Abstract
Background
Centenarians are exceptionally long living individuals who escaped the most common age-related diseases. In particular they appear to be effectively protected from cancers. The mechanisms that underlie this protection are quite complex and still largely unclear.
Aim
To critically analyse the literature in order to propose a unifying hypothesis that can account for this cancer protection in centenarians.
Methods
Review of the scientific literature regarding three main players in tumourigenesis such as IGF-1, inflammation and p53, and centenarians.
Results
Centenarians appear to be characterised by low IGF-1-mediated responses and high levels of anti-inflammatory cytokines such as IL-10 and TGF-β, a condition that results in protection from cancer. Both inflammation and IGF-1 pathway converge on the tumour suppressor p53. Accordingly, some studies indicate that genetic variants of p53 are associated with human longevity by providing protection from cancer mortality.
Conclusions
The available data let us to hypothesise that among other possible mechanisms, well-preserved p53-mediated responses are likely a key factor contributing to protection from cancer in centenarians.
Keywords: Centenarians, Cancer, p53, IGF-1, Inflammation
Introduction
Centenarians are the best living example of successful ageing, as they largely avoided, postponed or survived the majority of life-threatening, age-related diseases such as cancer, diabetes, and cardiovascular diseases [1]. As far as neoplastic diseases are concerned, studies on autopsy records revealed that centenarians are characterised by a lower than expected incidence of cancer, together with a decline of metastatic rate, and a decrease in mortality due to cancer (see [2] for references). Accordingly, it has been reported that the mortality due to cancer constantly decreases after the age of 85–90 years [3]. Therefore, it seems that centenarians are endowed with a peculiar resistance to cancer. The reason for this phenomenon is still largely unclear. It is likely that protection from cancer is not determined by a single molecular mechanism, but rather by the interaction among different ones, and between these mechanisms and the environment. In this regard, we reported some years ago that a well-maintained NK activity, as it is found in centenarians, can be one of these mechanisms [4]. In this review we will take in consideration other possible mechanisms and in particular we will briefly summarise the data indicating the involvement of IGF-1 pathway and inflammation in this phenomenon. These two pathways both converge on p53 protein, the well-known “guardian of the genome” that is widely recognised as an efficient anti-cancer defence [5]. Given the inherent importance of p53 for human cancers as well as its functional links with IGF-1 pathway and inflammation, it can be hypothesised that p53 is a key element in protecting centenarians from cancers. However, it is not precisely understood how p53 determines the level of protection from cancer in aged people. Given the complex network of interactions that p53 has with a high number of proteins, it is possible that genetic pool and epigenetic modifications of the components of such a network can play a role. In this regard, the age-related changes in p53 interactome are currently the subject of studies. Another interesting hypothesis can be also put forward, i.e. that p53 itself undergoes a series of age-related post-translational modifications that alter its functionality. A possible decrease in p53 activity seems to be a plausible explanation for the correlation between tumourigenesis and the ageing process. No data are available in this regard on humans. Nevertheless, recently published data suggest that an age-related accumulation of a conformational mutant form of p53 occurs in human peripheral blood mononuclear cells (PBMC) [6]. This mutant-like form of p53 lacks transcriptional activity and the reason/s of this accumulation is/are presently unclear, even if oxidative stress likely plays a crucial role. We think that these data are worth to be deepened and extended to centenarians, within a scenario of profound and important age-related modification of p53 and p53-mediated responses in humans.
Insulin/IGF-1: a trade-off between cancer and longevity
Insulin-like growth factors (IGFs) comprise a family of peptides that play important roles in mammalian growth and, among them, IGF-1 is a polypeptide mediating many of the growth-promoting hormone effects, being essential for a normal development [7]. In yeast, worms, and flies, the evolutionary conserved glucose or insulin/IGF-1-like pathways down-regulate antioxidant enzymes and heat shock proteins, reduce the accumulation of glycogen or fat, and control growth together with mortality [8]. Mutations that reduce the activity of these pathways appear to extend animal longevity [9]. In animal models like mouse, the down-regulation of IGF-1 pathway is associated with an extension of life span [10, 11], whereas high levels of IGF-1 are associated with a shortened life span [11]. Also in humans this pathway appears to be crucial for longevity and data obtained in long-lived individuals, such as centenarians, showed that longevity is associated with decreased plasma levels of IGF-1 and preserved insulin sensitivity [12, 13]. On the basis of these data, we tested the hypothesis that the IGF-1 plasma level could be genetically controlled, and we investigated the role of genetic variability at some human loci that share similarities with the genes that regulate the insulin/IGF-1 pathway in invertebrates [14]. The major findings emerging from these studies were that Italian long-lived people (aged >85 years of age) had IGF-1 plasma levels lower than younger controls (that can be an expected but not trivial result, considering the association between low IGF-1 levels and frailty) and that these levels were affected by polymorphisms at insulin-like growth factor type 1 receptor (IGF-1R) and phosphoinositide 3-kinase (PI3KCB) genes that were found more frequently in centenarians [15]. This study was the first indication that genetic variability in the genes responsible for IGF-1 regulation plays a role in human longevity as well, indicating that the impact of these genes on longevity is an evolutionary conserved property, from yeast to humans. Other authors had confirmed our results by finding a reduced functionality of Insulin/IGF-1 signaling in different populations such as elderly Dutch people [16] and Ashkenazi Jewish centenarians [17]. Thus, it seems that a reduction in Insulin/IGF-1 pathway function is an important determinant of longevity also in humans and not only in animals, but the reasons for the survival advantage brought by this reduction are not immediately evident. Indeed, an efficient IGF-1 signaling should provide the individual with greater body mass and one could think that the biggest and strongest individuals should be favoured in the struggle for survival and protected against disabilities and mortality. To this regard, it has been shown that low serum levels of IGF-1 are detrimental in aged people, especially in terms of physical performance and muscle strength maintenance [14]. Furthermore, a relationship has been observed between IGF-1 and inflammation, and in particular IL-6, one of the best-known pro-inflammatory cytokines. Pro-inflammatory cytokines often act as negative regulatory signals that temper the action of hormones and growth factors. We found that, not only muscle strength is inversely correlated with IL-6 plasma levels [14], but it also appears that IGF-1 and IL-6 have an inverse impact on other important outcomes such as disability and mortality. In a longitudinal survey it has been shown that older women with low serum levels of IGF-1 and high serum levels of IL-6 have the highest risk of disability and mortality, in comparison with women who have low levels of IL-6 and high levels of IGF-1 [18]. Such a beneficial effect of high IGF-1 serum level in the elderly is in contrast with the above reported data showing that reduced IGF-1 plasma levels are associated with longevity [15]. How can these data be reconciled? It has to be considered that IGF-1 has been implicated in several chronic age-associated diseases, including cancer, heart disease, and osteoporosis [19]. In particular, both IGF-1 and IGF-1R have been reported to play important roles in the establishment and maintenance of the transformed phenotype [20]. It can be hypothesised that the decrease of plasmatic IGF-1 observed in nonagenarians and centenarians might minimise the risk of cancer brought by IGF-1 by decreasing global mitogenic stimulation. The price to pay is frailty and massive reduction of muscle strength, two characteristics of such very old people [18, 19]. In this perspective, a sort of trade-off between cancer risk and frailty can be envisaged. On one hand, elderly people with elevated IGF-1 are protected against sarcopenia and disability but have a higher risk of cancer, while on the other hand elderly people with lower IGF-1 levels are at risk of disability and frailty but have a lower risk of cancer. This trade-off is likely an evolutionarily conserved “survival” response that shifts the organism’s resources from growth to maintenance, as an adaptation to stresses such as DNA damage. To this regard, studies on mice defective for the DNA repair gene Ercc1 have shown that the GH-IGF-1 axis is suppressed likely as a defense response toward DNA damage accumulation [21]. The mechanism by which Ercc1 deficiency induces the suppression of the somatotroph axis is yet unknown. These Ercc1−/− animals display a dramatic premature senescence, but still this IGF-1 suppression can be considered beneficial for longevity since it leads to metabolic changes that shift energy usage from growth and proliferation to protective maintenance, aimed at counteracting the accumulation of stochastic DNA damages. This shift includes a series of gene expression changes shared by a number of animal strains independently on how long they can live (progeroid, normal, or long-living) [22] and protects the organism from cancer and promotes late life survival, likely also in a context of a chronic pro-inflammatory status typical of the elderly that we will discuss in the next section. In this perspective centenarians, who have very low levels of serum IGF-1, could be seen not as IGF-1-deficient subjects, but rather as the people who have the most tightly preserved connection between DNA damage sensing and suppression of somatotroph axis.
Inflammation and cancer in the oldest old
It is known that inflammatory diseases increase the risk of developing many types of cancer (including bladder, cervical, gastric, intestinal, oesophageal, ovarian, prostate, and thyroid cancer), and that inflammatory cells and mediators are present in the microenvironment of most, if not all, tumours, irrespective of the trigger for development [23]. Cancer-related inflammation has been recently described as “extrinsic” (driven by inflammatory conditions that increase the risk of cancer) or “intrinsic” (due for example to the activation of oncogenes that induce a transcriptional pattern similar to that which occurs during inflammation) [23]. On the other side, it has been observed that ageing is characterised by an increase in the level of pro-inflammatory markers, and this state of sub-clinical, chronic inflammation has been called “inflamm-ageing” [24]. Thus it would be expected that inflamm-ageing can be a trigger for a series of diseases with an inflammatory pathogenesis such as diabetes, neurodegeneration, cardiovascular pathologies and, as discussed, also cancer. This is in fact the general picture found in elderly people. To double-check this hypothesis, it should be demonstrated that centenarians who escaped these diseases, on the contrary, have no signs of inflamm-ageing. Quite surprisingly, centenarians have been found to display elevated markers of inflammation [25]. This apparent paradox is blunted by the fact that at the same time centenarians display high levels of anti-inflammatory agents such as IL-10, IL1-Ra, and TGF-β, that likely counteract the effect of the pro-inflammatory ones [26–28]. Moreover, it is also possible that some genetic findings obtained from centenarians indicative of the presence of inflammation risk factors have in fact a different biological meaning. As an example, it has been reported that centenarians have higher frequencies of the 4G allele and of the homozygous 4G4G genotype of the plasminogen activator inhibitor 1 (PAI-1) gene with respect to young subjects [29]. The 4G allele is associated with high serum levels of PAI-1, which in turn is considered a predictor of myocardial infarction and also a marker of poor prognosis for a series of cancers. Thus, it is paradoxical that genotypes associated with high levels of such a risk factor are more represented in centenarians than in young people. It is possible that other actions of PAI-1 might overcome in very old age the detrimental ones (pro-inflammatory, inhibition of fibrinolysis, etc.). As an example, it has been reported that PAI-1 is necessary and sufficient to induce cell senescence [30]. In particular, PAI-1 knockdown results in sustained activation of the PI(3)K-AKT-GSK3β pathway and nuclear retention of cyclin D1, consistent with a role for PAI-1 in regulating growth factor signalling. In agreement with this, it has been found that the PI(3)K-AKT-GSK3β-cyclin D1 pathway is also causally involved in cellular senescence [30]. This pathway, at least in its upstream part, is the same that is activated by IGF-1 and, as we have discussed above, the down-regulation of this pathway seems to be correlated with longevity. Thus it is plausible that high levels of PAI-1 can have a double-sided role for longevity: negative on one side, as risk factors for cardiovascular diseases and cancers, but positive on the other side, as inhibitor of the PI(3)K-AKT pathway. At present it is unclear what elements control and decide what has to be the prevalent effect of high levels of PAI-1 (negative or positive for longevity).
The plasma levels of some cytokines important for inflammation and anti-inflammation (such as IL-6 and IL-10) have been found affected by common genetic polymorphisms in the correspondent genes (reviewed in [2, 31]). Accordingly, we and other authors observed that genotypes associated with production of high amounts of pro-inflammatory cytokines such as IL-6 and of low amounts of anti-inflammatory ones such as IL-10 are less represented in long-lived people and centenarians, thus supporting the hypothesis that inflammation in old age represents a jeopardy for the survival. In fact, those individuals who are genetically predisposed to produce high levels of IL-6 have a reduced capacity to reach the extreme limits of human life [32], whereas the high IL-10-producer genotype is increased among centenarians [26]. The gene of another important pro-inflammatory cytokine, TNF-α, involved in cancer onset and metastasis [23], has a (A/G) polymorphism at position −308 that is associated with age-related diseases, and we found that the frequency of the A allele is decreased with age in old men, including centenarians, but not in women [33].
This opposite effect of IL-6 and IL-10 common gene polymorphisms in longevity is intriguing, indeed, inflammatory genotypes may be both friends and foes. In fact, inflammation is an important and necessary part of the normal host responses to pathogens, but the overproduction of inflammatory cytokines might cause immune-inflammatory diseases and eventually death. In fact, our immune system has evolved to control pathogens, so pro-inflammatory responses are likely to be evolutionarily programmed to resist fatal infections in the early phases of life. However, in the post-reproductive period of life, strong inflammatory responses may represent a risk factor for many non-infectious diseases including cancer, while weak responses may on the contrary hinder their onset [25]. These conditions might result in an increased chance of long-life survival in an environment with a reduced antigen (i.e. pathogens) load.
We observed that IL-6 genetic variability influence the amount of the cytokine in patients with colorectal cancer, in particular in presence of hepatic metastasis [34]. Another interesting aspect of the complex role of inflammation results from recent data showing opposite roles played by IL-6 as both cancer promoter and inhibitor. Indeed, it has been recently reported that as a paracrine factor, secreted IL-6 contributes to tumourigenesis by promoting angiogenesis [35]. Furthermore IL-6 is a determinant factor for the acquisition of a malignant phenotype in an in vitro model of breast cancer [36], and it is capable to down-regulate p53 by maintaining TP53 promoter methylation [37]. On the other side, IL-6 has been observed to act in the autocrine way in the induction and maintenance of oncogene-induced cell senescence, an efficient mechanism to suppress tumourigenesis [38] and it appears to be down-regulated by p53 likely via a p53-dependent reduction in the expression of the NF-κB subunit p65 [39]. It is thus possible that cytokines such as IL-6 can play a complex double-edged role in neoplastic diseases and represent a bridge between inflammation, cell senescence and cancer, with a feedback loop with another key player for the control of these phenomena, that is p53, which will be the topic of the next paragraphs.
P53: a connection point between IGF-1 and inflammation
As discussed in the previous paragraphs, IGF-1 pathway and inflammation are deeply involved in tumourigenesis and centenarians seem to be protected against cancer through mechanisms involving low levels of circulating IGF-1, genetic variants predisposing to low IGF-1-mediated responses, and low inflammatory responses. Recent studies have demonstrated that both IGF-1/insulin signalling pathway and inflammation are interconnected with p53 protein. p53 gene, TP53, is a critical tumour suppressor gene that is mutated in about half of all human cancers [40, 41] and loss of TP53 predisposes mice and humans to increased morbidity and mortality (reviewed in [42]). Apart from its well known action on cell cycle, DNA repair, apoptosis and cell senescence, it has been demonstrated that p53 can exert its tumour suppressive activity by inhibiting IGF-1 pathway at various stages (it suppresses Igf-1R transcription, it regulates the levels PTEN and TSC2, which antagonise the signalling of IGF-1 to Akt and mTOR, respectively, and it stimulates the transcription of IGFBP-3, which inhibits the activation of IGF-1R) (reviewed in [43]).
As for inflammation, it is known that inflammatory responses induce reactive oxygen and nitrogen species including hydrogen peroxide, nitric oxide, and reactive intermediates such as hydroxyl radicals, superoxide and peroxynitrite. To avoid the damaging effects of these compounds several defence mechanisms have evolved. One of the genes at the cross-roads of cellular stress response networks is TP53, which, in response to inflammatory stress, can trans-activate or trans-repress a number of genes [44]. Indeed, p53 function not only as a sequence-specific transcriptional activator in the G1 and G2 phases of the cell cycle [45], but it also mediates the repression of a number of target genes [46]. In fact, it is estimated that up to 80% of p53-responsive genes are repressed rather than activated [47]. Accordingly, loss of p53 function is reported to be associated with increased inflammatory responses and increased T cell responses in mice [48]. In particular, it has been reported to down-regulate the transcription of genes such as metalloproteinase-13, IL-6, IL-2, c-fos, and c-jun and, as discussed, competitively inhibits others transcription factors such as NF-κB p65 (discussed in [48]). These factors are important mediators of inflammation, such as IL-6, as discussed in the previous paragraph, or promote the activation of immune responses, responsible in turn for inflammation to occur.
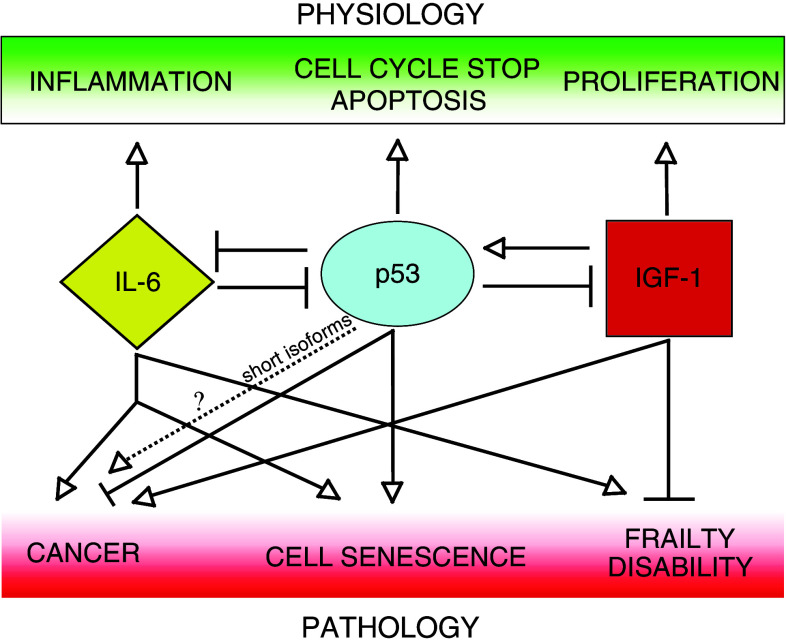
Thus, on the basis of what has been discussed in the first two paragraphs, the overall picture emerging from the available experimental evidence, summarised in Fig. 1, is that IGF-1 pathway activation and inflammatory responses represent a risk factor for tumourigenesis and p53 can negatively regulate both of them, so that at least a part of the anti-cancer activity of p53 can be attributed to this capability.
Fig. 1.
The complex interplay between IL-6, p53 and IGF-1 and its effect on physiology and pathology. The dashed line indicates a possible role for some short p53 isoforms in tumourigenesis (see text). Nevertheless, this role has yet to be clarified
P53 activity in ageing
It has long been considered that TP53 is a longevity-assurance gene in the sense that it acts on longevity by preventing tumours, since p53-deficient mice live shorter than normal counterpart because of rapid development of tumours [49]. Nevertheless, in the case of p53-competent animals, the situation was considered a little bit more complicated and controversial. Indeed, it has been observed that in mice the expression of a truncated form of p53 coding for the C-terminal fragment of the protein (called M protein) led to increased tumour suppression but also decreased longevity, likely because of a deleterious effect on stem cell proliferative capability [50]. Thus it was hypothesised that p53 activity against cancer was at the expenses of longevity [18]. It has been proposed that in TP53+/m mice the age-associated accumulation of mutations provokes augmented cell cycle arrest or apoptosis responses, thus preventing tumour formation, but also decrease the number of division-competent stem cells to a point in which their functional capacity is so reduced that sufficient numbers of mature cells cannot be provided to maintain organ homeostasis [51]. Recently, it has been shown that such a truncated form of p53 leads to an alteration of wild type (wt) p53 stability, localisation and activity [52]. In particular the M protein interacts with wt p53, increases its stability, and facilitates its nuclear localisation even in the absence of stress.
Nevertheless, a different TP53 transgenic mouse model displays an increased activity of tumour suppression and ages normally [53]. These “Super-p53 mice” are provided with supernumerary copies of the TP53 gene in the form of large genomic transgenes. These supernumerary copies of TP53 are surrounded by regulatory sequences that are identical to those at the endogenous TP53 locus, thus TP53 gene is under normal regulatory control and this avoids abnormal activity, likely responsible for the observed deleterious side effects (premature ageing). More recently, a new model of genetically manipulated mice with increased, but otherwise normally regulated levels of Arf and p53, presents strong cancer resistance and has decreased levels of ageing-associated damage [54].
These experimental evidences on mice are in agreement with the hypothesis that TP53 acts as a longevity gene in mammals basically by shutting down tumourigenesis. If this is true, it is possible that the increased age-related incidence of cancer can be due not only to an accumulation of mutations, but also to a possible age-related decrease in p53-mediated responses. In agreement with this hypothesis, it has been reported that the efficiency of the p53 response to gamma-irradiation was found to decline significantly in various tissues of ageing mice, including lower p53 transcriptional activity and p53-dependent apoptosis [55]. This decline resulted from a decreased stabilisation of the p53 protein after stress. The function of the Ataxia-telangiectasia mutated (ATM) kinase, which activates p53 by phosphorylating it at Ser-15 [56], declined significantly with age, which may then be responsible for the decline of the p53 response to radiation in mice [55]. Declining p53 responses to other stresses were also observed in the cultured splenocytes from ageing mice. Interestingly, the time of onset of this decreased p53 response correlated with the life span of mice; mice that live longer delay their onset of decreased p53 activity with time [55]. On the basis of their results, these authors suggest that an enhanced fixation of mutations in older mice should occur because of the declining fidelity of p53-mediated apoptosis or senescence in response to stress [55]. Furthermore, this age-related decline in p53-mediated responses suggests a plausible explanation for the correlation between tumourigenesis and the ageing process. No evidence in this regard has been reported to date in human ageing, where a correlation between age and tumours is also present. Lack of p53 functional activity, beside gene mutations, can be due to conformational changes in its tertiary structure. In fact, p53 DNA-binding domain exhibits a high degree of conformational flexibility. Such feature permits the existence of at least two different conformational isoforms in human cells, reported as “wild type” and “mutant-like.” This latter does not present sequence mutations and it is transcriptionally inactive due to the oxidation of the DNA-binding domain [57]. It has been recently reported that this mutant-like form of p53 (p53mut) does accumulate in dermal fibroblasts and PBMC of patients with Alzheimer Disease (AD) [6, 58]. Some years ago it was also demonstrated that fibroblasts of AD patients display a profound impairment in the H2O2-activated, p53-dependent pathway, which results in a lack of activation of p53 or p53-target genes, including p21, GADD45 and bax [59]. As a whole, these data suggest that there is a decrease in p53 activity in at least some tissues of AD patients. Interestingly, it has been observed that the percentage of PBMC highly positive for monoclonal antibodies specific for p53mut increases with age even in non-demented people [6]. Thus, it could be hypothesised that the accumulation of p53mut with age impacts on p53-mediated responses, leading to their decrease similarly to what has been observed in mice. The mechanisms that lead to this age-dependent decrease in p53 functionality would thus be different between mice and humans (mediated by ATM decline in mice and by accumulation of conformational mutant form in humans) but with a similar final result. Further studies are needed to confirm this hypothesis, also taking into account that this p53mut is very abundant in fibroblasts and PBMC from AD patients, as mentioned [58] and that an inverse correlation seems to exist between AD and cancer incidence [60]. This is an apparent paradox, since it would be expected that, if “mutant-like” p53 accumulation is really indicative of a progressive decrease in p53 activity, then cancer incidence should be high in AD patients, while in fact it is not. There can be different explanations for this paradox. First of all, it has to be considered that nanomolar concentrations of Amyloid-β1-40 peptide are able to induce the conformational change of p53 [61], and the increased amount of p53mut in cells from AD patients may simply reflect the presence of such peptide in the cellular environment. Further, it is possible that p53 is substituted for by other similar ones such as p63 and p73, and/or that other molecular pathways can substitute for the p53 one in AD. The amount of the remaining wt p53 should be also taken into consideration and the ratio between wt p53 and p53mut should be considered in order to better understand what it is really going on during ageing, i.e. to understand whether an increase in the percentage of cells positive for the p53mut means a real increase in the expression of this isoform or rather merely reflects a general increase of p53 expression that leaves unchanged, or even increases, the amount of wt p53.
It is at present not known whether in centenarians there is a high or low percentage of cells positive for p53mut. Studies in this direction are actually ongoing. If these percentages would result to be lower with respect to old people and more similar to young people, one could interpret this datum as an indication that centenarians have a well-preserved p53 function that can justify the observed decrease in cancer incidence. On the contrary, if this percentage would result to be similar to that of old people, thus the protection from cancer should likely be explained by other mechanisms, which in any case are not excluded by our p53-based hypothesis.
An age-related change in p53 interactome should also be considered as a determinant of a possible decrease of p53 activity. Indeed, many proteins interact with p53 and activate or inhibit it. It is at present unknown if these interactors undergo modification(s) in their expression during ageing thus affecting p53 activity as observed in mice [55].
It is known since many years that p53 can exist in different isoforms, shorter than the full length [62]. Recently it has been reported accordingly that TP53 gene has an unforeseen complex regulation similar to that of p63 and p73 genes [63]. This includes multiple splicing at intron 2 and 9, alternative promoter and alternative initiation of translation. This complex regulation leads to the formation of nine possible short isoforms of p53, which can modulate p53 activity as far as gene transcription, apoptosis, and capability to bind to DNA is concerned [64]. Some of these isoforms can enhance p53 target gene expression, while others, such as the so-called Δ133p53 are dominant-negative toward full-length p53 [63]. Δ133p53 is found to be expressed in many breast tumours [63], so that it is possible that a failure of appropriate regulation of p53 isoforms plays role in tumourigenesis, as indicated in Fig. 1 (dashed line). To date, it is not yet completely understood the precise role and relevance of such isoforms, especially for the ageing process. It is nevertheless possible to hypothesise that a genetic-based capability to modulate the expression of such short p53 isoforms can have some importance in the protection from cancer and thus impact on longevity, for example by stabilizing wt p53, or on the contrary by competing with wt p53 at the level of p53 responding elements on the DNA. Some suggestions of a possible relevance for ageing of the short p53 isoforms come indirectly from the mouse model described by Tyner et al. [50] and discussed above, in which a truncated form of p53 is expressed together with a wt one. This mouse model exhibits premature ageing, but it is unknown whether the truncated p53 isoform it expresses (the M protein) is similar to those occurring in wt animals or in human cells. Moreover, it is not clear if these alternative p53 variants are always expressed at the same extent during lifetime or rather if they can undergo an age-related modulation. All these questions need to be answered by future studies.
Conclusion
As discussed along this paper, it appears that centenarians are protected from cancer by at least two different mechanisms: low IGF-1-mediated responses and elevated production of anti-inflammatory mediators. We summarised here the main experimental data obtained in this regard and discussed also the possibility that a central link between these two pathways is constituted by p53. In particular, we hypothesise that centenarians are endowed with a high protection from cancer because of a well-preserved p53-mediated response able to block tumourigenesis or to decrease cancer aggressiveness. This can be likely due to individual genetic variants (e.g. TP53 gene variants more active in inducing apoptosis or cell senescence) and epigenetic modifications (p53 protein modifications such as acetylation, phosphorylation, ubiquitination, or redox modifications that impinge upon protein stability, activation, turn-over, and functionality). To this regard, early studies from our group indicated that a genetic variant of TP53 due to a polymorphism at codon 72 leading to two functionally different isoforms of p53 protein (one with an arginine residue at position 72, the other with a proline residue) was not differently distributed among age classes, including centenarians, in the Italian population [4]. Nevertheless, a more recent study reported a meta-analysis indicating that subjects carrying the TP53 codon 72 proline allele have a survival advantage in spite of a higher risk of cancer in long living people [65], even if the association appears to be dependent on the type of tumour and on the genetic background of the population studied. Indeed, we recently reported that the TP53 codon 72 arginine allele is more frequent in patients with colorectal cancer with respect to age-matched controls and centenarians [66]. The results obtained on the Dutch population have been partially confirmed by another study in Danish population, in which the authors suggest that increase longevity of TP53 codon 72 proline allele carriers may be due to increased survival after a diagnosis of cancer or other life-threatening diseases [67]. However, it is to mention that p53 has a crucial role not only in cancer but also in a number of other age-related pathologies, such as cardiovascular diseases that are among the major causes of death in western Countries [68]. It is thus possible that the difference between young and long-living people observed in different populations, such as, for example in Italians, Danish and Dutch regarding the frequency of TP53 codon 72 genotype can be explained by hypothesising that different TP53 genotypes may predispose to different age-related diseases, and thus the genotype frequencies observed in centenarians or long-living people could be the result of a population-specific balancing between opposite tendencies towards an increased or decreased risk of many different life-threatening diseases (cancer, cardiovascular diseases, etc.). We have reported that the two p53 isoforms resulting from this polymorphism at codon 72 differ in the capability to induce apoptosis and cell senescence in ex vivo experiments only when cells were obtained from aged individuals [69, 70]. All together, these results suggest that the activity of p53 changes with age due to genetic polymorphisms and/or epigenetic age-related modifications. Consequently, it can be hypothesised that centenarians are endowed with a well preserved p53 activity as a result of the combination of genetics and epigenetics that allow them to be protected from cancer, and in particular p53 can play a direct role as a tumour suppressor, or it may act indirectly through its involvement in the IGF-1 pathway and in inflammation, as discussed above. Of course it is not excluded that other mechanisms can co-exist in order to ensure protection from cancer in these exceptional subjects. Further studies are needed to verify this hypothesis by means of genomic and proteomic analysis on cells obtained from subjects of different age, including centenarians, focused on p53 and its downstream transcriptional targets as well as its interactors, also in the light of the possibility in the near future to inhibit or enhance p53 functions pharmacologically for therapeutic purposes, such as cancer treatment.
Acknowledgments
This work was supported by: EU (European Union) Grant “PROTEOMAGE” Contract n. FP6-518230; the PRRIITT program of the Emilia-Romagna Region (and Fondi Strutturali Obiettivo 2); Italian Ministry of Health Grant “Progetto Finalizzato «Studio delle differenze uomo-donna nei meccanismi patogenetici delle malattie cardiovascolari»” to C. Franceschi; Italian Ministry of University and Research (MiUR) PRIN 2006 Project to C. Franceschi (no. 2006061707), and S. Salvioli (no. 2006063387); University of Bologna Grant “Ricerca Fondamentale Orientata (RFO ex 60%) 2005”; Roberto and Cornelia Pallotti Legacy for Cancer Research Grants to C. Franceschi and S. Salvioli. University of Bologna “Progetti Strategici” 2006 grant (“p53 e patologie non neoplastiche nell’anziano: uno studio multidisciplinare sul ruolo del polimorfismo al codone 72 del gene TP53”) to S. Salvioli.
Footnotes
This article is part of the Symposium in Writing on “Impact of Ageing on Cancer Immunity and Immunotherapy”.
References
- 1.Franceschi C, Motta L, Motta M, Malaguarnera M, Capri M, Vasto S, Candore G, Caruso C, CE IMUS. The extreme longevity: the state of the art in Italy. Exp Gerontol. 2008;43:45–52. doi: 10.1016/j.exger.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 3.Piantanelli L. Cancer and aging: from the kinetics of biological parameters to the kinetics of cancer incidence and mortality. Ann N Y Acad Sci. 1988;521:99–109. doi: 10.1111/j.1749-6632.1988.tb35268.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonafè M, Barbi C, Storci G, Salvioli S, Capri M, Olivieri F, Valensin S, Monti D, Gonos ES, De Benedictis G, Franceschi C. What studies on human longevity tell us about the risk for cancer in the oldest old: data and hypotheses on the genetics and immunology of centenarians. Exp Gerontol. 2002;37:1263–1271. doi: 10.1016/S0531-5565(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 5.Donehower LA. p53: Guardian AND suppressor of longevity? Exp Gerontol. 2005;40:7–9. doi: 10.1016/j.exger.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Lanni C, Racchi M, Mazzini G, Ranzenigo A, Polotti R, Sinforiani E, Olivari L, Barcikowska M, Styczynska M, Kuznicki J, Szybinska A, Govoni S, Memo M, Uberti D. Conformationally altered p53: a novel Alzheimer’s disease marker? Mol Psychiatry. 2008;13:641–647. doi: 10.1038/sj.mp.4002060. [DOI] [PubMed] [Google Scholar]
- 7.Lund PK. Insulin-like growth factor I: molecular biology and relevance to tissue-specific expression and action. Recent Prog Horm Res. 1994;49:125–148. doi: 10.1016/b978-0-12-571149-4.50010-6. [DOI] [PubMed] [Google Scholar]
- 8.Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo V, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/S0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 11.Dozmorov I, Bartke A, Miller RA. Array-based expression analysis of mouse liver genes: effect of age and of the longevity mutant Prop1df. J Gerontol A Biol Sci Med Sci. 2001;56:B72–B80. doi: 10.1093/gerona/56.2.b72. [DOI] [PubMed] [Google Scholar]
- 12.Paolisso G, Gambardella A, Ammendola S, D’Amore A, Balbi V, Varricchio M, D’Onofrio F. Glucose tolerance and insulin action in healthy centenarians. Am J Physiol. 1996;270:E890–E894. doi: 10.1152/ajpendo.1996.270.5.E890. [DOI] [PubMed] [Google Scholar]
- 13.Paolisso G, Barbieri M, Rizzo MR, Cartella C, Rotondi M, Bonafè M, Franceschi C, Rose G, De Benedictis G. Low insulin resistance and preserved beta-cell function contribute to human longevity but are not associated with TH-INS genes. Exp Gerontol. 2001;37:149–156. doi: 10.1016/S0531-5565(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 14.Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafè M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 15.Bonafè M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- 16.Van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RG. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 17.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappola AR, Xuem QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 19.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 20.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 21.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher B, van der Pluijm I, Moorhouse MJ, Kosteas T, Robinson AR, Suh Y, Breit TM, van Steeg H, Niedernhofer LJ, van Ijcken W, Bartke A, Spindler SR, Hoeijmakers JH, van der Horst GT, Garinis GA. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS Genet. 2008;4:e1000161. doi: 10.1371/journal.pgen.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevità emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafé M, Cavallone L, Marchegiani F, Olivieri F, Franceschi C, Caruso C. Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10–1082 promoter SNP and its interaction with TNF-alpha -308 promoter SNP. J Med Genet. 2003;40:296–299. doi: 10.1136/jmg.40.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavallone L, Bonafè M, Olivieri F, Cardelli M, Marchegiani F, Giovagnetti S, Di Stasio G, Giampieri C, Mugianesi E, Stecconi R, Sciacca F, Grimaldi LM, De Benedictis G, Lio D, Caruso C, Franceschi C. The role of IL-1 gene cluster in longevity: a study in Italian population. Mech Ageing Dev. 2003;124:533–538. doi: 10.1016/S0047-6374(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Carrieri G, Marzi E, Olivieri F, Marchegiani F, Cavallone L, Cardelli M, Giovagnetti S, Stecconi R, Molendini C, Trapassi C, De Benedictis G, Kletsas D, Franceschi C. The G/C915 polymorphism of transforming growth factor beta1 is associated with human longevity: a study in Italian centenarians. Aging Cell. 2004;3:443–448. doi: 10.1111/j.1474-9728.2004.00129.x. [DOI] [PubMed] [Google Scholar]
- 29.Mannucci PM, Mari D, Merati G, Peyvandi F, Tagliabue L, Sacchi E, Taioli E, Sansoni P, Bertolini S, Franceschi C. Gene polymorphisms predicting high plasma levels of coagulation and fibrinolysis proteins. A study in centenarians. Arterioscler Thromb Vasc Biol. 1997;17:755–759. doi: 10.1161/01.atv.17.4.755. [DOI] [PubMed] [Google Scholar]
- 30.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, Salvioli S, Valensin S, De Benedictis G, Di Iorio A, Caruso C, Paolisso G, Monti D. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev. 2005;126:351–361. doi: 10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Bonafè M, Olivieri F, Cavallone L, Giovagnetti S, Mayegiani F, Cardelli M, Pieri C, Marra M, Antonicelli R, Lisa R, Rizzo MR, Paolisso G, Monti D, Franceschi C. A gender—dependent genetic predisposition to produce high levels of IL–6 is detrimental for longevity. Eur J Immunol. 2001;31:2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::AID-IMMU2357>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Cardelli M, Cavallone L, Marchegiani F, Oliveri F, Dato S, Montesanto A, Lescai F, Lisa R, De Benedictis G, Franceschi C. A genetic-demographic approach reveals male-specific association between survival and tumor necrosis factor (A/G)-308 polymorphism. J Gerontol A Biol Sci Med Sci. 2008;63:454–460. doi: 10.1093/gerona/63.5.454. [DOI] [PubMed] [Google Scholar]
- 34.Belluco C, Olivieri F, Bonafè M, Giovagnetti S, Mammano E, Scalerta R, Ambrosi A, Franceschi C, Nitti D, Lise M. 174 G>C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clin Cancer Res. 2003;9:2173–2176. [PubMed] [Google Scholar]
- 35.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, Munroe DJ, Farrar WL. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65:4673–4682. doi: 10.1158/0008-5472.CAN-04-3589. [DOI] [PubMed] [Google Scholar]
- 38.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Dijsselbloem N, Goriely S, Albarani V, Gerlo S, Francoz S, Marine JC, Goldman M, Haegeman G, Vanden Berghe W. A critical role for p53 in the control of NF-kappaB-dependent gene expression in TLR4-stimulated dendritic cells exposed to Genistein. J Immunol. 2007;178:5048–5057. doi: 10.4049/jimmunol.178.8.5048. [DOI] [PubMed] [Google Scholar]
- 40.Ko LJ, Prives C. p53: Puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 41.Levine AJ. p53, The cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 42.Attardi LD, Donehower LA. Probing p53 biological functions through the use of genetically engineered mouse models. Mutat Res. 2005;576:4–21. doi: 10.1016/j.mrfmmm.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 43.Scrable H, Medrano S, Ungewitter E (2008) Running on empty: how p53 controls INS/IGF signaling and affects lifespan. Exp Gerontol [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 44.Staib F, Robles AI, Varticovski L, Wang XW, Zeeberg BR, Sirotin M, Zhurkin VB, Hofseth LJ, Hussain SP, Weinstein JN, Galle PR, Harris CC. The p53 tumor suppressor network is a key responder to microenvironmental components of chronic inflammatory stress. Cancer Res. 2005;65:10255–10264. doi: 10.1158/0008-5472.CAN-05-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 46.Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, Tom E, Mack DH, Levine AJ. Analysis of p53- regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000;14:981–993. doi: 10.1101/gad.827700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Wu Q, Qiu P, Mirza A, McGuirk M, Kirschmeier P, Greene JR, Wang Y, Pickett CB, Liu S. Analyses of p53 target genes in the human genome by bioinformatic and microarray approaches. J Biol Chem. 2001;276:43604–43610. doi: 10.1074/jbc.M106570200. [DOI] [PubMed] [Google Scholar]
- 48.Leech M, Xue JR, Dacumos A, Hall P, Santos L, Yang Y, Li M, Kitching AR, Morand EF. The tumour suppressor gene p53 modulates the severity of antigen-induced arthritis and the systemic immune response. Clin Exp Immunol. 2008;152:345–353. doi: 10.1111/j.1365-2249.2008.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 50.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 Mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 51.Donehower LA. Does p53 affect organismal aging? J Cell Physiol. 2002;192:23–33. doi: 10.1002/jcp.10104. [DOI] [PubMed] [Google Scholar]
- 52.Moore L, Lu X, Ghebranious N, Tyner S, Donehower LA. Aging-associated truncated form of p53 interacts with wild-type p53 and alters p53 stability, localization, and activity. Mech Ageing Dev. 2007;128:717–730. doi: 10.1016/j.mad.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Cao I, García-Cao M, Martín-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Viña J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 55.Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci USA. 2007;104:16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Méplan C, Richard MJ, Hainaut P. Redox signalling and transition metals in the control of the p53 pathway. Biochem Pharmacol. 2000;59:25–33. doi: 10.1016/S0006-2952(99)00297-X. [DOI] [PubMed] [Google Scholar]
- 58.Uberti D, Lanni C, Carsana T, Francisconi S, Missale C, Racchi M, Govoni S, Memo M. Identification of a mutant-like conformation of p53 in fibroblasts from sporadic Alzheimer’s disease patients. Neurobiol Aging. 2006;27:1193–1201. doi: 10.1016/j.neurobiolaging.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Uberti D, Carsana T, Bernardi E, Rodella L, Grigolato P, Lanni C, Racchi M, Govoni S, Memo M. Selective impairment of p53-mediated cell death in fibroblasts from sporadic Alzheimer’s disease patients. J Cell Sci. 2002;115:3131–3138. doi: 10.1242/jcs.115.15.3131. [DOI] [PubMed] [Google Scholar]
- 60.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64:895–898. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- 61.Lanni C, Uberti D, Racchi M, Govoni S, Memo M. Unfolded p53: a potential biomarker for Alzheimer’s disease. J Alzheimers Dis. 2007;12:93–99. doi: 10.3233/jad-2007-12109. [DOI] [PubMed] [Google Scholar]
- 62.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP. p53 Isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourdon JC. p53 Family isoforms. Curr Pharm Biotechnol. 2007;8:332–336. doi: 10.2174/138920107783018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Heemst D, Mooijaart SP, Beekman M, Schreuder J, de Craen AJ, Brandt BW, Slagboom PE, Westendorp RG, Group LongLifeStudy. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol. 2005;40:11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 66. Mammano E, Belluco C, Bonafé M, Olivieri F, Mugianesi E, Barbi C, Mishto M, Cosci M, Franceschi C, Lise M, Nitti D (2008) Association of p53 polymorphisms and colorectal cancer: modulation of risk and progression. Eur J Surg Oncol (Epub ahead of print) [DOI] [PubMed]
- 67.Ørsted DD, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med. 2007;204:1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Regula KM, Kirshenbaum LA. p53 Activates the mitochondrial death pathway and apoptosis of ventricular myocytes independent of de novo gene transcription. J Mol Cell Cardiol. 2001;33:1435–1445. doi: 10.1006/jmcc.2001.1405. [DOI] [PubMed] [Google Scholar]
- 69.Bonafé M, Salvioli S, Barbi C, Trapassi C, Tocco F, Storci G, Invidia L, Vannini I, Rossi M, Marzi E, Mishto M, Capri M, Olivieri F, Antonicelli R, Memo M, Uberti D, Nacmias B, Sorbi S, Monti D, Franceschi C. The different apoptotic potential of the p53 codon 72 alleles increases with age and modulates in vivo ischaemia-induced cell death. Cell Death Differ. 2004;11:962–973. doi: 10.1038/sj.cdd.4401415. [DOI] [PubMed] [Google Scholar]
- 70.Salvioli S, Bonafé M, Barbi C, Storci G, Trapassi C, Tocco F, Gravina S, Rossi M, Tiberi L, Mondello C, Monti D, Franceschi C. p53 Codon 72 alleles influence the response to anticancer drugs in cells from aged people by regulating the cell cycle inhibitor p21WAF1. Cell Cycle. 2005;4:1264–1271. doi: 10.4161/cc.4.9.1978. [DOI] [PubMed] [Google Scholar]