Abstract
Multiple investigators have reported the presence of defects in the immune response of the elderly [Castle In: Clin Infect Dis 31:578, 2000; Ortqvist et al. In: Eur Respir J 30:414–422, 2007; Saurwein-Teissl et al. In: J Immunol 168:5893, 2002; Haynes et al. In: Proc Natl Acad Sci USA 100:15053–15058, 2003]. These defects reduce the magnitude of the immune response to infection and to vaccination. In individuals greater than 55 years of age, the probability of developing a fully protective neutralizing antibody response to the yearly multivalent particle inactivated influenza vaccine is less than 20% [Jefferson et al. In: Lancet 264:1165–1174, 2005; Goodwin et al. In: Vaccine 24:1159–1169, 2006; Jackson et al. In: Lancet 372:398–405, 2008; Simonsen and Taylor In: Lancet 7:658–666, 2007]. The defects in the aged immune system that are responsible for this limited response to vaccination in the older age groups include functional defects of the antigen presenting cells, functional defects in CD4 helper CD4 T cells and monocytes, and an altered microenvironment [Eaton et al. In: J Exp Med 200:1613–1622, 2004; Dong et al. In: J Gen Virol 84:1623–1628, 2003; Deng et al. In: Immunology 172:3437–3446, 2004; Cella et al. In: J Exp Med 184:747–752, 1996]. Starting at puberty, the involution of the thymus and the consequent reduction of the export of naïve T cells specific to neo-antigens leads to the reduction of the ratio of antigen naïve to memory cells as chronological age advances [Prelog In: Autoimmun Rev 5:136–139, 2006; McElhaney et al. In: J Immunology 176:6333–6339, 2006]. Changes in glycosylation of T cells and target antigens acquired during the aging process and the antibodies to these new glycopeptides and glycoproteins may also contribute to a reduction in the functioning of the adaptive immune response [Ishii et al. In: J Clin Neurosci 14:110–115, 2007; Shirai et al. In: Clin Exp Immunol 12:455–464, 1972; Adkins and Riley In: Mech Ageing Dev 103:147–164, 1998; Ben-Yehuda and Weksler In: Cancer Investigation 10:525–531, 1992]. One of the more interesting examples of the functional defects in the cells of the adaptive immune response is a reduced level of expression in the surface cytoadhesion and activation receptor molecules on CD4 helper T cells undergoing activation during vaccination. Upon infection or vaccination, CD40L is typically increased on the surface of CD4 helper T cells during activation, and this increased expression is absolutely essential to the CD40L promotion of expansion of antigen-specific B cells and CD 8 effector T cells in response to infection or vaccination [Singh et al. In: Protein Sci 7:1124–1135, 1998; Grewal and Flavell In: Immunol Res 16: 59–70, 1997; Kornbluth In: J Hematother Stem Cell Res 11:787–801, 2002; Garcia de Vinuesa et al. In: Eur J Immunol 29:3216–3224, 1999]. In aged human beings and mice, the reduced levels of expression of CD40 ligand (CD40L) in activated CD4 helper T cells is dramatically reduced [Eaton et al. In: J Exp Med 200:1613–1622, 2004; Dong et al. In: J Gen Virol 84:1623–1628, 2003]. To circumvent the reduction in CD40L expression and the subsequent reduction in immune response in the elderly, we have developed a chimeric vaccine comprised of the CD40L linked to the target antigen, in a replication incompetent adenoviral vector and in booster protein. This review will discuss the implementation the potential use of this approach for the vaccination of the older populations for cancer and infection.
Keywords: Vaccines, Immunoconjugates, MUC-1, CD40L
Introduction
The CD40L/CD40 receptor complex is composed of a homotrimeric ligand and receptor molecules, which belong to the TNF family of receptors and ligands [19–22]. The expression of CD40L on the surface of CD4 helper T cells is a requisite step for the activation of the adaptive immune response by vaccination [19, 20]. The goal of vaccination is to increase the frequency representation of antigen-specific CD8 effector T cells, or antigen-specific B cells, from one in a million to one in a hundred to one in a thousand. Extensive work has documented the permissive effect of the engagement of the CD40 receptor on dendritic cells (DCs) or antigen presenting cells, B cells and T cells by the CD40L on activated CD4 helper T cells on the induction of the activation and expansion of target-associated antigen (TAA)-specific CD8 effector cells and the induction of increases in the levels of TAA-specific antibodies these cells by vaccination. The binding of the CD40L to the CD40 receptor on the DCs promotes presentation of TAAs on Class I MHC molecules, and migration of the DCs to regional lymph nodes following antigen or virus exposure [19–22]. Among older test subjects (human beings as well as test mice), the expression of the CD40L on CD4 helper cells during activation is delayed and reduced in absolute level [9, 10]. The absence of the CD40 ligand on activated CD4 helper T cells in the older age group may result in a reduced magnitude of response to infections and vaccinations.
One way to overcome this functional defect in CD4 helper T cells in the older age groups has been to administer the CD40L protein at the time of vaccination (see Refs. [23–25] for examples of reports of clinical trials in human subjects using CD40L to induce the immune response). Intravascular administration of CD40L could be very dangerous due to the presence of CD40 receptors on monocytes, which are present in parenchymal tissue sites (such as the liver and lung) as well as in atherosclerotic plaques in the luminal endothelium of the vasculature of older individuals, which could result in the release of cytokines and resultant cytokine storm and thrombosis [21, 22]. In contrast, injection of the CD40L or of its immunoconjugates into the sc space, or into tumors, is not accompanied by any significant side effects [23–25]. Insertion of the CD40L gene into the transcription units of replication incompetent adenoviral vectors has been shown to induce an increase in the induction of an anti-tumor response in young test mice and in human subjects [23]. The intravenous administration to human subjects of chronic lymphocytic leukemia cells infected with replication incompetent adenoviral vectors carrying the CD40L embedded in an adenoviral vector transcription unit [24] or the injection of an adenoviral vector into tissue carrying CD40L transcription unit [23, 25] has been shown not to be associated with significant side effects. Xiang et al. [26] reported that the oral administration of bacterial cells transformed with a plasmid carrying a transcription unit encoding the carboxyl terminus of CD40L linked to the aminoterminus of TAA induced an antitumor response to carcinoembryonic antigen when administered to young mice. The response induced by this vaccination was weak since it required the co-administration of the IL2 protein in order to completely suppress the growth of colorectal cancer cells injected subcutaneously [26]. One reason for the observed weakness of this vaccine was the attachment of the carboxyl terminal end of the CD40L to the TAA since the carboxyl terminus of CD40L is the end, which is necessary for CD40 receptor binding.
Therefore, we have created a chimeric molecule composed of the carboxyl terminal end of the target antigen linked to the amino terminal end of the extracellular domain (ecd) of the CD40L by means of a 9 amino acid linker (Fig. 1). We chose to link the aminoterminal end of CD40L to the carboxyterminus of TAA, in contrast to Xiang et al. [26] who linked the carboxylterminus of CD40L to the target antigen, as the carboxylterminus of CD40L is the end of CD40L, which engages the CD40 receptor. Since all of the sequences necessary for the assembly of the homotrimeric CD40L [27] are contained within the ecd, and the carboxyl terminal domain engages the CD40L receptor, we restricted the sequences to be attached to the target antigen to the aminoterminal domain of the CD40L that includes the carboxyl terminal end of the ecd of the CD40L, but not the transmembrane domain nor the cytoplasmic domain of the CD40L [28–32]. This vaccine consists of two sc injections of the Ad-sig-TAA/ecdCD40L vector, or a single injection of that vector following by two sc injections of the TAA/ecdCD40L protein as a booster. The advantages of creating a CD40L-target antigen chimera are as follows:
The provision of the CD40L as the composition of matter of a recombinant protein vaccine provides the CD40L signal, which is missing in older individuals that is necessary for the expansion of antigen-specific B cells and CD8 effector cells as well as activation of DCs. Engagement of the CD40 receptor on DCs carrying TAA leads to an increase in expression of the surface cytoadhesion molecules described as “secondary signals” that are necessary for sustained induction of proliferation of antigen-specific CD8 effector T cells. This also induces expression of CCR 7 mRNA in these DCs which leads to their migration to the regional lymph nodes [28]. This solves the problem of reduced expression of CD40L on CD4 helper T cells.
The engagement of the CD40L to the CD40 receptor leads to the internalization of the chimeric TAA-CD40L chimeric molecule into the cytoplasmic compartment. This has been shown to lead to the presentation of the TAA peptides on the Class I MHC molecule [28]. Thus, administration of this chimeric molecule solves another of the important problems with vaccination: the need to secure presentation of the TAA by the Class I MHC.
A third problem solved by attaching the TAA to the CD40L (in the case of weakly immunogenic TAA) is to increase the immunogenicity of the TAA.
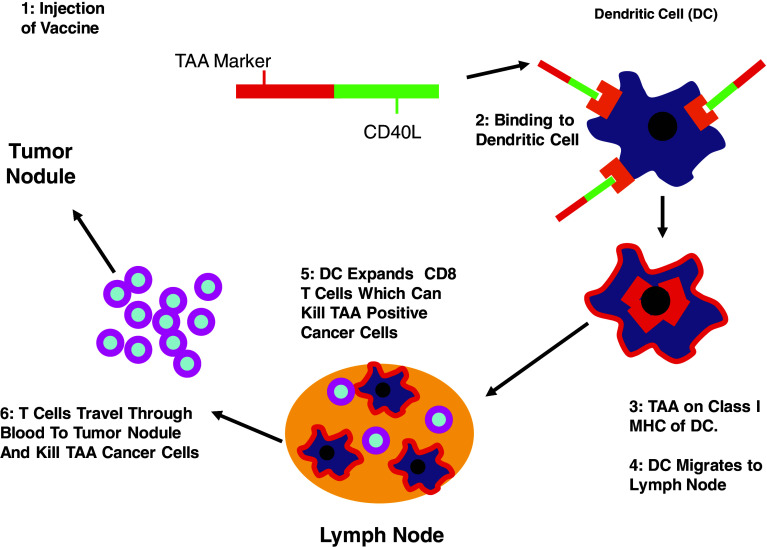
Fig. 1.
Induction of the adaptive immune response with the TAA/ecdCD4L vaccine. The TAA/ecdCD40L is injected subcutaneously. The CD40L end binds the CD40L receptor on the DCs leading to the internalization of the TAA, which then becomes presented by Class I MHC molecules. The TAA antigen-loaded activated DCs then migrate to the regional lymph nodes. The TAA-loaded CD40L stimulated DCs activate the expansion of the TAA-specific T cells, which then leave the lymph node, course through the peripheral blood, and then enter into the extravascular sites of infection, inflammation or tumor
As is well known, there are multiple factors that diminish the intensity of the adaptive immune response induced by vaccination in test subjects which are bearing tumor cells. One example is that most tumor-associated antigens are self-antigens, resulting in the deletion of T cells with high affinity antigen recognition receptors for these self-antigens in the thymus gland. A second example is the anergy to TAA that results from presentation of TAA on cancer cells, which are imperfect antigen presenting cells. In order to provide additional signals that can facilitate and promote the activation of the immune response, we have also embedded the transcription unit for the chimeric TAA/ecdCD40L protein in a replication incompetent adenoviral vector. The TAA/ecdCD40L transcription unit is preceded by a signal sequence for secretion (sig) and a cytomegalovirus (CMV) promoter. The reasons for using the adenoviral vector for the delivery of the TAA/ecdCD40L recombinant molecule are as follows:
The use of the adenoviral vector induces the release of cytokines from the antigen presenting cells due to the binding of viral-specific DNA and RNA motifs to the Toll like receptor (TLR) 9 on DCs.
In addition, the injection of the adenoviral vector to deliver the TAA/ecdCD40L results in the in vivo synthesis of the recombinant TAA/ecdCD40L protein.
The expression of the TAA/ecdCD40L induced by the infection of the cells surrounding the subcutaneous (sc) injection site of the Ad-sig-TAA/ecdCD40L vector lasts for 10–14 days, thus further amplifying the aggregate impact of the vaccination on the immune response induced by the Ad-sig-TAA/ecdCD40L vector vaccine.
The infection of the DCs by the adenoviral vector carrying the chimeric TAA/ecdCD40L transcription unit results in the release of the CD40L molecule in the regional lymph node following migration of the DCs to this site. This provides CD40L for the activation of CD40 receptor on CD8 T cells to induce the proliferation of the antigen (TAA)-specific T cells. In order to maximize the effect of infection of cells by the Ad-sig-TAA/ecdCD40L vector, the TAA/ecdCD40L transcription unit in the Ad vector is preceded by a secretory sequence (sig) and the transmembrane domain of the CD40L has been removed. This ensures that the TAA/ecdCD40L protein will be released by the Ad-sig-TAA/ecdCD40L infected cells. We have shown that the effect of the TAA/ecdCD40L vector on induction of the immune response is dependent on the secretion of the TAA/ecdCD40L from the adenoviral infected cells.
These advantages of the use of an adenoviral system to deliver the TAA/ecdCD40L transcription unit will make it useful in disease states like cancer in which there is acquisition of peripheral tolerance to TAA in addition to the existence of central tolerance to self-antigen TAA acquired early in life (which deletes the T cells bearing high affinity antigen recognition receptors to tumor-associated “self” antigens). One more measure that could be taken that would expand the magnitude of the immune response induced by the Ad-sig-TAA/ecdCD40L vector would be the use of sc injections of the TAA/ecdCD40L protein.
Summary of pre-clinical results on cancer
Ad-sig-TAA/ecdCD40L vector vaccines
The first type of experiment that we carried out with the TAA/ecdCD40L chimeric vaccine is the use of two sc injections of the Ad-sig-TAA/ecdCD40L vector 7 days apart [28–32]. We have tried the Ad-sig-TAA/ecdCD40L vector vaccine with the following tumor-associated self-antigens: the hMUC-1 (human MUC-1 antigen) that is overexpressed in over 80% of recurrent epithelial neoplasms, the rat Her-2-Neu (rH2N) growth factor receptor antigen which is overexpressed in 30% of breast cancers at diagnosis, the tyrosinase related protein-2 (TRP-2) melanoma antigen, a junctional peptide from the bcrabl gene for chronic myelogenous leukemia (Bcr-Abl), and the E7 protein expressed in human papilloma virus (HPV)-associated cervical cancer cells. All of these antigens are self-antigens except for E7 that is a viral antigen.
Two of the self-antigens tested (hMUC-1 and rH2N), which are overexpressed in epithelial malignancies with a poor prognosis, were tested in mouse strains transgenic for the human Mucin-1 gene (hMUC-1.Tg) or the rat Her-2-Neu gene (rH2N.Tg). These mouse strains were therefore anergic to the hMUC-1 or rH2N target xeno-antigens. These mouse models are very relevant to the situation encountered in the human cancer subject in which there is usually an anergic state to the tumor-associated target antigen, which are self-antigens. Two sc injections of the Ad-sig-TAA/ecdCD40L vaccination completely prevented the growth of sc deposits of cancer cells positive for the target antigen [28–32]. The magnitude of the cellular immune response induced in the mouse by two sc injections of the Ad-sig-TAA/ecdCD40L by ELISPOT assay is in the 50–100 antigen-specific T cells/100,000 spleen cell range [28–30]. This is true for the rH2N and hMUC-1 antigens as well as the rest of the other self-antigens tested (the P210Bcr-Abl junctional peptide, or TRP-2 antigens) as well as the HPV-associated cervical cancer antigen. Two Ad-sig-TAA/ecdCD40L vector injections are sufficient to induce antigen-specific memory cells [28]. This claim is based on the fact that two sc injections of the Ad-sig-TAA/ecdCD40L in tumor-bearing mice produces spleen cells, which can be collected 1 year after vaccination and then used to passively transfer protection to syngeneic nude mice. The intra-peritoneal injection of the spleen cells collected from the mice vaccinated with the Ad-sig-TAA/ecdCD40L 1 year earlier, suppressed the growth of TAA-specific cancer cells. The vaccine also induces an increase in the release of IL-12 and interferon gamma from the CD8 effector T cells, but does not change the level of IL6, IL10 or TNF alpha in the plasma of test mice.
Vector prime-protein boost vaccination in young mice
We have also tested the use of a single injection of the Ad-sig-TAA/ecdCD40L vector followed in 7 and 21 days with sc injections of the TAA/ecdCD40L recombinant protein as booster injections. The use of the booster injections increases the level of the TAA-specific CD8 effector T cells and the TAA-specific antibodies by sixfold when compared with mice injected twice with the Ad-sig-TAA/ecdCD4L vector alone. We call this the TAA/ecdCD40L VPP vaccination [30]. We have also tested the use of the TAA/ecdCC40L VPP vaccination in mice in which there was progressive cancer. The TAA/ecdCD40L VPP vaccine completely suppresses the growth of pre-existing established and growing sc nodules of cancer even in old mice [30]. In addition, the VPP vaccination induces regressions of established pulmonary metastases [30–32]. Finally, the VPP vaccine has been shown to prevent the development of spontaneous mammary cancer in the rat Her-2-Neu transgenic cancer mouse model (rH2N.Tg mice), thus suggesting that the TAA/ecdCD40L VPP vaccine is successful with slowly developing spontaneous tumors which take months to develop [30]. The spontaneous tumors are different from sc nodules that develop in 10 days from sc injection of cancers cells derived from established cell lines. The development of spontaneous tumors involves a multistep process requiring months of increasing severity of epithelial dysplasia from the second to the sixth months of life. The development of mammary tumor nodules and the development of metastatic tumor are only seen at 8–10 months of life in the rH2N.Tg mice, where as only 10 days are required for tumors to form following the sc injection of cells derived from established cancer cell lines.
Vector prime-protein boost vaccine in old mice
As outlined in the introduction, the vaccine was designed to overcome the defects that exist in the aged immune response system. We have therefore tested the Ad-sig-TAA/ecdCD40L vector prime-TAA/ecdCD40L protein boost vaccine in 18-month-old mice. The same defects in expression of the CD40L that were reported in the fifth and sixth decades of life in the aged human subjects, are also found in the activated CD4 helper T cells of 18-month-old mice. Our experiments [30] showed that the combination of the Ad-sig-TAA/ecdCD40L vector prime-TAA/ecdCD40L protein boost vaccine induced a 10-fold increase in the levels of the TAA-specific CD8 effector T cells in the sc tumor nodules of 18-month-old mice. Furthermore, the use of the Ad-sig-TAA/ecdcD40L vector prime-TAA/ecdCD40L protein boost vaccine induced a robust TAA-specific humoral immune response in the aged mouse test subjects that induced regressions in sc tumor nodules, which were progressing in size prior to the administration of the vaccine [30]. This effect was accompanied by an increase in the release of IL-12 and interferon gamma from the CD8 effector T cells, but no change the level of IL6, IL10 or TNF alpha in the plasma of test mice were detected.
Clinical trials in women with recurrent carcinoma of the breast and in men with recurrent carcinoma of the prostate
On the basis of these pre-clinical results, the FDA gave its permission for patient entry on phase I trials to evaluate the toxicity and efficacy of the Ad-sig-hMUC-1/ecdCD40L vaccine in women with recurrent carcinoma of the breast, which is only partially responsive to front line salvage chemotherapy. This trial was opened for accrual of women with recurrent breast cancer in 2008. In addition, a phase I trial for the toxicity of the Ad-sig-hMUC-1/ecdCD40L vector vaccine in men with recurrent prostate cancer which has failed hormonal therapy and only partially responded to chemotherapy has also been opened for patient accrual by the FDA.
We chose the human mucin-1 protein (hMUC-1) as the tumor- or target-associated antigen because:
Overexpression of hMUC-1 correlates with adverse therapy outcome in carcinomas of the prostate, ovary and breast;
hMUC-1 is hypoglycosylated in cancer thereby producing a tumor-specific antigen;
In cancer, overexpression of the transmembrane subunit of MUC-1 correlates with reduced expression of p53 [33], leading to chemotherapy resistance, and to activation of NFkappaB [34], which leads increased proliferation and metastasis;
hMUC-1 is expressed on the “cancer stem cells” as well as in the more mature cells in epithelial cancer thus making MUC-1 a target, which will direct the immune response to the most aggressive cells in the tumor population when it is included in a cancer vaccine [35].
The hMUV-1ecdCD40L TAA chimeric vaccine has been shown to decrease the level of the hMUC-1 CD44 + CD24neg/low cells, which are MUC-1 positive by over 20-fold.
In this phase I clinical trial, we are measuring endpoints of toxicity as well as endpoints of the immune response to the vaccine. In addition, we are using pheresis to collect peripheral blood cancer cells before and after vaccination. We are purifying these cancer cells from the vast excess of hematopoietic cells with magnetic bead conjugated antibodies. We are able to generate populations of peripheral blood cancer cells that are over 90% pure and number in the 150,000 range. We then subject them to the following assays: gene expression microarray profiling; in vitro chemotherapy resistance; in vitro migration; tumorigenicity in NODXSCID mice; and self-renewal by multiple passage in NODXSCID mice. We are testing for correlations between individual and combination gene expression signatures and the outcome of in vitro assays for chemotherapy resistance, in vitro migration (a surrogate for metastasis), and in vivo assays of self-renewal and tumorigenicity of the human peripheral blood cancer cells in NODXSCID mice. We plan on using the data to develop RT-CR tests for gene expression signatures in peripheral blood cancer cells which can predict prognosis, response to therapy, and provide new targets for therapy development.
Pre-clinical studies of the TAA/ecdCD40L vaccine with avian influenza antigens
As outlined in the introduction, the percent of human subjects vaccinated with the yearly multivalent particle inactivated human influenza vaccine who develop a fully protective neutralizing antibody response to vaccination is 20% in individuals above the age of 55 years, and 80% among individuals less than 55 years of age [5–8]. Moreover, the clinical experience with vaccination to avian influenza antigens is that avian influenza antigens appear to be only very weakly stimulatory to the human immune response even in young human subjects [36–39] Therefore, we compared the immune response induced the TAA/ecdCD40L VPP vaccine when the TAA was an antigen that was strongly immunostimulatory antigen (like the hemagglutinin (HA) antigen of the H5N1 avian influenza virus), with that induced by an antigen that is known to be only weakly immunostimulatory, like to M2 antigen of the H5N1 avian influenza virus.
Comparison of the levels of H5N1 HA-specific CD8 T cells and antibodies in 18-month-old (aged) versus 2-month-old (young) test mice
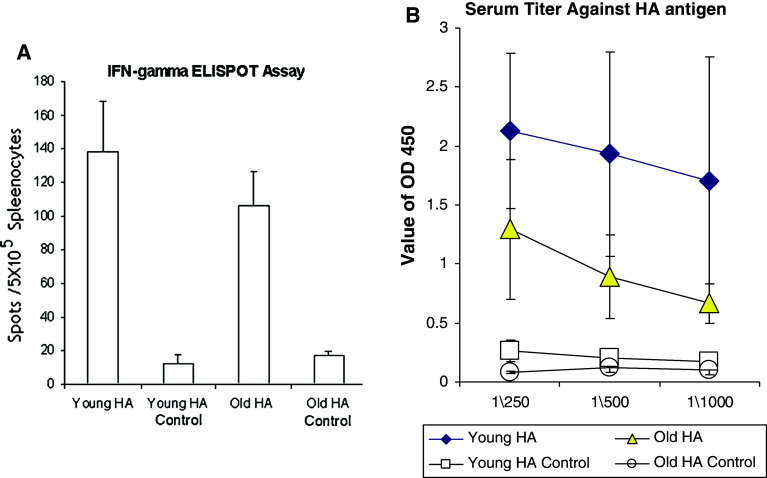
We injected the Ad-sig-HA/ecdCD40L vector sc once followed by 3 HA/ecdCD40L protein boost injections at 7 day intervals starting 7 days after the initial vector injection in 2 month-old C57BL6 mice (n = 4) and in 18-month-old C57BL6 mice (n = 4). We compared the levels of HA-specific splenic CD8 T cells (ELISPOT assay) and the levels of the HA-specific serum antibodies (ELISA assay) of vaccinated versus unvaccinated mice. As shown in Fig. 2a, the levels of HA-specific splenic CD8 T cells of the vaccinated mice were statistically significantly increased in both the 2 month old (p = 0.0004) as well as the 18-month-old mice (p = 0.0001). In addition, as shown in Fig. 2b, the level of serum antibodies specific for the H5N1 HA antigen was statistically significantly increased in the vaccinated versus the control mice at the p = 0.004 level in the 2-month-old mice and at the p = 0.015 level in the 18-month-old mice (1/250 dilution).
Fig. 2.
Study of the Ad-sig-HA/ecdCD40L vector prime-HA/ecdCD40L protein boost vaccine for the hemagglutinin antigen of the avian influenza virus. a HA/ecdCD40L protein boost injections at 7 day intervals starting 7 days after the initial vector injection in 2-month-old C57BL6 mice (n = 4) and in 18-month-old C57BL6 mice (n = 4). We compared the levels of HA-specific splenic CD8 T cells (ELISPOT assay) and the levels of the HA-specific serum antibodies (ELISA assay) of vaccinated versus unvaccinated mice. The levels of HA-specific splenic CD8 T cells of the vaccinated mice were statistically significantly increased in both the 2-month-old (p = 0.0004) as well as the 18-month-old mice (p = 0.0001). b The level of serum antibodies specific for the H5N1 HA antigen was statistically significantly increased in the vaccinated versus the control mice at the p = 0.004 level in the 2-month-old mice and at the p = 0.015 level in the 18-month-old mice (1/250 dilution)
Conclusions
It is feasible to use the Ad-sig-HA/ecdCD40L vector prime-HA/ecdCD40L protein boost strategy to induce a potent immune response to the H5N1 HA antigen in 18-month-old mice as well as in young mice. These data suggest that the vaccine is feasible and potentially of high impact for influenza antigens.
Testing of the feasibility of using the Ad-sig-M2/ecdCD40L vector prime-M2/ecdC40L protein boost vaccine strategy (VPPP) to induce a potent immune response for a weak antigen (M2) for a universal vaccine for avian influenza in young and aged mice
The amino acid structure of the H5N1 M2 protein does not change in strains with influenza harboring transitions of amino acid sequence in the HA antigen. However, M2 has been shown to be a weak antigen during vaccination. To overcome this, we linked the CD40L to the M2 gene in the Ad-sig-M2/ecdCD40L. We hypothesized that the Ad-sig-M2/ecdCD40L vector prime-M2/ecdCD40L protein boost vaccine strategy might create a potent “universal vaccine” for all strains of H5N1 influenza virus. This would be a vaccine which could be produced and stockpiled prior to a H5N1 pandemic and before each year’s H3N2 human influenza viruses epidemics as well.
As shown in Fig. 3a and in Fig. 3b, the sc injection of the Ad-sig-M2/ecdCD40L vector followed at 7 and 21 days with the sc injection of the M2/ecdCD40L protein (VPPP) induced an increase in the level of the M2-specific splenic CD8 T cells and M2-specific serum antibodies in the vaccinated mice. This was the case for both the 2-month-old (n = 5) as well as the 18-month-old (n = 5) C57BL6 mice. The levels of the M2-specific splenic CD8 T cells in unvaccinated versus vaccinated mice was statistically significantly different in the 2-month-old mice (young) at the p = 0.0006 level and in the 18-month-old mice at the p = 0.0009 level. In addition, as shown in Fig. 3b, the level of serum antibodies specific for the H5N1 M2 antigen was statistically significantly increased in the vaccinated versus the control mice, both in the 2-month-old mice (p = 0.0028) and in the 18-month-old mice (p = 0.0025) at a dilution of 1/250. The magnitude of the cellular immune response to the M2 vaccine was equivalent to that seen with the HA vaccine. However, the increase of M2-specific antibodies with the M2 vaccine (Fig. 3b) was less than that seen with the HA vaccine (Fig. 2b).
Fig. 3.
Study of the Ad-sig-M2/ecdCD40L vector prime-M2/ecdCD40L protein boost vaccine for the avian influenza virus. a The sc injection of the Ad-sig-M2/ecdCD40L vector was followed at 7 and 21 days with the sc injection of the M2/ecdCD40L protein (VPPP). This induced an increase in the level of the M2-specific splenic CD8 T cells and M2-specific serum antibodies in the vaccinated mice. This was the case for both the 2-month-old (n = 5) as well as the 18-month-old (n = 5) C57BL6 mice. The levels of the M2-specific splenic CD8 T cells in unvaccinated versus vaccinated mice was statistically significantly different in the 2-month-old mice (young) at the p = 0.0006 level and in the 18-month-old mice at the p = 0.0009 level. b We tested for the level of serum antibodies specific for the H5N1 M2 antigen using an ELISA assay and peptides for M2 adherent to the plastic. The level of the M2-specific antibodies in the vaccinated group was statistically significantly increased in the vaccinated versus the control mice, both in the 2-month-old mice (p = 0.0028) and in the 18-month-old mice (p = 0.0025) at a dilution of 1/250
Conclusions
This data shows that the linkage of the CD40L to the M2 protein in the Ad-sig-M2/ecdCD40L vaccine prime-M2/ecdCD40L protein boost vaccine makes it possible to make a weak antigen a strongly immunostimulatory antigen as measured by its induction of a significant immune response. Thus, it may be possible to develop a “universal vaccine” for all strains of H5N1 using the M2/ecdCD40L VPPP vaccine. This could be mass produced in cells (not eggs) and stockpiled prior to an epidemic.
Neutralization antibody levels in mice vaccinated with the HA/ecdCD40L PPP vaccine
We then tested whether these antibodies induced by 3 sc injections of the HA/ecdCD40L protein vaccine in test mice contained neutralizing activity to the H5N1 influenza virus (avian influenza virus). The sera from the vaccinated mice were pooled from several mice and sent to a contract laboratory, which could work with live H5N1 virus. These studies showed that levels of neutralizing antibodies were present at a 1/4,000 dilution.
Conclusions
The experimental results with the TAA/ecdCD40L vaccine show that the TAA/ecdCD40L vaccine induces memory, overcomes the defective response in older test subjects, overcomes the anergy present in TAA.Tg transgenic mice, prevents development of tumor formation and growth in spontaneous cancer transgenic mouse models, and induces complete regressions in the older “tumor progressor” mice. Having shown that the vaccine works in the cancer setting as well as for weak foreign viral antigens like M2, it is appropriate to speculate in what other infectious disease settings the Ad-sig-TAA/ecdCD40L vector prime-TAA/ecdCD40L protein boost vaccine or the TAA/ecdCD40L protein boost might have importance. There are many instances in which chronic viral infections, like hepatitis B, induce tolerance to the viral antigens so that the existing vaccines work in the preventative but not the therapeutic setting. Tuberculosis, hepatitis, influenza, malaria, dengue fever, and HPV are examples of infectious diseases in which chronic infection induces a state of tolerance that prevents therapeutic vaccines from being effective. Our laboratory plans to develop the TAA/ecdCD40L vaccine for infectious diseases as well as for cancer applications. Since the majority of individuals afflicted with epithelial neoplasms are above the age of 60, and these older patients are less able to withstand the rigors of chemotherapy, the TAA/ecdCD40L vaccine strategy which can induce an increase in the level of antigen-specific CD8 T cells in the tumor masses, as well as the level of the antigen-specific antibodies in the serum, will fill an unmet need in these older cancer patients. We plan to pursue the combination of this vaccine strategy with chemotherapy for the treatment of recurrent disease, and to employ the vaccine alone and in combination with other modalities in the setting of adjuvant therapy to prevent recurrence.
Acknowledgments
The authors are indebted to the Department of Defense (Grants 17999457 and BC022063), the California Breast Cancer Research Program (CBCRP 121B-0159), the Breast Cancer Research Foundation, and the Woltman Foundation for funding of this vaccine development program. The views expressed are the result of independent work and do not represent the views of the US Food and Drug Administration of the United States Government.
References
- 1.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 2.Ortqvist A, Granath F, Askling J, Hedlund J. Influenza vaccination and mortality: prospective cohort study of the elderly in a large geographical area. Eur Respir J. 2007;30:414–422. doi: 10.1183/09031936.00135306. [DOI] [PubMed] [Google Scholar]
- 3.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Lasko I, Parson W, Bock G, Schnoitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: association with CD8+ CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 4.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naïve cells functions well into old age, but memory generated from aged naïve cells functions poorly. Proc Natl Acad Sci USA. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferson T, et al. Efficacy and effect of influenza vaccines in the elderly. Lancet. 2005;264:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin K, Vibou C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 7.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. The Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 8.Simonsen L, Taylor RJ. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 9.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cells cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong L, Mori I, Hossain J, Liu B, Kimura Y. An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J Gen Virol. 2003;84:1623–1628. doi: 10.1099/vir.0.19029-0. [DOI] [PubMed] [Google Scholar]
- 11.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 12.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on DCs triggers production of high levels of IL-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 15.Ishii W, Matsuda M, Hanyuda M, Momose M, Nakayama J, Ehara T, Ikeda S. Comparison of the histological and immunohistochemical features of the thymus in young and elderly onset myasthenia gravis without thymoma. J Clin Neurosci. 2007;14:110–115. doi: 10.1016/j.jocn.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Shirai T, Yoshiki T, Mellors RC. Age-decrease of cells sensitive to an autoantibody-specific for thymocytes and thymus-dependent lymphocytes in NZB mice. Clin Exp Immunol. 1972;12:455–464. [PMC free article] [PubMed] [Google Scholar]
- 17.Adkins B, Riley RL. Autoantibodies to T-lineage cells in aged mice. Mech Ageing Dev. 1998;103:147–164. doi: 10.1016/S0047-6374(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Yehuda A, Weksler ME. Immune senescence: mechanisms and clinical implications. Cancer Invest. 1992;10:525–531. doi: 10.3109/07357909209024815. [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Garber E, Van Vlijmen H, Karpusas M, Hsu YM, Zheng Z, Maismith JH, Thomas D. The role of polar interactions in the molecular recognition of CD40L with its receptor CD40. Protein Sci. 1998;7:1124–1135. doi: 10.1002/pro.5560070506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal LS, Flavell RA. The CD40L: at the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 21.Kornbluth RS. An expanding role for CD40L and other tumor necrosis factor superfamily ligands in HIV infection. J Hematother Stem Cell Res. 2002;11:787–801. doi: 10.1089/152581602760404595. [DOI] [PubMed] [Google Scholar]
- 22.Garcia de Vinuesa C, MacLennan IC, Holman M, Klaus GG. Anti-CD40 antibody enhances responses to polysaccharide without mimicking T cell help. Eur J Immunol. 1999;29:3216–3224. doi: 10.1002/(SICI)1521-4141(199910)29:10<3216::AID-IMMU3216>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Tahara M, Pergolizzi RG, Kobayashi H, Krause A, Luettich K, Lesser ML, Crystal RG. Trans-splicing repair of CD40L deficiency results in naturally regulated correction of a mouse model of hyper-IgM X-linked immunodeficiency. Nat Med. 2004;10:835–841. doi: 10.1038/nm1086. [DOI] [PubMed] [Google Scholar]
- 24.Wierda WG, Cantwell JM, Woods SJ, Rassenti LZ, Preussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for CLL. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- 25.Vanderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, Ghalie R, Caron DA, Gribben JG. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 26.Xiang R, Primus FJ, Ruchlmann JM, Niethammer AG, Silletti S, Lode HN, Dolman CS, Gillies SD, Reisfeld RA. Dual function DNA vaccine encoding carcinoembryonic antigen and CD40L trimer induces T cell-mediated protective immunity against colon cancer in carcinoembryonic antigen-transgenic mice. J Immunol. 2001;167:4560–4566. doi: 10.4049/jimmunol.167.8.4560. [DOI] [PubMed] [Google Scholar]
- 27.Karpus M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, Thomas D. A crystal structure for an extracellular fragment of human CD40 ligand. Structure. 1995;3:1031–1039. doi: 10.1016/S0969-2126(01)00239-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Tang Y, Linton PJ, Deisseroth A. Injection of Ad vector encoding secretable form of TAA/CD40L fusion protein induces T cell dependent immune response against tumor cells. Proc Natl Acad Sci USA. 2003;100:15101–15106. doi: 10.1073/pnas.2135379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Zhang L, Yuan J, Maynard J, Deisseroth A. Vector mediated activation and tumor antigen loading of APC by CD40 ligand/tumor antigen secretory protein generates protection from cancer cell lines. Blood. 2004;104:2704–2713. doi: 10.1182/blood-2003-12-4319. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Akbulut H, Maynard J, Petersen L, Xiangming Fang, Zhang WW, Xia XQ, Koziol J, Linton PJ, Deisseroth A. Vector prim/protein boost vaccine which overcomes defects acquired during aging and cancer. J Immunol. 2006;177:5697–5707. doi: 10.4049/jimmunol.177.8.5697. [DOI] [PubMed] [Google Scholar]
- 31.Akbulut H, Tang YC, Akbulut KG, Maynard J, Zhang L, Deisseroth A. Antitumor immune response induced by i.t. injection of vector activated dendritic cells and chemotherapy suppresses metastatic breast cancer. Mol Cancer Ther. 2006;5:1975–1985. doi: 10.1158/1535-7163.MCT-06-0049. [DOI] [PubMed] [Google Scholar]
- 32.Akbulut H, Akbulut KG, Tang YC, Maynard J, Deisseroth A. Chemotherapy targeted to cancer tissue potentiates antigen specific immune response induced by vaccine for in vivo antigen loading and activation of dendritic cells. Mol Ther. 2008;10:1753–1760. doi: 10.1038/mt.2008.158. [DOI] [PubMed] [Google Scholar]
- 33.Wei XL, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress responses. Cancer Cell. 2004;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Ahman R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, Kufe D. MUC1 oncoprotein activates the IkB kinase beta complex and constitutive NF-kB signaling. Nat Cell Biol. 2007;9:1419–1435. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelmann K, Shen HM, Finn OJ. CDF7 side population cells with characteristics of stem/progenitor cells express the tumor antigen MUC-1. Cancer Res. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- 36.Treanor JJ, Campbell JD, Zhangwill KM, Rowe T, Wolff M. Safety and immunogenicxity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;254:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 37.Stephensen I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, Zamon M. Boosting immunity to influenza H5N1 with MF 59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–1693. doi: 10.1016/S0264-410X(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 38.Treanor JJ, Schiff GM, Couch RB, Cate TR, Brady RC, Hay M, Wolff M, She D, Cox MMJ. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006;193:1223–1228. doi: 10.1086/503050. [DOI] [PubMed] [Google Scholar]
- 39.Lin JT, Zhang JS, Dong XP, Fang HH, Chen JT, Su N, Gao Q, Zhang ZS, Liu YX, Wang ZH, Yang M, Sun RG, Li CGH, Lin S, Ji M, Liu Y, Want X, Wood HJ, Geng ZJ, Wang Y, Yin WD. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomized controlled trial. Lancet. 2006;368:9912–9997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]