Abstract
About 30% of renal cell carcinomas (RCC) will develop recurrence after surgery. Despite evidence for a significantly improved survival by autologous tumour cell vaccination therapy, the procedure has not become standard. Between August 1993 and December 1996, 1,267 RCC patients undergoing radical nephrectomy in 84 German hospitals were subsequently treated by autologous tumour cell vaccination therapy. The study group comprised 692 patients with complete follow-up (stages pT2-3, pNx-2, M0 based on the TNM classification, 4th edition). Subsequent propensity-score matching according to 7 defined criteria with 861 control patients undergoing nephrectomy alone without adjuvant treatment at the Carl-Thiem-Hospital Cottbus, resulted in 495 matched pairs. Overall and stage-specific survival rates were analysed after a median follow-up of 131 months. The 5- and 10-year overall survival (OS) rates were 80.6 and 68.9% in the vaccine group and 79.2 and 62.1% in the control group (p = 0.066). Patients with pT3 stage RCC revealed 5- and 10-year OS rates of 71.3 and 53.6% in the study group and 65.4 and 36.2% in the control group (p = 0.022). In multivariable analysis, patients in the vaccine group showed a significantly improved survival both in the whole study group (HR = 1.28, p = 0.030) and in the subgroup presenting with pT3 stage tumours (HR = 1.67, p = 0.011). Adjuvant treatment with autologous vaccination therapy resulted in a significantly improved overall survival in pT3 stage RCC patients, suggesting benefit especially in this subgroup. However, controlled clinical trials integrating the recent TNM classification and further risk constellations are required to define additional patient groups that may derive benefit from this treatment.
Keywords: Renal cell carcinoma, Nephrectomy, Autologous tumour vaccine, Adjuvant treatment, Overall survival
Introduction
The annual worldwide incidence of renal cell carcinoma (RCC) is 209,000 new cases resulting in 102,000 RCC-related deaths per year [16]. Over the recent years, nephron-sparing surgery, laparoscopic approaches, and minimally invasive treatment options such as cryotherapy and radiofrequency ablation have advanced treatment in patients with RCC. Furthermore, in several controlled clinical trials, novel targeted therapies have established their efficacy as systemic treatment options in metastasizing RCC [12, 18].
In a trial conducted at a number of hospitals and including more than 2,500 patients who underwent nephrectomy, the cancer-specific survival rate after 5 and 10 years was 74.2 and 67.2%, respectively [9]. Data derived from this and further studies clearly demonstrate the need for adjuvant treatment options in addition to surgery alone for tumour and metastasis control, adjusted to the individual oncological risk profile of each patient.
There are several externally validated prognostic nomograms and models for risk assessment by determining each patient’s individual progression-free survival (PFS), cancer-specific survival (CSS), and overall survival (OS) after surgery [2, 9, 19, 20]. However, no proven and widely available adjuvant treatment revealing definitive efficacy in RCC has yet been available.
Adjuvant vaccination strategies employed in RCC can be divided into autologous tumour cell-based vaccines, genetically modified tumour cell-based and dendritic cell-based vaccines, and peptide-based vaccines. Autologous tumour cell vaccines are based on the concept that RCCs themselves express tumour-associated antigens that will in turn start a cytotoxic T lymphocyte response. Additional treatment has to be employed to enhance the immune response necessary for a strong therapeutic effect [1].
In 2004, a multicenter phase-III study published by Jocham et al. [8] demonstrated a significantly reduced risk of tumour progression after radical nephrectomy using an autologous tumour cell lysate vaccination therapy (Reniale®). The PFS 70 months after vaccination therapy was found to be 72% in the study group, while it was 59.3% in the control group (p = 0.0204). The incidence rate of side effects was minimal at <1%. Quality of life was not impaired by the treatment itself or its application. The authors recommended the use of this autologous vaccination therapy in patients with M0 RCC and a tumour diameter of >2.5 cm. In a second survival analysis, a significantly increased OS was also reported for the vaccine group [4].
In the present investigation, the influence of this adjuvant autologous tumour cell lysate vaccine on overall survival was evaluated in patients with RCC in a Compassionate Use Program (CUP) [10]. After performing propensity-score matching with a historical control group of patients who underwent nephrectomy alone without any adjuvant treatment, survival data of both groups were compared.
Materials and methods
Study groups
The study group comprised 1,267 patients out of 84 German hospitals who received tumour vaccination therapy with the respective autologous tumour cell lysate (Reniale®) after undergoing radical nephrectomy between 1993 and 1996. Out of these, 575 patients were excluded from further analysis according to different reasons that are summarized in Table 1.
Table 1.
Exclusion criteria for patients in both study groups
| Vaccine starting group (n = 1,267) | Control starting group (n = 861) | ||
|---|---|---|---|
| No. of patients | Exclusion criteria | No. of patients | Exclusion criteria |
| 391 | Missing consent for the use of data for scientific purposes or data are used already for other clinical trials [14, 15] | 11 | Lost to follow-up |
| 52 | Lost to follow-up | 12 | Death within the first 4 month post surgery |
| 5 | Histopathological data incomplete | 2 | Tumour stage pT2, cM0 and partial nephrectomy with R1 status and without a final R0 status |
| 27 | Additional medication with other immune modulatory properties in the adjuvant indication | 63 | Tumour stage pT1-4, cM1 |
| 77 | Tumour stage pT1-4, cM1 | 104 | Tumour stage pT1, cM0 |
| 13 | Tumour stage pT1, cM0 | 8 | Tumour stage pT4, cM0 |
| 10 | Tumour stage pT4, cM0 | ||
| 692 | Patients left from the vaccine starting group for the propensity-score matching | 661 | Patients left from the control starting group for the propensity-score matching |
| After matching reduced down to 495 patients (vaccine group) | After matching reduced down to 495 patients (control group) | ||
Total 2,128 patients with a histologically verified renal cell carcinoma after surgery, Vaccine starting group patients treated with the autologous tumour cell lysate vaccine, Control starting group patients of the Carl-Thiem Hospital Cottbus
The control group consisted of 861 patients with RCC who underwent either radical (n = 732) or partial (n = 129) nephrectomy between 1992 and 2006 at the Carl-Thiem-Hospital Cottbus. Table 1 summarizes reasons for exclusion of control patients from further data evaluation. Finally, after applying exclusion criteria, the study group and the control group included 692 and 661 patients, respectively.
Both groups were matched according to oncological and demographic criteria in order to simulate a randomized clinical trial. Propensity scores, including the variables age, gender, pT stage, pN stage, grading, histological cell type, and UICC stage were calculated (Fig. 1). As the exact tumour size was not available for 282 patients in the vaccine group (40.5%), this variable was not included. Patients with comparable propensity scores were then matched from each group, which resulted in 495 matched pairs (72% of the vaccine group and 75% of the control group). By pooling the criteria developed by Rubin and Rosenbaum [17], only up to a 25% deviation of the propensity scores was accepted. The resulting group of patients for analysis contained 990 matched patients presenting with RCC stages pT2-3N0-2M0G1-3 (4th edition of the TNM classification 1992). The respective oncological and demographic data are summarized in Table 2.
Fig. 1.
Mirror histogram with the frequencies of propensity scores for the vaccine (n = 692) and control groups (n = 661) before matching
Table 2.
Demographical and oncological criteria in the matched study groups (propensity-score matching)
| Parameter | Total (n = 990) | Vaccine group (n = 495) | Control group (n = 495) | p value |
|---|---|---|---|---|
| Age, average, (range) (in years) | 60.8 (18–86) | 60.3 (21–86) | 61.1 (18–84) | 0.141 |
| Gender | ||||
| Male | 608 (61.4%) | 295 (59.6%) | 313 (63.2%) | 0.229 |
| Female | 382 (38.6%) | 200 (40.4%) | 182 (36.8%) | |
| pT stage | ||||
| pT2 | 780 (78.8%) | 387 (78.2%) | 393 (79.4%) | 0.417 |
| pT3 | 210 (21.2%) | 108 (21.8%) | 102 (20.6%) | |
| pN stage | ||||
| N0/pNx | 967 (97.7%) | 480 (97.0%) | 487 (98.4%) | 0.210 |
| pN1 | 23 (2.3%) | 15 (3.0%) | 8 (1.6%) | |
| Grading | ||||
| G1 | 176 (17.8%) | 94 (19.0%) | 82 (16.6%) | 0.419 |
| G2 | 694 (70.1%) | 336 (67.9%) | 358 (72.3%) | |
| G3 | 120 (12.1%) | 65 (13.1%) | 55 (11.1%) | |
| Histological cell type | ||||
| Clear cell carcinoma | 782 (79.0%) | 381 (76.9%) | 401 (81.0%) | 0.414 |
| Papillary carcinoma | 133 (13.4%) | 75 (15.2%) | 58 (11.7%) | |
| Chromophobic carcinoma | 66 (6.7%) | 34 (6.9%) | 32 (6.5%) | |
| Pleomorphic carcinoma | 9 (0.9%) | 5 (1.0%) | 4 (0.8%) | |
| UICC stage | ||||
| Stage 2 | 770 (77.8%) | 381 (77.0%) | 389 (78.6%) | 0.302 |
| Stage 3 | 220 (22.2%) | 114 (23.0%) | 106 (21.4%) | |
| Average maximal tumour diametera (range) (in cm) | 6.1 (2.6–23) | 6.4 (2.6–23) | 5.9 (2.6–20) | 0.003 |
The p value demonstrates the range of variables between each group
aAverage maximal tumour diameter was only available for 800 patients (vaccine group, n = 305; control group, n = 495)
Therapy
Surgery was carried out in both groups using three different surgical techniques: (a) transperitoneal tumour nephrectomy including optional regional lymphadenectomy, (b) lumbodorsal tumour nephrectomy using intercostal or subcostal access, and (c) lumbodorsal nephron-sparing nephrectomy by intercostal access. The ratio of procedures (a) and (b) did not differ in both groups. Procedure (c) was used merely in the control group (all R0 status). In patients with tumours close to the upper kidney pole an ipsilateral adrenalectomy was performed.
All patients signed a contract prior to nephrectomy, including information on the manufacturing of the autologous tumour vaccines as well as informed consent regarding subsequent treatment in the CUP and approval of the use of the basic data for statistical analysis.
The vaccine was used as an extemporaneous mixture. It was manufactured according to Good Manufacturing Practice (GMP) rules from the tumour material removed under sterile conditions (5–10 g) and immediately shipped by courier to the manufacturer (Macropharm GmbH, Hannover, Germany). After macroscopic preparation of the malignant tissue, a single tumour cell suspension was incubated with 1,500 IE interferon-γ (Imukin; Boehringer, Ingelheim, Germany) and 750 mg Tocopherolacetate (E-Vicotrat; Heyl, Berlin, Germany) for 120 min. A complete devitalisation was accomplished by threefold freezing (−82°C) and thawing. After testing the specification of the product by a set of different assays, the product was released for clinical use. The vaccine units were stored until use at −82°C and were shipped frozen to the treating physician 1 day before the planned vaccination. The autologous vaccine contained about 5 × 106 autologous devitalised tumour cells per 1 ml. In total 81.2% (403/495) of the patients in the vaccine group received a minimum of six doses as intradermal injections into the upper arm over a 4-week interval (average 16.1, range 3–30).
Study variables, study goal, and statistical analysis
Demographic information (age, gender), therapy-related variables (date of surgery, number and date of vaccination), and oncological parameters (TNM classification, grading according to WHO, maximum tumour diameter, histologic subtype, UICC stage) were retrospectively analysed (Table 2). To achieve comparability, the 1992 TNM classification (4th edition) was used for all patients. Therefore, patients from the Cottbus hospital who had been classified using the fifth or sixth edition of the TNM classification were reclassified according to their tumour diameter to the fourth edition of the TNM classification. The primary endpoint of this study was OS (time between surgery and death).
Patient data for the control group patients were retrieved from the prospective nephrectomy register of the Carl-Thiem-Hospital, Cottbus, whereas patient data for the vaccine group were sourced from the GMP. Survival data were obtained by written request from the local authorities. Due to the German Data Protection Act, only survival data could be requested, but not reasons for death. Additional data were obtained by written request from the treating urologists, tumour centres, and the register of death of the German States.
After matching both treatment groups (Table 2), the balance of the covariates was assessed by using the t test for paired samples and the Wilcoxon test or McNemar test for the categorical variables. The cumulative probability of survival was calculated according to the Kaplan–Meier method. For the survival differences between both study groups, the log-rank test was used. In all analyses p ≤ 0.05 was considered to indicate statistical significance. Using the Cox proportional hazards model, the influence of different criteria on OS was determined. After univariably analysing all parameters, those revealing significant influence on OS (α < 10%) were included in the multivariable analysis. All statistical analyses were carried out by SPSS, Version 15.0.
At the end of the study 323 out of 990 patients (32.6%) had died. The mean follow-up of all surviving patients was 113.5 months (median 131 months). The mean follow-up time of patients still alive at the end of the study related to the vaccine group was 139.4 months (median 140 months) and 89.1 months (median 84 months) in the control group, respectively.
Results
After performing propensity-score matching, the study and control group did not significantly differ within the parameters age, gender, pT stage, pN stage, tumour grade, histological subtype, and UICC stage. Seventy-nine percent of all patients had clear cell carcinoma. Twenty-three patients (2.3%) showed histologically verified lymph node involvement, and 120 patients (12.1%) presented with G3-RCC. Tumour stages pT2 and pT3 were present in 780 (78.8%) and 210 (21.2%) out of all patients (Table 2).
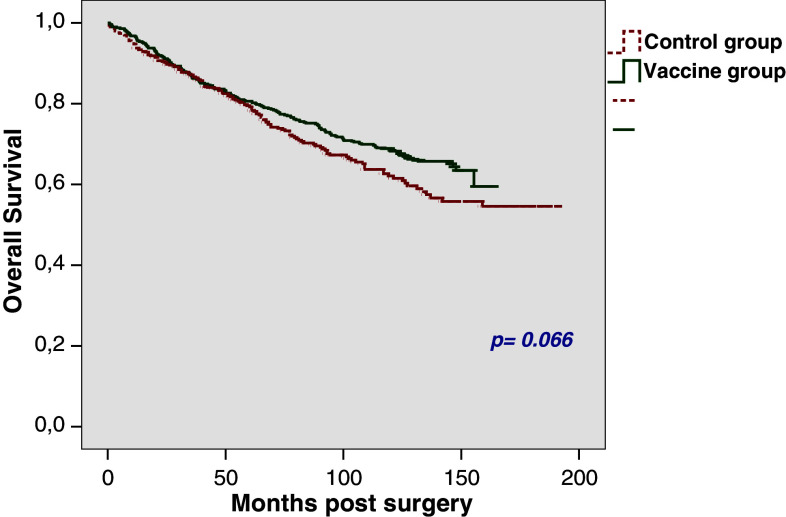
OS rates after 5 and 10 years were found to be 80.6 and 68.9%, respectively, in the vaccine group and 79.2 and 62.1%, respectively in the control group (p = 0.066; Fig. 2). In the vaccine group, 25% of the patients had died within 88.1 months (SE 8.6) in contrast to 68 months (SE 6.3) in the control group.
Fig. 2.
Overall survival in the total study group (n = 990)
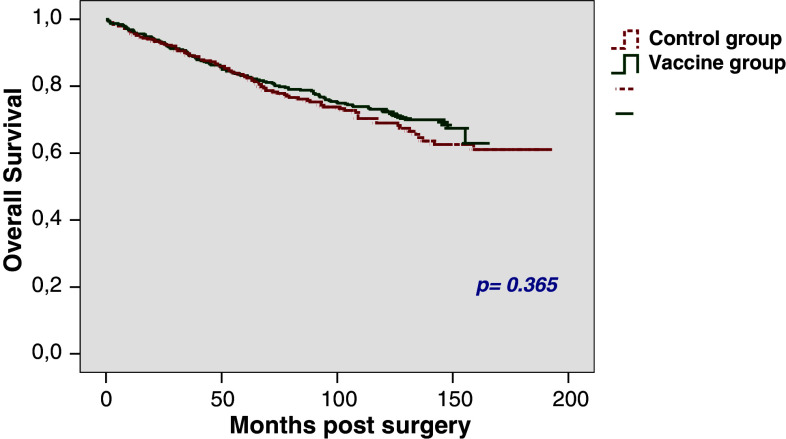
In patients with pT2 tumours the 5-year OS rates were found to be 83.2% in the vaccine group and 82.8% in the control group (p = 0.365), respectively. OS rates after 10 years were 73.1 and 69%, respectively (Fig. 3); 25% of the patients had died by 100.2 months (SE 14.6) in the vaccine group and 93 months (SE 11.3) in the control group.
Fig. 3.
Overall survival rate in patients with pT2 stage (n = 780)
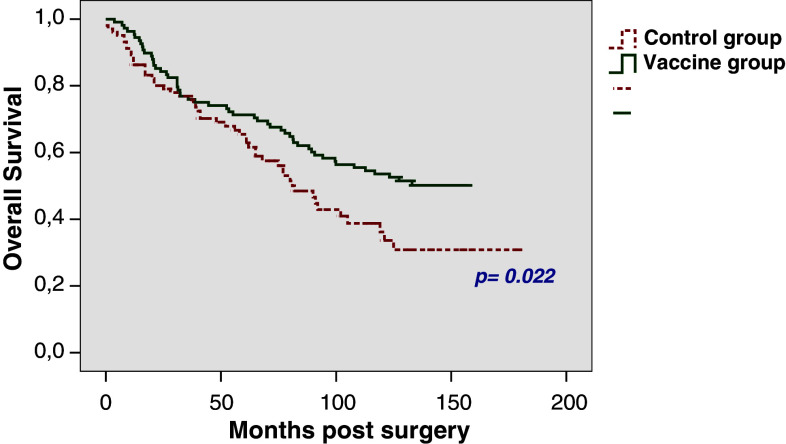
The 5-year OS rates for patients with pT3 tumours were found to be 71.3% in the vaccine group and 65.4% in the control group (p = 0.022), respectively. OS after 10 years could be calculated as 53.6 and 36.2%, respectively (Fig. 4). The median time to death was 81 months (SE 7.8) in the control group and was not reached in the vaccine group; 25% of the pT3 patients had died within 38.7 months (SE 13) in the vaccine group, compared to 38 months (SE 10.7) in the control group.
Fig. 4.
Overall survival rates in patients with pT3 stage (n = 210)
No difference regarding survival was found between both groups of patients presenting with RCC UICC stage 2 (p = 0.472). In patients with UICC stage 3 RCC, which includes all pT3 and pT2 tumours with pN1 status, OS in the vaccine group was significantly increased in comparison with the control group (5-year OS rates, 71.9 vs. 60.3%; p = 0.008).
A separate analysis including only patients with clear cell RCC and N0 status yielded analogous results to those of the whole group of patients (data not shown).
The influence of different parameters on OS was investigated using Cox proportional hazards model. Table 3 demonstrates the results of the univariable and multivariable analysis. Tumour size was excluded from multivariable analysis as it was not available for 190 patients in the vaccine group. Furthermore, UICC stage was excluded due to a highly positive correlation with pT stage (ρ = 0.971, p < 0.001). In addition to age, pT stage, pN stage, and tumour grading, application of tumour cell vaccination therapy was identified to show a significant independent influence on OS in the whole study group (HR 1.28, p = 0.030). For patients presenting with pT3 tumours, the absence of tumour cell vaccination resulted in a significantly impaired OS (multivariable regression analysis, HR 1.67; p = 0.011) (Table 4).
Table 3.
Cox proportional hazards model (univariate and multivariate) demonstrating the influence of different parameters on the overall survival of the total study group (n = 990)
| Parameter | Standard error | Hazard ratio | 95% CI | p value |
|---|---|---|---|---|
| Univariate analysis | ||||
| Age (1-year steps) | 0.006 | 1.038 | 1.026–1.051 | <0.001 |
| Gender (male vs. female) | 0.116 | 0.927 | 0.739–1.162 | 0.509 |
| pT stage (pT2 vs. pT3) | 0.119 | 2.180 | 1.727–2.751 | <0.001 |
| pN stage (pN0/pNx vs. pN+) | 0.250 | 3.780 | 2.318–6.165 | <0.001 |
| Grading (G1 vs. G2 vs. G3) | 0.107 | 1.522 | 1.233–1.878 | <0.001 |
| Histological cell type (clear cell vs. other) | 0.157 | 1.129 | 0.831–1.535 | 0.437 |
| UICC stage (stage 2 vs. stage 3) | 0.117 | 2.288 | 1.819–2.878 | <0.001 |
| Tumour cell lysate vaccine (yes vs. no) | 0.113 | 1.231 | 0.986–1.536 | 0.067 |
| Tumour sizea (1-cm steps) | 0.020 | 1.098 | 1.055–1.143 | <0.001 |
| Multivariate analysis | ||||
| Age (1-year steps) | 0.006 | 1.036 | 1.023–1.049 | <0.001 |
| pT stage (pT2 vs. pT3) | 0.122 | 1.846 | 1.453–2.346 | <0.001 |
| pN stage (pN0/pNx vs. pN+) | 0.255 | 3.201 | 1.942–5.277 | <0.001 |
| Grading (G1 vs. G2 vs. G3) | 0.108 | 1.363 | 1.104–1.683 | 0.004 |
| Tumour cell lysate vaccine (yes vs. no) | 0.115 | 1.282 | 1.024–1.605 | 0.030 |
n The multivariate analysis of all except the UICC stage were available and in the univariate (with a 10% probability of error), significant evaluated parameters were included. The UICC stage was here excluded due to the highly positive correlation with the pT stage (ρ = 0.971, p < 0.001)
aAverage maximal tumour diameter was available for only 800 patients (vaccine group, n = 305; control group, n = 495)
Table 4.
Cox proportional hazards model (univariate and multivariate) demonstrating the influence of different parameters on the overall survival of the study group in the pT3 stage (n = 210)
| Parameter | Standard error | Hazard ratio | 95% CI | p value |
|---|---|---|---|---|
| Univariate analysis | ||||
| Age (1-year steps) | 0.011 | 1.006 | 0.984–1.029 | 0.588 |
| Gender (male vs. female) | 0.208 | 0.845 | 0.562–1.271 | 0.419 |
| pN stage (pN0/pNx vs. pN+) | 0.333 | 2.553 | 1.329–4.905 | 0.005 |
| Grading (G1 vs. G2 vs. G3) | 0.182 | 1.353 | 0.947–1.933 | 0.096 |
| Histological cell type (clear cell vs. other) | 0.186 | 1.235 | 0.857–1.778 | 0.257 |
| Tumour cell lysate vaccine (yes vs. no) | 0.198 | 1.567 | 1.063–2.310 | 0.023 |
| Tumour sizea (1-cm steps) | 0.033 | 1.050 | 0.983–1.121 | 0.145 |
| Multivariate analysis | ||||
| pN stage (pN0/pNx vs. pN+) | 0.337 | 2.877 | 1.486–5.573 | 0.002 |
| Grading (G1 vs. G2 vs. G3) | 0.192 | 1.372 | 0.942–1.999 | 0.099 |
| Tumour cell lysate vaccine (yes vs. no) | 0.200 | 1.666 | 1.125–2.468 | 0.011 |
In the multivariate analysis all except the UICC stage were available and in the univariate (with a 10% probability of error), significant evaluated parameters were included. The UICC stage was here excluded due to the high positive correlation with the pT stage (ρ = 0.971, p < 0.001)
aAverage maximal tumour diameter was only obtainable for 155 patients (vaccine group, n = 53; control group, n = 102)
Discussion
Considering the high progression rates in patients with locally advanced disease, efficient adjuvant therapies represent an unmet medical need in RCC [6, 9].
Cytokine therapy with IFN-α and IL-2, classically used in the treatment of metastatic RCC, did not confer an advantage regarding progression-free survival in the adjuvant setting. Additionally, severe side effects occurred in more than 30% of patients, resulting in interruption of immunotherapy [11, 13].
The first randomized trial of a specific immune therapy, using not genetically modified tumour cells in an adjuvant indication, did not show any significant difference regarding PFS and OS compared to a control group after 5 years of observation [7]. The immune therapy was carried out using autologous tumour cells and the attenuated mycobacterial Bacillus Calmette-Guérin/BCG vaccine [7]. Repmann et al. [14, 15] investigated the efficacy of an autologous tumour cell lysate vaccine successfully in a non-randomized clinical trial, showing a significant advantage regarding PFS and OS in the vaccine group in comparison with a historical control group.
Until now, only one positive phase-III trial has been published demonstrating an improvement in PFS in patients with RCC following tumour nephrectomy. Jocham et al. [8] evaluated the efficacy of an adjuvant autologous tumour cell lysate vaccine (Reniale®) in patients with stage pT2-3bN0-3M0 (equal to a tumour size of >2.5 cm with regard to the fourth edition of the TNM classification). Five hundred and fifty-eight patients from 55 German centres were included between 1997 and 1998. A number of patients were excluded due to protocol violations and lack of follow-up, leaving 379 patients that were finally included in the “Intention-to-treat”-analysis (comprising 177 patients in the vaccine group). The primary goal of the study, i.e. reduction of the risk of progression in the therapy group, was reached for the whole study group (70 month PFS: 72 vs. 59.3%, p = 0.0204). In a subgroup analysis, patients presenting with pT2 tumours showed a not significantly differing PFS after 5 years (p = 0.216) of 81% in the vaccine group (n = 119) and 75% in the control group (n = 145), respectively. The PFS rates after 5 years in patients with pT3 tumours were found to be 67.5% in the vaccine group (n = 58) and 49.7% in the control group (n = 57), respectively (p = 0.039). Due to this improvement in the vaccine group, backed up by additional subgroup analyses, the authors concluded that patients with an increased risk (pT3 stage, G3 tumour, higher Störkel score) had more benefit from vaccination therapy. However, the observed increase in PFS barely reached statistical significance. In addition, there were some methodological questions, and OS data were not reported. An extremely low rate of side effects was shown in this trial. Out of 177 patients in the vaccine group who received 1,053 doses, merely 12 drug-related side effects (all mild to moderate) were observed in 2 patients. No abnormalities in the blood count or other parameters were observed [8]. Reniale has currently not received approval as an adjuvant medication in RCC.
Recently reported updated results include secondary intention-to-treat analysis (n = 477) and OS data. The report shows a statistically significant benefit in favour of the vaccine group for PFS (p = 0.0476). OS was not statistically significant different between the groups (p = 0.1185). A per-protocol analysis (n = 352) revealed a statistically significant PFS and OS in favour of the vaccine (p = 0.0224 and p = 0.0356, respectively) [5].
In an attempt to evaluate the therapeutic mechanism of action of their autologous tumour vaccine, the same group of authors used syngenic RENCA cells implanted into kidneys in a mouse model [3]. Interestingly, in the follow-up all mice in the control group developed kidney cancer and lung metastasis, whereas in the vaccine group, only 9.7% (7/72) of mice developed histologically detectable tumours.
A number of studies are currently investigating the value of heat shock protein vaccine HSPPC-96 (Oncophage®), monoclonal antibody against carbonic anhydrase (G250, Rencarex®), and treatment with the tyrosine kinase inhibitors sunitinib (Sutent®) or sorafenib (Nexavar®) [4]. While results from the latter studies have to be awaited, intermediate results from the Oncophage®-trial have been published recently [21]. This phase-3 study compared adjuvant vaccination with the autologous tumour-derived heat shock protein peptide complex (HSPPC-96, vitespen, Oncophage) (n = 361) with observation (n = 367) in RCC patients at high risk for recurrence after nephrectomy. After a median follow-up of 1.9 years in the intention-to-treat population, recurrence events were reported in 136 (38%) patients in the vaccine group and 146 (40%) in the observation group (HR: 0.923, 95% CI 0.73–1.17, p = 0.506). Autologous HSPPC-96 could not significantly improve recurrence-free survival in RCC patients at high risk for recurrence after nephrectomy, but did so in a population that correlates with ECOG’s intermediate-risk category (stage 1–2 high grade or stage 3 T1-3a low-grade diseases). Among the 362 patients who were at intermediate risk, there were significantly fewer recurrence events in the vitespen group (28/184) than in the observation group (47/178) (15.2 vs. 26.4%, respectively; p = 0.026). The authors concluded that the improvement in recurrence-free survival in patients with earlier-stage disease who received vitespen requires further validation. In a recent update of the OS data of this study, a favourable trend in the vitespen arm versus observation was demonstrated in all analysis sets, but especially among patients with earlier-stage disease (stage 1/2 low grade; n = 118) or at intermediate risk for recurrence (stage 1/2 high grade, stage 3 low grade; n = 184), with 10.4 versus 18.3%, and 9.8 versus 18.5% deaths reported in the vitespen and observation arms, respectively [22]. Again, this trend towards improved OS for the vitespen group needs to be confirmed with prolonged follow-up.
In contrast to this study, but in keeping with the study by Jocham et al., we observed a better OS in patients with locally advanced tumours. The reasons for the conflicting results from these studies remain speculative. On the one hand, in patients with locally advanced RCC, tumours may have adopted immune escape mechanisms that render them more resistant to immunotherapy compared to those with localized tumours. On the other hand, pT3 patients have a higher probability of progression and the effect of a vaccine preventing or slowing progression may be demonstrated earlier and in a smaller cohort of patients than would be required to show the same effect in T2 patients. Further results from prospective trials are clearly required to answer these questions.
The present investigation displays many known limitations of retrospective multicenter studies. As primary therapy, the nephrectomy data of selected patients from 84 German hospitals (vaccine group) and the data of consecutive patients from a single hospital (as control group) were compared, this may have led to a selection bias regarding surgical strategies. In total 63/2.128 (2.9%) of patients were lost to follow-up and excluded from the analysis and further five patients had an incomplete histological diagnosis. One established prognostic factor, tumour size [2, 9, 19, 20], was not included in the matching criteria as it was not documented in the GMP documentation of the vaccine group. Therefore, the 1992 TNM classification (4th edition) was used. In the TNM classification of 2002 (6th edition) pT1 tumours are differentiated into pT1a (≤4 cm) and pT1b (>4–7 cm). Thus, patients with RCC stages pT1a or pT1b according to the current TNM edition have also been treated. Accordingly, it is not possible to exclude a significant improvement by vaccination therapy in patients with RCC stages pT1b and pT2 according to the recent TNM classification. Although the different TNM classifications have identical definitions with regard to T3 tumours, for which a significant OS advantage was seen in our study, our results must be interpreted with caution. The comorbidity of patients, which may influence OS, was not available for the majority of patients in the vaccine group. Furthermore, PFS and CSS could not be evaluated due lack of relevant data related to the retrospective study design and legal reasons. Patients with tumour progression were treated according to the pattern of metastasis and their comorbidities with metastasectomy, cytokine-based immunotherapy or best supportive care, resulting in a variable influence on the individual prognosis. Respective follow-up information was not available. However, since treatment patterns were relatively uniform in all German urological departments, only a small probability of bias should be expected. No information about side effects of the vaccination was available, but based on the data from the phase-III trial and other publications revealing a very low toxicity profile, a significant impact on outcome seems unlikely [8, 14, 15]. In our study, 16.1 intradermal injections were applied on average (median 15; range 3 up to 30). No reliable information about the optimal number of vaccine doses to be applied to patients can be derived from our study. Repmann et al. [14, 15] showed no correlation between the number of doses applied to patients and survival rates.
Conclusion
By applying stringent matching criteria, the current study allows a comparison of OS in RCC patients treated with an adjuvant autologous tumour cell lysate vaccine with untreated patients in a control group. The findings of our analysis, demonstrating a significant OS advantage by treatment with autologous tumour vaccine especially in patients with tumour stages pT3 and UICC stage 3, are in line with the results of a previous phase-III study using the same vaccine in an adjuvant setting [8].
Currently, it is not possible to draw firm conclusions about the effect of this vaccine on OS in all patients with localized RCC. Controlled trials, using the recent TNM classification and incorporating known risk factors for prognosis, are required to identify further patient groups that may benefit from treatment with autologous tumour cell vaccination.
Acknowledgments
The authors wish to thank Mrs. Sandra Pflanz (study coordinator of the project group renal cell carcinoma, Department of Urology, Cottbus), many (but not forgotten) urologists and GPs from all over Germany as well as the regional tumour centres for their help in data acquisition. Without their help it would not have been possible to keep the “lost-to-follow-up” group in this multicentre trial so small.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Asemissen AM, Brossart P. Vaccination strategies in patients with renal cell carcinoma. Cancer Immunol Immunother. 2009;58(7):1169–1174. doi: 10.1007/s00262-009-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B, Zigeuner RE, Artibani W, Guillé F, Abbou CC, Salzano L, Gallo C. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer. 2005;104(7):1362–1371. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 3.Doehn C, Esser N, Pauels HG, Kießig ST, Stelljes M, Grossmann A, Jocham D, Drevs J (2008) Mode-of-action, efficacy, and safety of a homologous multi-epitope vaccine in a murine model for adjuvant treatment of renal cell carcinoma. Eur Urol Jun 4 [Epub ahead of print] [DOI] [PubMed]
- 4.Doehn C, Merseburger AS, Jocham D, Kuczyk MA. Is there an indication for neoadjuvant or adjuvant systemic therapy in renal cell cancer? Urologe A. 2007;46(10):1371–1372. doi: 10.1007/s00120-007-1540-1. [DOI] [PubMed] [Google Scholar]
- 5.Doehn C (2006) Prolongation of progression-free and overall survival following an adjuvant vaccination with Reniale in patients with non-metastatic renal cell carcinoma: secondary analysis of a multicenter phase-3 trial. Abstract presented at Deutscher Krebskongress, 22–26 March 2006, Berlin, Germany
- 6.Ficarra V, Galfano A, Guillé F, Schips L, Tostain J, Mejean A, Lang H, Mulders P, De La Taille A, Chautard D, Descotes JL, Cindolo L, Novara G, Rioux-Leclercq N, Zattoni F, Artibani W, Patard JJ. A new staging system for locally advanced (pT3-4) renal cell carcinoma: a multicenter European study including 2,000 patients. J Urol. 2007;178(2):418–424. doi: 10.1016/j.juro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 7.Galligioni E, Quaia M, Merlo A, Carbone A, Spada A, Favaro D, Santarosa M, Sacco C, Talamini R. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumour cells and bacillus Calmette-Guèrin: five-year results of a prospective randomized study. Cancer. 1996;77(12):2560–2566. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2560::AID-CNCR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, Schmitt E, Dannenberg T, Lehmacher W, von Wietersheim J, Doehn C. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy phase III randomised controlled trial. Lancet. 2004;363(9409):594–599. doi: 10.1016/S0140-6736(04)15590-6. [DOI] [PubMed] [Google Scholar]
- 9.Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, Cindolo L, De la Taille A, Tostain J, Mulders PF, Salomon L, Zigeuner R, Prayer-Galetti T, Chautard D, Valeri A, Lechevallier E, Descotes JL, Lang H, Mejean A, Patard JJ. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25(11):1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 10.May M, Kendel F, Hoschke B, Gilfrich C, Kiessig S, Pflanz S, Seidel M, Brookman-Amissah S. Adjuvant autologous tumour cell vaccination in patients with renal cell carcinoma. Overall survival analysis with a follow-up period in excess of more than 10 years. Urologe A. 2009;48(9):1075–1083. doi: 10.1007/s00120-009-2044-y. [DOI] [PubMed] [Google Scholar]
- 11.Messing EM, Manola J, Wilding G, Propert K, Fleischmann J, Crawford ED, Pontes JE, Hahn R, Trump D, Eastern Cooperative Oncology Group/Intergroup trial Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. 2003;21(7):1214–1222. doi: 10.1200/JCO.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 13.Pizzocaro G, Piva L, Colavita M, Ferri S, Artusi R, Boracchi P, Parmiani G, Marubini E. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol. 2001;19(2):425–431. doi: 10.1200/JCO.2001.19.2.425. [DOI] [PubMed] [Google Scholar]
- 14.Repmann R, Goldschmidt AJ, Richter A. Adjuvant therapy of renal cell carcinoma patients with an autologous tumour cell lysate vaccine: a 5-year follow-up analysis. Anticancer Res. 2003;23(2A):969–974. [PubMed] [Google Scholar]
- 15.Repmann R, Wagner S, Richter A. Adjuvant therapy of renal cell carcinoma with active-specific-immunotherapy (ASI) using autologous tumour vaccine. Anticancer Res. 1997;17(4B):2879–2882. [PubMed] [Google Scholar]
- 16.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics. 1985;41(1):103–116. doi: 10.2307/2530647. [DOI] [PubMed] [Google Scholar]
- 18.Ryan CW, Goldman BH, Lara PN, Jr, Mack PC, Beer TM, Tangen CM, Lemmon D, Pan CX, Drabkin HA, Crawford ED, Southwest Oncology Group Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J Clin Oncol. 2007;25(22):3296–3301. doi: 10.1200/JCO.2007.11.1047. [DOI] [PubMed] [Google Scholar]
- 19.Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, McKiernan J, Russo P. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173(1):48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML, Frank I. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: the D-SSIGN score. J Urol. 2007;177(2):477–480. doi: 10.1016/j.juro.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 21.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, Isakov L, Flanigan R, Figlin R, Gupta R, Escudier B. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;272:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 22.Wood C, Srivastava P, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Teofilovici F, Isakov L, Escudier B (2009). Survival update from a multicenter, randomized, phase III trial of vitespen versus observation as adjuvant therapy for renal cell carcinoma in patients at high risk of recurrence. ASCO no. 3009 [DOI] [PubMed]