Abstract
Type I interferon (IFN) possesses antiviral and antitumor activities and also having an immune regulatory effect, activating cellular immune response and upregulating several cytokines. Recent study has shown that type I IFN upregurates the dendritic cell production of IL-15 capable of activating natural killer cells and CD8+ memory T lymphocytes. However, it is still unknown if type I IFN induces IL-15 production in non-immune cells and if type I IFN affects IL-15 production in vivo. The present study investigated the effect of type I IFNs on IL-15 expression in hepatocellular carcinoma (HCC) cell lines in vitro and in patients with chronic hepatitis C in vivo. When three HCC cell lines, Huh7, HepG2, and JHH4 were cultured in vitro, IFN upregulation of IL-15 expression was observed at both the mRNA and protein levels. In experiments using Huh7 cells, upregulation of IL-15 expression occurred within 24 h of the start of IFN stimulation, and both IFN-α and -β dose-dependently increased IL-15 production in the range from 100 U/ml to 10,000 U/ml of concentration. IFN-β showed stronger activity in IL-15 production induction in vitro than IFN-α. For in vivo examination, sera were obtained from 21 chronic hepatitis C patients treated with IFN and 29 healthy individuals, and the serum IL-15 level was quantified by ELISA. The serum IL-15 level of chronic hepatitis C patients before IFN treatment was similar to that of the healthy controls and significantly increased only during the IFN administration period. These results confirm that IFN-α/β induce IL-15 production and also suggest that IL-15 may be associated with type I IFN-induced immune response.
Keywords: Chronic Hepatitis, Huh7 Cell, Liver Biopsy Sample, JHH4 Cell, Huh7 Culture
Introduction
Interferon (IFN)-α and -β are categorized as type I IFN and possess antiviral activity useful for the treatment of chronic hepatitis C. The hepatitis C virus (HCV), the pathogen of hepatitis C, causes a persistent infection in 80% of patients exposed and leads to chronic hepatitis and hepatic fibrosis that progresses in some patients to liver cirrhosis and hepatocellular carcinoma (HCC) [1–5]. A sustained elimination of serum HCV RNA is observed in 30–40% of patients administered IFN-α or -β [6–11]. Type I IFNs also have antitumor activity and are used for the treatment of chronic myelogenous leukemia and renal cell carcinoma. Moreover, IFN treatment decreases the HCC carcinogenesis rate of chronic hepatitis C patients [12–14].
Type I IFN directly affects cells to induce the antiviral proteins 2′–5′ oligo adenyl synthetase, Mx protein and PKR protein kinase [15], and also affects tumor cells to elicit a cell-cycle arrest or an apoptosis [16–18]. Recently, it has been reported that both type I IFN-induced antiviral defense and tumor suppression are related to p53 gene expression [19]. It has also been shown that type I IFNs activate cytolytic T lymphocytes (CTL) and natural killer cells (NK) [20–22], and that they upregulate production of several T cell-derived cytokines, such as IL-1β, IL-6 and TNF-α [11, 23, 24]. This suggests that the antiviral and antitumor activity of type I IFN is associated with the immune system in vivo. However, the immunological mechanisms of type I IFN are still largely unknown.
It has been reported that type I IFN upregurates IL-15 expression from dendritic cells in vitro [25]. IL-15 is a four-helix bundle cytokine related to IL-2, and its receptor consists of a unique α-chain and shared IL-2 receptor β- and γ-chains. In contrast to IL-2, which is mainly expressed in activated T cells, IL-15 is produced by various cells such as monocytes/macrophages, epitherial and fibroblast cells, placenta, skeletal muscle, heart, lung, kidney, and liver, but not by normal resting or activated T cells [26]. It is considered that IL-15 is essential for NK and NK-T cell development [27–29] and that it is capable of promoting proliferation, long-term survival and activation of CD8 memory T cells [30–32], suggesting that IL-15 plays a pivotal role in protective immune response. Thus, it is possible that IL-15 may be involved in type I IFN-induced immune response, but the relationship remains to be clarified. To better understand the immunobiological function of type I IFNs and to develop new therapeutic methods, it is important to investigate implications of how type I IFNs affect IL-15 production.
In this study, we attempted to determine if and how IFN-α and -β upregulate IL-15 production in human HCC cell lines. These results confirmed that IFN-α/β induce IL-15 production and show the first evidence of IFN-α/β induced IL-15 production in non-immune cells. Our study also indicated that serum IL-15 levels increase in chronic hepatitis C patients during the IFN-α or -β administration period. These data suggest the clinical significance of IL-15 in type I IFN-induced immune response.
Materials and methods
Cell culture and IFNs
HCC cell lines Huh7, HepG2 and JHH4 were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated FBS and antibiotic agents (100 U/ml penicillin G and 100 μg/ml streptomycin) in a humidified atmosphere of 5% CO2 at 37°C. In all experiments, 2×105 cells were seeded in a 6-well cell-culture plate and cultured for 24 h to allow the cells to stick to the culture plate and enter their logarithmic growth phase. After 24 h of preincubation, the medium was changed to fresh medium with or without IFNs. Natural human IFN-α (Sumiferon) and natural human IFN-β (Feron) were kindly provided by Sumitomo Pharmaceutical (Japan) and Dai-Ichi Pharmaceutical (Japan), respectively.
Patients with chronic HCV infection and controls
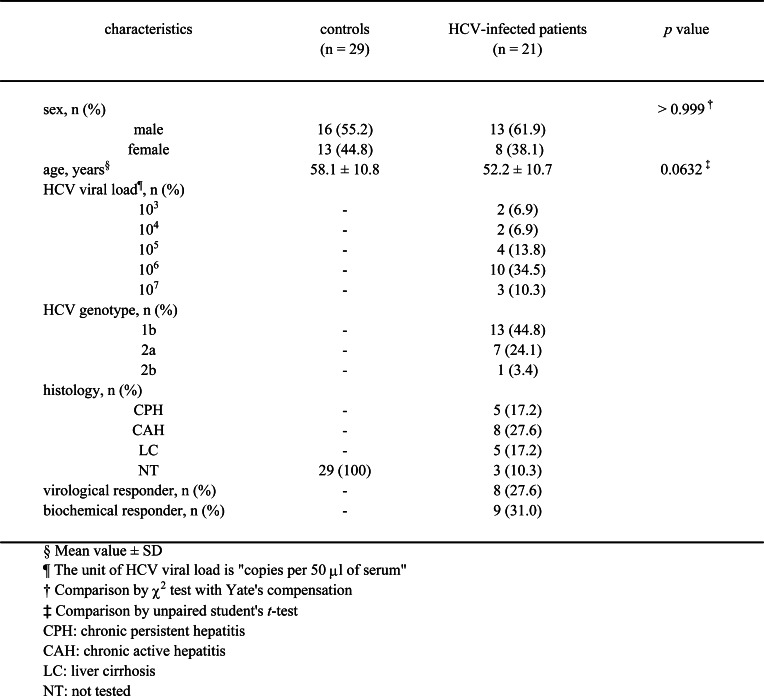
Twenty-one Japanese patients (12 men and 9 women; age range, 31–76 years; mean, 52.2 years) with chronic hepatitis C were studied. All patients were positive for serum HCV RNA and had elevated serum alanine aminotransferase (ALT). No patient was positive for hepatitis B surface antigen (HBsAg) or anti-human immunodeficiency virus (HIV) antibody. Twenty-nine healthy volunteers (16 men and 13 women; age range, 42–74 years; mean, 58.1 years) negative for serum HCV RNA, HBsAg, and anti-HIV antibody and without a clinical history or symptoms of liver disease were recruited as controls. Before any treatment was given, a liver biopsy was done for chronic hepatitis C patients and histological changes were evaluated. Quantification of serum HCV RNA was done for all patients before treatment by competitive polymerase chain reaction (PCR), as described previously [33]. Eleven chronic hepatitis C patients were given 6 million units of natural IFN-α (Sumiferon, Sumitomo Pharmaceutical Co., Japan) by intramuscular injection daily for 14 days, then three times weekly for 22 weeks. Another ten chronic hepatitis C patients were given 6 million units of natural IFN-β (Feron, Dai-ichi Pharmaceutical Co., Japan) by intravascular drip infusion daily for 56 days. The serum ALT level of the chronic hepatitis C patients treated with IFN was tested monthly during the observation period. A qualitative HCV-RNA examination of the serum from the chronic hepatitis C patients was done 6-months after the cessation of IFN treatment. Response to IFN treatment was classified as follows: virological responders were defined as patients in whom serum HCV-RNA was negative at 6 months after the cessation of IFN treatment and biochemical responders were defined as patients in whom serum ALT was continuously normal for 6 months after the cessation of IFN treatment. Informed consent was obtained from all patients and healthy volunteers.
RNA extraction and cDNA synthesis
Total RNA was extracted from cultured cells and liver biopsy samples using the RNeasy Mini kit (QIAGEN, Germany) and treated with RNase-Free DNase Set (QIAGEN, Germany) to remove contaminated DNA, according to the manufacturer’s instruction. A concentration of isolated RNA was measured by spectrophotometer, and 200 ng of total RNA was applied to reverse transcription using Superscript II RNase H− Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) with 50 ng of random hexamers according to the manufacturer’s instructions. The complementary DNA (cDNA) solution obtained was used for the subsequent PCR.
Preparation of DNA standards
To prepare DNA standards for real-time PCR, IL-15 and β-actin genes were amplified from cDNA by PCR. The forward and reverse primers for human IL-15 were hIL-15F2: 5′-GCAGGGCTTCCTAAAACAGA-3′and hIL-15R2: 5′-GTTGTTTGCTAGGATGATCAG-3′, and those for human β-actin were hβ-actinF1: 5′-GGTCACCCACACTGTGCCCAT-3′ and hβ-actinR1: 5′-GGATGCCACAGGACTCCATGC-3′. For the PCR of the IL-15 gene, 0.5 μmol of Tris-HCl (pH 8.4), 1.25 μmol of KCl, 37.5 nmol of MgCl2, 5 nmol of dNTPs, 10 pmol each of the forward and reverse primers, 0.5 U of Platinum Taq DNA (Invitrogen, Carlsbad, USA), and 1 μl of cDNA solution were mixed with distilled water to a 50 μl final volume in a 0.5 ml tube. The mixture was incubated in a thermal cycler at 94°C for 3 min, followed by 36 cycles at 94°C for 1 min, 55°C for 1 min, 72°C for 1 min, and 72°C for a final 3 min. The PCR for β-actin was performed using the same mixture condition as the PCR for IL-15, except for the primer sets. Thermocycling conditions for the β-actin PCR consisted of an initial 94°C for 3 min, followed by 20 cycles at 94°C for 1 min, 57°C for 1 min, 72°C for 1 min, and an additional 72°C for 3 min. PCR products were applied to electrophoresis on 1% agarose gel. Specific amplification was verified according to the predicted size of each amplicon (IL-15 PCR product, 240 bp; β-actin PCR product, 350 bp). IL-15 and β-actin PCR products were then extracted from the gel using the MinElute Gel Extraction Kit (QIAGEN, Germany), according to the manufacturer’s instruction. The concentration of the gel-extracted PCR products was measured by spectrophotometer. Finally, the gel-extracted IL-15 and β-actin PCR products were diluted with 0.1 × Tris-EDTA buffer.
Real-time PCR
Human IL-15 and β-actin mRNAs were quantified by real-time PCR. The primer sets for real-time PCR of the IL-15 and β-actin genes were the same used in normal PCR for a standard preparation, as described above. PCR was done using the Light Cycler (Roche, Mannheim, Germany) with LightCycler-FastStart DNA Master SYBR Green I (Roche, Mannheim, Germany). The PCR condition for IL-15 was as follows: after an initial denaturing at 95°C for 10 min, the amplification was done by 40 cycles of denaturing at 95°C for 10 s, annealing at 55°C for 10 s, and extension at 72°C for 10 s. The PCR condition for β-actin was as follows: after an initial denaturing at 95°C for 10 min, the amplification was done by 40 cycles of denaturing at 95°C for 10 s, annealing at 57°C for 10 s, and extension at 72°C for 10 s. The amplified products were monitored directly by measuring the increase of the dye intensity of the SYBR Green I that binds to the double-strand DNA amplified by PCR. The copy number of mRNA in the cDNA samples was calculated using standard amplification curves.
Assay for IL-15 concentration
The IL-15 concentration of supernatants collected from the cell cultures and serum samples was determined by use of a human IL-15 ELISA kit (Genzyme, Cambridge, MA, USA), according to the manufacturer’s instructions. The absorbance at 450 nm (reference at 540 nm) was measured. The assay was done in duplicate.
Statistical analysis
Statistical analysis was done using the StatView software package (SAS Institute Inc., Cary, NC, USA). Unpaired Student’s t-test was used to assess the statistical significance of differences in pre-treatment serum IL-15 levels between sera from controls and chronic hepatitis C patients. Paired Student’s t-test was used to compare the serially assayed serum IL-15 of chronic hepatitis C patients. The χ2 test was used for gender comparison of the controls and chronic hepatitis C patients. Ages differences between the controls and chronic hepatitis C patients were compared by unpaired Student’s t-test. P<0.05 was considered significant.
Results
IFN-α and -β upregulation of IL-15 production in Huh7 cells
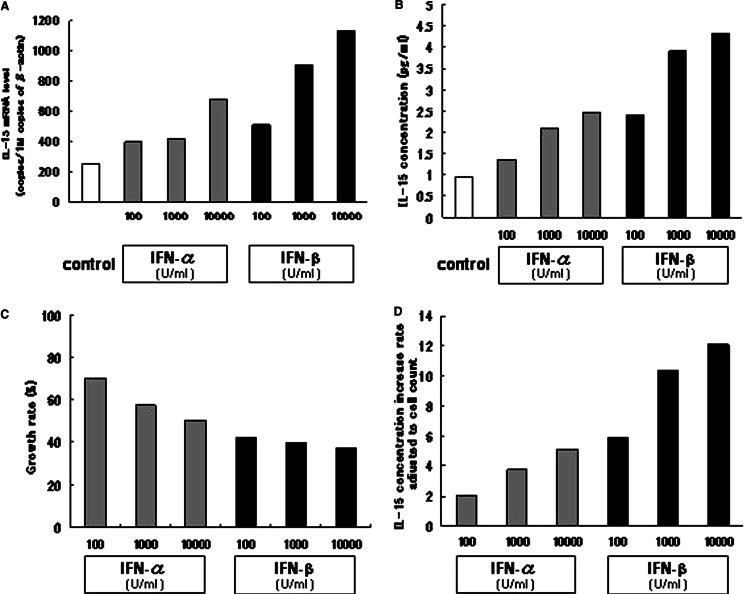
To determine if IFN-α and -β upregulate IL-15 transcription in a HCC cell line, IL-15 mRNA expression level was quantified by RT-PCR in Huh7 cells cultured for 72 h with various concentrations of IFN-α or -β. The β-actin mRNA expression level was also determined for use in adjusting the IL-15 mRNA expression level (Fig. 1a). In comparison with the controls, the IL-15 mRNA level increased in Huh7 cells cultured with IFN-α or -β. This IL-15 increase in the Huh7 cells cultured with both IFNs was dose-dependent in the range from 100 U/ml to 10,000 U/ml of IFN concentration. The IL-15 transcription induction activity was higher in IFN-β than in IFN-α, when compared at the same concentration. These data suggest that IFN-α/β upregulated IL-15 gene transcription in this Huh7 cell line.
Fig. 1.
The effects on IL-15 expression in Huh7 cells of IFN-α and -β at various concentrations. Huh7 cells were cultured for 72 h with or without IFN-α/β at consentrations of 100, 1,000, or 10,000 U/ml. a The IL-15 Expression level and the β-actin gene were quantified by reverse-transcription real-time PCR. The IL-15 mRNA expression level was adjusted to the β-actin mRNA expression level. b The IL-15 concentration in the culture supernatant was quantified by ELISA. c Cell numbers were counted by flow cytometry after detachment with trypsinization, and the cell growth rate of IFN-treated cells in comparison with the controls was calculated. d The supernatant IL-15 concentration was adjusted to the cell number, and the IL-15 production increase rate of IFN-treated cells in comparison with the controls was calculated. All experiments were repeated three times and representative results are depicted
To verify IL-15 upreguration of type I IFNs at the protein level, IL-15 concentration in the supernatant of the Huh7 culture was determined by ELISA. Huh7 cells were cultured with or without IFNs at various concentrations. After a 72 h-culture, the IL-15 concentration in the supernatant was examined by ELISA. Figure 1b shows the IL-15 concentration in the culture supernatant of each condition. As expected from the results of IL-15 mRNA quantification, the IL-15 concentration increased in comparison with the controls in the supernatants of the Huh7 cells cultured with IFN-α or -β. The IL-15 concentration dose-dependently increased in both the IFN-α and -β cultures, and the concentration was higher in the IFN-β than in the IFN-α culture.
The Huh7 cell number was determined by flow cytometry after a 72-h culture period to eliminate the possibility that the increase in IL-15 concentration in Huh7 culture with IFN-α/β might have been caused by an increase in cell number. The cell count data showed that type I IFNs suppressed Huh7 proliferation in a dose dependent manner and that the suppression was stronger in Huh7 cultured with IFN-β than with IFN-α (Fig. 1c). Moreover, the IL-15 concentration of the culture supernatant was adjusted to the cell number of the corresponding culture, and the ratio of IFN-treated conditions to controls was calculated (Fig. 1d). Because the adjustment reflects the IL-15 production level of each cell, it could be shown conclusively that IFNs promote IL-15 production from Huh7 cells. Thus, these results confirmed that IFN-α/β upregulate IL-15 production from Huh7 cells.
We also examined the IL-15 mRNA expression level of Huh7 cells cultured with IFN at different time points. Huh7 cells were cultured with or without 1,000 U/ml of IFN-α/β, the cells were harvested at 24, 48 and 72 h, and the IL-15 mRNA expression level was determined (Fig. 2a). A control culture without IFN showed almost the same IL-15 mRNA expression level throughout the period of observation. In the cells cultured with IFN-α, the IL-15 mRNA level increased at 24 h, and maintained this level to 72 h In the cells cultured with IFN-β, the IL-15 mRNA level also increased at 24 h. However, an even higher level was noted at 72 h. These data suggest that upregulation of IL-15 transcription occurs within 24 h after the start of stimulation by either IFN-α or -β, but that the manner of IL-15 induction may differ between IFN-α and -β.
Fig. 2.
The effects of IFN-α and -β on IL-15 expression in Huh7 cells at various time points. Huh7 cells were cultured with or without 1,000 U/ml IFN-α/β for 24, 48, or 72 h. a IL-15 and β-actin gene expression levels were quantified by reverse-transcription real-time PCR. The IL-15 mRNA expression level was adjusted to the β-actin mRNA expression levels. b The IL-15 concentration in the culture supernatant was quantified by ELISA. c Cell numbers were counted by flow cytometry after detachment with trypsinization, and the cell growth rate of IFN-treated cells in comparison with the controls was calculated. d The supernatant IL-15 concentration was adjusted to the cell number, and the IL-15 production increase rate of IFN-treated cells in comparison with the controls was calculated. All experiments were repeated three times and representative results are depicted
The IL-15 concentration of the supernatant was determined, by ELISA, in a Huh7 culture with or without 1,000 U/ml of IFN at 24, 48, and 72 h. At each time point, the IL-15 concentration was higher in Huh7 cultured with IFN than in the control (Fig. 2b). The Huh7 cell-growth rate was determined by cell count, and the data showed that Huh7 cell-growth was time-dependently suppressed by IFNs (Fig. 2c). To compare the IL-15 production level in each cell among different culture conditions, we adjusted the IL-15 concentration of the culture supernatant to the cell number. Figure 2d shows the IL-15 production increase rate of IFN-treated cells in comparison with the controls. At each time point, an increase in the IL-15 level was observed, even after adjustment to the cell number, and the value was higher in the culture with IFN-β than in that with IFN-α. As observed in mRNA quantification, the increase in IL-15 production from cells cultured with IFN-β continued at 72 h. These results confirm that both IFN-α and -β increase IL-15 production from Huh7 cells within 24 h and that the IFN-β activity is stronger than that of IFN-α.
IFN-α and -β upregulattion of IL-15 production in other HCC cell lines
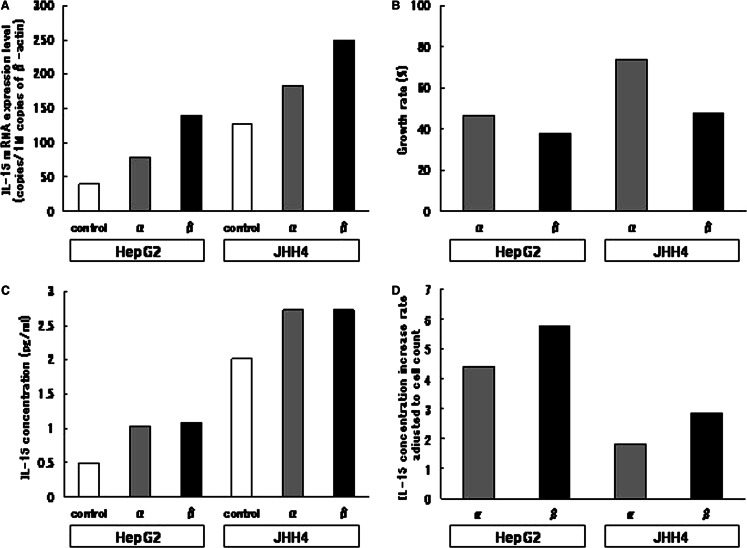
To clarify if type I IFNs upregulate IL-15 expression in other HCC cell lines, we quantified IL-15 mRNA expressed in HepG2 and JHH4 cells cultured with or without type I IFNs. The cells were cultured with or without IFN-α or -β at a concentration of 1,000 U/ml and, after a 72-h culture, the IL-15 mRNA expression level was determined by RT-PCR. Figure 3a shows the IL-15 mRNA levels of HepG2 and JHH4 cells after culture with or without type I IFNs. In both types of cells, the IL-15 mRNA expression level was increased by type I IFNs and, at same concentration, IFN-β showed stronger IL-15 mRNA expression induction activity than IFN-α.
Fig. 3.
The effects of IFN-α and -β on IL-15 expression in HepG2 and JHH4 cells. HepG2 cells and JHH4 cells were cultured with or without 1,000 U/ml IFN-α/β for 72 h. a Expression levels of IL-15 and β-actin gene were quantified by reverse-transcription real-time PCR. The IL-15 mRNA expression level was adjusted to the β-actin mRNA expression level. b Cell numbers were counted by flow cytometry after detachment with trypsinization, and the cell growth rate of IFN-treated cells in comparison with the controls was calculated. c The IL-15 concentration in culture supernatant was quantified by ELISA. d The supernatant IL-15 concentration was adjusted to the cell number, and the IL-15 production increase rate of IFN-treated cells in comparison with the controls was calculated. All experiments were repeated three times and representative results are depicted
The IL-15 concentration of the supernatant and the number of cells were determined by ELISA and flow cytometry, respectively, in the experiments on HepG2 and JHH4 cultured for 72 h with or without type I IFNs at a concentration of 1,000 U/ml (Fig. 3b, c). The IL-15 concentration in the supernatant of both types of cells cultured with type I IFNs, increased in comparison with their respective controls and was higher in cells cultured with IFN-β than IFN-α. A suppression of cell growth was observed in both types of cells cultured with type I IFNs, and the suppression activity of IFN-β was stronger than that of IFN-α. Figure 3d shows the cell count-adjusted IL-15 level, which reflects IL-15 production from each cell. The adjusted IL-15 data clearly showed that HepG2 and JHH4 cells cultured with IFN-β produced more IL-15 than those cultured with IFN-α. These results indicate that type I IFNs upregulate IL-15 production, not only in Huh7 but also in other HCC cell lines such as HepG2 and JHH4.
IL-15 mRNA expression in liver biopsy samples from chronic hepatitis C patients
To investigate if IL-15 is expressed in human liver, we examined IL-15 mRNA expression in liver biopsy samples obtained from six chronic hepatitis C patients. The amplification of IL-15 transcript by PCR showed that IL-15 mRNA was expressed in all the liver biopsy samples examined (Fig. 4a). We also quantified IL-15 transcripts in liver samples, and the data adjusted by β-actin mRNA expression levels is shown in Fig. 4b. The IL-15 mRNA expression level in the liver showed little valiation between the samples, but generally, it was similar to that of Huh7 cells. These results suggest that IL-15 is expressed in the human liver.
Fig. 4.
Interleukin-15 mRNA expression in liver biopsy samples of chronic hepatitis C patients. a The IL-15 transcripts were amplified from the liver biopsy samples of six patients by reverse-transcription PCR. DEPC-treated water was used as the negative control. lanes 1–6; liver biopsy samples, lane 7; negative control, M; DNA marker. b Expression levels of IL-15 and the β-actin gene were quantified by reverse-transcription real-time PCR. The IL-15 mRNA expression level was adjusted to the β-actin mRNA expression level
Increased serum IL-15 level in chronic hepatitis C patients during Type I IFN treatment
To investigate the induction of IL-15 production by type I IFNs in vivo, we examined alterations of the serum IL-15 level in 21 chronic hepatitis C patients during treatment with IFN-α or -β. Table 1 shows the clinical characteristics of 11 patients treated with IFN-α, 10 patients treated with IFN-β, and 29 control subjects. No significant difference in sex or age was found between the control subjects and chronic hepatitis C patients. No significant pretreatment difference in IL-15 level (Fig. 5) was found between the samples from controls and the chronic hepatitis C patients. In the chronic hepatitis C patients, the IL-15 level significantly increased during the IFN treatment period in comparison with pretreatment (week 2; P<0.0001, week 4; P<0.0001, end of treatment; P<0.0001). At 6 months after cessation of treatment, the serum IL-15 had returned to a level similar to the pretreatment. No significant differences in the serum IL-15 level of patients by type of IFN received or by virological or biochemical response were found during the observation period (data not shown). These results suggest that both IFN-α and -β increased the serum IL-15 level of the chronic hepatitis C patients during the administration period.
Table 1.
Clinical characteristics of 21 patients treated with IFN-α or -β and 29 control subjects
Fig. 5.
The serum IL-15 level of IFN-treated patients with type C chronic liver disease and controls. The IL-15 level was quantified by ELISA. Unpaired Student’s t-test was used to assess the statistical significance of differences in serum IL-15 levels between sera from controls and HCV-infected patients before treatment. Paired Student’s t-test was used to compare serially assayed serum IL-15 of the HCV-infected patients. Circles indicate the individual serum IL-15 levels of the controls (open circle) and HCV-infected patients (closed circle). Horizontal bars show mean ± SD in each sample group. *P<0.0001
Discussion
Interleukin-15 is considered a key factor for both innate and adaptive immune response. IL-15 expression has been implicated in clinical studies to be associated with graft rejection in transplantations[34–36] and with synovial T cell activation in rheumatoid arthritis[37, 38], suggesting that IL-15 plays an important role in local immune reaction. It has also been reported that IL-15 enhances antitumor immune response by NK cells and CTLs [39–42], and it is expected that IL-15 can be applied to antitumor immunotherapy in the future [43, 44]. The present study revealed that IFN-α and -β upregulate IL-15 production from HCC cell lines in vitro. The finding suggests a possibility that type I IFNs may induce IL-15 production in HCC cells in vivo leading to proliferation and activation of surrounding immune cells, such as NK and CTL. It has been demonstrated that some cancer cells can regulate immune response and lead to inhibition of antitumor immunity [45]. Type I IFNs may elicit the opposite immune regulatory effect, i.e. activation of antitumor immunity through IL-15 production from cancer cells. This may be one of the mechanisms contributing to the antitumor effect of IFN-α and -β.
The serum IL-15 levels of the chronic hepatitis C patients in this study significantly increased during the IFN administration period, indicating that IFN-α and -β stimulate IL-15 production in humans in vivo. In the present study, IL-15 production was increased in human HCC cell lines by IFN-α/β, and a comparable level of IL-15 mRNA expression was observed in liver biopsy samples. It has been reported that multiple STAT signals are activated by IFN-α in hepatocytes [46]. The IL-15 promotor includes two enhancer elements, the binding sites of NF-κB and IRF-1 which are transcription factors in the downstream of type I IFN signals [47]. These findings led us to believe that IFN-α and -β upregulate IL-15 production in hepatocytes in vivo, which in turn contributes to the elevation of serum IL-15 in chronic hepatitis C patients during IFN administration. It is also possible that IL-15 production is increased in immune cells, although a recent report suggested that type I IFN-mediated production of IL-15 was impaired in monocyte-derived dendritic cells of chronic hepatitis C patients[48]. Which cells or tissues were involved in type I IFN-mediated production of IL-15 in vivo remains unknown. The present study, however, demonstrated that type I IFNs upregulate IL-15 production in vivo, and the results suggest that IL-15 is associated with type I IFN-induced immune response.
In the present study, in vitro IFN-β showed a stronger activity than IFN-α in IL-15 upregulation of HCC cell lines. IFN-β also inhibited the growth of HCC cell lines more efficiently than IFN-α, as reported previously [49]. It is unclear what causes these differences between IFN-α and -β, since IFN-α and -β bind to the same receptor. The receptor, type I IFN-R, is composed of at least two subunits; α chain, IFNAR1 and β subunit, IFNAR2 which has short (βS) and long (βL) forms. In a previous report, it was shown that IFN-α2 and IFN-β require a distinct intracytoplasmic region of the βL subunit for their antiviral response, suggesting activation of a distinct signaling pathway by IFN-β [50]. In another report, it was shown that expression of CXCL11 (alias β-R1, I-TAC), a CXC chemokine ligand for CXCR3, was selectively induced by IFN-β, but not by IFN-α in a fibrosarcoma cell line [51, 52]. These findings suggested differences in biological activity between IFN-α and -β, and that it may be associated with the differences in the level of activity in IL-15 upregulation. We have also reported differences in clinical outcome between patients treated with IFN-α and -β. Sustained virological response was predicted by disappearance of HCV viremia early in the course of IFN administration in patients treated with IFN-α [53], but not in patients treated with IFN-β [11]. Moreover, by IFN-α treatment, the risk of HCC was significantly reduced in chronic hepatitis C patients with a biochemical response. In contrast, in patients treated with IFN-β, there was no difference in the incidence of HCC between patients with and without biochemical response, and there was a tendency for the risk to be reduced irrespective of the biochemical response of patients treated with IFN-β [14]. Thus, differences in biological activity between IFN-α and -β have been suggested by both basic and clinical studies, and these differences may be associated with the differences in the level of activity in IL-15 upregulation.
There was no significant difference in the serum IL-15 level of the patients treated with IFN-α and -β during the administration period. One possible explanation is that the administration routes are different for IFN-α and -β, i.e. intramuscular and intravenous injection, respectively. This difference would affect the drug concentration in tissues including liver. Another possibility is varied response to type I IFN among different cell types, or that only some cells or organs show differences in IL-15 expression response as between IFN-α and -β, resulting in the differences being overshadowed by other aspects of systemic response in vivo.
The present study provided the first evidence that type I IFN induces IL-15 production in vitro in HCC cell lines and also suggests a mechanism by which IL-15 production from non-immune cells mediates IFN-α/β-induced immune response. Our study also demonstrated that IFN-α and -β induce IL-15 production in human in vivo. These findings may help us better understand the immune regulatory mechanism of IFN-α/β and its implication in IL-15 expression. However, the precise roles of IFN-α/β are still unclear and further study is needed to clarify these issues. Such study will be useful for the development of selective and effective therapies for viral infection and cancer.
References
- 1.Alter HJ. To C or not to C: these are the questions. Blood. 1995;85:1681–1695. [PubMed] [Google Scholar]
- 2.Tong MJ, el-Farra NS, Reikes AR, et al. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Ikematsu H, Hirohata T, et al. Hepatitis C virus infection and risk of hepatocellular carcinoma among Japanese: possible role of type 1b (II) infection. J Natl Cancer Inst. 1996;88:742–746. doi: 10.1093/jnci/88.11.742. [DOI] [PubMed] [Google Scholar]
- 4.Tagger A, Donato F, Ribero ML, et al. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695–699. doi: 10.1002/(SICI)1097-0215(19990531)81:5<695::AID-IJC4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi J, Furusyo N, Ariyama I, et al. A relationship between the evolution of hepatitis C virus variants, liver damage, and hepatocellular carcinoma in patients with hepatitis C viremia. J Infect Dis. 2000;181:1523–1527. doi: 10.1086/315431. [DOI] [PubMed] [Google Scholar]
- 6.Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- 7.Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506–1510. doi: 10.1056/NEJM198911303212204. [DOI] [PubMed] [Google Scholar]
- 8.Kakumu S, Arao M, Yoshioka K, et al. Recombinant human alpha-interferon therapy for chronic non-A, non-B hepatitis: second report. Am J Gastroenterol. 1990;85:655–659. [PubMed] [Google Scholar]
- 9.Hayashi J, Ohmiya M, Kishihara Y, et al. A statistical analysis of predictive factors of response to human lymphoblastoid interferon in patients with chronic hepatitis C. Am J Gastroenterol. 1994;89:2151–2156. [PubMed] [Google Scholar]
- 10.Hayashi J, Kishihara Y, Ueno K, et al. Age-related response to interferon alfa treatment in women vs men with chronic hepatitis C virus infection. Arch Intern Med. 1998;158:177–181. doi: 10.1001/archinte.158.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Furusyo N, Hayashi J, Ohmiya M, et al. Differences between interferon-alpha and -beta treatment for patients with chronic hepatitis C virus infection. Dig Dis Sci. 1999;44:608–617. doi: 10.1023/A:1026625928117. [DOI] [PubMed] [Google Scholar]
- 12.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–1055. doi: 10.1016/S0140-6736(95)91739-X. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT study group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwagi K, Furusyo N, Kubo N, et al. A prospective comparison of the effect of interferon-alpha and interferon-beta treatment in patients with chronic hepatitis C on the incidence of hepatocellular carcinoma development. J Infect Chemother. 2003;9:333–340. doi: 10.1007/s10156-003-0271-5. [DOI] [PubMed] [Google Scholar]
- 15.Stark GR, Kerr IM, Williams BR, et al. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 16.Meurs EF, Galabru J, Barber GN, et al. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano H, Iemura A, Haramaki M, et al. Interferon alfa receptor expression and growth inhibition by interferon alfa in human liver cancer cell lines. Hepatology. 1999;29:1708–1717. doi: 10.1002/hep.510290624. [DOI] [PubMed] [Google Scholar]
- 18.Murphy D, Detjen KM, Welzel M, et al. Interferon-alpha delays S-phase progression in human hepatocellular carcinoma cells via inhibition of specific cyclin-dependent kinases. Hepatology. 2001;33:346–356. doi: 10.1053/jhep.2001.21749. [DOI] [PubMed] [Google Scholar]
- 19.Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 20.von Hoegen P. Synergistic role of type I interferons in the induction of protective cytotoxic T lymphocytes. Immunol Lett. 1995;47:157–162. doi: 10.1016/0165-2478(94)00208-9. [DOI] [PubMed] [Google Scholar]
- 21.Hiroishi K, Tuting T, Lotze MT. IFN-alpha-expressing tumor cells enhance generation and promote survival of tumor-specific CTLs. J Immunol. 2000;164:567–572. doi: 10.4049/jimmunol.164.2.567. [DOI] [PubMed] [Google Scholar]
- 22.Biron CA, Nguyen KB, Pien GC, et al. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 23.Ohzato H, Monden M, Yoshizaki K, et al. Systemic production of interleukin-6 following acute inflammation. Biochem Biophys Res Commun. 1993;197:1556–1562. doi: 10.1006/bbrc.1993.2655. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami Y, Hayashi J, Ueno K, et al. Elevation of serum soluble interleukin-2 receptor levels in patients with hepatitis C virus infection. Fukuoka Igaku Zasshi. 1997;88:274–282. [PubMed] [Google Scholar]
- 25.Mattei F, Schiavoni G, Belardelli F, et al. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 26.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 27.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharif-Askari E, Fawaz LM, Tran P, et al. Interleukin 15-mediated induction of cytotoxic effector cells capable of eliminating Epstein-Barr virus-transformed/immortalized lymphocytes in culture. J Natl Cancer Inst. 2001;93:1724–1732. doi: 10.1093/jnci/93.22.1724. [DOI] [PubMed] [Google Scholar]
- 30.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/S1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Sun S, Hwang I, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 32.Liu K, Catalfamo M, Li Y, et al. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci USA. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi J, Yoshimura E, Kishihara Y, et al. Hepatitis C virus RNA levels determined by branched DNA probe assay correlated with levels assessed using competitive PCR. Am J Gastroenterol. 1996;91:314–318. [PubMed] [Google Scholar]
- 34.Strehlau J, Pavlakis M, Lipman M, et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci USA. 1997;94:695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith XG, Bolton EM, Ruchatz H, et al. Selective blockade of IL-15 by soluble IL-15 receptor alpha-chain enhances cardiac allograft survival. J Immunol. 2000;165:3444–3450. doi: 10.4049/jimmunol.165.6.3444. [DOI] [PubMed] [Google Scholar]
- 36.Conti F, Frappier J, Dharancy S, et al. Interleukin-15 production during liver allograft rejection in humans. Transplantation. 2003;76:210–216. doi: 10.1097/01.TP.0000067530.95852.67. [DOI] [PubMed] [Google Scholar]
- 37.McInnes IB, al-Mughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 38.McInnes IB, Leung BP, Sturrock RD, et al. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 39.Lu J, Giuntoli RL, 2nd, Omiya R, et al. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3877–3884. [PubMed] [Google Scholar]
- 40.Lewko WM, Smith TL, Bowman DJ, et al. Interleukin-15 and the growth of tumor derived activated T-cells. Cancer Biother. 1995;10:13–20. doi: 10.1089/cbr.1995.10.13. [DOI] [PubMed] [Google Scholar]
- 41.Yajima T, Nishimura H, Ishimitsu R, et al. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168:1198–1203. doi: 10.4049/jimmunol.168.3.1198. [DOI] [PubMed] [Google Scholar]
- 42.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/S1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 44.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 46.Radaeva S, Jaruga B, Hong F, et al. Interferon-alpha activates multiple STAT signals and down-regulates c-Met in primary human hepatocytes. Gastroenterology. 2002;122:1020–1034. doi: 10.1053/gast.2002.32388. [DOI] [PubMed] [Google Scholar]
- 47.Azimi N, Shiramizu KM, Tagaya Y, et al. Viral activation of interleukin-15 (IL-15): characterization of a virus-inducible element in the IL-15 promoter region. J Virol. 2000;74:7338–7348. doi: 10.1128/JVI.74.16.7338-7348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jinushi M, Takehara T, Tatsumi T, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 49.Damdinsuren B, Nagano H, Sakon M, et al. Interferon-beta is more potent than interferon-alpha in inhibition of human hepatocellular carcinoma cell growth when used alone and in combination with anticancer drugs. Ann Surg Oncol. 2003;10:1184–1190. doi: 10.1245/ASO.2003.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Domanski P, Nadeau OW, Platanias LC, et al. Differential use of the betaL subunit of the type I interferon (IFN) receptor determines signaling specificity for IFNalpha2 and IFNbeta. J Biol Chem. 1998;273:3144–3147. doi: 10.1074/jbc.273.6.3144. [DOI] [PubMed] [Google Scholar]
- 51.Rani MR, Foster GR, Leung S, et al. Characterization of beta-R1, a gene that is selectively induced by interferon beta (IFN-beta) compared with IFN-alpha. J Biol Chem. 1996;271:22878–22884. doi: 10.1074/jbc.271.37.22878. [DOI] [PubMed] [Google Scholar]
- 52.Rani MR, Gauzzi C, Pellegrini S, et al. Induction of beta-R1/I-TAC by interferon-beta requires catalytically active TYK2. J Biol Chem. 1999;274:1891–1897. doi: 10.1074/jbc.274.4.1891. [DOI] [PubMed] [Google Scholar]
- 53.Yamaji K, Hayashi J, Kawakami Y, et al. Hepatitis C viral RNA status at two weeks of therapy predicts the eventual response. J Clin Gastroenterol. 1998;26:193–199. doi: 10.1097/00004836-199804000-00009. [DOI] [PubMed] [Google Scholar]