–Meier survival plots for melanoma patients based on the amount of CD3+ cells (d), CD16+ cells (e) and CD20+ cells (f). Patients were divided in two groups stratified based on the median value for CD3+, CD16+ and CD20+ cells, respectively
Dividing the patients by the type of growth phase, being radial or vertical growth phase, a higher amount of peritumoral CD8+ (P = 0.045), CD16+ (P = 0.001), CD20+ (P = 0.049) cells and intratumoral CD3+ (P = 0.030), CD16+ (P = 0.001), CD68+ (P = 0.040) cells is shown in patients with vertical growth phase. All other subsets of leukocytes showed the same trend in more leukocytes in patients with a vertical growth phase.
The presence of regression did not show any correlation with intratumoral or peritumoral leukocytes, but was mostly observed in thin melanomas (15/18 tumor samples with regression had a Breslow thickness <1.5 mm). Kaplan–Meier analysis did not show any predictive value of regression in patient with primary cutaneous melanoma.
The amount of CD16+ cells and T cells predict short survival
Survival analysis was performed by dividing the patient population on basis of the median value of leukocyte infiltration. Kaplan–Meier analysis revealed that a higher amount of intratumoral CD3+ cells or CD16+ cells predicted shorter survival, both for 10 and 5 years of follow up (respectively, P = 0.003 and P = 0.01, Fig. 3d, e). For the other subsets (Fig. 3f) and for the peritumoral infiltrates (not shown), no significant relationship was found with survival (Table 1). Multivariate analysis indicated that none of these parameters outperformed Breslow depth as a predictor for survival. However, for meaningful multivariate analysis the patient numbers were too low.
Table 1.
Kaplan–Meier analysis for leukocyte infiltration and PAS patterns
| Log rank χ 2 | P-value | |
|---|---|---|
| CD45+ peritumoral | 0.58 | 0.4466 |
| CD45+ intratumoral | 1.09 | 0.2967 |
| CD3+ peritumoral | 1.08 | 0.2981 |
| CD3+ intratumoral | 8.70 | 0.0032* |
| CD8+ peritumoral | 1.20 | 0.2731 |
| CD8+ intratumoral | 0.26 | 0.6131 |
| CD16+ peritumoral | 1.75 | 0.1859 |
| CD16+ intratumoral | 6.08 | 0.0137* |
| CD20+ peritumoral | 0.34 | 0.5582 |
| CD20+ intratumoral | 0.92 | 0.3387 |
| CD68+ peritumoral | 0.01 | 0.9283 |
| CD68+ intratumoral | 0.29 | 0.5922 |
| FOXP3+ peritumoral | 1.51 | 0.2186 |
| FOXP3+ intratumoral | 0.00 | 0.9788 |
| CD69+ peritumoral | 0.43 | 0.5128 |
| CD69+ intratumoral | 2.07 | 0.1504 |
| PAS patterns | 10.01 | 0.0016* |
* Significant p-values
The amount of immunosuppressive T regulatory cells does not predict survival, while the amount of activated lymphocytes is positively correlated to survival
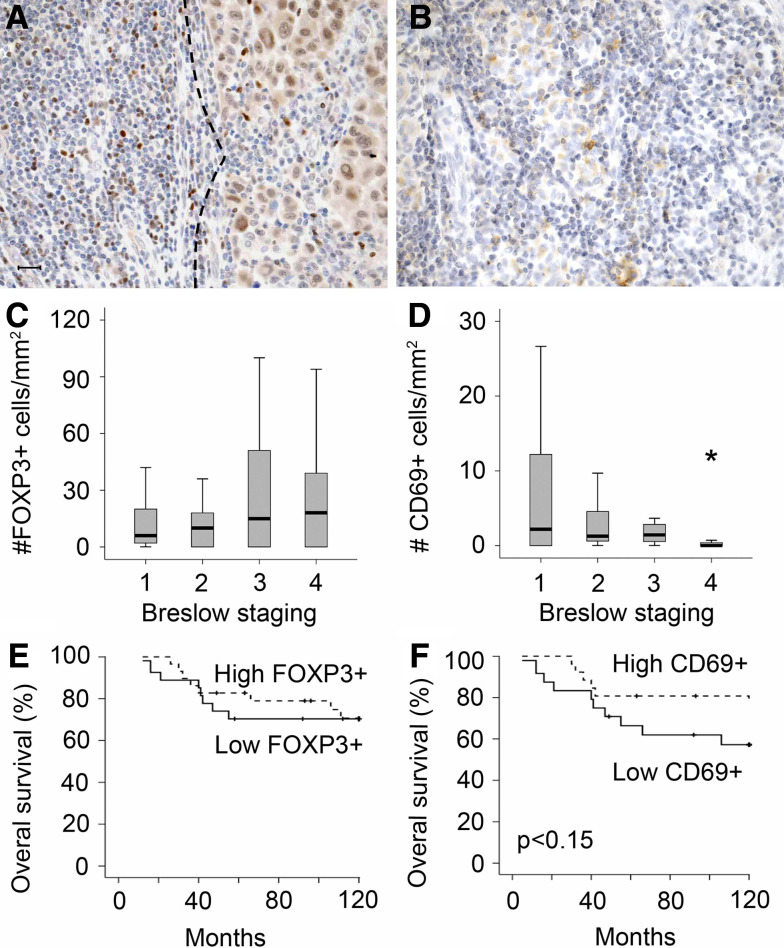
To analyze the activation status and immunosuppressive regulatory T lymphocytes in our patient population, we quantified the amount of FOXP3+ and CD69+ cells, both in intratumoral and peritumoral areas (Fig. 4a, b).
Fig. 4.
The activation status and presence of immunosuppressive regulatory T lymphocytes. Examples of immunostainings of peri-and intratumoral FOXP3+ (a) and intratumoral CD69+ (b) lymphocytes. The dotted lines indicate the border between the tumor and the surrounding tissue. Scale bar, 20 μm. The amount of FOXP3+ (c) and CD69+ (d) cells classified according to Breslow depth (group 1, ≤0.75 mm; group 2, 0.76–1.5 mm; group 3, 1.51–3 mm and group 4, >3 mm). Significant difference (P < 0.05) is indicated by an asterisk. Black lines in the box plots present the median, upper and lower quartiles and the error bars show minimum and maximum data values. Univariate survival analysis based on the amount of FOXP3+ cells (e) and CD69+ cells (f). Patients were divided in two groups stratified based on the median value for FOXP3+ and CD69+ cells, respectively
The presence of intratumoral FOXP3 regulatory T cells was not significantly different between the different stages of melanoma (Fig. 4c). The amount of intratumoral FOXP3+ T lymphocytes did not predict survival (Fig. 4e), but correlated to the amount of peritumoral CD3+ (P = 0.003), CD8+ (P = 0.001), CD16+ (P = 0.049), CD68 (P = 0.020) cells and the amount of intratumoral CD3+ (P = 0.006), CD8+ (P = 0.032), CD16+ (P = 0.043) and CD68+ (P = 0.034) cells.
The amount of CD69+ cells was significantly lower in the tumor samples with a Breslow depth of more than 3 mm (Fig. 4d). Univariate analysis showed a trend of a shorter survival for patients with a low amount of CD69+ cells, though this was not significant (Fig. 4f, P = 0.15). The amount of CD69+ cells was positively correlated to survival (P = 0.025) and negatively correlated to lymph node metastasis (P = 0.049), ulceration (P = 0.022) and the histological subtype (P = 0.020).
Leukocyte infiltration is not correlated with parameters of angiogenesis and tumor cell plasticity
Since leukocytes home to the tumor by extravasation from the intratumoral blood vessels, we were interested in the relationship between angiogenesis and the amount of leukocytes. We previously reported that angiogenic potential of melanoma tissue, as measured by the number of proliferating endothelial cells, is associated to tumor progression, predicting short survival [19]. In human melanoma we did not find a correlation between neovascularization, being the amount of Ki67 positive endothelial cells, and the presence of infiltrated leukocytes.
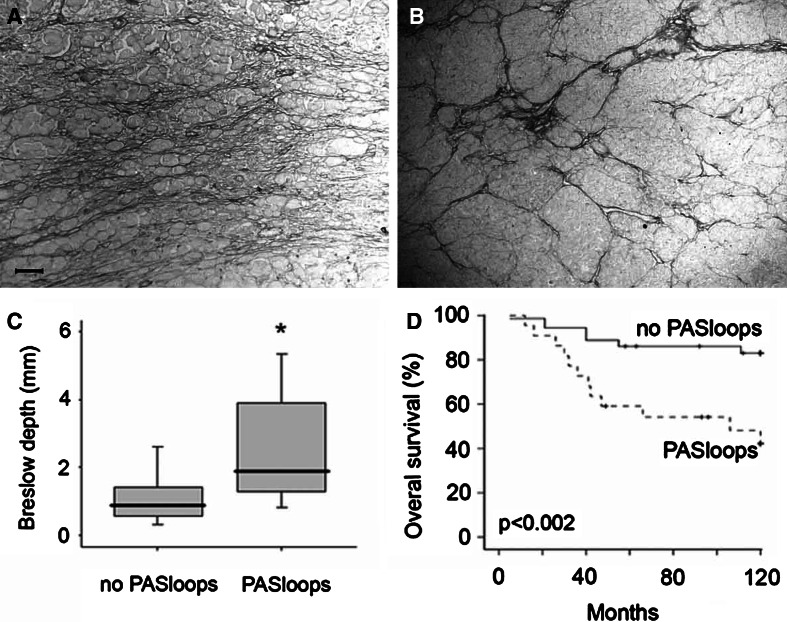
We and others recently demonstrated that plastic tumor cells, that are dedifferentiating into an endothelial cell phenotype, can form vessel-like structures that contribute to blood circulation [18], a process also referred to as vasculogenic mimicry [21]. This urged us to investigate a relationship between vasculogenic like structures and leukocyte infiltration. We evaluated the amount of tumor cell plasticity by the presence of patterned networks of interconnected loops of extracellular matrix that were stained with PAS reagent. Two clinical samples of patients with cutaneous melanoma with PAS positive patterns are shown in Fig. 5a, b. We found that 38% of the tissues presented these patterns. The presence of these loops was significantly higher in the patients with a higher Breslow depth (Fig. 5c). In addition, Kaplan–Meier analysis revealed a strong prognostic value for the presence of PAS positive loops (Fig. 5d, P = 0.002), which confirms earlier results [20, 22]. Similar to the angiogenesis parameters of conventional blood vessels, no significant correlations were found between PAS loops and leukocyte infiltration. However, for all leukocyte subsets a trend towards more infiltration in melanoma samples with PAS-positive loops was observed [e.g., intratumoral CD16+ cells (P = 0.094) and intratumoral CD8+ T cells (P = 0.059), Fig. 2g, h].
Fig. 5.
The presence of tumor cell plasticity is a prognostic factor in human cutaneous melanoma. a, b Two clinical samples of patients with cutaneous melanoma with PAS positive patterns in intratumoral regions. Scale bar, 50 μm. c Presence of PAS positive loops in the tumor tissue was related to a higher Breslow thickness. Significant difference between the two groups is indicated by an asterisk. Black lines in the box plots present the median and the error bars show quartiles. d Kaplan–Meier survival curve for patients with melanoma with or without PAS loops present inside the tumor tissue
Discussion
Cutaneous melanoma is a highly malignant tumor type. It is one of the most frequent malignant tumors in young adults and its incidence is still rising [23]. Among the potential prognostic markers, the American Joint Committee on Cancer staging system for melanoma incorporates tumor thickness, level of invasion, ulceration, satellite metastases, lymph node metastases [24], but not tumor infiltrating leukocytes. Because most melanomas are characterized by a heavy infiltrate, several attempts have been made to develop immunotherapies in melanomas. In animal models, transfer of high numbers of tumor infiltrating lymphocytes could stimulate tumor rejection [25–27]. However, only some clinical responses could be established in the many clinical immunotherapy protocols and vaccination strategies [28–31].
In an attempt to verify the presence of different subsets of tumor infiltrating leukocytes as an independent histological parameter, we evaluated tumor tissues of 58 patients diagnosed with primary nodular or superficial spreading cutaneous melanoma for the amount of infiltrated CD45, CD3, CD8, CD20, CD16 and CD68 positive cells. We found that leukocyte infiltration is associated with markers of tumor progressiveness, as evidenced by the following observations. (1) Dividing the patients by Breslow thickness, we found that high Breslow depth was significantly associated with a higher amount of intratumoral CD45+, CD8+ and CD16+ cells. The other subsets of leukocytes showed a same trend, although not significantly, of more leukocyte infiltration in more progressed tumors, both intratumorally and peritumorally. (2) In nodular melanomas, more leukocytes were present than in superficial spreading melanoma and (3) almost all subsets of leukocytes where more abundantly presented in patient with a higher proliferation rate of tumor cells. This suggests that immune cell reactions are more pronounced in aggressive tumors. A recent paper of Hussein et al. [32] reported the same for CD3+ and CD20+ cells. Brocker et al. described also that an increased amount of intratumoral T lymphocytes is associated with progression in melanoma, though, in contrast to our observations, they observed in their patient population that peritumoral densities of T lymphocytes was lower in more progressed melanomas [33].
In contrast to a recent publication [34], we did not find a positive correlation between patient survival and the presence of CD3+ and CD16+ cells present in the tumor.
Our results show that CD16+ cells, macrophages and B cells are better able to infiltrate into the tumor itself, while T cells tend to reside in the peritumoral areas (Fig. 1g). Little is known on the exact contribution of these leukocytes in the process of melanoma rejection.
Some effects were observed with the use of CD16+ cells in therapeutic applications in melanoma. Additional infiltration of NK cells (CD56+) and monocytes during treatment with a therapy consisting of histamine, interferon-α and interleukin-2 was seen only in patients responding to the treatment [35]. Impaired activity of NK cells has also been observed. Jovic et al. reported that the dysfunction of NK cells in patients with metastatic melanoma was not associated with a decrease in amount of these cells but in a defect detected in NK cell perforin-mediated cytotoxic activity [36].
Tumor-associated macrophages have an important role in presenting antigens, but their clinical application as immunotherapy has limited success. This may be due to the paradox concerning tumor associated macrophages. On the one hand, tumor associated macrophages are able destroy neoplastic cells thereby reducing tumor growth. On the other hand, both macrophages and tumor cells can produce growth factors that can stimulate tumor growth [37–39].
Several in vivo studies in mice revealed an attenuating effect on B lymphocytes by unknown mechanisms leading to cytotoxic T-lymphocyte anergy and in that way to a less effective immune response [40–42]. On the other hand, normal human peripheral B cells were activated in vitro through CD40 ligation and were able to stimulate autologous T cells in the presence of melanoma cell line lysate [43]. Unfortunately, patient studies have also been contradictory. CD80+ B cells in combination with a higher amount of CD8+ cytotoxic cells correlated with a good prognosis in melanoma [44], while another study was not able to improve the response rate by a B cell depletion therapy [45].
We observed that a high amount of CD3 and CD16 expressing leukocytes predicts short survival. This suggests the inability of tumor infiltrating leukocytes to mount an effective immune response. These findings are supported by several papers that report possible escape mechanisms of melanoma cells from immunological responses. This escape from tumor immunity can be explained by several mechanisms: (1) the secretion of immunosuppressive cytokines by melanoma cells [46, 47], (2) the defective or incomplete activation status of the infiltrating T cells [48], (3) the immunosuppressive effect of melanoma cell expressing proteins [49, 50] and a variety of other escape mechanisms [51].
In our study, we analyzed the amount of FOXP3+ cells. FOXP3 is a unique functional marker of regulatory T cells [52]. Cancer patients have been described to have an enlarged pool of immunosuppressive regulatory T cells in the peripheral blood and/or the tumor. Only a few papers correlated this to the progression of the tumor [53–61]. Data on this correlation in cutaneous melanoma are lacking until know. We report the absence of the significant correlation between FOXP3+ cells and the different Breslow stages of melanoma and that it is not a prognostic factor in this type of tumors. The amount of FOXP3+ cells was positively correlated to the amount of several subsets of leukocytes. It appears that regulatory T cells are as much recruited as the other subsets of lymphocytes and other leukocytes. This observation was already reported in several earlier papers. Immunotherapy in melanomas with a Melan-A peptide vaccine resulted in more CD8+ cells, however, clinical benefit was limited due to a simultaneous induction of regulatory T cells, that suppressed the activity of the recruited CD8+ cells [62]. Similarly, the administration of IL-2 resulted in an increase of FOXP3+ cells with a high suppressive activity in both melanoma and renal cancer patients [63]. Some promising results were described in a recent paper of Nair et al. where targeting FOXP3 by vaccination resulted in an enhanced tumor immunity in a mouse model [64].
The activation status of lymphocytes was studied by the quantification of CD69+ cells. CD69 is among the earliest inducible cell surface glycoproteins during lymphoid activation [65]. Several manuscripts describe the decrease of CD69+ cells in tumors with a more progressed stage and a higher amount of CD69+ cells predicted better survival [11, 66, 67]. We are the first to report on the prognostic value of CD69+ cells in primary cutaneous melanoma. We found a significantly higher amount of CD69+ cells in melanomas with the most progressed stage. Univariate analysis showed an inverse pattern in comparison to the other subsets of leukocytes. There was a clear trend that a higher amount of CD69+ cells predicts a better prognosis for melanoma patients. It is clear that the detection of only infiltrating leukocytes is not a sensitive marker to evaluate tumor immune response.
The impact of regression is still unclear. As in most studies regression was mainly observed in thin melanomas. Some studies indicated that regression is a negative prognostic factor [14, 68, 69], while others could not find a relationship between regression and survival [70–73]. We could not find a relationship with survival nor a correlation with intratumoral and peritumoral leukocytes.
Since leukocytes home into tumors by extravasation from blood vessels, we investigated the relationship between leukocyte infiltration and parameters of angiogenesis [39, 74]. We did not find any correlation. Therefore, we studied the plasticity of the melanoma cells, which has been discovered in melanoma to contribute to circulation [18, 75]. We did not find any significant evidence that this alternative way of blood circulation in the tumor [18] contributes to the formation of an inflammatory infiltrate. However, we did find a trend in this direction. This might be due to the fact that vasculogenic mimicry is a marker of tumor aggressiveness, resulting in the attraction of more immune cells. Since it is known that leukocyte infiltrate can recognize and eradicate tumor mass, this might be seen as a contradictory observation. However, it can be argued that the high number of infiltrated leukocytes reflects the enhanced antigenicity of more aggressive tumor cells [32].
In summary, we demonstrated that leukocyte infiltration in melanoma predicts a short survival. In our study, we included all stages of Breslow depth. We conclude that more advanced melanomas are characterized by more tumor infiltrating leukocytes, maybe because of the more aggressive behaviour of the tumors. From survival analysis it is concluded that this is insufficient for an effective immune response. Our observation that all stages of Breslow depth are infiltrated with regulatory T cells but that the amount of CD69+ activated lymphocytes is a possible prognostic factor, should be taken into account in future studies. It is clear that more research on the immunosuppressive mechanisms and the stimulation of antigen driven leukocyte responses is necessary in order to improve immunotherapeutic applications.
References
- 1.Ramirez-Montagut T, et al. Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene. 2003;22(20):3180–3187. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, Wang WC, Evans SS. Tumor microvasculature as a barrier to antitumor immunity. Cancer Immunol Immunother. 2003;52(11):670–679. doi: 10.1007/s00262-003-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vesalainen S, et al. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A(12):1797–1803. doi: 10.1016/0959-8049(94)E0159-2. [DOI] [PubMed] [Google Scholar]
- 4.Marrogi AJ, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74(5):492–501. doi: 10.1002/(SICI)1097-0215(19971021)74:5<492::AID-IJC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Chao HT, et al. Lymphocyte-infiltrated FIGO Stage IIB squamous cell carcinoma of the cervix is a prominent factor for disease-free survival. Eur J Gynaecol Oncol. 1999;20(2):136–140. [PubMed] [Google Scholar]
- 6.Naito Y, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494. [PubMed] [Google Scholar]
- 7.Schumacher K, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–3936. [PubMed] [Google Scholar]
- 8.Cho Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63(7):1555–1559. [PubMed] [Google Scholar]
- 9.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 10.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badoual C, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka K, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94(2):275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussein MR. Tumour-infiltrating lymphocytes and melanoma tumorigenesis: an insight. Br J Dermatol. 2005;153(1):18–21. doi: 10.1111/j.1365-2133.2005.06629.x. [DOI] [PubMed] [Google Scholar]
- 14.Clark WH, Jr, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 15.Clemente CG, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Thorn M, et al. Trends in tumour characteristics and survival of malignant melanoma 1960–84: a population-based study in Sweden. Br J Cancer. 1994;70(4):743–748. doi: 10.1038/bjc.1994.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnhill RL, et al. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. 1996;78(3):427–432. doi: 10.1002/(SICI)1097-0142(19960801)78:3<427::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.van der Schaft DW, et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65(24):11520–11528. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- 19.Hillen F, et al. Proliferating endothelial cells, but not microvessel density, is a prognostic parameter in human cutaneous melanoma. Melanoma Res. 2006;16:453–457. doi: 10.1097/01.cmr.0000232291.68666.4c. [DOI] [PubMed] [Google Scholar]
- 20.Warso MA, et al. Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res. 2001;7(3):473–477. [PubMed] [Google Scholar]
- 21.Hendrix MJ, et al. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 22.Thies A, et al. PAS-positive loops and networks as a prognostic indicator in cutaneous malignant melanoma. J Pathol. 2001;195(5):537–542. doi: 10.1002/path.988. [DOI] [PubMed] [Google Scholar]
- 23.Levi F, et al. Cancer mortality in Europe, 1995–1999, and an overview of trends since 1960. Int J Cancer. 2004;110(2):155–169. doi: 10.1002/ijc.20097. [DOI] [PubMed] [Google Scholar]
- 24.Carlson JA, et al. Malignant melanoma 2003: predisposition, diagnosis, prognosis, and staging. Am J Clin Pathol. 2003;120(Suppl):S101–S127. doi: 10.1309/J9M2NUM9MHYLN3DQ. [DOI] [PubMed] [Google Scholar]
- 25.Eberlein TJ, Rosenstein M, Rosenberg SA. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982;156(2):385–397. doi: 10.1084/jem.156.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 27.Overwijk WW, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussein MR, et al. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol. 2006;59(3):316–324. doi: 10.1136/jcp.2005.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brocker EB, et al. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988;41(4):562–567. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- 34.Piras F, et al. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer. 2005;104(6):1246–1254. doi: 10.1002/cncr.21283. [DOI] [PubMed] [Google Scholar]
- 35.Jorkov AS, et al. Immune response in blood and tumour tissue in patients with metastatic malignant melanoma treated with IL-2, IFN alpha and histamine dihydrochloride. Anticancer Res. 2003;23(1B):537–542. [PubMed] [Google Scholar]
- 36.Jovic V, et al. Impaired perforin-dependent NK cell cytotoxicity and proliferative activity of peripheral blood T cells is associated with metastatic melanoma. Tumori. 2001;87(5):324–329. doi: 10.1177/030089160108700509. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, et al. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 38.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 39.Dirkx AEM, et al. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 40.Bennett SR, et al. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188(11):1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perricone MA, et al. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27(4):273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Shah S, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117(4):574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 43.Lapointe R, et al. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63(11):2836–2843. [PubMed] [Google Scholar]
- 44.Martinez-Escribano JA, et al. Changes in the number of CD80(+), CD86(+), and CD28(+) peripheral blood lymphocytes have prognostic value in melanoma patients. Hum Immunol. 2003;64(8):796–801. doi: 10.1016/S0198-8859(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 45.Aklilu M, et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15(7):1109–1114. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 46.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 47.Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444–3451. [PubMed] [Google Scholar]
- 48.Ladanyi A, et al. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10(2):521–530. doi: 10.1158/1078-0432.CCR-1161-03. [DOI] [PubMed] [Google Scholar]
- 49.Rubinstein N, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5(3):241–251. doi: 10.1016/S1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 50.Le QT, et al. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23(35):8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pawelec G. Tumour escape from the immune response. Cancer Immunol Immunother. 2004;53(10):843. doi: 10.1007/s00262-004-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 53.Woo EY, et al. Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 54.Woo EY, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168(9):4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 55.Sasada T, et al. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 56.Wolf AM, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 57.Viguier M, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173(2):1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 58.Alvaro T, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 59.Wolf D, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11(23):8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 60.Hiraoka N, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 61.Bates GJ, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 62.Appay V, et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177(3):1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 63.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nair S, et al. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67(1):371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 65.Cambiaggi C, et al. Constitutive expression of CD69 in interspecies T-cell hybrids and locus assignment to human chromosome 12. Immunogenetics. 1992;36(2):117–120. doi: 10.1007/BF00215288. [DOI] [PubMed] [Google Scholar]
- 66.Healy CG, et al. Impaired expression and function of signal-transducing zeta chains in peripheral T cells and natural killer cells in patients with prostate cancer. Cytometry. 1998;32(2):109–119. doi: 10.1002/(SICI)1097-0320(19980601)32:2<109::AID-CYTO6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 67.Koch M, et al. Tumor infiltrating T lymphocytes in colorectal cancer: tumor-selective activation and cytotoxic activity in situ. Ann Surg. 2006;244(6):986–992. doi: 10.1097/01.sla.0000247058.43243.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slingluff CL, Jr, et al. Lethal “thin” malignant melanoma. Identifying patients at risk. 1988;208(2):150–161. doi: 10.1097/00000658-198808000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20(4):315–322. doi: 10.1111/j.1365-2559.1992.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 70.Trau H, et al. Metastases of thin melanomas. Cancer. 1983;51(3):553–556. doi: 10.1002/1097-0142(19830201)51:3<553::AID-CNCR2820510332>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 71.Wanebo HJ, Cooper PH, Hagar RW. Thin (less than or equal to 1 mm) melanomas of the extremities are biologically favorable lesions not influenced by regression. Ann Surg. 1985;201(4):499–504. doi: 10.1097/00000658-198504000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw HM, et al. Thin malignant melanomas and recurrence potential. Arch Surg. 1987;122(10):1147–1150. doi: 10.1001/archsurg.1987.01400220057011. [DOI] [PubMed] [Google Scholar]
- 73.Fontaine D, et al. Partial regression of primary cutaneous melanoma: is there an association with sub-clinical sentinel lymph node metastasis? Am J Dermatopathol. 2003;25(5):371–376. doi: 10.1097/00000372-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Molema G, Griffioen AW. Rocking the foundations of solid tumor growth by attacking the tumor’s blood supply. Immunol Today. 1998;19(9):392–394. doi: 10.1016/S0167-5699(98)01314-0. [DOI] [PubMed] [Google Scholar]
- 75.Maniotis AJ, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]