Abstract
The aim of this study is to identify the phenotype of resistant oral tumors, and to delineate the contribution of immune effectors to resistance of oral tumors. UCLA-1 oral tumors which were resistant to NK cell mediated cytotoxicity secreted increased amounts of IL-6, IL-1β, GM-CSF, and IL-8 when cultured with or without immune effectors. In addition, the levels of vascular endothelial growth factor (VEGF) secretion in the co-cultures of naïve immune effectors with UCLA-1 rose significantly when compared to tumor cells alone. IL-2 activated NK cells decreased VEGF secretion in all tumor cells. However, NK cells which were induced to undergo cell death with anti-CD16 antibody were not only unable to decrease VEGF secretion, but they also contributed further to the increase in VEGF secretion by oral tumors. Overall, we show in this paper that naïve as well as non-viable immune effectors may contribute to the growth and resistance of oral tumors by triggering the secretion of key tumor cell growth factors.
Keywords: NK cells, IFN-γ, IL6, UM-SCC-1, Primary oral tumors, VEGF
Introduction
Survival of patients with head and neck tumors has not improved in the last several decades despite numerous technological advances in surgical procedures and the availability of well-designed radio and chemotherapeutic modalities. Failure to stop tumor growth and prevent tumor progression has been hypothesized to relate in part to a generalized immune dysfunction observed in patients [3, 16, 25–27]. Deficiencies in the functions of main immune effectors such as T, NK and dendritic cells (DC) have been suggested to account for decreased immunity and increased tumor survival and metastasis [20]. However, the mechanisms by which immune effectors become incapacitated or the tumor cells become more resistant and aggressive have not been fully explored. It is quite clear that immune effectors can either promote or inhibit tumor progression depending on the status of their activation and the nature of cytokines they produce. Indeed, effective tumor immunity, and clearance of many tumors have been suggested to relate to the activation and promotion of Th1 type cytokine profiles such as IL-2 and IFN-γ, whereas Th2 type cytokine secretion has been attributed to tumor progression and lack of effective immunity [1, 2, 15, 19]. However, it is likely that elements contributed by both the immune effectors and the tumor cells are responsible for immune inactivation and increased survival of transformed tumor cells.
Increased secretion of interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-1β (IL-1β), granulocyte monocyte-colony stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF) has been shown to be the profile of resistant or progressing oral tumors [5, 17, 23]. Many of the above mentioned factors have been shown to inhibit the function of immune effectors. Indeed, our recent work established that NFκB is an important modulator of immune function in tumor cells [11, 13]. Inhibition of NFκB in tumor cells was found to increase the function of immune effectors through decreased secretion of IL-6 [11]. Therefore, blocking NFκB in oral tumors switched the immune-inhibitory profile to an immune-enhancing profile in the co-cultures of immune effectors with oral tumors by reversing the ratio between the secreted IL-6 and IFN-γ. Furthermore, significant decrease in the secretion of IL-8, IL-1β and GM-CSF in addition to IL-6 could be seen when NFκB was inhibited in oral tumors (please see below). Indeed, the ability of either tumor cells or immune cells to upregulate NFκB in the co-cultures of tumor cells with immune effectors will determine which cell population will eventually survive and proliferate. However, despite this knowledge base many important questions still remain unanswered with regard to survival of either immune effectors or tumor cells. It is still unclear whether immune effectors which are not able to eliminate tumor cells either contribute to or inhibit tumor progression or whether they do not play an important role in the course of tumor progression. Recent in vivo studies indicated that both the nature of the tumor cells and their interacting myeloid immune effectors have significant roles in determining the size and the extent of tumor progression [6]. However, the details of such interaction have not been established.
In this paper we present evidence that immune effectors, including cytotoxic cells, can be contributory or inhibitory to tumor survival and proliferation depending on whether the tumor cells are resistant or sensitive to the cytotoxic function of immune effectors. Since VEGF is primarily secreted by tumor cells and not immune effectors, by using the secreted levels of VEGF in the co-cultures of immune effectors with tumor cells we have found a contributory role for the function of naïve immune effectors to the resistance of oral tumor cells, whereas in sensitive tumor cells the contribution of naïve effectors to the resistance of the tumors remains lower.
Materials and methods
Cell lines, reagents, and antibodies
RPMI 1640 supplemented with 10% FCS was used for the cultures of NKs and peripheral blood mononuclear cells (PBMCs). UCLA-1 and UCLA-2 primary oral tumors were prepared by Dr. Christian Head at UCLA using freshly resected squamous cell carcinoma tongue tumors and were cultured in RPMI 1640 supplemented with 10% FCS. UM-SCC-1 oral tumor lines were a generous gift from Dr. Christian Head and were cultured in DMEM supplemented with 1% sodium pyruvate, 1% non-essential amino acids, 1% glutamine, 1% penicillin-streptomycin. Recombinant IL-2 was obtained from Hoffman La Roche, NJ. The NK purification kit was obtained from Milteny Biotech (Auburn, CA).
Isolation of PBMCs and NK cells
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated as described before [9, 12]. Purified NK cells were negatively isolated using an NK purification kit (Miltenyi Biotech) and consisted of 85–95% of CD16+ cells. Approximately 10–15% of NK cell population was previously shown to have no or low CD16 expression on the surface of NK cells [14]. The percentage of CD3+ T and CD19+ B cells determined by anti-CD3 and anti-CD19 antibodies in the purified NK samples was 2.3 ± 3.2 and 3 ± 4, respectively. These levels of antibody staining in purified NK samples were also observed after staining with isotype control antibodies indicating the levels of nonspecific binding of antibodies to purified NK cells.
ELISA, surface staining, 51Cr release assay and DNA staining of ethanol fixed NK cells for apoptosis were all described previously [10].
Multiplex cytokine array
Fluorokine MAP cytokine multiplex kits were purchased from R&D Systems and the procedures were conducted as suggested by the manufacturer. To analyze and obtain the cytokine concentration, a standard curve was generated by threefold dilution of recombinant cytokines provided by the manufacturer. Analysis was performed using the software provided by Luminex.
Statistics
Statistical analyses were performed using a Student’s t test, with significance levels set at P < 0.05.
Results
Characteristics of NK resistant versus sensitive primary oral tumor cells
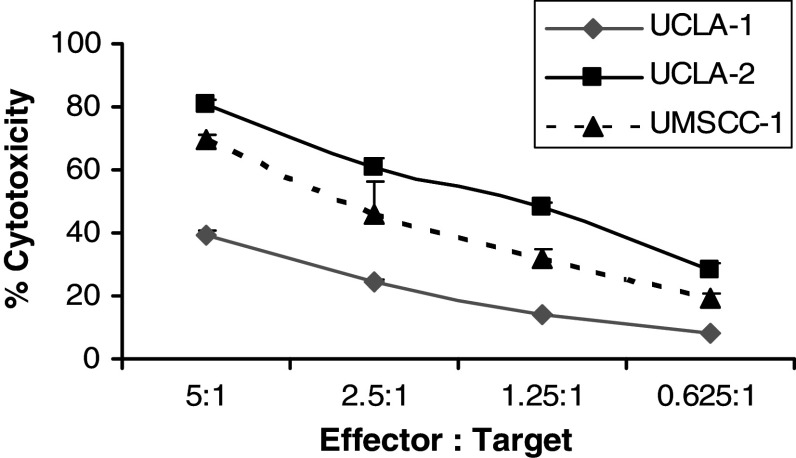
To establish the characteristics of NK cell sensitive and resistant oral tumors, we tested several established tumor lines in addition to primary oral tumors obtained from patients with oral cancers. Based on the profiles obtained for NK cell resistance we selected two primary oral tumors (UCLA-1 and UCLA-2) and one established cell line (UM-SCC-1) for subsequent studies (Fig. 1 and Table 1). UCLA-1 was found to be relatively resistant to NK cell cytotoxicity as compared to NK sensitive UCLA-2 and UM-SCC-1 cells (Fig. 1 and Table 1). Likewise, UCLA-1 triggered lower secretion of IFN-γ from NK cells when compared to either UCLA-2 or UM-SCC-1 cells (Table 1). Only IL-2 activated NK cells were able to lyse or secrete IFN-γ when co-cultured in the presence of oral tumors (Table 1). Similarly, only IL-2 activated PBMCs were able to lyse or secrete IFN-γ significantly when co-cultured with oral tumors (Table 1). However, as expected the levels of cytotoxicity or IFN-γ secretion were much lower in PBMCs than in NK cells (Table 1). Untreated PBMCs or NK cells were unable to mediate significant cytotoxicity or secretion of IFN-γ when co-cultured in the presence of all the oral tumor cells tested (Table 1).
Fig. 1.
Increased cytotoxicity of IL-2 treated PBMCs against UCLA-2 and UM-SCC-1 but not UCLA-1 oral tumors. PBMCs were treated with IL-2 (500 units/ml) for 8–12 h before they were added to 51Cr labeled oral tumors at the effector to target ratios indicated in the figure. After 4–6 h of incubation at 37°C, supernatants were harvested and the levels of released 51Cr radioactivity were determined by a γ counter. One of ten representative experiments is shown in this figure
Table 1.
Inability to lyse oral tumors results in synergistic induction of VEGF secretion by oral tumor cells
| Lytic units (30/10 7) | Interferon-γ levels (pg/ml) | VEGF levels (pg/ml) | |
|---|---|---|---|
| UCLA-1 alone (resistant) | – | 0 | 625.4 ± 6.7 (100%) |
| +PBMC control | 0.06 ± 0.01 | 0.87 ± 0.3 | 1,211.5 ± 28.7 (194%) |
| +PBMC IL-2 | 2.3 ± 0.1 | 399 ± 16.1 | 878.4 ± 57 (140%) |
| +NK control | 3.3 ± 0.0 | 0 | 799 ± 21.2 (128%) |
| +NK IL-2 | 27.85 ± 0.8 | 887.5 ± 95 | 145.25 ± 0.35 (23%) |
| UCLA-2 alone (sensitive) | – | 0 | 1,781.7 ± 51.4 (100%) |
| +PBMC control | 0.3 ± 0.06 | 5.15 ± 0.91 | 1,585 ± 262 (89%) |
| +PBMC IL-2 | 4.73 ± 0.1 | 988 ± 32 | 1,210.9 ± 64.6 (68%) |
| +NK control | 9.5 ± 0.0 | 0 | 1,281.4 ± 35.8 (72%) |
| +NK IL-2 | 153 ± 19.8 | 1,561.7 ± 238 | 166.5 ± 12.0 (9.3%) |
| UM-SCC-1 alone (sensitive) | – | 0 | 832.3 ± 46.6 (100%) |
| +PBMC control | 0.3 ± 0.05 | 1.7 ± 0.0 | 925.1 ± 53.6 (111%) |
| +PBMC IL-2 | 3.7 ± 0.3 | 993.6 ± 72 | 457.1 ± 31.6 (55%) |
| +NK control | 3.3 ± 0.0 | 0 | 338.3 ± 31 (41%) |
| +NK IL-2 | 89.75 ± 16 | 1,211.2 ± 312 | 75.1 ± 3.5 (9%) |
| PBMC control | – | 0 | 25.04 |
| PBMC IL-2 | – | 342 ± 14 | 12.5 |
| NK control | – | 0 | 0 |
| NK IL-2 | – | 289 ± 16 | 12.5 |
PBMCs and NK cells were left untreated or treated with IL-2 (500 units/ml) for 8–12 h before they were added to primary oral tumors at an effector to target ratio of 10:1 and 5:1 respectively. The levels of VEGF and IFN-γ secretion in each sample were determined using a multiplex ELISA array kit specific for each cytokine. PBMC and NK cell cytotoxicity was determined using a standard 51Cr release assay and the lytic units 30/107 were determined using the inverse number of effectors required to lyse 30% of the tumor cells X 100. Data is presented as mean ± SD. Percentages in parenthesis represent relative increase or decrease in VEGF secretion compared to each tumor cell alone. Differences were significant at P value of <0.05 between the control and IL-2 activated immune effector function (cytotoxicity and IFN-γ secretion) against all tumor lines. Similarly, P < 0.05 is for the difference in VEGF secretion between UCLA-1 tumors alone and those co-cultured in the presence of naïve immune effectors with UCLA-1 tumor cells. One of eight representative experiments is shown in this table
Contribution of naïve immune effectors to induction of VEGF secretion by NK cell resistant oral tumors
Untreated or IL-2 treated PBMCs and purified NK cells were unable to secrete significant amounts of VEGF (Table 1). In contrast, oral tumors secreted significant amounts of VEGF in the supernatants (Table 1). Untreated PBMCs and NK cells contributed substantially to the secretion of VEGF by NK resistant but not NK sensitive oral tumors cells (Table 1). Indeed, nearly two fold increase in tumor cell mediated VEGF secretion could be observed when untreated PBMCs were co-cultured with NK resistant tumor cells whereas either equal or lower amounts of VEGF secretion could be observed in the supernatants obtained from the co-cultures of NK sensitive oral tumors with naïve immune effectors as compared to those secreted only by the tumor cells alone (Table 1). In all cases IL-2 activated NK cells were able to decrease the secreted amounts of VEGF from oral tumors, however, the levels of secreted VEGF remained higher in NK cell resistant tumors co-cultured with IL-2 treated PBMCs or NK cells when compared to those co-cultured with NK cell sensitive oral tumors (Table 1). Therefore, there is a direct relationship between lysis of the oral tumors and a decrease in VEGF secretion (Table 1).
NK cell resistant oral tumors secreted higher levels of GM-CSF, IL-1β, IL-6 and IL-8
We next identified several important characteristics of oral tumor cells, which correlated with their resistance to NK cell cytotoxicity. NK cell resistant UCLA-1 cells secreted higher levels of GM-CSF, IL-1β, IL-6 and IL-8 when compared to either UCLA-2 or UM-SCC-1 (Table 2). Either no differences or lower secretion of TNF-α, IL-10, IL-4 or VEGF were observed between UCLA-1 and those of UCLA-2 and UM-SCC-1 tumor cells (data not shown).
Table 2.
Cytokine secretion profiles of NK resistant UCLA-1 and those of NK sensitive UCLA-2 and UM-SCC-1 oral tumors
| GM-CSF | Interleukin-1β | Interleukin-6 | Interleukin-8 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (MFI) | (pg/ml) | (MFI) | (pg/ml) | (MFI) | (pg/ml) | (MFI) | (pg/ml) | |||||||
| UCLA-1 (resistant) | 65 ± 19 | 86.5 ± 19 | 104 ± 40 | 14.5 ± 4 | 1,555 ± 170 | 312 ± 45 | 7,378 ± 289 | 1,928 ± 106 | ||||||
| UCLA-2 (sensitive) | 31.7 ± 1.06 | 0.0 ± 0.0 | 7 ± 0.0 | 0.0 ± 0.0 | 11 ± 2 | 0.0 ± 0.0 | 3,005 ± 341 | 507 ± 42 | ||||||
| UM-SCC-1 (sensitive) | 32 ± 1.4 | 0.0 ± 0.0 | 7 ± 0.0 | 0.0 ± 0.0 | 27 ± 5.7 | 0.0 ± 0.0 | 1,432 ± 60.8 | 121 ± 18 | ||||||
MFI mean fluorescence intensity
Supernatants from the cultures of the oral tumor cells, UCLA-1, UCLA-2 and UM-SCC-1 at (1 × 105/ml), were collected after an overnight incubation and the levels of secreted GM-CSF, IL-1β, IL-6 and IL-8 were determined using a multiplex ELISA array kit specific for each cytokine. Data is presented as mean ± SD. Differences between UCLA-1 and those of UCLA-2 and UM-SCC-1 oral tumor cells were significant at a P value of <0.05. One of five representative experiments is shown in this table
Relative contribution of each of the immune effectors and oral tumors to the secretion of IL-6 and IL-8 in the co-cultures of immune effectors with oral tumors
Untreated and IL-2 treated NK cells were co-cultured with UCLA-1, UCLA-2 and UM-SCC-1 cells and the relative contribution of each of the immune effectors and oral tumors to the secretion of cytokines was assessed. Each of the immune effectors or the tumor cells secreted some levels of IL-6 and IL-8. However, the co-culture of the immune effectors with the oral tumors had synergistic effect on the secretion of IL-6 and IL-8 (Table 3). UCLA-1 tumor cells secreted higher levels of IL-6 when compared to NK cells. The secretion was much higher in the co-cultures of naïve NK cells with UCLA-1 oral tumors indicating the contribution of NK cells to tumor induced IL-6 secretion. The relative resistance of UCLA-1 to NK cell cytotoxicity is also reflected in the ability of these tumor cells to contribute more to the secretion of IL-6 when cultured in the presence of untreated and IL-2 treated NK cells as compared to UCLA-2 and UM-SCC-1 (Table 3). There is a decrease in the levels of IL-6 secreted in the IL-2 treated NK cells with UCLA-2 and UM-SCC-1 cells when compared to co-cultures of tumor cells with untreated NK cells (Table 3, exp. 1). The levels of secreted IL-6 remained significantly higher in the supernatants recovered from the co-cultures of IL-2 treated NK cells with UCLA-1 oral tumors when compared to co-cultures with UCLA-2 or UM-SCC-1 oral tumors (Table 3). Similar observations could be obtained for secretion of IL-8 in the supernatants recovered from the co-cultures of NK cells with oral tumor cells (Table 3). UCLA-1 tumor cells constitutively secreted several fold higher amounts of IL-8 when compared to UCLA-2 and UM-SCC-1 tumor cells (Tables 2 and 3). Each of the NK cells and tumor cells secreted moderate amounts of IL-8, however, synergistic secretion of IL-8 was obtained after the co-culture of immune effectors with tumor cells (Table 3). Induction of IL-8 secretion remained significantly higher in the co-cultures of both untreated or IL-2 treated NK cells with UCLA-1 when compared to UCLA-2 and UM-SCC-1 tumor cells (Table 3). Naïve NK cells contribute significantly to the induction of IL-8 by the oral tumors; whereas IL-2 treated NK cells diminish significantly the secreted IL-8 in tumor cells (Table 3). However, the overall secreted amounts of IL-8 remained significantly lower in the co-cultures of IL-2 treated NK cells with UCLA-2 and UM-SCC-1 as compared to those co-cultured with UCLA-1 (Table 1). The relative differences obtained for IL-8 secretion between IL-2 treated NK cells co-cultured with those of UCLA-2 and UM-SCC-1 as compared to UCLA-1 could largely be due to the higher lysis of those tumors by IL-2 activated NK cells (Tables 1 and 3). Overall, these results indicated that naïve NK cells can significantly amplify the secretion of IL-6 and IL8 from oral tumors, and that the levels are significantly higher in the co-cultures of NK cells with NK resistant tumors when compared to NK sensitive tumors.
Table 3.
Synergistic induction of IL-6 and IL-8 secretion in co-cultures of naïve NK cells with oral tumors
| Tumors alone | NK control | NK IL-2 | |
|---|---|---|---|
| Interleukin-6 (pg/ml) | |||
| Exp. No. 1 | |||
| No tumors | – | 0 | 0 |
| UCLA-1 | 114 ± 5 | 213 ± 10 | 281 ± 9 |
| UCLA-2 | 0 | 56 ± 3 | 0 |
| UM-SCC-1 | 0 | 18 ± 3 | 0 |
| Exp. No. 2 | |||
| No tumors | – | 0 | 0 |
| UCLA-1 | 79.6 ± 5 | 153.7 ± 10 | 203.9 ± 12 |
| UCLA-2 | 0 | 0 | 0 |
| UM-SCC-1 | 0 | 0 | 0 |
| Interleukin-8 (pg/ml) | |||
| Exp. No. 1 | |||
| No tumors | – | 246 ± 12 | 166 ± 6 |
| UCLA-1 | 2,161 ± 63 | 2,518 ± 140 | 2,382 ± 45 |
| UCLA-2 | 162 ± 10 | 2,428 ± 6 | 261 ± 19 |
| UM-SCC-1 | 69 ± 0 | 2,390 ± 110 | 226 ± 13 |
| Exp. No. 2 | |||
| No tumors | – | 0 | 3.14 ± 3 |
| UCLA-1 | 314.5 ± 174 | 702.7 ± 29 | 229 ± 75 |
| UCLA-2 | 26 ± 4 | 77 ± 15 | 11 ± 1.5 |
| UM-SCC-1 | 59 ± 1.5 | 93 ± 2.6 | 2.3 ± 1 |
NK cells were left untreated or treated with IL-2 (500 units/ml) for 8–12 h before they were added to primary oral tumors at an effector to target ratio of 5:1. The levels of IL-6 and IL-8 secretion in each sample were determined using a multiplex ELISA array kit specific for IL-6 and IL-8. Differences between resistant and sensitive oral tumor cells were significant at a P value of <0.05. Two of five representative experiments for each of IL-6 and IL-8 are shown in this table
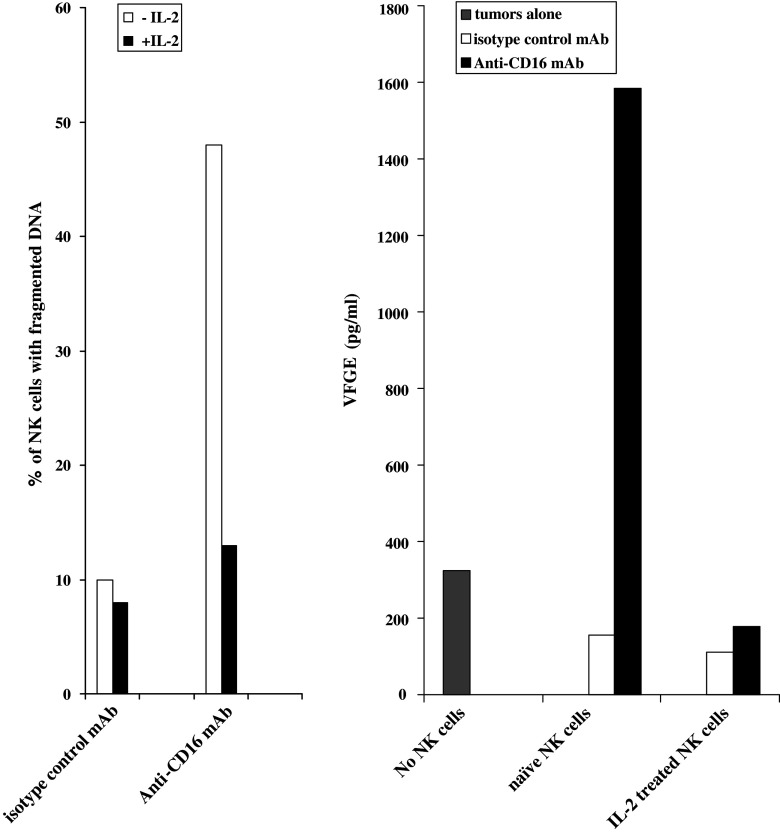
Increased secretion of VEGF in co-cultures of NK cells with sensitive oral tumors when NK cells were triggered to undergo cell death
Oral cancer patients exhibit increased rate of spontaneous apoptosis in their circulating lymphocytes and dendritic cells (DCs) [7]. Thus, we tested the effect of immune cell apoptosis on tumor induced VEGF secretion. Well-characterized receptor mediated trigger for cell death was used to induce apoptosis in NK cells [8, 10, 12]. Addition of intact anti-CD16 antibody as well as F(ab′)2 fragment, was previously shown by our laboratory to induce significant cell death of NK cells [8]. Thus, we used the same strategy to induce cell death of NK cells (Fig. 2). NK cells were left untreated or treated with IL-2 in the presence and absence of anti-CD16 antibody treatment and immediately added to NK cell sensitive UCLA-2 tumor cells and the levels of VEGF secretion were determined in the co-cultures after an overnight incubation (Fig. 2). As indicated above no significant induction of VEGF secretion could be observed by NK cells alone, therefore, the amounts of VEGF secretion obtained in each sample were primarily from the tumor cells (Fig. 2). As expected, untreated and IL-2 treated NK cells were able to suppress VEGF secretion by UCLA-2 tumor cells. Addition of anti-CD16 antibody induced significant apoptosis in NK cells, and this was reflected on the inability of apoptotic NK cells to decrease VEGF secretion (Fig. 2). IL-2 was able to prevent NK cells from undergoing apoptosis in the presence of anti-CD16 antibody, and this resulted in the ability of NK cells to decrease tumor induced VEGF secretion (Fig. 2). Therefore, apoptotic immune effectors observed in the blood of oral cancer patients may contribute to the increased release of tumor growth factors.
Fig. 2.
Addition of anti-CD16 antibody to NK cells induces NK cell death and blocks inhibitory function of NK cells on tumor derived VEGF secretion. NK cells were treated with isotype control antibody (6 μg/ml), anti-CD16 antibody (3 μg/ml) or IL-2 (500 units/ml) alone or in combination as indicated in the figure, and either left in the absence of tumors or immediately added to UCLA-2 primary oral tumors at an effector to target (E:T) ratio of 1:1. After an overnight incubation the levels of NK cell apoptosis were determined using flow cytometric analysis of ethanol fixed NK cells. In addition, the supernatants were removed from the co-cultures of NK cells with oral tumors after an overnight incubation and assayed for VEGF secretion using a specific ELISA assay. One of four representative experiments is shown in this figure
Discussion
In this paper we have identified several characteristics of oral tumor cells which could be used to differentiate between NK cell resistant and sensitive oral tumors. Additionally, the conditions under which immune effectors could contribute to tumor cell resistance have been delineated. In general, naïve untreated NK cells do not have the ability to lyse any of the oral tumors tested significantly. This conclusion was based on a significant panel of oral tumor cells tested in our laboratory (data not shown). IL-2 treated NK cells, on the other hand, are capable of lysing some but not all oral tumors tested. Those oral tumor cells that are highly sensitive to IL-2 activated NK cells are sometimes minimally lysed by naïve untreated NK cells when compared to K562 NK sensitive tumor cell lysis (data not shown). The levels of lysis of oral tumors differ between NK donors and among different oral tumor cells. Immune cells which are not able to lyse the tumor cells may further contribute to tumor cell resistance by triggering production of different tumor resistance factors. The signature of an NK cell resistant tumor cell was found to be the increased synthesis and secretion of GM-CSF, IL-1β, IL-6 and IL-8 in the presence and absence of naïve immune effectors. Although IL-2 activated NK cells were able to reduce some of the resistance factors in NK resistant tumor cells, the levels of those factors remained substantially higher in the co-cultures of NK cells with resistant oral tumors when compared to sensitive tumor cells. Indeed, squamous cell carcinomas of head and neck which secreted higher levels of IL-1α, IL-6 and GM-CSF were previously found to be a progressor tumor in immune-competent animals [23]. Downregulation of CD80 by the above mentioned cytokines was found to be the mechanism of tumor resistance [23]. Likewise, the production of IL-6 and IL-8 in oral tumors was hypothesized to be the principal mechanism of inducible radio-resistance and chemo-resistance in oral tumors [21]. However, no studies thus far have established the principal in vivo or in vitro mechanism for the induction of resistance in oral tumor cells. Our studies indicate that naïve immune effectors or the immune effectors that are signaled to undergo cell death may be the principal contributors to tumor resistance.
Immune effectors that are unable to lyse oral tumors contributed significantly to VEGF secretion by resistant oral tumors. Indeed, 1.25–1.96 fold induction of VEGF secretion could be observed when no or little NK cell cytotoxicity could be seen. However, decreased secretion of VEGF even in the presence of NK cell resistant oral tumors could be seen when IL-2 activated NK cells lysed a small proportion of the tumor cells. Inhibition of VEGF secretion by immune cells was even more dramatic when NK sensitive oral tumor cells were used in the co-cultures. Even in the presence of moderate lysis, decreased rather than an increased secretion of VEGF could be observed in the co-cultures of naïve PBMCs and NK cells with sensitive tumor cells. The inhibitory effect of IL-2 activated NK cells on VEGF secretion was more dramatic in sensitive oral tumor cells as compared to resistant oral tumor cells and this correlated with decreased secretion of both IL-6 and IL-8 in the co-cultures of immune effectors with sensitive oral tumors. Both IL-6 and VEGF secretion have been shown to contribute to immune inactivation in a variety of tumor model systems [4, 5, 18, 22, 24]. Thus, IL-6 and VEGF secretion may not only be growth promoting for resistant oral tumors, they may also contribute to tumor resistance by inactivating the immune cell function which could further contribute to tumor resistance. Indeed, when NFκB was inhibited in oral tumors significant decrease in IL-6 secretion was found to relate to the increased immune cell function [11]. In addition, the cytotoxic immune cells were able to survive longer, and expand when exposed to NFκB knock down tumor cells [11, 13]. However, when the immune cells were co-cultured with oral tumors with high NFκB expression they were able to inactivate the function of the immune cells and induce cell death in a large proportion of the immune effectors [10, 13]. Thus, resistance to cell death either in immune cells or the tumor cells serves as the determining factor for the sensitivity or resistance of the tumor cells respectively. Therefore, strategies to increase survival of the immune effectors in the presence of decreased survival of the tumor cells should be substantially more effective in eradication of oral cancers when either subpopulation is targeted individually. Indeed, blocking of NFκB in the tumor cells should in principle achieve this objective since it will not only decrease the survival of tumor cells, but it should also increase the survival of interacting immune effectors due to their increased function and survival as observed previously [11, 13].
In conclusion, even though mechanisms governing tumor resistance are significantly more complex and may involve participation of a number of other cells, such as dendritic cells and other cells, not present in our simple model of PBMCs and purified NK cells, the studies reported in this paper point to the crucial role of immune effectors in induction of resistance factors in oral tumors. Thus, in this paper we have identified several important targets which may be used to establish prognosis as well as treatment modalities to enhance patient survival.
Footnotes
This work was supported by RO1-12880 from NIH-NIDCR.
References
- 1.Agarwal A, Rani M, Saha GK, Valarmathi TM, Bahadur S, Mohanti BK, Das SN. Disregulated expression of the Th2 cytokine gene in patients with intraoral squamous cell carcinoma. Immunol Invest. 2003;32:17–30. doi: 10.1081/IMM-120019205. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Verma S, Burra U, Murthy NS, Mohanty NK, Saxena S. Flow cytometric analysis of Th1 and Th2 cytokines in PBMCs as a parameter of immunological dysfunction in patients of superficial transitional cell carcinoma of bladder. Cancer Immunol Immunother. 2006;55(6):734–743. doi: 10.1007/s00262-005-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.CIR.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 5.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 6.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT, Storkus WJ, Whiteside TL. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res. 2002;8:1787–1793. [PubMed] [Google Scholar]
- 8.Jewett A, Bonavida B. MHC-Class I antigens regulate both the function and the survival of human peripheral blood NK cells: role of endogenously secreted TNF-alpha. Clin Immunol. 2000;96:19–28. doi: 10.1006/clim.2000.4871. [DOI] [PubMed] [Google Scholar]
- 9.Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156:907–915. [PubMed] [Google Scholar]
- 10.Jewett A, Cacalano NA, Head C, Teruel A. Coengagement of CD16 and CD94 receptors mediates secretion of chemokines and induces apoptotic death of naïve natural killer cells. Clin Cancer Res. 2006;12:1994–2003. doi: 10.1158/1078-0432.CCR-05-2306. [DOI] [PubMed] [Google Scholar]
- 11.Jewett A, Cacalano NA, Teruel A, Romero M, Rashedi M, Wang M, Nakamura H. Inhibition of nuclear factor kappa B (NFkappaB) activity in oral tumor cells prevents depletion of NK cells and increases their functional activation. Cancer Immunol Immunother. 2006;55:1052–1063. doi: 10.1007/s00262-005-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jewett A, Cavalcanti M, Bonavida B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J Immunol. 1997;159:4815–4822. [PubMed] [Google Scholar]
- 13.Jewett A, Wang MY, Teruel A, Poupak Z, Bostanian Z, Park NH. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor kappaB in HEp2 tumor cell line: effect on the function of natural killer cells. Hum Immunol. 2003;64:505–520. doi: 10.1016/S0198-8859(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 14.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 15.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang S, Tiwari S, Andratschke M, Loehr I, Lauffer L, Bergmann C, Mack B, Lebeau A, Moosmann A, Whiteside TL, Zeidler R (2007) Immune restoration in head and neck cancer patients after in vivo COX-2 inhibition. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed]
- 17.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 18.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 19.Rayman P, Wesa AK, Richmond AL, Das T, Biswas K, Raval G, Storkus WJ, Tannenbaum C, Novick A, Bukowski R, Finke J. Effect of renal cell carcinomas on the development of type 1 T-cell responses. Clin Cancer Res. 2004;10:6360S–6366S. doi: 10.1158/1078-0432.CCR-050011. [DOI] [PubMed] [Google Scholar]
- 20.Sakakura K, Chikamatsu K, Takahashi K, Whiteside TL, Furuya N. Maturation of circulating dendritic cells and imbalance of T-cell subsets in patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2006;55(2):151–159. doi: 10.1007/s00262-005-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamatani T, Azuma M, Ashida Y, Motegi K, Takashima R, Harada K, Kawaguchi S, Sato M. Enhanced radiosensitization and chemosensitization in NF-kappaB-suppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J Cancer. 2004;108:912–921. doi: 10.1002/ijc.11640. [DOI] [PubMed] [Google Scholar]
- 22.Tanner J, Tosato G. Impairment of natural killer functions by interleukin 6 increases lymphoblastoid cell tumorigenicity in athymic mice. J Clin Invest. 1991;88:239–247. doi: 10.1172/JCI115283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas GR, Chen Z, Leukinova E, Van Waes C, Wen J. Cytokines IL-1 alpha, IL-6, and GM-CSF constitutively secreted by oral squamous carcinoma induce down-regulation of CD80 costimulatory molecule expression: restoration by interferon gamma. Cancer Immunol Immunother. 2004;53:33–40. doi: 10.1007/s00262-003-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vredevoe DL, Widawski M, Fonarow GC, Hamilton M, Martinez-Maza O, Gage JR. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am J Cardiol. 2004;93:1007–1011. doi: 10.1016/j.amjcard.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 25.Whiteside TL. Apoptosis of immune cells in the tumor microenvironment and peripheral circulation of patients with cancer: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A46–A51. doi: 10.1016/S0264-410X(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 26.Whiteside TL, Chikamatsu K, Nagashima S, Okada K. Antitumor effects of cytolytic T lymphocytes (CTL) and natural killer (NK) cells in head and neck cancer. Anticancer Res. 1996;16:2357–2364. [PubMed] [Google Scholar]
- 27.Whiteside TL, Letessier E, Hirabayashi H, Vitolo D, Bryant J, Barnes L, Snyderman C, Johnson JT, Myers E, Herberman RB, et al. Evidence for local and systemic activation of immune cells by peritumoral injections of interleukin 2 in patients with advanced squamous cell carcinoma of the head and neck. Cancer Res. 1993;53:5654–5662. [PubMed] [Google Scholar]