Abstract
To study DNA vaccination directed against human HER-2 in the HHD mouse Tg strain, we created a novel HER-2-expressing syngeneic tumor transplantation model. We found that a DNA vaccine encoding the full length HER-2 DNA protected HHD mice from HER-2+ tumor challenge by a CTL independent mechanism. A more efficient approach to induce HLA-A2 restricted CTLs, through immunization with a multi-epitope DNA vaccine expressing the HLA-A2 restricted HER-2 369–377, 435–443 and 689–697 epitopes, resulted in high numbers of peptide specific T cells but failed to induce tumor protection. Subsequently we discovered that HER-2 transfected tumor cells down-regulated MHC class I antigen expression and exhibited a series of defects in the antigen processing pathway which impaired the capacity to produce and display MHC class I peptide-ligands to specific CTLs. Our data demonstrate that HER-2 transfection is associated with defects in the MHC class I presentation pathway, which may be the underlying mechanism behind the inability of CTLs to recognize tumors in this HLA-A2 transgenic model. As defective MHC class I presentation may be a common characteristic of HER-2 expressing tumors, vaccines targeting HER-2 should aim at inducing an integrated immune response where also CD4+ T cells and antibodies are important components.
Keywords: DNA vaccine, HER-2, MHC class I, Antigen processing
Introduction
HER-2 is a 185-kDa glycoprotein member of the epidermal growth factor receptor family of tyrosine kinases which is over-expressed in 25–40% of all breast cancers and in a variety of other tumors such as ovarian, gastric and colorectal carcinomas. Due to the selective overexpression in malignant tissue, HER-2 is considered one of the most attractive targets for immunotherapeutic interventions [14].
DNA immunization is a powerful method to stimulate tumor antigen-specific immune responses directed against tumor associated antigens [9]. DNA-based vaccines directed against HER-2 have proven to be successful in the prevention of tumor growth in transplantable tumor models as well as in HER-2 transgenic mice [18, 24, 25, 33]. The induction of an effective HER-2 anti-tumor immunity seems to rely on different mechanisms depending on the tumor model, and there is evidence that both antibodies and CTLs play a role in HER-2 tumor protection after DNA vaccination [18, 25].
HHD mice are a valuable tool to study specific HLA-A2-restricted CTL responses, which may be relevant for human vaccine development. CTL responses directed against HER-2 have been analyzed in this model using peptide- or DC-based vaccines [1, 4], and some of the epitopes used in these studies have already been the research focus of several clinical trials [15, 22]. Here we wanted to evaluate the effects of HER-2 DNA vaccination in the HHD mouse model in terms of (1) generation of HER-2 specific CTLs and (2) the nature of the mechanism of tumor protection. For this purpose we generated a syngeneic HER-2-expressing tumor cell line which developed tumors in HHD mice, thus providing a suitable model to test the effect of HER-2 based tumor vaccines and allowing the analysis of HLA class I restricted CTLs in the tumor protection. Our results demonstrate that while a full length HER-2 pDNA vaccine protected mice from tumor challenge via a CTL independent, apparently antibody-mediated mechanism, a HER-2 “mini-gene” vaccine based on 3 HLA-A2 restricted HER-2 epitopes was unable to induce tumor protection, even when high numbers of specific CTLs were induced. This result argues strongly against a role for CTLs in tumor protection and prompted us to analyze the MHC class I and APM pathway in the syngeneic HHD mouse derived tumor cell lines. We found that HER-2 expression induces down regulation of MHC class I and APM components, as supported by previous studies [3, 10]. Our data show the functional consequences in vivo of HER-2-mediated down regulation of MHC class I and APM components, demonstrating that impaired processing and presentation of HLA-A2 peptide complex prevents tumor recognition by specific CTLs.
These findings underline the importance of designing vaccines which will induce both antibodies and CTLs to overcome HER-2 induced tumor resistance to specific T cell effector mechanisms.
Materials and methods
Plasmids and peptides
pDNA encoding HER-2 (pCMV/E2) and pCMV vector were kindly provided by Dr. Wei-Zen Wei (Karmanos Cancer Center, Detroit, MI, USA).
The plasmid vector pVAX/HER-2 encoding the mutated full length HER-2 was constructed as described [18]. The multi-epitope DNA vaccine designed as HER-2/mini-gene was constructed inserting into pVax (Invitrogen, Carlsbad, CA, USA) between EcoRI and XhoI sites the following DNA sequence: AA TTC GAG ATG GCG GCC GCT AAA ATC TTT GGT TCC CTG GCG TTT CTG GCA GCA GCA ATT CTG CAC AAT GGC GCC TAC TCG CTA GCA GCA GCG AGA CTG CTC CAG GAA ACG GAG CTC GTG GCG GCT GCG TTT CTG CCT TCT GAC TTC TTT CCC AGC GTT TAG C. The sequence translation is: M A A A K I F G S L A F L A A A I L H N G A Y S L A A A R L L Q E T E L V A A A F L P S D F F P S V and encodes for HER-2 369–377, 435–443, 689–697 epitopes, and an additional HBV core 18–27 control epitope. The AAA spacer between the epitopes is to allow a proper peptide processing [5]. E. coli (TOP10, Invitrogen) were transformed with this construct and the recombinant plasmid was extracted from the cultured bacteria using Qiagen MiniPrep Kit (Qiagen GMBH, Hilden, Germany) and sequenced.
In the Ub/HER-2 mini-gene, the sequence encoding for ubiquitin was added at the EcoRI site before the start codon of the sequence encoding the 4 epitopes. Plasmids needed for all immunizations were extracted from E. coli cultured in Luria-Bertani medium supplemented with 50 mg/l Kanamycin, using Qiagen GigaPrep Endofree Kit.
The HER-2-derived synthetic peptides 369–377 (KIFGSLAFL), 435–443 (ILHNGAYSL), 689–697 (RLLQETELV); the HBV core-derived 18–27 (FLPSDFFPSV) and the HIV1-gag derived 77–85 (SLYNTVATL) HLA-A2 restricted peptides were purchased from GenScript Corporation (Piscataway, NJ, USA). The peptides were dissolved in DMSO at 5 mM and stored at −20°C.
Mice and cell lines
The homozygote HHD mice [21] were kindly provided by Dr. F. A. Lemonnier. Mice were bred and maintained at the animal facilities of the Microbiology and Tumor Biology Center at the Karolinska Institute. All animal studies have been reviewed and approved by the Swedish National board for Laboratory Animals.
MC (methylcholanthrene) induced sarcomas generated in HHD mice were isolated and passaged in vitro as described previously [11]. The derived cell line, MC-2, was co-transfected with full length HER-2 pDNA (pCMV/E2) or pCMV and pCDNA3/neo, using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions.
The TAP-defective HLA-A2.1 T2 cell line derived from the human T cell leukemia/B cell LCL hybrid 174 was a gift from Dr. P. Cresswell (Yale University School of Medicine, New Haven, CT, USA). Cell lines were maintained in IMDM supplemented with 10% heat-inactivated FCS (GibcoBRL, Life Technologies, Grand Island, NY, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin (complete medium). Selective antibiotic G418 (Sigma Aldrich, St. Louis, MO, USA) was added at 800 μg/ml to the complete medium for the MC-2/HER-2 cell line culture.
DNA immunizations and tumor challenge
Six- to eight-week-old HHD mice received 2 immunizations at days 0 and 14 of full length HER-2 plasmid pVAX/HER-2 or 1 immunization of HER-2 mini-gene plasmids.
Mice, anaesthetized with 4% isofluorene, received 20 μg pDNA dissolved in 20 μl of PBS in each flank by intradermal injections, using a 29-gauge insulin-grade syringe (Micro-Fine U-100, BD Consumer Healthcare, Franklin Lakes, NJ, USA). Immediately after DNA administration, a needle array electrode was placed over the area of injection, and two groups of pulses of different voltages were applied [28]. The electrode and the PA-4000S–Advanced PulseAgile Rectangular Wave Electroporation System and software were purchased from Cyto Pulse Sciences, Inc. (Glen Burnie, MD, USA).
Challenges, blood sampling or spleen removal were performed 13 days after immunization (HER-2 minigene groups) or 2 weeks after the second immunization (pVAX/HER-2 groups). Mice were challenged s.c. in the right flank with 70,000 MC-2 or MC-2/HER-2 tumor cells. Tumors were measured twice a week by a caliper, and mean tumor volume was calculated. Animals were sacrificed when tumor volume reached 103 mm.
T cells depletion
To deplete CD8+ T cells, immunized mice were injected i.p. with 200 μg of anti-CD8 TIB 105 antibody (Mabtech, Nacka Strand, Sweden) 1 day before tumor challenge and then every three days thereafter until the completion of the experiment. Specific depletion was confirmed 10 days after the first injection by FACS analysis of splenocytes by staining with the CD8-α PE conjugated specific antibody (Becton Dickinson, Sparks, MD, USA).
Ex vivo detection of peptide-specific CD8+ T cells
Blood samples from the tail vein were collected in the presence of CPD-A anticoagulant (Sigma-Aldrich). Red blood cells were lysed with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2–7.4). Effector cells (106/well) were stimulated for 4 h in a V-bottom 96 wells plate with MC-2 or MC-2/HER-2 cells (105/well), irrelevant or relevant peptide (100 nm) and PMA/ionomycin (Sigma-Aldrich) as positive control. Specific CD8+ T cells were quantified by ICCS for IFN-γ using BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit with BD GolgiPlug™ according to the manufacturer’s instructions.
The following antibodies were used for stainings: FITC-labeled anti-mouse CD8α and PE-labeled anti-mouse IFN-γ (Becton Dickinson). Purified rat IgGs (Sigma-Aldrich) diluted 1:10 in PBS were added before starting the staining steps in order to block non-specific binding. The samples were acquired with a FACSCalibur cytometer and analyzed with CellQuest Pro software (both from Becton Dickinson).
In vitro restimulation of peptide-specific T cells
Splenocytes (42 × 106/14 ml/T25 bottle) were cultured in the presence of 1 μg/ml of peptides in complete medium supplemented with 1 mM 2-mercaptoethanol, 1% non essential amino acids and 1% sodium pyruvate for 5 days. The T cells were harvested 5 days later and tested for peptide or tumor specificity in ICCS as described above.
Cytotoxicity assays
Standard 4-h 51Cr-release assays were performed as previously described [31]. 51Cr-release in the supernatants was measured by a γ-counter (Wallac Sverige AB, Stockholm, Sweden).
Flow cytometry analysis and antibody detection
The RPE-labeled HLA-ABC specific antibody W6/32 and relevant isotype control (IgG2a) were obtained from DAKOPATTS AB (Älvsjö, Sweden); the mouse monoclonal anti HER-2 specific antibody conjugated to PE and the IgG1-PE conjugated isotype control were purchased from BD Biosciences Pharmingen (San Diego, CA, USA).
Cells were incubated with an excess of the relevant antibody or with isotype control for 30 min on ice, washed twice and analyzed by flow cytometry (Becton Dickinson, Mountain View, CA, USA).
For the measurement of anti HER-2/neu antibodies in mice sera, anti HER-2 IgG were measured by a flow cytometry assay. Serum samples were diluted 1:50, added to MC-2 or MC-2/HER-2 cells and left to incubate 30 min on ice. Cells were washed and stained with FITC-conjugated rabbit anti-mouse IgG antibody and with FITC-conjugated rabbit IgG1 isotype control (Becton-Dickinson). The MFI was measured with a FACS-Calibur cytometer.
Western blot analysis
For Western blot analysis cell pellets were lysed in electrophoresis sample buffer. Aliquots of total cell lysates corresponding to 105 cells were separated by SDS-PAGE followed by transfer onto nitro-cellulose membrane (Millipore AB, Sweden). Membranes were blocked in PBS containing 5% milk and 0.1% Tween-20 and probed with the specific antibodies. The mouse mAb clone W6/32 [32] directed against HLA ABC was purchased from Serotec (Oxford, UK) and the mouse mAb HC10 recognizing HLA class I heavy chains was a kind gift of Dr. Soldano Ferrone (Hillman Cancer Center, Pittsburgh, USA). Rabbit anti-mouse IgGs conjugated to horseradish peroxidase (Amersham Pharmacia Biotech AB, Uppsala, Sweden) were used as secondary antibodies. The reaction was visualized by enhanced chemiluminescence according to the manufacturers protocol (Amersham Pharmacia Biotech AB, Uppsala, Sweden).
RT-PCR analysis
Total cellular RNA from MC2 and MC2/Her-2 cells was isolated using the Qiagen kit and then subjected to real time RT-PCR using the following mouse primers: β-actin (5′ TCT GCT GGA AGG TGG ACA GT 3′ and 5′ CCT CTA TGC CAA CAC AGT GC 3′; 187 bp), Tapasin (5′ ACA CTG CGA GAT GAG CCG CTT C 3′ and 5′ TGA GGA CGG TCA GCA CCA CTG T 3′; 221 bp), TAP1 (5′ TGC CTA AGA AGC TGG GAA AA 3′ and 5′ GTA AGC CAA GGC CTC CTT CT 3′; 202 bp), TAP2 (5′ CGG TGC TAA AGG AGA TCC AG 3′ and 5′ CCA TCA CCC TCC GTA TGA CT 3′; 203 bp), PSX (5′ TCC CAG ACG GTG AAG AAA GT 3′ and 5′ CCC CAT GCC TTT GTA CTG AT 3′; 200 bp), PSY (5′ TGA CCA AGG ACG AAT GTC TG 3′ and 5′ CCT AAA ACA CCA GGC CTC AG 3′; 194 bp), PSZ (5′ GCA ACT GAA GGG ATG GTT GT 3′ and 5′ ATC TGC TTC AGC ATC CGA TT 3′; 199 bp), LMP2 (5′ TCT TCT GTG CCC TCT CAG GT 3′ and 5′ TGG TCC CAG CCA GCT ACT AT 3′; 193 bp), LMP7 (5′ GGA ACG CAT CTC CGT GTC TG 3′ and 5′ CTG CCG GTA ACC ACT GTC CA 3′; 223 bp), LMP10 (5′ ACC CAC ATG GTT CCT ACA GC 3′ and 5′ GTG ATC ACA CAG GCA TCC AC 3′; 196 bp), β2-microglobulin (5′ GCC GAA CAT ACT GAA CTG CT 3′ and 5′ GCC ATA CTG GCA TGC TTA AC 3′; 207 bp), PA28-α (5′ GGA GCC AGC TCT CAA TGA AG 3′ and 5′ GTT GCA GGA GGA CCA CAA TC 3′; 212 bp), PA28-β (5′ CTT CTC AGA ACG AGG GGA TG 3′ and 5′ TCG GGT TGA CGA TTT TCT CT 3′; 190 bp), EERAP (5′ CAC AGA AGG CTG GGA TTT TC 3′ and 5′ GGG TTT CTG CCA ATG AGT GT 3′; 194 bp), calnexin (5′ GCC TGA AGA TTG GGA TGA AA 3′ and 5′ CAA TCC TCT GGC TTC TCT GC 3′; 177 bp) and calreticulin (5′ GGA AGA CTG GGA TGA ACG AG 3′ and 5′ TCA ATT TGA CGT GGT TTC CA 3′; 194 bp).
Statistics
Comparison of data for statistical analysis was performed with Single Factor ANOVA with a significance level of at least P ≤ 0.05.
Results
A human HER-2 DNA vaccine protects HHD mice from HER-2 positive tumor challenge
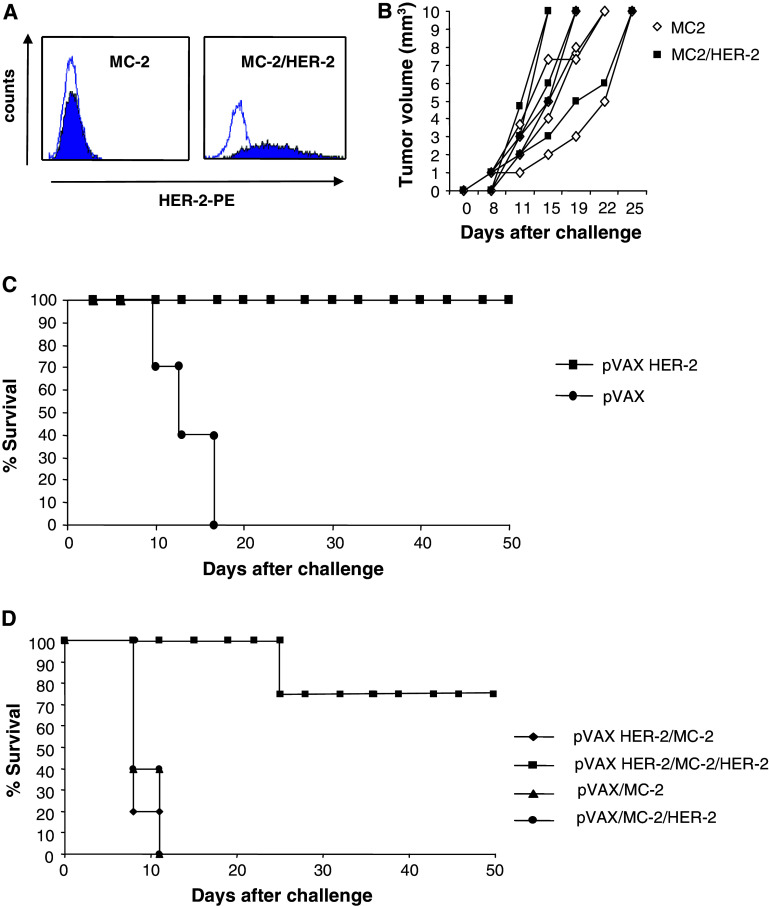
To study the effect of HER-2 specific DNA vaccination in the HHD mouse strain, we generated a syngeneic HER-2 positive transplantable tumor which expresses both the chimeric HHD molecule and HER-2. To this end, a primary MC-induced tumor was induced in HHD mice and the in vitro derived MC cell line was transfected with a full length HER-2 plasmid or with an “empty” vector plasmid. HER-2 expression in the MC-2/HER-2 cell line was confirmed by flow cytometry (Fig. 1a). When naïve HHD mice were given a s.c. tumor challenge of either MC-2 or MC-2/HER-2 cells, the two tumors grew at the same rate in 100% of the animals (Fig. 1b) which allowed us to test the efficacy of HER-2 DNA vaccines in this new HHD tumor model. When HHD mice were immunized twice by i.d. injection of a DNA plasmid encoding a non-transforming full length human HER-2 vaccine (pVAX/HER-2) [18] followed by electroporation and then challenged subcutaneously with MC-2/HER-2 tumor cells, all mice were protected (Fig. 1c). To examine whether the tumor protective immune response was HER-2 specific, a group of HHD mice received the same DNA vaccine before challenge with MC-2 or MC2/HER-2 tumor cells. As shown in Fig. 1d, 80% of full length HER-2 vaccinated mice were completely protected against challenge with the HER-2 positive MC-2/HER-2 cell line while no protection was observed in the group challenged with MC-2. We therefore surmise that it is possible to induce a specific and strong tumor protection in this new HHD tumor model.
Fig. 1.
Tumor protection induced by HER-2 plasmid vaccination in the HHD syngeneic tumor transplantation model. a Surface HER-2 expression by the transplantable tumor cell lines MC-2 and MC-2/HER-2 was measured by flow cytometry using the HER-2 specific antibody or relevant isotype control. b HHD mice (n = 5/group) were given a s.c. tumor challenge with 70.000 MC-2 or MC-2/HER-2 cells, and animals were monitored for the development of palpable tumors. c HHD mice were immunized two times at 2-week intervals by i.d. injection and electroporation with HER-2 plasmid (n = 12 mice/group) or pVAX control vector (n = 12 mice/group). Fourteen days after the final immunization, mice were challenged with 70.000 MC-2/HER-2 cells s.c. in the right flank. d HHD mice (n = 12/group) vaccinated with HER-2 plasmid or pVAX control were challenged with MC-2 or MC-2/HER-2 tumor cells. The data shown are combined from two separate experiments
Tumor protection is independent of CTLs and associated with induction of HER-2 specific antibodies
We asked whether CTL responses specific for 3 HLA-A2 restricted HER-2 derived epitopes known to be naturally processed on HER-2 expressing tumors (HER-2 369–377, HER-2 435–443 and HER-2 689–697) [7, 13, 27] were involved in the tumor protection of HHD HER-2 vaccinated mice. We did not detect significant levels of IFN-γ secretion by CD8+ T cells upon restimulation with HER-2 specific peptides or tumor cells directly ex vivo (Fig. 2a). After 5 days of culture the number of peptide specific CD8+ T cells increased significantly against all 3 HLA-A2-restricted HER-2 peptides (Fig. 2b) in the pVAX/HER-2 vaccinated mice but not in the pVAX control mice. Thus HER-2 vaccination appears to generate a T cell response against these peptides, demonstrating that all 3 epitopes are correctly processed and presented in vivo upon vaccination with pVAX/HER-2. These T-cell cultures were however not able to recognize the HER-2+-HLA-A2+ tumor cells (Fig. 2b).
Fig. 2.
CTLs do not contribute to tumor protection induced by HER-2 plasmid vaccination. HHD mice (n = 6/group) received two immunizations at 2-week intervals with pVAX control (grey square) or HER-2 plasmid (filled square) by i.d. injection and electroporation. a Fourteen days after the second immunization blood was collected from individual mice and pooled. Effector cells were stimulated for 4 h either with 100 nM of peptides or with MC-2 and MC-2/HER-2 cells. The activated CD8+ T cells were quantified by intracellular cytokine staining for IFN-γ and analyzed by flow cytometry. b Fourteen days after the second immunization splenocytes from the same mice were re-stimulated for 5 days in vitro with the different HER-2-derived peptides. Each CTL culture was tested against relevant-peptide, HIV-derived irrelevant peptide and MC-2 or MC-2/HER-2 cells as described in A. Mean ± SD of three independent experiments is shown for a and b. c HHD mice (n = 6/group) immunized twice with pVAX or HER-2 plasmid received anti-CD8 Ab (TIB 105) 1 day before and 2, 6, 9 and 12 days after challenge with MC-2/HER-2 tumor cells. d HHD mice (n = 10/group) were immunized two times at 2-week intervals with control vector or HER-2 DNA as indicated. Sera were collected after the second vaccination and diluted 50-fold with PBS. Anti-HER-2 specific Abs were measured using a secondary Ab specific for murine IgG by flow cytometry using MC-2/HER-2 cells. Whole anti-HER-2 IgG was detected. The amount of anti-HER-2 Abs in each group is expressed as MFI (***P < 0.001)
To further investigate the role of CD8+ T cells in our tumor model, the pVAX/HER-2 vaccinated HHD mice were depleted of CD8+ T cells 1 day before tumor challenge. Immune responses induced by tumor challenge in pVAX/HER-2 immunized mice in the absence of CD8+ T cells were as protective against tumor as those induced in their presence, indicating that the tumor protection in this model is not dependent on CTLs (Fig. 2c). The same result was obtained after depletion of CD4+ T cells or NK cells 1 day before tumor challenge (data not shown) suggesting that these T cell subsets is not involved in tumor protection at the effector stage. Taken together, these data indicate that tumor protection after pVAX/HER-2 vaccination is independent of CD8+ or CD4+ effector T cells.
We then examined if vaccination induced HER-2 specific antibodies. Serum samples from pVAX/HER-2 vaccinated mice, but not from pVAX vaccinated ones, collected 2 weeks after the first vaccination contained high levels of anti-HER-2 specific antibodies (Fig. 2d). We therefore conclude that although vaccination with the full length HER-2 pDNA vaccine induces low levels of specific CTLs, these appear not to be necessary for the tumor protection, and that high levels of specific antibodies are induced in the vaccinated mice.
A multi-epitope HER-2 DNA vaccine induces high numbers of HER-2 peptide-specific CTLs but fails to elicit tumor reactive CTLs in vitro and tumor protection in vivo
Since these data showed that a full length HER-2 DNA vaccine was unable to confer tumor protection in HHD mice by a CTL dependent mechanism, we next asked if a more efficient approach of inducing HLA-A2 restricted CD8+ T cell responses would be able to do so. To this end, we designed a multi-epitope DNA vaccine encoding the amino acid sequence of the 3 HLA-A2-restricted HER-2 derived epitopes 369–377, 435–443 and 689–697 and additionally encoding an HBV-derived HLA-A2 restricted epitope as a positive control (HER-2 mini-gene). In order to improve the processing of the translated DNA, we generated another construct with the same sequence and in addition encoding for Ubiquitin (Ub/HER-2 mini-gene) [17] (Fig. 3a). The multi-epitope vaccine was administered once since in preliminary experiments we have shown that the highest CTL number is obtained after one vaccination (data not shown). Immunization with this Ub/HER-2 mini-gene plasmid efficiently induced peptide-specific CTL responses as observed both ex vivo and in CTL cultures stimulated with peptide for 5 days, whereas immunization with the corresponding non-ubiquinated HER-2 construct was unable to do so (Fig. 3b, c). The Ub/HER-2 construct elicited much higher peptide-specific CD8+ T cell responses as compared to the full length HER-2 DNA vaccine which did not induce any ex vivo reactivity. Between 2 and 8% of CD8+ T cells in peripheral blood from the Ub/HER-2 immunized mice were HER-2 peptide-specific after 4 h of peptide stimulation and after 5 days of in vitro restimulation the frequency increased to 25–80%. However, the same CD8+ T cells were unable to recognize the MC-2/HER-2 tumor line (Fig. 3b, c). Similar results were obtained when these in vitro restimulated and HER-2 peptide specific CD8+ T cells were tested in a 4-h cytotoxicity assay (Fig. 4).
Fig. 3.
Effect of HER-2 and Ub/HER-2 mini-gene plasmids vaccination. a The multi-epitope HER-2 mini-genes were constructed inserting into a pVAX vector, between EcoRI and XhoI sites, the sequences encoding the three HER-2 CTL epitopes p369, p435, p689 and a HBV dominant epitope, with (1) or without (2) an Ubiquitin coding sequence at the 5′ end. HHD mice (n = 6/group) received one immunization with pVAX empty vector (open square), HER-2 mini-gene (grey square) or Ub/HER-2 mini-gene (filled square) by i.d. injection and electroporation. b Thirteen days after the immunization blood was collected from individual mice and pooled. Effector cells were stimulated for 4 h with 100 nM of peptides or with MC-2 and MC-2/HER-2 cells. The activated CD8+ T cells were quantified by intracellular cytokine staining for IFN-γ and analyzed by flow cytometry. c Fourteen days after the immunization splenocytes from the same mice were re-stimulated for 5 days in vitro with the different HER-2-derived peptides. Each CTL culture was tested against relevant-peptide, irrelevant-peptide and MC-2 or MC-2/HER-2 cells as described in b. Mean ± SD of three independent experiments is shown for b and c. d Plot of tumor-free survival in HHD mice (n = 12/group) immunized as described in Materials and methods and subsequently challenged with MC-2 or MC-2/HER-2 tumor cells. The data shown are combined from two separate experiments
Fig. 4.
CTLs generated by Ub/HER-2 mini-gene vaccination are cytolytic. CTL cultures were generated from splenocytes of Ub/HER-2 mini-gene immunized mice restimulated 5 days in vitro with the different HER-2-derived peptides. Each CTL culture was tested against T2 cells (a) or MC-2 cells (b) pre-pulsed with relevant or irrelevant peptides or against MC-2 and MC-2/HER-2 tumor cells (c). One representative experiment out of three is shown, and mean ± SD of the triplicates is indicated
The high efficiency by which the Ub/HER-2 mini-gene induced peptide-specific CTLs compared to the inefficiency of the non-ubiquitinated HER-2 clearly showed that ubiquitin is an absolute requirement for efficient generation of CTL responses, probably by allowing correct processing of the peptides. Despite the induction of high numbers of CTLs specific for all three HLA-A2 restricted HER-2 peptides, no tumor protection was observed after Ub/HER-2 mini-gene vaccination (Fig. 3d). When we tested serum samples from HER-2 mini-gene or Ub/HER-2 mini-gene vaccinated mice for the presence of HER-2 specific antibodies they were negative (data not shown). Hence, also vaccination with this “mini gene” vaccine demonstrates that CTLs are not sufficient for tumor protection in this HHD model.
The HER-2 transfected, but not the control transfected MC-2 tumor, displays multiple defects in the MHC class I pathway
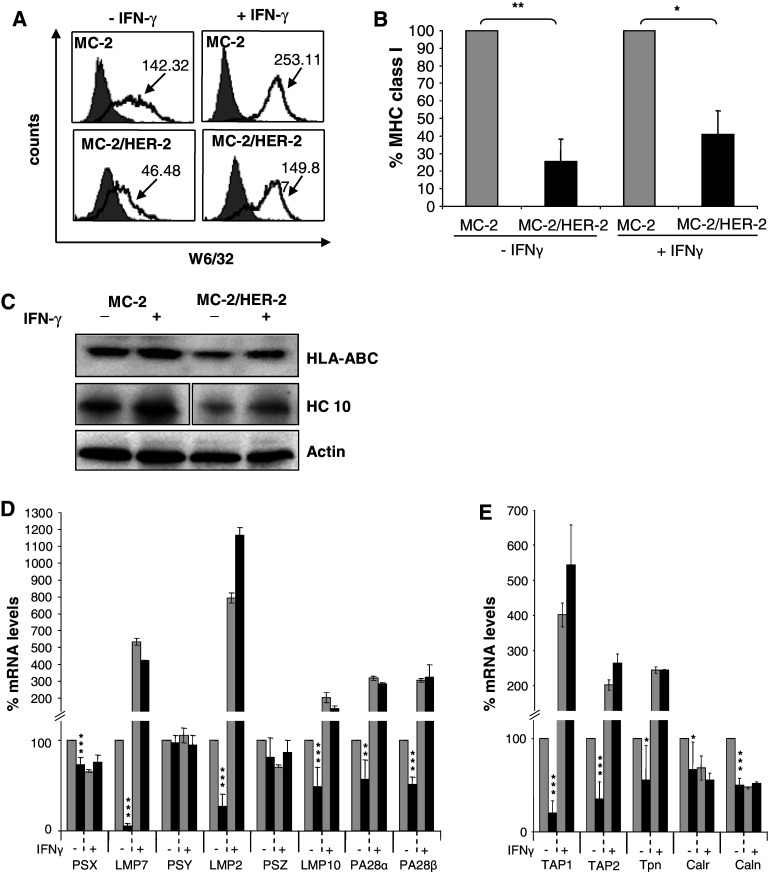
The lack of recognition of the MC-2/HER-2 tumor even in the presence of high numbers of peptide-specific T cells lead us to ask whether this tumor had a decreased HLA class I antigen expression, which may impair the display of HLA-A2 restricted HER-2 epitopes. We found that MC-2/HER-2 cells expressed very low levels of cell surface and intracellular MHC class I antigen as measured by flow cytometry and Western Blot, respectively, compared to the control transfectant MC-2 (Fig. 5a–c). In order to investigate whether the deficient MHC class I antigen surface expression on MC-2/HER-2 cells would be associated with down-regulation of different components of the antigen processing pathway, the MC-2/HER-2 transfectants and the MC-2 were analyzed for the expression of the constitutive and IFN-γ inducible proteasome subunits and proteasome activator PA28, the peptide transporters TAP1 and TAP2 as well as chaperones such as calnexin, calreticulin and tapasin, using real time RT-PCR analysis. As demonstrated in Fig. 5d, HER-2 over-expression strongly diminished the expression of the IFN-γ inducible subunits, LMP2, LMP7 and LMP10, and PA28α and β, whereas the constitutive proteasome subunits (X, Y, Z) were not (Y, Z) or much less (X) affected. In addition, TAP1, TAP2, tapasin (Tpn), calreticulin (Calr) and calnexin (Caln) were also significantly suppressed in these cells. Nevertheless, the down-regulation of MHC class I and APM components could be restored by IFN-γ treatment, whereas the constitutive proteasome subunits were not affected upon treatment (Fig. 5d). These findings confirm our earlier observations that HER-2 expression affects MHC class I and APM pathway [3, 10, 12]. We produced another tumor cell line from an independent HER-2 plasmid transfection. Since this transfectant showed the same defects in MHC class I and APM (data not shown), this argues against the possibility of an artifact due to clonal selection upon pDNA transfection.
Fig. 5.
Down regulation of MHC class I and APM components in MC-2/HER-2 cells. a MHC class I expression at the cell surface of MC-2 and MC-2/HER-2 cells untreated or treated with 500 U/ml of IFN-γ for 48 h was measured by staining with the W6/32 mAb and analyzed by flow cytometry. One representative experiment out of three is shown. b MC-2/HER-2 MHC class I level is expressed as a percentage compared to MC-2 class I expression considered as 100%. Mean ± SD of three independent experiments (*P < 0.01, **P < 0.001) is shown. c The expression of MHC class I in total cell lysates of MC-2 and MC-2/HER-2 cells was monitored by Western blot before and after IFN-γ treatment. Expression of β-actin was used as a control for loading. d Total RNA from MC-2 (grey square) or MC-2/HER-2 (filled square) cells either untreated or treated with 500 U/ml IFN-γ for 48 h was extracted and subjected to RT-PCR analysis using the APM-specific primers as described in the section “Materials and methods”. Mean ± SD of three independent experiments is shown (*P < 0.05, **P < 0.01, ***P < 0.001)
Peptide-loaded MC-2/HER-2 tumor cells exhibit impaired ability to sensitize T-cells compared to MC-2 cells
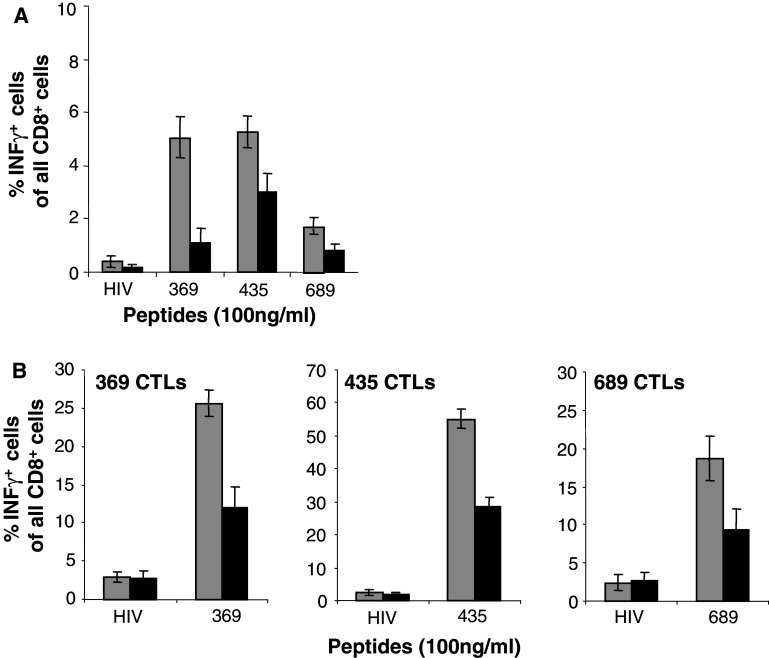
We asked if the lower MHC class I antigen expression could affect the capacity of MC-2/HER-2 cells to present exogenously loaded peptides. For this reason MC-2 or MC-2/HER-2 cells pre-pulsed with the different HLA-A2 HER-2 peptides were used as stimulators to induce IFN-γ production by splenocytes from Ub/HER-2 mini-gene immunized mice. MC-2/HER-2 cells prepulsed with HER-2 peptides were less efficient in activation of T cells ex vivo (Fig. 6a). The same results were obtained using MC-2 and MC-2/HER-2 cells pre-pulsed with peptides for induction of IFN-γ production by 5 days peptide-restimulated CTL cultures (Fig. 6b).
Fig. 6.
Impaired presentation by MC-2/HER-2 cells of HLA-A2 restricted epitopes. MC-2 (grey square) or MC-2/HER-2 (filled square) cells were pre-pulsed for 1 h at 37°C with control HIV or relevant HER-2 peptides and subsequently used to stimulate for 4 h ex vivo isolated (a) or 5 days in vitro peptide-re-stimulated splenocytes (b) from Ub/HER-2 mini-gene immunized HHD mice. The activated CD8+ T cells were quantified by intracellular cytokine staining for IFN-γ and analyzed by flow cytometry. Mean ± SD of three independent experiments is shown
Discussion
Here we investigated the effects of DNA vaccination in (1) the generation of HLA-A2-restricted HER-2-specific CTL responses and in (2) the induction of tumor protection in HHD mice. The results, contrary to our initial expectations, demonstrate that protection in this model is independent of CTLs. Furthermore, we confirm our earlier findings that HER-2 induces multiple defects in antigen presentation.
Earlier results have demonstrated the contribution of both humoral and cellular components induced by full-length or truncated HER-2 pDNA vaccines, as analyzed in transgenic or orthotopic mouse tumor models [18, 24]. In these models the evidence for MHC class I restricted CTLs in protection is however scarce. Yet, HLA-A2-restricted HER-2-derived T cell epitopes have been identified which are naturally processed and presented by various tumor types including ovarian, breast and renal cell carcinoma [2, 16, 30] and these epitopes can be efficiently recognized by tumor specific-CTLs [2, 27]. Some of these epitopes have been used for immunization of HHD mice in peptide, DC-based, or DNA-based vaccines. Multiple immunizations with DC pulsed with the 369–377 or 773–782 peptides in the presence of IL-2 retarded tumor growth in HLA-A2 × neu tg mice [20]. Vaccination of HHD mice with a HER-2-derived peptide 435–443 increased the frequency of HER-2-peptide-specific CTLs and also induced protective and therapeutic immunity against a transplantable tumor cell line transfected to co-express HLA-A2 and HER-2 [8]. Vaccinating HHD mice with an anti-HER2 poly-epitope DNA construct induced a broad CTL-based immune response, which correlated with a delay in tumor onset upon EL-4/HHD/ErbB2 thymoma tumor cell line challenge [29].
In the current investigation, we have produced the first example of a syngeneic HER-2-expressing tumor in the HHD mouse strain. To efficiently target a HER-2-specific DNA vaccine into the MHC class I processing pathway we adopted the poly-epitope mini-gene approach where single T cell epitopes are linked together in a string-of-beads fashion [17]. In our study we designed a plasmid encoding 3 HER-2 immunogenic peptides (p369, p435, p689) and a second plasmid containing a ubiquitin monomer fused with the sequence encoding the 3 HER-2 peptides to enhance proteosomal delivery [26]. These 3 epitopes were chosen as they were earlier shown to be immunodominant and naturally processed in several different human and mouse tumors [14], and two of the epitopes (p369 and p689) were used in the poly-epitope DNA vaccine used in the study by Scardino et al. [29]. A high number of HER-2 peptide specific CTLs was induced by the ubiquitin containing version of this construct, but not by the non-ubiquitinated one, yet no tumor protection was observed.
We hypothesized that the HER-2 epitopes encoded for by this vaccine may not have been efficiently processed on the MC-2/HER-2 tumor cells. Several observations indicate that HER-2 expression is associated with an immune escape phenotype. Spontaneous mammary carcinomas from transgenic animals expressing the rat HER-2/neu proto-oncogene have lost or down-regulated MHC class I antigen surface expression which could be corrected by IFN-γ treatment [19]. In line with these findings, murine osteosarcoma tumor cell lines which expressed mouse HER-2/neu were poorly recognized by CTLs, associated with inefficient antigen processing which could be enhanced by IFN-γ treatment [12]. Furthermore, HER-2 over-expression in murine fibroblast lines resulted in approximately 70% down-regulation of surface MHC class I antigen expression, attributed to impaired functional expression of TAP, tapasin, and the LMP proteasome subunits [10]. Also, a study by Choudhury et al. [3] showed that silencing the HER-2 gene by siRNA in HER-2 + tumor cell lines in vitro increases the expression of HLA class I. When we, in the present study, analyzed MHC class I and APM components in the HER-2 + tumor cell line compared to the HER-2- one, we found a strong down regulation of the APM components which could be restored by IFN-γ treatment. More significantly, we show for the first time that the defects in APM components displayed by the HER-2 positive tumor hamper the generation of HLA-A2-restricted HER-2-derived epitopes, therefore preventing recognition by specific T cells.
These findings may seem at odds with those where tumor protective CTLs were induced against defined HER-2 epitopes, as reviewed above, but several possible explanations may account for these discrepant findings. The effects of HER-2 expression on antigen presentation may vary between different types of tumors. As an example, we have failed to observe an effect of HER-2 on MHC class I antigen expression by the D2F2E2 mammary carcinoma [33]. Also, the method of vaccination and the resulting quality of CTLs may be of importance. As the CTLs induced by the ubiquitinated “mini gene” in the present study were able to recognize peptides down to the nano-molar concentration (data not shown), generation of low avidity T cells is not likely to explain the failure of tumor recognition.
We have recently found that the effect on MHC class I antigen presentation may be observed only when a critical “threshold” of HER-2 is reached, while in tumor cell lines which express HER-2 at intermediate levels, no or low effects on MHC class 1 are present (manuscript in preparation). The high levels of HER-2 in our MC-2/HER-2 cells may therefore more efficiently have down-regulated antigen presentation as compared to other published studies.
Seemingly discordant outcomes can be observed also comparing results from clinical trials in which HER-2 peptide-specific CTLs were obtained in patients upon vaccination. Peoples et al. [23] recently showed how they could elicit p369-specific CTLs in previously treated, disease free, nodule-positive breast cancer patients, and in turn obtain a significant increase in the disease-free survival. These results are in contrast with a study by Zaks and Rosenberg [34] where immunization of a group of HLA-A2+ HER-2 overexpressing tumor patients with the HER-2 p369 leads to generation of peptide-specific cytotoxic T cells that failed to recognize HER-2+ tumors. In this study IFN-γ treatment of the tumor cells failed to confer reactivity by p369-reactive-T cells.
In contrast, the full length HER-2 pDNA vaccine in our study efficiently protected mice from tumor challenge, arguing against the possibility that the MC-HER-2 tumor is inherently resistant to immune rejection. Also this full length HER-2 construct induced CTLs, but at considerably lower level as compared to the “mini-gene” construct vaccine, demonstrating that a full-length HER-2 molecule is naturally processed by the MHC class I pathway. Yet, depletion of CTLs in vivo did not affect tumor rejection, proving that the protective mechanism is CTL independent. All the immunized mice developed high levels of HER-2 specific antibodies, arguing for the possible involvement of an antibody mediated mechanism in the protection. Since mice with defects in B cells or Fc receptors are not available on the HHD mouse background, the importance of antibodies could however not be finally evaluated.
Regardless of the underlying mechanism of HER-2 induced defects in antigen presentation, the present study argues for the advantage of a HER-2 based vaccination method that targets both MHC class I and II dependent pathways, which will efficiently induce both a T cell and an antibody response. Clinical trials based on longer HER-2 peptides which contain both MHC class I and II epitopes [6] or HER-2 pDNA full length or truncated constructs may therefore have an advantage as compared to administration of short CTL peptide epitopes.
Acknowledgments
This work was supported by grants to R. K from the Swedish Cancer Society, the Cancer Society of Stockholm, the European Union (Grants "ENACT" and "DC-THERA"), the Karolinska Institutet, and "ALF-Project" grants from the Stockholm City Council.
Abbreviations
- APM
Antigen processing machinery
- HER-2
ErbB-2
- HHD
HLA-A2-Db
- ICCS
Intra-cellular cytokine staining
- MC
Methylcholanthrene
Footnotes
S. Vertuani and C. Triulzi contributed equally to this work.
References
- 1.Baxevanis CN, Sotiriadou NN, Gritzapis AD, Sotiropoulou PA, Perez SA, Cacoullos NT, Papamichail M. Immunogenic HER-2/neu peptides as tumor vaccines. Cancer Immunol Immunother. 2006;1:85–95. doi: 10.1007/s00262-005-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brossart P, Stuhler G, Flad T, Stevanovic S, Rammensee HG, Kanz L, Brugger W. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 1998;4:732–736. [PubMed] [Google Scholar]
- 3.Choudhury A, Charo J, Parapuram SK, Hunt RC, Hunt DM, Seliger B, Kiessling R. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer. 2004;1:71–77. doi: 10.1002/ijc.11497. [DOI] [PubMed] [Google Scholar]
- 4.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4–1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;6:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 5.Del Val M, Schlicht HJ, Ruppert T, Reddehase MJ, Koszinowski UH. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991;6:1145–1153. doi: 10.1016/0092-8674(91)90037-Y. [DOI] [PubMed] [Google Scholar]
- 6.Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol. 2004;5:571–578. doi: 10.1023/B:JOCI.0000040928.67495.52. [DOI] [PubMed] [Google Scholar]
- 7.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;6:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gritzapis AD, Mahaira LG, Perez SA, Cacoullos NT, Papamichail M, Baxevanis CN. Vaccination with Human HER-2/neu (435–443) CTL peptide induces effective antitumor immunity against HER-2/neu-expressing tumor cells in vivo. Cancer Res. 2006;10:5452–5460. doi: 10.1158/0008-5472.CAN-05-4018. [DOI] [PubMed] [Google Scholar]
- 9.Haupt K, Roggendorf M, Mann K. The potential of DNA vaccination against tumor-associated antigens for antitumor therapy. Exp Biol Med (Maywood) 2002;4:227–237. doi: 10.1177/153537020222700403. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;1:215–220. doi: 10.1158/0008-5472.CAN-2522-2. [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi S, Petersson M, Nakazawa T, Kanda M, Zea AH, Ochoa AC, Kiessling R. Primary chemically induced tumors induce profound immunosuppression concomitant with apoptosis and alterations in signal transduction in T cells and NK cells. Cancer Res. 1999;12:2950–2956. [PubMed] [Google Scholar]
- 12.Kaplan BL, Norell H, Callender GG, Ohlum T, Kiessling R, Nishimura MI. Interferon-gamma renders tumors that express low levels of Her-2/neu sensitive to cytotoxic T cells. Cancer Immunol Immunother. 2006;6:653–662. doi: 10.1007/s00262-005-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawashima I, Hudson SJ, Tsai V, Southwood S, Takesako K, Appella E, Sette A, Celis E. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum Immunol. 1998;1:1–14. doi: 10.1016/S0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 14.Kiessling R, Wei WZ, Herrmann F, Lindencrona JA, Choudhury A, Kono K, Seliger B (2002) Cellular immunity to the Her-2/neu protooncogene. Adv Cancer Res 101–144 [DOI] [PubMed]
- 15.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;4:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kono K, Halapi E, Hising C, Petersson M, Gerdin E, Vanky F, Kiessling R. Mechanisms of escape from CD8+ T-cell clones specific for the HER-2/neu proto-oncogene expressed in ovarian carcinomas: related and unrelated to decreased MHC class 1 expression. Int J Cancer. 1997;1:112–119. doi: 10.1002/(SICI)1097-0215(19970106)70:1<112::AID-IJC17>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Leifert JA, Rodriguez-Carreno MP, Rodriguez F, Whitton JL (2004) Targeting plasmid-encoded proteins to the antigen presentation pathways. Immunol Rev 40–53 [DOI] [PubMed]
- 18.Lindencrona JA, Preiss S, Kammertoens T, Schuler T, Piechocki M, Wei WZ, Seliger B, Blankenstein T, Kiessling R. CD4+ T cell-mediated HER-2/neu-specific tumor rejection in the absence of B cells. Int J Cancer. 2004;2:259–264. doi: 10.1002/ijc.11654. [DOI] [PubMed] [Google Scholar]
- 19.Lollini PL, Nicoletti G, Landuzzi L, De Giovanni C, Rossi I, Di Carlo E, Musiani P, Muller WJ, Nanni P. Down regulation of major histocompatibility complex class I expression in mammary carcinoma of HER-2/neu transgenic mice. Int J Cancer. 1998;6:937–941. doi: 10.1002/(SICI)1097-0215(19980911)77:6<937::AID-IJC24>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;3:752–761. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 21.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2 m) HLA-A2.1 monochain transgenic H-2Db beta2 m double knockout mice. J Exp Med. 1997;12:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;30:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Peoples GE, Holmes JP, Hueman MT, Mittendorf EA, Amin A, Khoo S, Dehqanzada ZA, Gurney JM, Woll MM, Ryan GB, Storrer CE, Craig D, Ioannides CG, Ponniah S. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;3:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 24.Piechocki MP, Ho YS, Pilon S, Wei WZ. Human ErbB-2 (Her-2) transgenic mice: a model system for testing Her-2 based vaccines. J Immunol. 2003;11:5787–5794. doi: 10.4049/jimmunol.171.11.5787. [DOI] [PubMed] [Google Scholar]
- 25.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;8:2858–2864. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez F, An LL, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller JT, Kincaid C, Campbell IL, Whitton JL. DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;6:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiessling R. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol. 1999;2:1037–1044. [PubMed] [Google Scholar]
- 28.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;2:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Scardino A, Alimandi M, Correale P, Smith SG, Bei R, Firat H, Cusi MG, Faure O, Graf-Dubois S, Cencioni G, Marrocco J, Chouaib S, Lemonnier FA, Jackson AM, Kosmatopoulos K. A polyepitope DNA vaccine targeted to Her-2/ErbB-2 elicits a broad range of human and murine CTL effectors to protect against tumor challenge. Cancer Res. 2007;14:7028–7036. doi: 10.1158/0008-5472.CAN-06-3998. [DOI] [PubMed] [Google Scholar]
- 30.Seliger B, Rongcun Y, Atkins D, Hammers S, Huber C, Storkel S, Kiessling R. HER-2/neu is expressed in human renal cell carcinoma at heterogeneous levels independently of tumor grading and staging and can be recognized by HLA-A2.1-restricted cytotoxic T lymphocytes. Int J Cancer. 2000;3:349–359. doi: 10.1002/1097-0215(20000801)87:3<349::AID-IJC7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Torsteinsdottir S, Masucci MG, Ehlin-Henriksson B, Brautbar C, Ben Bassat H, Klein G, Klein E. Differentiation-dependent sensitivity of human B-cell-derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci USA. 1986;15:5620–5624. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran TM, Ivanyi P, Hilgert I, Brdicka T, Pla M, Breur B, Flieger M, Ivaskova E, Horejsi V. The epitope recognized by pan-HLA class I-reactive monoclonal antibody W6/32 and its relationship to unusual stability of the HLA-B27/beta2-microglobulin complex. Immunogenetics. 2001;6:440–446. doi: 10.1007/s002510100353. [DOI] [PubMed] [Google Scholar]
- 33.Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;5:748–754. doi: 10.1002/(SICI)1097-0215(19990531)81:5<748::AID-IJC14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;21:4902–4908. [PubMed] [Google Scholar]