Abstract
We have demonstrated that coupling an immunoregulatory segment of the MHC class II-associated invariant chain (Ii), the Ii-Key peptide, to a promiscuous MHC class II epitope significantly enhances its presentation to CD4+ T cells. Here, a series of homologous Ii-Key/HER-2/neu(776-90) hybrid peptides, varying systematically in the length of the epitope(s)-containing segment, are significantly more potent than the native peptide in assays using T cells from patients with various types of tumors overexpressing HER-2/neu. In particular, priming normal donor and patient PBMCs with Ii-Key hybrid peptides enhances recognition of the native peptide either pulsed onto autologous dendritic cells (DCs) or naturally presented by IFN-γ-treated autologous tumor cells. Moreover, patient-derived CD4+ T cells primed with the hybrid peptides provide a significantly stronger helper effect to autologous CD8+ T cells specific for the HER-2/neu(435-43) CTL epitope, as illustrated by either IFN-γ ELISPOT assays or specific autologous tumor cell lysis. Hybrid peptide-specific CD4+ T cells strongly enhanced the antitumor efficacy of HER-2/neu(435-43) peptide-specific CTL in the therapy of xenografted SCID mice inoculated with HER-2/neu overexpressing human tumor cell lines. Our data indicate that the promiscuously presented vaccine peptide HER-2/neu(776-90) is amenable to Ii-Key-enhancing effects and supports the therapeutic potential of vaccinating patients with HER-2/neu+ tumors with such Ii-Key/HER-2/neu(776-90) hybrid peptides.
Keywords: Ii-Key hybrid peptides, HER-2/neu peptides, Cancer vaccines, T helper cells, CTL
Introduction
Since CTL play an essential role in protection against tumor growth, improving methods of CTL induction are of paramount importance for cancer immunotherapy. Optimal CTL induction and long-term immunological memory depend on strong responses from T helper cells [8, 9, 30, 32, 34, 39, 42, 43], which recognize MHC class II epitopes of tumor-associated antigens (TAA) [7, 17, 22]. Many vaccine TAA peptides for CTL (MHC class I epitope peptides) and for T helper cells (MHC class II epitope peptides) have been identified [47, 49], and some have entered clinical trials [53]. In particular for breast carcinoma, peptide vaccines are being developed with epitopes of the HER-2/neu receptor, which is overexpressed in some breast cancers and serves as an established target for immunotherapy [7]. A major issue in clinical trials with cancer peptide vaccines is the relatively weak presentation of MHC class II epitope peptides [12]. A novel method to boost the potency of MHC class II epitope peptide presentation has become available in the form of Ii-Key/MHC class II hybrid peptides [1, 2]. Such Ii-Key hybrid peptides use an immunoregulatory segment of the Ii protein (the Ii-Key peptide) to loosen the epitope-binding groove of MHC class II molecules to permit insertion of a tethered MHC class II epitope [51]. The first Ii-Key peptide (Ii77-2; LRMKLPKPPKPVSQMR) was synthesized to test for biological activity related to regulation of MHC class II antigenic peptide binding, because its primary sequence suggested a regulatory structure signal (six positive amino acids, no negative amino acids, four spaced prolines and recurrent cationic-hydrophobic doublets reminiscent of protease-cleavage sites) [33]. In vitro peptide presentation assays showed that this “Ii-Key-peptide greatly enhanced the presentation of I-E-restricted antigenic peptides to murine T hybridomas [1, 2]. Structure activity relationship studies of 160 homologs revealed a shorter core sequence (LRMKLPK) with significantly greater activity than the original 16-amino acid peptide. The shortest active sequence constituted four amino acids (LRMK) [1, 52]. Covalent linkage of that Ii-Key peptide (LRMK) to MHC class II epitopes strongly potentiated presentation of the tethered peptides in vitro [18, 21, 24] and in vivo [23, 25]. The mechanistic hypothesis has been that the Ii-Key moiety binds initially to an allosteric site just outside the MHC class II binding groove inducing a conformational change in the trough for more accessible antigenic epitope charging. The affinity of the antigenic epitope in the MHC class II binding groove far exceeds the affinity of the Ii-Key moiety to the allosteric site. Thus, it is speculated that the allosteric effector (LRMK) is pulled away from its action site as the antigenic epitope settles into a functional conformation in the antigenic peptide-binding site.
The objective of this study was to assess pre-existing immunity in patients with HER-2/neu+ tumors not receiving immunomodulating therapies, to a homologous series of Ii-Key/HER-2/neu(776-90) vaccine peptides as therapeutic candidates for phase I clinical trials. Members of this series differed in the linear extent of native epitope containing segment N-terminal to the P1 site residue. This HER-2/neu(776-90) native peptide sequence is “promiscuously presented-by many HLA-DR alleles [41, 44, 46]. Such presentation might reflect one epitope, which binds to many HLA-DR alleles and/or multiple, closely overlapping, but slightly offset MHC class II peptides [44]. Here, we examine the immunogenicity of the clinically important HER-2/neu(776-90) MHC class II epitope [16, 41, 44, 46] in a novel strategy to enhance the potency of presentation to T helper cells and consequently the quality of CTL response to the HER-2/neu(435-43), [HER-2(9435)], MHC class I peptide [18, 19, 26, 39] mediating therapeutic antitumor immunity. In particular, we report that the Ii-Key/HER-2/neu(776-90) hybrid peptides compared to the native peptide induced higher levels of activation of CD4+ T cells rendering them able to stimulate higher HER-2/neu(435-43) peptide-specific CTL responses. Peptide-specific CD4+ T cells derived from patients with different types of cancer were also able to recognize their autologous tumors demonstrating that HER-2/neu(776-90) is a naturally presented epitope expressed by a variety of tumors. Furthermore, hybrid peptide-specific CD4+ T cells strongly enhanced the antitumor efficacy of HER-2/neu(435-43) peptide-specific CTL in the therapy of xenografted SCID mice inoculated with human tumor cell lines. Thus, Ii-Key/MHC class II epitope hybrids of TAA might have significant therapeutic value in the treatment of cancer.
Materials and methods
Tumor cell lines
The SKOV3 (ovarian adenocarcinoma) and SKBR3 (breast carcinoma) cell lines overexpressing HER-2/neu (44) were maintained in McCoy’s medium supplemented with 10% FCS. SKOV3.A2 cells were produced upon transfection with pcDNA3.1-A2-neoR construct (the generous gift of Prof. Jotereau, Institut de Biologie, Nantes) encoding the HLA-A2 gene. The MDA-231 cells were propagated in RPMI with 10 % FCS while the MCF-7 cell line was cultured in DMEM supplemented with 1% insulin and 10 % FCS. All tumor cell lines were purchased from American Type Collection (Manassas, VA).
Blood samples
Patients in this study had, histologically confirmed, HER-2/neu overexpressing cancers. Tumor cells were isolated from pleural or ascitic fluids (prostate Ca, n = 2; breast Ca, n = 6; ovarian Ca, n = 3; lung Ca, n = 2; pancreas Ca, n = 2), which had been collected during routine therapeutic aspirations. The selection of patients for this study was dependent on having HER-2/neu overexpression (+3) on the primary tumor or metastasis and expressing at least one of the following HLA-DR alleles previously shown [41] to bind the native peptide with high affinity: DRB1*0101, DRB1*1501, DRB1*0404, DRB1*1101, DRB1*1302, DRB1*0701, DRB1*0802, DRB1*0901, DRB4*0101, DRB1*0401 and DRB5*0101. In addition, three normal donor samples with predicted allelic reactivity (DR0404/1601, DR1101/1602, DR0404/DR1101) to the native peptide were assessed to detect enhanced immunological responses to the hybrid peptides. Patients whose CD8+ T cells were tested for IFN-γ responses or cytotoxicity against their autologous tumor cells were HLA-A2.1 positive. DNA typing of MHC class II HLA alleles was determined with the Life Match HLA-SSO typing kit according to the manufacturer’s instructions (Tepnel, Stamford, CT). Samples were analyzed with the Luminex100 instrument (Luminex Corp. Austin, TX). Tumors were staged according to standard classifications, with all cases being either Stages III or IV. Biologic material was provided by the 1st Oncology Department of the Errikos Dunant Hospital and the Medical Oncology Unit of the Department of Pathophysiology of Laikon General Hospital in Athens under protocols approved by the Institutional Review Boards of both institutions. All patients and normal donors provided informed consent before entering these studies.
Isolation of tumor cells
Tumor cells were isolated from malignant effusions by described methods [5, 6]. Briefly, fluids were spun at 400 g for 10 min to sediment cells, which were placed on top of a 75% Ficoll Separation Solution (Biochrom, Berlin, Germany) gradient overlaid with 100% Ficoll Separation Solution and respun at 700 g for 25 min. Tumor cells collected from the top of the 75% Ficoll Separation Solution were cultured in A-MEM medium supplemented with 10% fetal bovine serum and 50 μg/ml gentamicin (all culture reagents were purchased from Life Technologies, Bochum, Germany).
HER-2/neu expression
The level of tumor cell expression of HER-2/neu was determined immunohistochemically by estimating the number and intensity of stained tumor cells per section of tumor specimen as previously reported [10] by using the DAKO’s 0- scoring system. Expression of HER-2/neu on single tumor cells isolated from malignant effusions was determined by flow cytometry using the PE-conjugated Neu24.7 mAb, which recognizes the extracellular domain of HER-2/neu (BD Pharmingen, Erembodegem, Belgium). For flow cytometry, the expression of HER-2/neu was controlled by comparing the mean fluorescence intensity (MFI) of primary tumor cells to the MFI of breast cancer cell lines expressing HER-2/neu at different levels [HER-2/neu expression of the MDA-231 cell line is scored as 1 (MFI: 192 ± 27), and of the MCF7 cells as 2 (MFI: 1010 ± 98), and of the SKBR3 cells as 3 (MFI: 3754 ± 177); mean values from three independent analyses]. In all cases, the MFI levels of the primary tumor cells were comparable to that of SKBR3 cells.
Isolation of PBMCs
PBMCs were collected from peripheral blood samples of normal donors and cancer patients. PBMCs were isolated by density gradient centrifugation using Ficoll Separating Solution. Cells were washed three times with HANKS-balanced salt solution (Biochrom) and either assayed immediately or kept frozen until analysis.
Isolation of T cell subsets
CD4+ or CD8+ T cells were isolated from freshly isolated total PBMCs or cell cultures using MACS CD4 or MACS CD8 Microbeads (Miltenyi Biotec, Bergish Gladbach, Germany) respectively, as described in detail previously [8]. The purity of isolated CD4+ or CD8+ T cells was in all cases > 97%, whereas, the negative fractions (i.e., CD4+ or CD8+ T cell-depleted cell cultures) were totally devoid of CD4+ or CD8+ T cells, respectively.
CD14+ cell isolation and generation of DCs
CD14+ monocytes were isolated from PBMCs by positive immunoselection by anti-CD14 mAb-coupled magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. Monocyte differentiation into mature DC was induced as described [37]. Briefly, CD14+ cells were cultured in X-VIVO 15 medium (Life Technologies) in the presence of rIL-4 (1000 IU/ml; R&D Systems, Europe) and rGM-CSF (1000 IU/ml; Immunex, Seattle, WA) for 6 days followed by another 24 h incubation with 10 ng/ml TNF-α (R&D Systems). DCs obtained from these cultures (> 60% mature, based on the expression of CD83, CD80 and CD86) were irradiated (3000 rads) and then pulsed for 4 h in CO2 incubators with the peptides.
Peptides
A homologous series of Ii-Key/HER-2/neu(776-90) epitope hybrids were synthesized by systematically varying the structure and length of the spacer which connects the Ii-Key (LRMK) segment to the promiscuous HER-2/neu(776-90) MHC class II epitope through a flexible polymethylene, ava, (5-aminovaleric acid = 5-aminopentanoic acid) chain (Table 1). All peptides were found to be > 98% pure by analytical HPLC and mass spectrometry (Commonwealth Biotechnologies, Richmond, VA). The above peptides were N-acetylated and C-amidated to inhibit exopeptidases. The HLA-A2.1-restricted CTL epitope peptides HER-2/neu(435-43) (ILHNGAYSL), and gp100(154-62) (KTWGQYWQV) were synthesized by the solid phase method with an Ecosyn P peptide synthesizer (Eppendorf-Biotronik, Hamburg, Germany) using the Fmoc strategy and 4-carboxybenzyl alcohol resin. Purity was > 95% as indicated by HPLC. In addition, a human Ii-Key/HIVgag(164-76; Ac-LRMK-ava-YVDRFYKTLRAEQ-NH2) hybrid peptide constituting a promiscuous MHC class II epitope recognized by most common HLA-DR alleles (26) was synthesized to > 95% purity (NeoMPS, Inc., San Diego, California) and used as an irrelevant control in our studies. All peptides were dissolved in sterile distilled water (5 mg/ml) and stored at -0°C.
Table 1.
Ii-Key/HER-2/neu (776-90) MHC class II epitope hybrid design
| Peptide | Position | Sequence |
|---|---|---|
| NP | 776-90 | Ac-GVGSPYVSRLLGICL-NH2 |
| B | 776-90 | Ac-LRMK-ava-GVGSPYVSRLLGICL-NH2 |
| C | 777-90 | Ac-LRMK-ava-VGSPYVSRLLGICL-NH2 |
| D | 778-90 | Ac-LRMK-ava-GSPYVSRLLGICL-NH2 |
| E | 779-90 | Ac-LRMK-ava-SPYVSRLLGICL-NH2 |
| F | 776-90 | Ac-LRMK-GVGSPYVSRLLGICL-NH2 |
NP is the native peptide HER-2/neu(776-90), whereas peptides B through F are the Ii-Key/HER-2/neu(776-90) hybrid peptides. The Ii-Key (LRMK) segment of the immunoregulatory Ii protein was linked through a simple polymethylene ava spacer to the promiscuous HER-2/neu(776-90) MHC class II epitope by systematically deleting amino acids N-terminal to the P1 (Y) site residue
ELISA assay
PBMCs from normal donors were cultured in X-VIVO 15 medium (105 cells per 200 μl) in 96-well U-bottomed plates with added peptide (50 μg/ml) in CO2 incubators. Seven days later, half of the medium was removed and replenished with 100 μl of fresh medium containing 104 autologous, irradiated (3000 rads) DCs pulsed with each respective peptide. Thereafter, the growing microcultures were restimulated at weekly intervals with 105 autologous CD14+ cells (3000 rads) pulsed with one respective peptide. The peptide concentration was decreased in each successive restimulation cycle (i.e., 25 μg/ml, 12.5 μg/ml). After the third restimulation, cells were tested for IFN-γ production in the presence of autologous DCs unpulsed or pulsed with the native peptide using an IFN-γ ELISA kit, according to the manufacturer’s instructions (Diaclone, Besancon, France).
ELISPOT assay
Cancer patients-unfractionated PBMCs (2.5 × 105 cells/well) or purified CD4+ T cells (105 cells/well) and autologous CD14+ cells (104 cells/well) were incubated with either Ii-Key/HER-2/neu or control Ii-Key/HIVgag hybrid peptides (50 μg/ml) or native peptide (50 μg/ml) in 96-well, U-bottomed plates (quadruplicate wells) in 200 μl of X-VIVO 15 medium supplemented with 100 pg/ml of human recombinant IL-12 (R&D Systems). A week later, peptide-stimulated responder cells (total T or CD4+ T cells) were incubated with autologous DCs (10:1 ratio) unpulsed or pulsed with 25 μg/ml of the native peptide for 48 h. Peptide-stimulated responder CD4+ T cells were also tested (48 h incubation) for recognition of the HER-2/neu(776-90) peptide naturally processed and presented by primary autologous tumor cells (10:1 ratio), which were induced to express HLA-DR molecules by rIFN-γ treatment. Tumor cells were induced to express MHC class II molecules in the presence of human recombinant IFN-γ (300 IU/ml; Boehringer Mannheim, Mannheim, Germany). After 72 h in a CO2 incubator, tumor cells were analyzed for HLA-DR expression using the L243 (IgG2a) monoclonal antibody conjugated with PE (BD Biosciences). Non-treated autologous tumor cells (being MHC class II negative) were used as stimulatory cells in control cultures. The peptide-stimulated responder cells were then transferred to a pre-coated IFN-γ ELISPOT plate and incubation was extended for another day. The ELISPOT assay was performed according to the manufacturer’s instructions (BD PharMingen, San Diego, CA).
Interactions between peptide-specific CD4+ and CD8+ T cells
The interactions between CD4+ and CD8+ T cells were determined by both IFN-γ-based ELISPOT and cytotoxicity assays. The patient-derived PBMCs were stimulated either with the hybrid peptides or the native peptide in 96-well, U-bottomed plates at 105 cells per well in 200 μl of X-VIVO 15 culture medium for a week. Cultures were then pooled from decaplicate wells and peptide-stimulated CD4+ T cells were isolated by immunomagnetic purification (Miltenyi Biotech). On the same day, autologous CD8+ T cells were isolated from thawed PBMCs by immunomagnetic procedures (Miltenyi Biotech). The CD4+ and CD8+ T cells (5 × 104 from each subset) were mixed with 5 × 103 autologous DCs pulsed with the respective helper peptide used for stimulation and with the CTL HER-2(435-43) peptide, in 200 μl of X-VIVO 15 medium per well in 96-well plates. Control cultures consisted of only CD8+ T cells and DCs pulsed with HER-2/neu(435-43) peptide. As for another control, HER-2/neu(435-43) pulsed DCs were added to CD8+ T cell cultures in the presence of nonprimed CD4+ T cells or CD4+ T cells primed with the control Ii-Key/HIVgag(164-76) hybrid peptide. Seven days later (i.e., day 14 from culture initiation), immunomagnetically purified CD8+ T cells were IFN-γ ELISPOT assayed against irradiated HLA-A2.1+ autologous tumor cells. For testing the enhancement of cytotoxicity mediated by HER-2/neu(435-43)-primed CTL through potentiation with hybrid peptide-primed autologous CD4+ cells, cultures were set up as above until day 14, and the recovered T cells were restimulated twice at 7-day intervals in the presence of irradiated autologous PBMCs (ratio 1:1) preincubated with HER-2(435-43). The CD8+ T cells were then separated from the CD4+ T cells by negative immunomagnetic purification and subsequently cultured in low doses of rIL-2 (20 IU/ml). Thereafter, the retrieved CD8+ T cells were tested for cytotoxicity against their autologous tumor targets at the designated ratios. Cytotoxicity assays were performed as described [19, 20].
Tumor rejection models
The CD4+ and CD8+ T cells from a single HLA-A2.1+ patient primed as previously described were tested for their capacity to synergistically induce antitumor activity against the HER-2/neu overexpressing SKOV3 ovarian cancer cell line transfected to express HLA-A2.1 (SKOV3.A2). Before adoptive transfer, both CD4+ and CD8+ T cells (1 × 106 cells/ml each) were expanded using the T cell expander kit according to the manufacturer’s instructions (Dynal, Biotech, Oslo, Norway). IL-2 (Proleukin; Chiron BV, Amsterdam, The Netherlands) at 100 U/ml was added to the cultures 3 days later. The cells were fed with fresh medium containing 100 U/ml IL2 twice a week. After a total of 20 days approximately 2 × 107 cells could be recovered from a single culture. SCID mice were inoculated subcutaneously (sc.) on the back with 5 × 05 tumor cells in 0.5 ml PBS. Injections with expanded CD4+ and CD8+ T cells (1 × 106 cells from each subset in 0.5 ml PBS) were administered intraperitoneally (ip.) at a time when the tumor became palpable (i.e. 12-6 days after inoculation). Tumor size was caliper-measured and recorded as the product of the perpendicular diameters of individual tumors every 4 days. The observation was terminated with euthanasia when the tumor mass grew to 200-50 mm2.
Statistical analyses
All functional assays, e.g., IFN-γ production in ELISA and ELISPOT assays, and cytotoxicity assays were performed in quadruplicate (unless differently indicated). Significance of differences among all groups was assessed with the Student’s t-test. A nonparametric Wilcoxon rank test was also used in the statistical analysis of the size of tumor in individual groups. The difference was considered statistically significant when P < 0.05.
Results
Normal human T cell responses to Ii-Key/HER-2/neu(776-90) hybrid peptides and the native peptide
Since natural cellular and humoral HER-2/neu immunity is evident in both normal donors and cancer patients [7], we first investigated the PBMCs of healthy volunteers in order to explore whether the normal T cell repertoire harbors precursors inducible by the hybrid peptides. Normal donors with predicted HLA-DR allelic reactivity based on promiscuity of the native HER-2/neu(776-90) sequence [41, 44] were thus assessed for upregulated hybrid peptide immunogenicity. More specifically, the enhanced immunostimulatory activity of Ii-Key/HER-2/neu MHC class II epitope hybrids (Table 1) comparable to the native peptide was evaluated in lymphocyte cultures of three healthy volunteers after the third round of priming with either native peptide or hybrid peptides. The pooled data from these experiments, shown in Fig.1, demonstrate the potency of hybrid peptides-B, -D and -F to more efficiently activate donor T cells for enhanced recognition of the native peptide pulsed onto autologous DCs. Priming with these peptides induced a modest (P < 0.05) enhancement of IFN-γ production in the three healthy donors tested compared to the native peptide (stimulation index: 1.52-.81), with a stimulation index ranging between 1.85 to 2.92 for peptide-B, and 2.37 to 3.01 and 2.15 to 3.47 for peptides-D and -F respectively. Hybrid peptides-C and -E induced IFN-γ responses, which, however, did not differ significantly from those induced by the native peptide. Overall, priming with the hybrid peptides-B, -D and -F induced a noteworthy increase of immune response comparable to the native peptide in peripheral low avidity normal donor T cells. Such T cell clones were in vitro stimulated via multiple rounds of stimulation, generating low IFN-γ responder cells to the native epitope. Healthy donors-T cells primed with a control Ii-Key/HIV gag(164-76) hybrid peptide, which binds to a series of HLA-DR alleles including the native peptide-binding ones (26, 41), produced similar levels of IFN-γ in response to autotologous DCs, either unpulsed or pulsed with the native peptide (stimulation index: 0.95 ± 0.25) (Fig. 1), thus demonstrating the specificity of the CD4+ T cell responses for the native peptide, upon priming with the hybrid peptides-B, -D and -F.
Fig. 1.
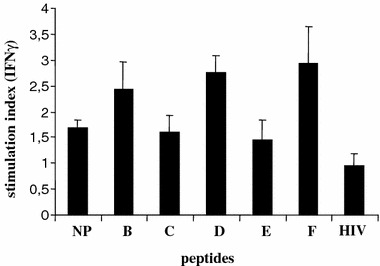
Stimulation of normal donor PBMC with hybrid peptides enhances recognition of native peptide presented onto autologous DC. Mean stimulation index ± SD from three normal donors is shown. NP native peptide, B-F hybrid peptides, HIV Ii-Key/HIV gag(164-76) control hybrid peptide
Enhanced T cell responses to autologous HER-2/neu + tumor cells following in vitro stimulation of cancer patient PBMCs with Ii-Key/MHC class II epitope hybrid vaccine peptides
We first extended our analysis to evaluate whether PBMCs from cancer patients whose tumors overexpress HER-2/neu might be more efficiently primed by Ii-Key hybrid peptides comparable to the native peptide in terms of recognizing the native HER-2/neu(776-90) sequence presented on autologous DCs, consistently, as with the previously demonstrated trend of hybrid peptide immunogenic sensitivity obtained from the normal donors. Stimulating patients-PBMCs with hybrid peptides-B, -D and -F generally enhanced the frequencies of T cells in recognizing the native peptide that is pulsed onto autologous DC (data not shown).
More interestingly, we then evaluated whether the hybrid peptide-primed T cells elicited stronger immune responses against autologous HER-2/neu + tumors presenting the native peptide in the context of HLA-DR molecules induced by rIFN-γ treatment (Table 2). Overall, hybrid peptides-B, -D and -F enhanced patients-T cell numbers responding to autologous tumors relative to the native peptide (Fig. 2a, b). Immune response was defined as the specific IFN-γ spots induced either by native peptide or hybrid peptide-primed T cells against autologous tumor cells pre-treated with rIFN-γ subtracting the levels of background activity observed with the untreated tumor cells. Enhanced immune responses (including intermediate and high responders, (Fig. 2a) were seen with T cells stimulated with hybrid peptide-B (4 of 9; 44%), -D (4 of 10; 40%) and -F (4 of 8; 50%) relative to T cells stimulated with the native peptide. Mean specific IFN-γ spots with these peptides were 283 for hybrid peptide-B, 252 for peptide-D and 319 for peptide-F, P = 0.0003, 0.0007 and 0.002, respectively compared to the native peptide (mean spots: 125) (Fig. 2b). As previously shown, hybrid peptides-E and -C were not active to any of the patients tested (Fig. 2a, b). Mean specific IFN-γ spots with peptide-C (n = 157) and peptide-E (n = 171) did not differ significantly compared to the native peptide-induced spots. The data from these type of experiments confirmed patients-correlation of enhanced hybrid peptides-B, -D and -F potency to more efficiently sensitize patients-T cells for increased recognition of the native peptide naturally expressed on autologous tumor cells similarly as when presented on DC.
Table 2.
HLA-DR expression on primary tumor cells upon pre-treatment with IFN-γ.
| Pat. No. | Type of cancer | Before IFN-γ treatment | After IFN-γ treatment |
|---|---|---|---|
| 1 | Prostate | 105a | 1.745 |
| 2 | Breast | 201 | 2.846 |
| 3 | Breast | 118 | 1.175 |
| 4 | Breast | 194 | 2.817 |
| 5 | Pancreas | 127 | 1.613 |
| 6 | Pancreas | 103 | 2.515 |
| 7 | Ovarian | 110 | 1.162 |
| 8 | Breast | 112 | 1.170 |
| 9 | Lung | 207 | 2.152 |
| 10 | Prostate | 281 | 2.914 |
aResults are given as net mean fluorescence intensities (MFI), obtained with the PE-conjugated anti-DR mAb L243, from which the background MFI [PE-labled isotype matched (IgG2a) antibody] was subtracted
Fig. 2.
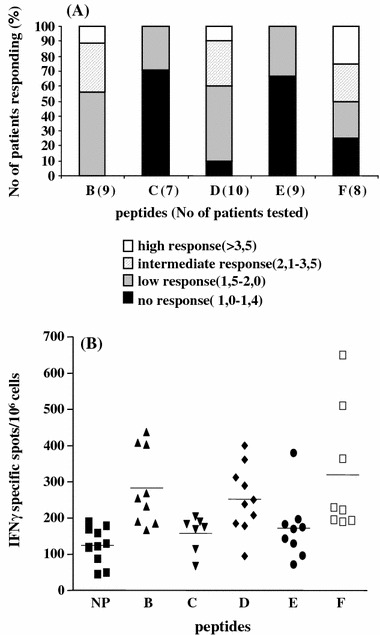
Levels of responses to autologous tumor cells after priming with the hybrid peptides. a Graphs were assigned as percent of no, low, intermediate and high responders based on the level of responses [i.e. number of IFN-γ specific spots generated against the autologous tumor cells, induced to express HLA-DR molecules upon IFN-γ treatment, after subtracting the control stimulations with non-IFN-γ treated autologous tumor cells]. The levels of responses are given as fold increase compared to those induced upon priming with the NP. The cut-off for each group was defined randomly. b Recognition of IFN-γ treated tumor cells by autologous CD4+ T cells primed with NP or hybrid peptides B–F. Each dot represents number of IFN-γ specific spots produced by a single patient tested. Bars represent mean values. P values compared to NP: peptide-B = 0,0003, -C = 0,0658, -D = 0,0007, -E = 0,3982, -F = 0,0002. P values for hybrid peptides -C and -E were non-significant compared to NP
CD4+ T cells are the target of Ii-Key hybrid peptides immunoenhancing effect
For certain patients with adequate blood supply, immunomagnetically isolated hybrid peptide-primed CD4+ T cells were assayed against both DC-pulsed with the native peptide and HER-2/neu overexpressing autologous tumor cells to ascertain Ii-Key/MHC class II hybrid peptide immune response enhancement specifically mounted to Th1 specificity. CD4+ T cells produced a similar pattern of immune response enhancement mediated by hybrid peptides-B, -D and -F in contrast to the native peptide as did total PBMCs cultures (Fig. 3). This increased IFN-γ production mediated by hybrid peptide-stimulated CD4+ T cells was demonstrated in response to the native peptide-pulsed DCs (Fig. 3a) as well as their autologous tumor cells (Fig. 3b). To confirm HER-2/neu antigen immune response specificity, the Ii-Key/HIVgag(164-76) hybrid peptide was used as an irrelevant control in the functional in vitro assays. More specifically, priming CD4+ T cells with such a non-specific control hybrid peptide and testing against DCs pulsed with native HER-2/neu(776-90) epitope or against autologous tumor cells, induced a low number of IFN-γ responder cells (Fig. 3a, b).
Fig. 3.
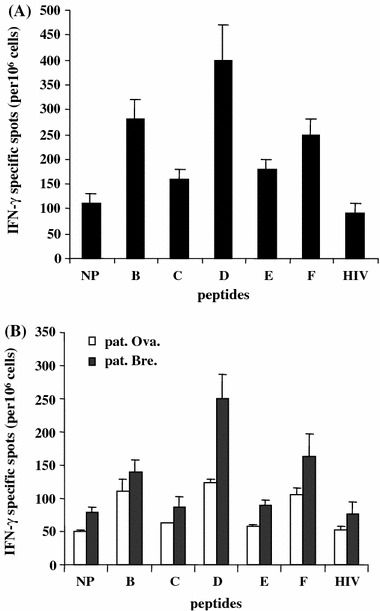
Stimulation of isolated CD4+ T cells with Ii-Key/HER-2/neu hybrid peptides enhances recognition of native HER-2/neu peptide presented onto autologous DCs in a breast cancer patient (a). In (b) similarly stimulated CD4+ cells from two patients with ovarian (pat. ova) and breast (pat. bre) cancer, showed enhanced recognition of their autologous tumor cells. Mean values of IFN-γ specific spots ± SD from quadruplicate wells are shown. Priming CD4+ T cells with the irrelevant control Ii-Key/HIVgag(164-76) hybrid peptide and testing against DCs pulsed with native HER-2/neu(776-90) epitope in (a) or against the autologous tumors in (b), induced a low number of IFN-γ responder cells. NP native peptide; B-F hybrid peptides, HIV irrelevant control peptide
CD4+ T cell-mediated enhancement of autologous CD8+ T cell IFN-γ production and cytolytic activity
Our next aim was to test whether enhanced hybrid peptide immunostimulatory activity boosts IFN-γ production and lytic activity of autologous CD8+ T cells. HER-2/neu(435-43), is an immunogenic CTL epitope, naturally presented by HER-2/neu overexpressing primary tumor cells and cell lines [20, 40, 45]. This epitope is recognized by HLA-A2.1+ CTL lines and clones. The PBMCs from an HLA-A2.1+ patient were evaluated in an in vitro CD4+/CD8+ T cell functional assay via an IFN-γ ELISPOT readout measuring cytokine recall response to patients-autologous tumor cells overexpressing HER-2/neu (Fig. 4). A significant increase in the number of specific IFN-γ spots was revealed after incubation of CD8+ T cells with hybrid peptides B- and D-stimulated autologous CD4+ T cells as compared to native peptide-stimulated CD4+ T cells [160 (B) and 205 (D) vs. 56 (native peptide) mean spots; P < 0.001]. A weaker, though noteworthy, enhancement of the CD8+ T cell response was observed upon incubation with hybrid peptide-F-primed CD4+ T cells (82 vs. 56 mean spots; P < 0.05 compared to native peptide). Mean specific IFN-γ spots produced by CD8+ CTL alone, or preincubated with non-stimulated CD4+ T cells were significantly less compared to those observed when the same CD8+ CTL were incubated with native peptide-primed CD4+ T cells (P < 0.01) (Fig. 4). In addition, HER-2(435-43)-specific CTL from the same patients lysed their autologous tumor cell targets more efficiently after incubation with hybrid peptide-B-stimulated CD4+ T cells than when preincubated with native peptide-stimulated CD4+ T cells (65 vs. 28% cytotoxicity at an E:T ratio of 40:1; P < 0.001) (Fig. 5a). Peptide D- and F-primed CD4+ T cells mediated a relatively weaker though significant helper effect (53 and 48% cytotoxicity; P < 0.005 compared to native peptide). Cytotoxicity with control CD8+ CTL (i.e., those preincubated with non-stimulated CD4+ T cells) was significantly less compared to that induced by native peptide-stimulated CD4+ T cells (Fig. 5a). In a similar fashion, HER-2/neu(435-43)-specific CTL from a second HLA2.1+ patient also lysed autologous tumor targets better after incubation with hybrid peptides than with the native peptide; approximately 55% specific lysis was observed with hybrid peptide-B-primed CD4+ T cells as compared to 22% when CD4+ T was primed with the native peptide. Peptide D- and F-stimulated CD4+ T cells induced almost a comparable augmentation of CD8+ T cell mediated cytotoxicity (47 and 52% specific lysis, respectively) (Fig. 5b). Cytotoxicity with control CD8+ CTL was significantly less compared to that induced by native peptide-stimulated CD4+ T cells. The CD4+ T cells primed with Ii-Key/HIVgag(164-76) hybrid peptide potentiated IFN-γ responses (Fig. 4) and cytotoxicity (Fig. 5a, b) of CD8+ T cells towards the autologous tumor cells at levels comparable to those induced by the native peptide-primed CD4+ T cells.
Fig. 4.
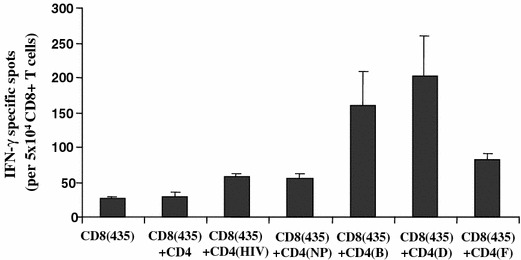
CD8+ T cells (from a lung cancer patient), primed with autologous CD4+ T cells specific for Ii-Key hybrid peptides, [CD4(B), CD4(D) and CD4(F) induced increased frequencies of HER-2/neu(435-43)-specific CTL as compared to those CD8+ T cells primed with the native peptide, [CD4(NP)], in an IFN-γ ELISPOT assay. HER-2/neu(435-43)-CTL which were either not preincubated with CD4+ T cells [CD8(435)] or were preincubated with non-stimulated CD4+ T cells [CD8(435) + CD4] or with CD4+ T cells primed with the control Ii-key/HIVgag(164-76) peptide [CD8(435) + CD4(HIV)], elicited comparable IFN-γ responses. Mean values ± SD from quadruplicate wells are illustrated
Fig. 5.
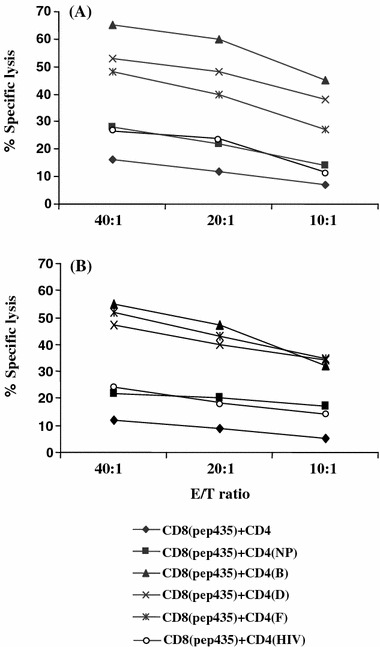
Increased cytotoxicity of HER-2/neu(435-43)-specific CD8+ T cells, [CD8(435)], from a lung (A) (the same donor as in Fig.4) and ovarian (B) cancer patient upon incubation with autologous CD4+ T cells stimulated with NP or hybrid peptides. Mean values from quadruplicate cultures are shown. The SD was always less than 15% of the means and thus omitted
These data suggested that B, D and F hybrid peptide-primed CD4+ T cells are capable of providing an augmented helper effect to their autologous HER-2/neu(435-43)-primed CD8+ T cells for lysing more efficiently the autologous tumor targets. In addition, they confirm that HER-2/neu(435-43) peptide is naturally processed and expressed by HER-2/neu overexpressing primary tumor cells.
The adoptive transfer of Ii-Key hybrid peptide-stimulated CD4+ T cells and autologous HER-2(435-43) CTL from an HLA-A2.1+ cancer patient protects SCID mice against the growth of the human SKOV3.A2 ovarian tumor cell line
We lastly sought to determine whether the hybrid peptide-stimulated CD4+ T cells were also more effective, as compared to the native peptide-stimulated CD4+ T cells in collaborating with autologous CTL, for the induction of antitumor responses in vivo. The transfer of HER-2/neu(435-43) CTL exerted high levels of antitumor activity against the growth of the transplanted SKOV3.A2 (HER-2/neu+, HLA-A2.1+) tumor cells in SCID mice. Solid tumor formation induced upon inoculation of SKOV3.A2 cells reached an area of 220 mm2 on day 84 as compared to a similar tumor size reached already on day 44 (Fig. 6) when mice were not treated. As also displayed in Fig. 6, transfer of HER-2/neu(435-43) CTL along with the autologous CD4+ T cells specific for the native peptide HER-2/neu(776-90) significantly improved the survival of SCID mice; on day 116, the tumor reached a size of 235 mm2 (P < 0.05). However, this improvement in survival was much greater when the HER-2/neu(435-43)-specific CTL was transferred with peptide-B, or peptide-F-specific CD4+ T cells (tumor size 225 mm2 by day 148 and 250 mm2 by day 140, respectively; P < 0.05 for both peptides compared to native peptides). As for controls, HER-2/neu(435-43) CTL transferred with non-stimulated CD4+ T cells gave similar results to those obtained with the transfer of HER-2/neu(435-43) CTL alone; tumor growth in SCID mice treated with CTL primed with an irrelevant HLA-A2.1-restricted peptide [gp(154-62)] along with peptide-B-specific CD4+ T cells was similar to the growth of untreated mice (Fig. 6). HER-2/neu(435-43)-specific CTL transferred either alone or together with hybrid peptide-stimulated CD4+ T cells remained without any effect against the growth of wild-type SKOV3 (not expressing HLA-A2.1) indicating the specificity of the in vivo CTL response (data not shown).
Fig. 6.
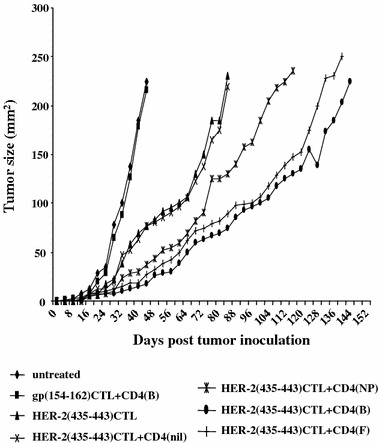
Therapeutic efficacy of HER-2/neu(435-43) CTL transferred along with peptide-specific autologous CD4+ T-cells in SCID mice xenografted with the HER-2/neu+ , HLA-A2.1+ human breast tumor cell line SKOV3.A2. CTL and CD4+ T cells (1 × 106 each subset) were ip. injected per mouse in groups of ten SCID mice, which were previously (12-6 days) inoculated sc. on the back with 5 × 105 SKOV3.A2 cells. Tumor growth was caliper-measured and recorded every 4 days in all groups until tumor area had reached a diameter > 200 mm2. Data are reported as the mean tumor area of 10 mice per group. The SD was always less than 25% of the means and thus, it was omitted. CTL primed with an irrelevant HLA-A2.1-restricted peptide [gp(154-62)] along with the CD4(B) T cells did not eradicate the growth of transplanted SKOV3. A2 tumor cells similarly as the untreated controls
Discussion
Toward the ultimate goal of enhancing peptide vaccines with MHC class II-epitopes of HER-2/neu, we have demonstrated that CD4+ T cells of patients with various HER-2/neu+ tumors are activated significantly better in vitro by Ii-Key/HER-2/neu MHC class II epitope vaccine therapeutic candidates than by the corresponding native peptide. The T cell repertoire from healthy donors harbored low level responder cells with modest sensitivity to the epitope represented in the context of hybrid peptides-B, -D and -F compared to the native peptide after three rounds of stimulation. The magnitude of the HER-2-specific cellular immune response in lymphocyte cultures from cancer patients was further amplified compared to the normal donor lymphocytes, being comprised of low as well as intermediate and high responders to hybrid peptides-B, -D and -F. Even though the trend of hybrid peptide potency in native epitope presentation is consistent with normal donor and cancer patients´ cells, the greater magnitude of immune response enhancement in patients might be attributed to HER-2/neu+ tumor-dependent immune activation [4, 38].
Immune responses were observed initially with total T cell cultures and confirmed with immunomagnetically purified CD4+ T cells, which were stimulated by autologous DC. Results demonstrated that hybrid peptides-B, -D and -F elicited the most potent T cell responses in the largest fraction of patients. Our studies further addressed whether hybrid peptide-primed antigen-specific reactive T cells are functionally active to better recognize HER-2/neu naturally processed and presented on autologous tumor cells that are induced to express HLA-DR for antigen presentation by IFN-γ treatment. Our data confirmed patient correlation of enhanced hybrid peptides-B, -D and -F potency to more efficiently sensitize patient’s T cells for increased recognition of the native peptide naturally expressed on autologous tumor cells similarly as when presented on DC. More specifically, these studies support the view that vaccinating patients with Ii-Key/MHC class II epitope hybrid peptides will elicit primed T cells that recognize the native HER-2/neu(776-90) peptide on the surface of the autologous tumor cells that are induced to express MHC class II by IFN-γ locally secreted from activated peptide-specific CD4+ or CD8+ T cells or even NK cells [13]. In addition, the primed T cells would also recognize the native HER-2/neu peptide processed from inclusion bodies of autologous HER-2/neu+ tumor cells undergoing apoptosis and presented by autologous DCs.
In addition to establishing the direct stimulatory potential of Ii-Key hybrids on CD4+ cells, it was important to demonstrate the functional significance of this antigen-specific stimulation in further potentiation of CTL enhancement. DCs are known to up-regulate the expression of MHC and co-stimulatory molecules that are required for efficient priming of CD8+ T cells upon interaction with peptide-specific CD4+ T helper cells. This interaction between CD4+ T cells and DC is mediated by cell surface molecules such as CD40 and CD40 ligand, as well as by soluble mediators [9, 39, 43]). These mediators, together with DC activation, facilitate the direct interaction between CD4+ and CD8+ T cells required for efficient generation of memory CD8+ T cells [11, 13] and thus, leading to long-term cytotoxic responses associated with better clinical outcomes. These and other studies [8, 17, 30, 32, 34, 42] demonstrate the crucial role of CD4+ T helper cells in the induction and maintenance of adequate CD8+ T cell-mediated anti-tumor response. To test the ability of hybrids to increase CD4+ T cell help in enhancing antitumor CTL responses, we co-cultured hybrid stimulated CD4+ T cells with CD8+ T cells primed by HER-2(435-43). This is an immunogenic CTL epitope naturally expressed by HER-2/neu overexpressing tumor cell lines and primary tumor cells from HLA-A2.1+ patients [20, 40, 45]. We showed that the CTL response is increased when CD4+ T cells are pre-stimulated with the hybrid peptides-B, -D or -F, the same hybrids found to optimally stimulate CD4+ T cells to recognize autologous tumor cells or the native peptide-pulsed onto autologous DCs. The CTL responses were enhanced by co-cultivation of CTL with hybrid stimulated immunopurified CD4+ cells, as measured by either IFN-γ ELISPOTs in response to the stimulatory autologous tumor cells or specific cytolysis against autologous tumor cell targets. More importantly, CD4+ T cells stimulated with hybrid peptides-B and -F displayed a higher degree of enhancement of the antitumor activity of autologous CD8+ T cells stimulated with HER-2(435-43) in xenografted SCID mice. CD4+ T cells primed with a control Ii-key/HIV gag(164-76) hybrid peptide, promiscuously binding to several HLA-DR alleles (25), could not recognize autologous tumor cells naturally expressing the HER-2/neu(776-90) epitope or autologous DCs pulsed with the HER-2(776-90) synthetic peptide. These demonstrated that the enhancing potency of the Ii-Key/HER-2/neu(776-90) hybrid, specifically affected CD4+ T cells recognizing the native HER-2/neu peptide and did not induce an overall stimulation via its Ii-Key moiety. In contrast, the Ii-Key/HIVgag hybrid peptide-induced CD4+ T cells did potentiate CD8+ T cell-mediated responses towards autologous tumor cells at levels similar to those induced by the native HER-2/neu(776-90) peptide. This is not surprising since helper epitopes unrelated to those recognized by CTL have been shown to activate CD4+ T cells for effective synergistic interactions with CD8+ T cells resulting in enhanced antitumor cytotoxic responses [14, 48]. However, the enhancing effect induced by the Ii-Key/HIVgag hybrid was inferior compared to those induced by hybrid peptides-B, -D, -F. This can be attributed to differences in (i) HLA-DR binding affinities and dissociation kinetics of HER-2/neu(776-90) versus HIVgag(164-76) and (ii) frequencies of CD4+ T cell clones induced by these peptides.
Our previous in vivo studies [23, 25] have so far indicated that the shorter the distance between the Ii-Key moiety and the epitope-containing segment, the better the potency of immune response enhancement; i.e., the peptides with a shorter distance between the C terminus of the 5-aminopentanoic acid spacer and the N terminus (P1 site residue) of the antigenic epitope were usually more potent than the homologs with additional amino acids N-terminal to the presented epitope. The outcome was consistent with the view that the additional spacer residues from the antigenic sequence are not needed and might compete in MHC desetope binding or permit the allosteric effector LRMK too much access to its site of action, thereby leading to an autorejection of the tethered MHC class II epitope. Hybrid peptide -D is consistent with this view. Hybrid peptide-F, which lacks the ava-spacer equivalent to 2.5 amino acids of the peptidyl backbone also fits the hypothesized theory of limited autorejection. However, this is not an established rule and may not be the case with promiscuous peptides. Considering the HLA-DR heterogeneity of humans, it is speculated that a promiscuously presented segment of the longer Ii-Key/HER-2/neu(776-90) hybrid peptide-B, i.e., a peptide presented by many different HLA-DR alleles, is comprised of multiple, closely overlapping, MHC class II epitopes, which are offset slightly in the linear sequence that is one, two or three adjacent amino acids near the N terminus of a longer, promiscuously presented peptide, might each constitute a P1 site residue of a shorter hybrid recognized by different HLA-DR alleles [44]. Whether the Ii-Key moiety can potentiate each of the respective epitopes equally well, depending upon the HLA-DR allele product that the hybrid encounters, or whether there are other factors that control the increased potency of Ii-Key hybrid peptides observed here will require larger studies.
At this point it is important to note that the levels of HLA-DR expression on tumor cells upon IFN-γ treatment, apparently did not influence the response profile of their autologous hybrid peptide-primed CD4+ T cells to the naturally processed peptide. For instance patients no. 5 and 6 (both with pancreatic Ca) whose tumors expressed significantly different levels of HLA-DR molecules (Table 2), both were high responders, and patients no. 1, 3 and 7 with almost equal levels of HLA-DR expression responded differently [intermediate (pat. no.1) and no responders]. Although the patterns of response are complex, certain conclusions appear to be well supported. Firstly, there is no obvious value in HLA-DR genotyping patients with HER-2/neu+ tumors prior to selecting Ii-Key hybrids for vaccination of such patients. The majority of the patients tested, regardless of HLA-DR genotype, responded equally well to hybrid peptides-B, -D and -F. Secondly, within a series of Ii-Key hybrids including sequences of a promiscuously presented peptide, the variance of response (intermediate and high responders) among potent members of Ii-Key homologous series is generally small, such that one potent hybrid, eliciting most consistently a strong response from the PBMCs of most patients can be justifiably selected for clinical trials. Given that it is difficult to find an individual who does not possess at least one DR allele that has affinity for the HER-2/neu epitope used in these studies, it may be of interest to establish whether a combination of the most potent hybrids identified here, (hybrid peptides-B, -D and -F) shows activity in a greater percent of PBMC samples than either alone. Perhaps of greater importance is the clinical evaluation of either of the most potent MHC class II epitope peptides alone with an MHC class I epitope peptide. To date, clinical responses to immunizations with either or both class I and MHC class II epitope peptides have been disappointing [3, 29, 54]. The data presented here show that linking MHC class II epitopes to the Ii-Key hybrid peptides augments their potency in terms of IFN-γ release by CD4+ T cells and that those cells are functionally more active as demonstrated by augmentation of CTL activity in vitro and in vivo. Recent data indicate the suppressive function of CD4+ CD25+ regulatory T cells on Th stimulation. Patients with various types of cancer have been demonstrated to express significantly different percentages of Tregs in their peripheral blood [50] or malignant effusion [15]. One must yet test whether the potent stimulation provided by the hybrid peptides-B, -D or -F allows Th cells to overcome the Treg-mediated suppression. Together with a growing body of evidence pointing to the importance of CD4+ T cell activity in successful tumor immunotherapy, these data support the view that the combination of an Ii-Key/MHC class II hybrid peptide and CTL epitope peptide (s) could become an important approach to the immunotherapy of cancer.
Footnotes
Nectaria N. Sotiriadou and Nikoletta L. Kallinteris have contributed equally to the work.
References
- 1.Adams S, Albericio F, Alsina J, Smith ER, Humphreys RE. Biological activity and therapeutic potential of homologs of an Ii peptide which regulates antigenic peptide binding to cell surface MHC class II molecules. Arzneimittelforschung. 1997;47:1069–1077. [PubMed] [Google Scholar]
- 2.Adams S, Humphreys RE. Invariant chain peptides enhancing or inhibiting the presentation of antigenic peptides by major histocompatibility complex class II molecules. Eur J Immunol. 1995;25:1693–1702. doi: 10.1002/eji.1830250632. [DOI] [PubMed] [Google Scholar]
- 3.Antonia S, Mule JJ, Weber JS. Current developments of immunotherapy in the clinic. Curr Opin Immunol. 2004;16:130–136. doi: 10.1016/j.coi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Baumgaertner P, Rufer N, Devevre E, Derre L, Rimoldi D, Geldhof C, Voelter V, Lienard D, Romero P, Speiser DE. Ex vivo detectable human CD8 T-cell responses to cancer-testis antigens. Cancer Res. 2006;66:1912–1916. doi: 10.1158/0008-5472.CAN-05-3793. [DOI] [PubMed] [Google Scholar]
- 5.Baxevanis CN, Dedoussis GV, Gritzapis AD, Stathopoulos GP, Papamichail M. Interleukin 1 beta synergises with interleukin 2 in the outgrowth of autologous tumour-reactive CD8 + effectors. Br J Cancer. 1994;70:625–630. doi: 10.1038/bjc.1994.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxevanis CN, Dedoussis GV, Papadopoulos NG, Missitzis I, Stathopoulos GP, Papamichail M. Tumor specific cytolysis by tumor infiltrating lymphocytes in breast cancer. Cancer. 1994;74:1275–1282. doi: 10.1002/1097-0142(19940815)74:4<1275::AID-CNCR2820740416>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Baxevanis CN, Sotiropoulou PA, Sotiriadou NN, Papamichail M. Immunobiology of HER-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol Immunother. 2004;53:166–175. doi: 10.1007/s00262-003-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164:3902–3912. doi: 10.4049/jimmunol.164.7.3902. [DOI] [PubMed] [Google Scholar]
- 9.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 10.Berger U, Wilson P, Thethi S, McClelland RA, Greene GL, Coombes RC. Comparison of an immunocytochemical assay for progesterone receptor with a biochemical method of measurement and immunocytochemical examination of the relationship between progesterone and estrogen receptors. Cancer Res. 1989;49:5176–5179. [PubMed] [Google Scholar]
- 11.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 12.Brinkman JA, Fausch SC, Weber JS, Kast WM. Peptide-based vaccines for cancer immunotherapy. Expert Opin Biol Ther. 2004;4:181–198. doi: 10.1517/14712598.4.2.181. [DOI] [PubMed] [Google Scholar]
- 13.Carlo ED, Cappello P, Sorrentino C, D’Antuono T, Pellicciotta A, Giovarelli M, Forni G, Musiani P, Triebel F. Immunological mechanisms elicited at the tumour site by lymphocyte activation gene-3 (LAG-3) versus IL-12: sharing a common Th1 anti-tumor immune pathway. J Pathol. 2005;205:82–91. doi: 10.1002/path.1679. [DOI] [PubMed] [Google Scholar]
- 14.Casares N, Lasarte JJ, de Lopez-Diaz Cerio, Sardoe P, Ruiz M, Melevo I, Prieto J, Borras-Cuesta F. Immunization with a tumor associated epitope plus a tumor-related or unrelated Th-helper peptide, elicits protective CTL immunity. Eur J Immunol. 2001;31:1780–1789. doi: 10.1002/1521-4141(200106)31:6<1780::AID-IMMU1780>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Chen YQ, Shi HZ, Qin XJ, Mo WN, Liang XD, Huang ZX, Yang HB, Wu C. CD4+ CD25+ regulatory T lymphocytes in malignant pleural effusion. Am J Respir Crit Care Med. 2005;172:1434–1439. doi: 10.1164/rccm.200504-588OC. [DOI] [PubMed] [Google Scholar]
- 16.Fisk B, Hudson JM, Kavanagh J, Wharton JT, Murray JL, Ioannides CG, Kudelka AP. Existent proliferative responses of peripheral blood mononuclear cells from healthy donors and ovarian cancer patients to HER-2 peptides. Anticancer Res. 1997;17:45–53. [PubMed] [Google Scholar]
- 17.Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T cell help is required to activate a memory CD8+ T cell to a fully functional tumor-killer cell. Cancer Res. 2002;62:6438–6441. [PubMed] [Google Scholar]
- 18.Gillogly ME, Kallinteris NL, Xu M, Gulfo JV, Humphreys RE, Murray JL. Ii-Key/HER-2/neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol Immunother. 2004;53:490–496. doi: 10.1007/s00262-003-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gritzapis AD, Mahaira LG, Perez SA, Cacoullos NT, Papamichail M, Baxevanis CN. Vaccination with human HER-2/neu(435-43) cytotoxic T lymphocyte peptide induces effective antitumor immunity against HER-2/neu expressing tumor cells in vivo. Cancer Res. 2006;66:5452–5460. doi: 10.1158/0008-5472.CAN-05-4018. [DOI] [PubMed] [Google Scholar]
- 20.Gritzapis AD, Sotiriadou NN, Papamichail M, Baxevanis CN. Generation of human tumor-specific CTLs in HLA-A2.1-transgenic mice using unfractionated peptides from eluates of human primary breast and ovarian tumors. Cancer Immunol Immunother. 2004;53:1027–1040. doi: 10.1007/s00262-004-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphreys RE, Adams S, Koldzic G, Nedelescu B, von Hofe E, Xu M. Increasing the potency of MHC class II-presented epitopes by linkage to Ii-Key peptide. Vaccine. 2000;18:2693–2697. doi: 10.1016/S0264-410X(00)00067-0. [DOI] [PubMed] [Google Scholar]
- 22.Jager E, Jager D, Karbach J, Chen YT, Ritter G, Nagata Y, Gnjatic S, Stockert E, Arand M, Old LJ, Knuth A. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101-103 and recognized by CD4(+) T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med. 2000;191:625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallinteris NL, Lu X, Wu S, Hu H, Li Y, Gulfo JV, Humphreys RE, Xu M. Ii-Key/MHC class II epitope hybrid peptide vaccines for HIV. Vaccine. 2003;21:4128–4132. doi: 10.1016/S0264-410X(03)00493-6. [DOI] [PubMed] [Google Scholar]
- 24.Kallinteris NL, Powell D, Blackwell CE, Kim M, Lu X, Wu S, Humphreys RE, Xu M, Von Hofe E (2006) Ii-Key/MHC class II epitope peptides as helper T cell vaccines for cancer and infectious disease. Frontiers in Bioscience:46-8 [DOI] [PubMed]
- 25.Kallinteris NL, Wu S, Lu X, Humphreys RE, von Hofe E, Xu M. Enhanced CD4+ T-cell response in DR4-transgenic mice to a hybrid peptide linking the Ii-Key segment of the invariant chain to the melanoma gp100(48-8) MHC class II epitope. J Immunother. 2005;28:352–358. doi: 10.1097/01.cji.0000170362.45456.00. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann DE, Bailey PM, Sidney J, Wagner B, Norris PJ, Johnston MN, Cosimi LA, Addo MM, Lichterfeld M, Altfeld M, Frahm N, Brander C, Sette A, Walker BD, Rosenberg ES. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J Virol. 2004;78:4463–4477. doi: 10.1128/JVI.78.9.4463-4477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawashima I, Hudson SJ, Tsai V, Southwood S, Takesako K, Appella E, Sette A, Celis E. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum Immunol. 1998;59:1–14. doi: 10.1016/S0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 28.Kiessling A, Schmitz M, Stevanovic S, Weigle B, Holig K, Fussel M, Fussel S, Meye A, Wirth MP, Rieber EP. Prostate stem cell antigen: Identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int J Cancer. 2002;102:390–397. doi: 10.1002/ijc.10713. [DOI] [PubMed] [Google Scholar]
- 29.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuball J, Schmitz FW, Voss RH, Ferreira EA, Engel R, Guillaume P, Strand S, Romero P, Huber C, Sherman LA, Theobald M. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–129. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee TV, Johnston DA, Thomakos N, Honda T, Efferson CL, Ioannides CG. Helper peptide G89 (HER-2:777-89) and G89-activated cells regulate the survival of effectors induced by the CTL epitope E75 (HER-2, 369-77). Correlation with the IFN-gamma: IL-10 balance. Anticancer Res. 2002;22:1481–1490. [PubMed] [Google Scholar]
- 33.Lu S, Reyes VE, Lew RA, Anderson J, Mole J, Humphreys RE, Ciardelli T. Role of recurrent hydrophobic residues in catalysis of helix formation by T cell-presented peptides in the presence of lipid vesicles. J Immunol. 1990;145:899–904. [PubMed] [Google Scholar]
- 34.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major “post-licensing-role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 35.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 36.Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez SA, Sotiropoulou PA, Sotiriadou NN, Mamalaki A, Gritzapis AD, Echner H, Voelter W, Pawelec G, Papamichail M, Baxevanis CN. HER-2/neu-derived peptide 884-99 is expressed by human breast, colorectal and pancreatic adenocarcinomas and is recognized by in-vitro-induced specific CD4(+) T cell clones. Cancer Immunol Immunother. 2002;50:615–624. doi: 10.1007/s002620100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rentzsch C, Kayser S, Stumm S, Watermann I, Walter S, Stevanovic S, Wallwiener D, Guckel B. Evaluation of pre-existent immunity in patients with primary breast cancer: molecular and cellular assays to quantify antigen-specific T lymphocytes in peripheral blood mononuclear cells. Clin Cancer Res. 2003;9:4376–4386. [PubMed] [Google Scholar]
- 39.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 40.Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiessling R. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol. 1999;163:1037–1044. [PubMed] [Google Scholar]
- 41.Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, Schiffman K, Disis ML. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9:5559–5565. [PubMed] [Google Scholar]
- 42.Schnell S, Young JW, Houghton AN, Sadelain M. Retrovirally transduced mouse dendritic cells require CD4+ T cell help to elicit antitumor immunity: implications for the clinical use of dendritic cells. J Immunol. 2000;164:1243–1250. doi: 10.4049/jimmunol.164.3.1243. [DOI] [PubMed] [Google Scholar]
- 43.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 44.Sotiriadou R, Perez SA, Gritzapis AD, Sotiropoulou PA, Echner H, Heinzel S, Mamalaki A, Pawelec G, Voelter W, Baxevanis CN, Papamichail M. Peptide HER2(776-88) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer. 2001;85:1527–1534. doi: 10.1054/bjoc.2001.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sotiropoulou PA, Perez SA, Voelter V, Echner H, Missitzis I, Tsavaris NB, Papamichail M, Baxevanis CN. Natural CD8+ T-cell responses against MHC class I epitopes of the HER-2/ neu oncoprotein in patients with epithelial tumors. Cancer Immunol Immunother. 2003;52:771–779. doi: 10.1007/s00262-003-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuttle TM, Anderson BW, Thompson WE, Lee JE, Sahin A, Smith TL, Grabstein KH, Wharton JT, Ioannides CG, Murray JL. Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer patients. Clin Cancer Res. 1998;4:2015–2024. [PubMed] [Google Scholar]
- 47.Wang RF. Human tumor antigens: implications for cancer vaccine development. J Mol Med. 1999;77:640–655. doi: 10.1007/s001099900042. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Miyahara Y, Kato T, Wang L, Aota T, Kuribayashi K, Shiku H. Essential roles of tumor-derived helper T cell epitopes for an effective peptide-based tumor vaccine. Cancer Immunity. 2003;3:16–29. [PubMed] [Google Scholar]
- 49.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999;170:85–100. doi: 10.1111/j.1600-065X.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 50.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 51.Xu M, Capraro GA, Daibata M, Reyes VE, Humphreys RE. Cathepsin B cleavage and release of invariant chain from MHC class II molecules follow a staged pattern. Mol Immunol. 1994;31:723–731. doi: 10.1016/0161-5890(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 52.Xu M, Jackson R, Adams S, Humphreys RE. Studies on activities of invariant chain peptides on releasing or exchanging of antigenic peptides at human leukocyte antigen-DR1. Arzneimittelforschung. 1999;49:791–799. [PubMed] [Google Scholar]
- 53.Yannelli JR, Wroblewski JM. On the road to a tumor cell vaccine: 20 years of cellular immunotherapy. Vaccine. 2004;23:97–113. doi: 10.1016/j.vaccine.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 54.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369-77) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–4908. [PubMed] [Google Scholar]


