Abstract
Immunotherapy based on T cell responses to the tumor is believed to involve killing of cancer cells by induction of apoptosis. The predominant mechanisms are death ligand-induced signaling mainly by TNF-related apoptosis-inducing ligand (TRAIL) mediated by CD4 T cells, monocytes and dendritic cells, and perforin/granzyme mediated apoptosis mediated by CD8 T cells and NK cells. Resistance against TRAIL involves loss of TRAIL death receptors and/or activation of the MEK and/or Akt signal pathways. Resistance to CD8 CTL responses also involves activation of the MEK and/or Akt pathways. Apoptosis induced by immune responses is regulated by the Bcl-2 family of proteins. Many reagents have been developed against the Bcl-2 antiapoptotic proteins and clinical trials combining them with immunotherapy are awaited. The second group of agents that regulate the Bcl-2 family of proteins are the signal pathway inhibitors. Clinical trials with inhibitors of RAS, RAF or MEK are in progress and would appear an exciting combination with immunotherapy. One of the main drivers of resistance to apoptosis are adaptive mechanisms that allow cancer cells to overcome endoplasmic reticulum (ER) stress. These adaptive mechanisms inhibit practically all known apoptotic pathways and create an acidic environment that may reduce infiltration of lymphocytes against the tumor. The signal pathway inhibitors may be effective against these adaptive processes but additional agents that target ER stress pathways are in development. In conclusion, combination of immunotherapy with agents that target antiapoptotic mechanisms in cancer cells offers a new approach that requires evaluation in clinical trials.
Keywords: Apoptosis, Bcl-2 proteins, Signal pathway inhibitors, Endoplasmic reticulum stress
Introduction
Immunological responses are believed to play a role in the natural history of cancers such as melanoma and can be demonstrated in immunohistological studies and by a variety of assays carried out ex vivo on lymphocytes from patients. Evidence from these sources has prompted clinical trials with vaccines using whole cells or cell lysates or more purified antigens known to be recognized by the immune system. The results of these studies have generally been disappointing, as reviewed elsewhere [47, 103, 125].
These results have posed somewhat of a dilemma as in many of the studies ex vivo assays have reported the induction of lymphocyte responses against the tumor but with little or no clinical benefits. Various explanations have been proposed to account for this lack of correlation, such as inhibition of antigen presentation, inhibition of cytokine production from T cells, anergy of T cells due to lack of costimulatory CD80, CD86 ligands, or interaction with PD1 ligands, induction of T regulatory cells, shift of T cell populations to TH2 or TH17 helper cells due to cytokines from tumor cells such as IL-6, TNF-α, IL-23, inhibition of leukocyte migration due to release of VEGF or PGE2 from tumor cells, selection of MHC and antigen loss variants [49]. Good studies and models supporting these explanations are numerous. To a large extent, however, they are focused on defects in the immune system and may be overlooking more fundamental properties of tumor cells that limit the effectiveness of immunotherapy even in the face of otherwise strong immune responses.
One of these properties is resistance to apoptosis, which is regarded as important as dysregulated cell division in development of tumors [41]. Given that the immune system kills tumor cells by induction of apoptosis, combining immunotherapy with agents that overcome apoptosis resistance pathways would appear a logical step in treatment. In the sections below, we indicate the principal mechanisms involved in resistance of cancer cells to apoptosis and new agents that may be effective in overcoming the resistance pathways.
How lymphocytes kill tumor cells
In general, there are two main killing pathways. The death ligand route utilized by CD4 T cells, monocytes and dendritic cells (DCs) involves interaction of members of the TNF family with their receptors in cell membranes of the cancer cells, which results in aggregation of the receptors, binding of adapter proteins such as Fas associated death domains (FADD) and activation of apical caspases such as caspase 8 through death effector domains (DED) [8, 9]. In some sensitive lymphoma cells the caspase cascade activated by caspase 8 can lead to direct activation of the effector caspases such as caspase 3 but in most solid cancers the pathway involves cleavage of the proapoptotic BH3 (Bcl-2 homology domain 3) protein Bid, which leads to activation of Bax/Bak proteins and mitochondrial outer membrane permeabilization (MOMP). This leads to release of aptogenic proteins from mitochondria and activation of the mitochondrial apoptosis pathway involving cytochrome c, APAF-1 and caspase 9, leading to formation of the apoptosome and activation of the effector caspases 3 and 7 [48]. This process is also facilitated by release of proteins such as Smac/DIABLO, which inhibit “inhibitor of apoptosis proteins” (IAPs), particularly XIAP, which inhibits events downstream of mitochondria [140].
The other main route utilized by CD8, CTL and NK cells depends on the transfer of cytotoxic granules into the immunological synapse formed between the effector and tumor cell. Proteases called granzymes are released from the granules and delivered to the tumor cell by perforin, which is a Ca2+-depending pore forming protein [14, 123], which multimerizes in the cell membrane [96]. This results in cleavage of substrates such as Bid by Granzyme B, resulting in MOMP and the events described above downstream from mitochondria that lead to apoptosis. This is an oversimplified view of killing by CD8 CTL in that granzyme B may also trigger other pathways to apoptosis such as cleavage of caspase 3 and 7 [131]. Additional granzymes such as granzyme A may contribute to cell death either by generation of reactive oxygen species (ROS) or by cleavage of proteins in the SET complex that leads to single strand breaks in DNA[123], leading to activation of p53. Killing by CTL appears to involve p53, which would be consistent with the above [87]. Granzymes H and K are called orphan enzymes as their substrates are unknown. They and granzyme M cause apoptosis by caspase-independent pathways [135].
Inhibitors of the cell death pathways
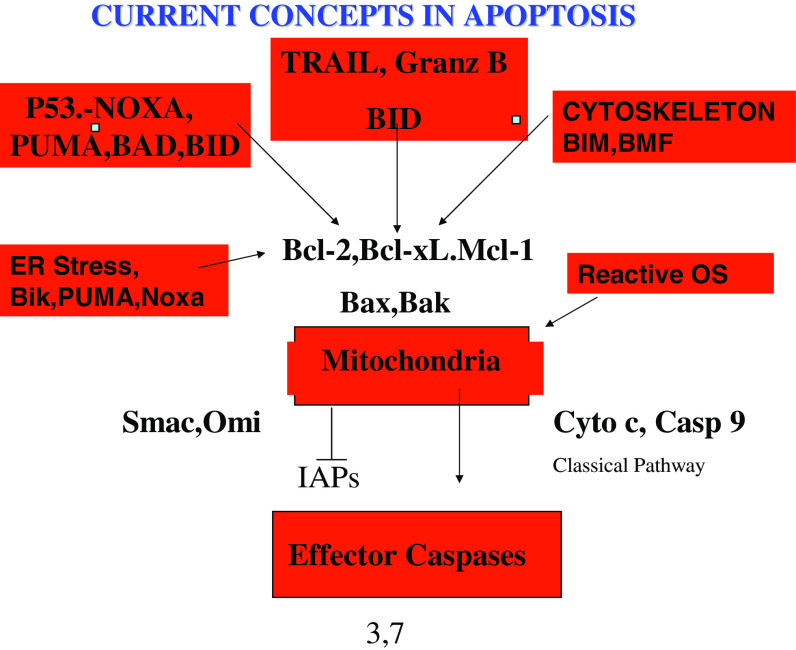
Mitochondrial dependent apoptotic pathways are regulated mainly by the Bcl-2 family of proteins, which, as reviewed elsewhere [21, 39, 51, 132] consist of a family of BH3 only proapoptotic proteins, two multidomain proapoptotic proteins (Bax and Bak) and several multidomain antiapoptotic proteins (Bcl-2, Bcl-XL, Bcl-w, Mcl-1 and A1) (see Fig. 1). In one model the antiapoptotic proteins bind the BH3 proteins and this displaces Bax or Bak from the antiapoptotic proteins, allowing them to bind to mitochondria and induce MOMP [18, 132]. Certain of the BH3 proteins have selectivity for different antiapoptotic proteins. In particular, Noxa binds selectively to Mcl-1. The latter also binds Bak and hence Noxa may displace Bak from Mcl-1, allowing it to bind to mitochondria [19, 133].
Fig. 1.
Current views of how the mitochondrial pathway to apoptosis is regulated
These findings are of particular interest in that immunohistological studies on tissue sections from melanoma have shown that Mcl-1 and Bcl-XL increase in expression with progression of the disease whereas Bcl-2 decreased during progression of the disease [142]. The factors determining the changes in the antiapoptotic proteins are not entirely clear but Mcl-1 and Bcl-XL appeared inversely correlated with expression of the transcription factor AP-2 and was weakly associated with Stat 3 expression. Further studies are needed to more closely define the regulators of these proteins, particularly Mcl-1 as current studies suggested it is upregulated as part of the unfolded protein response (UPR) to endoplasmic reticulum (ER) stress and is a major adaptive mechanism that prevents ER stress-induced apoptosis [50]. We [137] and others [70, 128] have also shown Mcl-1 is a critical factor in resistance to TNF-related apoptosis-inducing ligand (TRAIL).
These findings with respect to Mcl-1 in melanoma are important in design of treatment strategies in melanoma. As shown in Table 1, there are now a number of new agents that can be used clinically to target the antiapoptotic proteins. One of these is the Abbott ABT 737 agent, which has high affinity for Bcl-2 and Bcl-XL and Bcl-W. Preclinical studies have shown that many tumors were resistant to this agent due to Mcl-1 proteins in the tumor. Downregulation of Mcl-1 resulted in sensitivity to ABT 737 [20, 118]. These results are relevant to immunotherapy, particularly as ABT 737 was shown to sensitize tumor cells to killing by CTL [74]. ABT 263 is an orally active form of ABT 737 [116]. As shown in Table 1, however, there are a number of small mol. wt. inhibitors of the antiapoptotic proteins that have selectivity for all the antiapoptotic proteins, e.g. Obatoclax is now in preliminary trials in patients with hematological malignancies [105, 114]. At this stage, we would expect that these broad spectrum inhibitors would potentiate the effects of various forms of immunotherapy.
Table 1.
Targeting anti-apoptotic proteins
| Agent | Target protein(s) | Trial stage | References |
|---|---|---|---|
| Oblimersen (G3139) | Bcl-2 (specific) | Stage III | Bedikian et al. [13] |
| YM155 | Survivin | Phase II | Iwasa et al. [62] |
| LY2181308 (antisense) | Survivin | Phase II | Hunter et al. [58] |
| ABT-737 (ABT-263) | BH3-mimetic (inhibits Bcl-2 group: Bcl-2, Bcl-XL, Bcl-W, not Mcl-1) | Phase I | Chen et al. [20], Tse et al. [116], van Delft et al. [118] |
| Gossypol (AT-101) | BH3-mimetic (inhibits Bcl-2 group) | Phase I | MacVicar et al. [82] |
| Obatoclax (GX015-070) | BH3-mimetic (inhibits Bcl-2 group) | Phase I/II | Schimmer et al. [105] |
| SAHB | BH3 mimetic | Preclinical | Pitter et al. [97] |
| TW37 | Bim-mimetic (inhibits Bcl-2 group) | Preclinical | Wang et al. [130] |
| Smac mimetics | Inhibitor of IAP1, 2, XIAP | Preclinical | Zobel et al. [144] |
| Smac mimetic SM-164 | Inhibitor of IAP1, 2, XIAP | Preclinical | Lu et al. [78] |
| Smac mimetic Smac037 | Inhibitor of IAP1, 2, XIAP | Preclinical | Cossu et al. [22] |
Death ligand mediated killing: the Yin Yang of TRAIL death receptor signals
In the special case of TRAIL, the level of expression of death receptors, TRAIL-R1 and R2 were found to be major determinants of their susceptibility to killing by TRAIL [138]. Furthermore, progression of melanoma was associated with downregulation of receptors [143]. The cause of receptor downregulation is unknown but it is clear that immunotherapy that depends on administration of TRAIL or TRAIL mediated killing by CD4 T cells, DCs and monocytes, would be ineffective against TRAIL-R negative cancers. Upregulation of death receptors in some cancers can be achieved by chemotherapy that targets DNA and is p53 dependent [26]. However, in melanoma a wide range of chemotherapy agents and signal pathway inhibitors did not upregulate death receptors. As reported in studies on colon carcinoma cells [65] and prostate carcinoma [108], the ER stress inducer Tunicamycin was able to do so [63]. This was related to induction of the UPR response to stress. The transcription factor CCAAT/enhancer-binding protein homologous protein (CHOP) (Gadd153) was involved at late stages of receptor upregulation (36 h) but other factor(s) were involved at earlier periods [63]. Tunicamycin is considered too toxic for clinical use but several other agents that are in clinical use, such as Cox 2 inhibitors [68] or Dipyridamole, may be useful for this purpose [38]. They and curcumin from curry powder appear to act on CHOP to upregulate receptors [66].
One of the peculiarities of the TRAIL system is the concurrent delivery of opposing death and survival signals from its receptors [Yin (negative) and Yang (Positive)]. It has been known for some time that TRAIL receptors may associate with other adaptor proteins rather than or in addition to FADD and result in different outcomes rather than cell death. Principal among these is activation of NF-κB and JNK most likely through the receptor interacting protein (RIP) and TNF receptor associating factor 2 (TRAF2) [25]. We and others have shown that activation of NF-κB in melanoma by TRAIL is strongly anti-apoptotic [33]. One of the consequences of activation of NF-κB and of Akt [93] is upregulation of Flice inhibitory protein (cFLIP), which can bind to DED of FADD and caspase 8 and inhibit apoptosis. cFLIP may also bind to TRAF1, 2 and RIP, resulting in activation of NF-κB and ERK1/2 [122, 134]. Inhibitors of apoptosis proteins 1 and 2 (IAP1, 2) were also shown to be important in the activation of NF-κB (via ubiquitin domains) [43] by TNF-α and when IAPs were inhibited, activation of NF-κB did not occur [120]. Smac mimetics were shown to result in TNF-induced apoptosis by activation of caspase 8 either by inhibition of c-FLIP production or formation of a RIP/FADD/caspase 8 complex [127]. These observations have led to the development of a number of new agents, which target IAP1, 2 and XIAP, as shown in Table 1 [22, 78, 127, 144].
In addition, the MEK pathway may be strongly activated by TRAIL. This is rapid but relatively transient, peaking at 1 hour after exposure to TRAIL [137]. Activation of MEK is dependent on activation of protein kinase C (PKC), particularly PKC epsilon (ε), and the sensitivity of melanoma cells to TRAIL is inversely related to the activation of PKC-ε [37]. Activation of PKC is upstream of RAF but whether it is activated directly by TRAIL or by phospholipase C, as described for TNF-α [12], is not clear. The activation of MEK by TRAIL occurs irrespective of whether BRAF is mutated or not [137].
These results therefore imply that within a polyclonal population of melanoma cells there is a range of activation signals in response to TRAIL. Sensitive cells have predominant activation of the FADD/caspase 8 pathway whereas resistant cells have dominant activation of NF-κB and MEK pathways. These results clearly have implications in selecting treatment combinations.
Table 2 summarizes some of the experimental studies on combinations with TRAIL that may increase apoptosis. In general, they can be viewed as agents which upregulate TRAIL-R1–R2 receptors or which down-regulate anti-apoptotic proteins. Bortezomib appears to mediate its effect by down-regulation (directly or indirectly) of anti-apoptotic proteins such as cFLIP, Mcl-1 and NF-κB and upregulation of Noxa [90]. Clinical experience with the drug is mainly limited to hematologic malignancies such as multiple myeloma and mantle cell lymphoma, for which it has received FDA approval. Histone Deacetylase inhibitors have had relatively little effects when used as single agents but may be most effective when used as sensitizing agents to induce apoptosis [81, 139].
Table 2.
Treatment combinations with TRAIL or agonistic antibodies to TRAIL
| Agent | TRAIL/A.MAbs | Cancer | Mechanisms of action | References |
|---|---|---|---|---|
| Cox-2 inhibitors | TRAIL | Hepatocellular Ca | ↑TRAIL R1, R2 ↓Mcl-1 | Kern et al. [68] |
| Dipyridamole | TRAIL | Colon and prostate Ca | CHOP mediated ↑TRAIL R1, R2 | Goda et al. [38] |
| Curcumin | TRAIL | Renal Ca | ↑DR5 | Jung et al. [66] |
| Bortezomib | A.Mabs | NSCLC | ↓Mcl-1, ↓FLIP | Luster et al. [80] |
| Quercetin | TRAIL | Colon Ca | TRAIL R1, R2 in lipid rafts | Psahoulia et al. [98] |
| Sodium arsenite | TRAIL | Melanoma | TRAIL R1, R2↑, cFLIP↓ | Ivanov et al. [61] |
| Resveratol | TRAIL | Melanoma | NF-κB↓, Stat3↓, cFLIP, Bcl-XL | Ivanov et al. [60] |
| Vorinostat (HDACi) | MAb MD5 (murine) | Mouse breast Ca | cFLIP↓ | Frew et al. [34] |
| Quercetin | TRAIL | Human hepatoma | DR5↑, cFLIP↓ | Kim et al. [70] |
| Triterpenoid CDOO-Me | TRAIL | Human lung cancer | cFLIP↓ degradation | Zou et al. [145] |
Signal pathway inhibitors as partners for immunotherapy
Two survival pathways: the MEK/ERK and PI3-K Akt pathways are frequently upregulated in cancer cells [83, 107], as reported in melanoma [137, 141] and are potent inhibitors of apoptosis. We and others have shown that inhibition of the MEK/ERK pathway sensitizes cancer cells to TRAIL-induced apoptosis [137]. Similar results were obtained with inhibitors of Akt and when both inhibitors were used the results were additive [110]. In short-term assays inhibitors of the MEK pathway did not induce apoptosis when used alone but studies over longer periods resulted in killing of approximately 50% of melanoma lines due to upregulation of PUMA and Bim, and downregulation of Mcl-1 [129]. The multiple sites of action of the MEK pathway that inhibit apoptosis are shown in Table 3 and reviewed elsewhere [10]. The Akt pathway also has multiple sites of action that inhibit apoptosis, as shown in Table 4 from the review by Manning and Cantley [83].
Table 3.
The ERK1/2 pathway blocks apoptosis at multiple sites
Table 4.
Inhibition of apoptosis by activated Akt [83]
| Phosphorylates FOXO transcription factors resulting in decreased Bim |
| Phosphorylates and inhibits BAD |
| Phosphorylates and inhibits GSK3b |
| Activates NF-kB |
| Activates HDM2 and thereby decreases p53 |
MEK inhibitors or inhibitors of Ras and RAF upstream of MEK may therefore help in sensitizing melanoma cells to immunotherapy [126]. They may have other unexpected benefits in that MEK inhibitors were shown to upregulate melanoma differentiation antigens [71] and inhibit release of immunosuppressive factors IL-10, VEGF and IL-6 from melanoma [113]. RAF knockdown was shown to reduce ICAM-1 expression and IL-8 production from melanoma and to inhibit extravasation through blood vessels [73].
Table 5 shows the many RAS/BRAF/MEK signal pathway inhibitors are now available. They are reviewed in detail elsewhere [126] and as combinations with other therapies [112]. It is unknown whether they may have deleterious effects on immune function due to direct effects on lymphocytes. Relatively few studies have been conducted on their effects on the immune system but in this regard the RAF inhibitor PLX4032 (that is specific for mutated BRAF) should not have targets in lymphocytes. Holmstrom et al. [55] speculated the MEK pathway may prevent T cells from undergoing activation-induced death. Consistent with this, NRAS activating mutations were reported to be associated with autoimmune disease [91]. Sorafenib was found to inhibit the generation of vaccine specific CD8 T cells in mice. Sunitinib did not have these suppressive effects [52].
Table 5.
RAS/RAF/MEK signal pathway inhibitors
| Agent | Class of inhibitor | Target protein(s) | References |
|---|---|---|---|
| Sorafenib | Multikinase inhibitor | C-Raf; B-Raf; VEGF-2, -3; PDGF; Flt-3; c-Kit | Amaravadi et al. [2], Flaherty [31], Hauschild et al. [46], McDermott et al. [86] |
| Tanespimycin (KOS-953, 17-AGG) | Hsp90 inhibitor | Hsp90 (client proteins B-Raf, Akt, others) | Kefford et al. [67] |
| RAF265 | Multikinase inhibitor | Mutant B-Raf, VEGFR-2 | Fecher et al. [29] |
| PLX4032 | Selective B-Raf kinase inhibitor | Mutant B-Raf | Tsai et al. [115] |
| PD0325901 | Non-ATP-competitive specific MEK inhibitor | MEK1, 2 | Fecher et al. [29] |
| AZD6244 | Non-ATP-competitive specific MEK inhibitor | MEK1, 2 | Dummer et al. [24] |
| Tipifarnib (R115777) | Farnesyl transferase inhibitor | Prenylated proteins | Fouladi et al. [32], Haluska et al. [45] |
Development of inhibitors of Akt signaling is at an early stage (Table 6) and is discussed elsewhere [29, 59, 81]. The PI3K pathway was identified as critical for induction of a proliferative response to IL-2 in lymphocytes [16]. Treatment with an mTOR inhibitor, Rapamycin, was reported to sensitize cancer to adoptive immunotherapy in vivo [44], which is somewhat surprising given the role of Rapamycin as an immunosuppressive agent. It is highly likely that the effects of these drugs on immune responses will need to be evaluated in each system.
Table 6.
Akt, receptor tyrosine kinase (RTK), and Stat signal pathway inhibitors
| Agent(s) | Target protein | References |
|---|---|---|
| SF1126 (LY294002-prodrug) | PI3K | Garlich et al. [36] |
| Perifosine, PX-866 | Akt | Lopiccolo et al. [77] |
| CMEP | Akt | Zhang et al. [136] |
| GSK 690693 | Akt | |
| Temsirolimus (CCI-779) | mTOR | Abraham and Eng [1] |
| Everolimus (RAD001) | mTOR | Abraham and Eng [1] |
| Deforolimus (AP23573) | mTOR | Mita et al. [88] |
| XL765 | P13K/mTOR | Papadopoulos et al. [94] |
| PI 103 | PI3K/mTOR | Raynaud et al. [102] |
| SB216763, DW1/2 | GSK3β | Smalley et al. [111] |
| Imatinib, dasatinib, sunitinib, erlotinib | RTKs | Hodi et al. [53] |
| Dasatinib | Src | Guilhot et al. [42] |
| S31-M2001 | Stat3 | Siddiquee et al. [109] |
| SUI1274 | c-Met/HGF | Natali et al. [89], Puri et al. [99] |
Adaptation to ER stress as the driver of resistance to immunotherapy
Under stress conditions such as hypoglycemia or hypoxia, proteins in the ER may not undergo folding or glycosylation and accumulate as unfolded proteins. These bind to a chaperone protein called glucose-regulated protein 78 (GRP78), which is normally bound to the intraluminal domains of three sensor proteins called inositol-requiring transmembrane kinase and endonuclease 1α (IRE1α), activation of transcription factor 6 (ATF6), and protein kinase-like ER kinase (PERK). Release of these proteins results in their activation and initiation of three signal pathways called the UPR. As an adaptive response, the UPR is activated to alleviate the stress condition imposed on the ER and is orchestrated by transcriptional activation of multiple genes mediated by IRE1α and ATF6, a general decrease in initiation of translation and selective translation of specific mRNAs mediated by PERK. However, if the stress on ER remains unresolved, prolonged activation of the UPR can lead to apoptosis [69].
This is the outcome in normal cells but even under extreme ER stress cancer cells survive due to adaptive processes that are as yet not fully understood. Activation of the MEK/ERK and Akt pathways are involved [57, 63]. Induction of the chaperone protein GRP78 may sequester one of the BH3 proteins in the ER called Bik [35]. GRP78 also sequesters caspase 4 [63]. HDM2 becomes activated during ER stress either via Akt activation or direct phosphorylation by PERK [11]. It is possible but not yet proven that this may account for low p53 levels in some cancers such as melanoma. In addition to these direct effects on apoptosis, ER is also associated with metabolic changes resulting in glycolysis and lactic acid production [40, 92]. This results in an acidic environment that may inhibit the function or entry of immune cells into the tumor micro-environment. Table 7 summarizes some of the known antiapoptotic effects of ER stress.
Table 7.
ER stress induces antiapoptotic effects [50]
| Upregulation of Bcl-XL, Mcl-1. Downregulation of Bcl-2 |
| Activation of Akt, MEK/ERK |
| Upregulation of GRP78 |
| Downregulation of p53 (via ?HDM2) |
| Glycolysis and acidification of the microenvironment |
Agents that may reduce the effects of ER stress on resistance to apoptosis
Given the importance of ER stress in resistance to immunotherapy, agents that overcome the adaptive pathways may be important to combine with immunotherapy. We know from studies in vitro that MEK inhibitors may overcome resistance to ER stress-induced apoptosis most probably by downregulation of GRP78, which is a target for this pathway. One of the consequences of GRP78 downregulation is release of caspase 4 (that is normally bound by GRP78) so resulting in apoptosis of some melanoma [63]. Another approach is development of drugs that inhibit the activity of IRE1α so interfering with some of the downstream effects of the UPR. One such drug is referred to as Irestatin 9389 [30] (Table 8).
Table 8.
Additional agents to target ER stress-induced resistance to apoptosis [50]
| Agents that target HDM2 and increase p53, e.g. Nutlin 3a |
| Inhibitors of GSK3β that target S 315,376 on p53, DW 1/2 |
| Inhibitors of GRP78, IRE1α (epigallocatechin, Irestatin) |
| VEGF-R2 inhibitors (AZD 2171), proton pump inhibitors (Omeprazole) |
| Monocarboxylate transporter (MCT) inhibitors |
| Zinc protoporphyrin inhibitors of HO-1 |
Novel agents that target GRP78, such as epigallocatechin, are reviewed elsewhere [72, 100]. Other approaches involve targeting more downstream effects of the UPR such as upregulation of p53 by agents which interfere with binding of p53 with HDM2, such as Nutlin 3a [121]. Inhibitors of GSK3β, such as the organometallic protein kinase inhibitor DW1/2, were also shown to upregulate p53 perhaps due to effects on HDM2 [111] or by inhibiting the phosphorylation of p53 by GSK3β [101]. GSK3β inhibitors may, however, increase the stability of Mcl-1 as the latter was shown to be a target for phosphorylation by GSK3β in IL-3 dependent cells [85]. GSK3β inhibitors were also reported to increase TRAIL death receptor expression by activation of c-MYC [104].
Drugs that might exploit the metabolic consequences of ER stress such as excess lactate production may have a role in combination with immunotherapy. These include proton pump inhibitors such as Omeprazole, which target H(+)-ATPases [23] and monocarboxylate transporters (MCT) 1–4 [15, 84], as well as Na+/H+ exchangers. MCT transporters such as MCT-1 appeared more important in regulation of pH in melanoma cell lines [124] than Na+/H+ exchange pumps. MCT isoforms may, however, have opposing roles as studies on human cervical carcinoma suggested that MCT-4 transported lactate out of cells whereas MCT-1 transported lactate into cells where it was a source of energy under aerobic conditions [106]. Omeprazole was shown to increase the sensitivity of xenografts to cisplatin and this combination is the subject of ongoing clinical studies in melanoma.
Induction of ER stress is also believed to result in upregulation of heme oxygenase-1 (HO-1) due to induction of ROS [76] and has a cytoprotective effect. There is limited information about its role in protection of melanoma against these products of ER stress but inhibitors of HO-1 such as zinc protoporphyrin and polymeric derivatives [27] may have a role in increasing apoptosis to ER stress-inducing agents [28].
Targeting anti-apoptotic proteins for destruction by the immune system
Much interest also exists in decreasing the resistance of tumor (melanoma) cells to apoptosis by inducing T cell responses against the proteins concerned. This subject is well reviewed elsewhere [4]. In particular, several members of the IAP family were shown to be targets of the immune system. One of these, survivin, was referred to as a universal tumor antigen [7] and both MHC class I [54] and class II [95] epitopes identified. Phase I studies against survivin have been conducted or are in progress in patients with bladder cancer and melanoma [3, 56] and breast cancer [117]. T cell responses against ML-IAP (Livin) were also reported to be common in melanoma patients. Spontaneous CTL responses have also been identified against Mcl-1 [6] and Bcl-XL [5], indicating that these two proteins may also be effective targets for immunotherapy. It is also possible that immunotherapy against Mcl-1 may be complementary to treatment with ABT-737, which is limited in effectiveness due to lack of specificity against Mcl-1 (see above).
Conclusion
Increased knowledge concerning cell death pathways used by lymphocytes to kill cancer cells and the availability of many new agents that can inhibit resistance pathways against apoptosis have set the scene for new approaches to immunotherapy based on combinations with the new agents. This includes immunotherapy, not only with vaccines but logically also with adoptive immunotherapy and CTLA4 antibodies. Several agents appear particularly promising, e.g. agents which directly target the anti-apoptotic proteins in melanoma would appear to provide much scope for sensitization of melanoma to immunotherapy but it is also possible that inhibitors of signal pathways, which regulate expression of these proteins may be equally effective. Similarly, agents such as Bortezomib and Histone deacetylase inhibitors may find their true role as sensitizers of melanoma to immunotherapy. Evaluation of these drugs in patients undergoing immunotherapy with vaccines, cytokines, adoptive immunotherapy or CTLA4 antibodies in well-planned clinical trials is now needed.
References
- 1.Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008;12:209–222. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- 2.Amaravadi R, Schuchter LM, McDermott DF, Kramer A, Giles L, Troxel AB, Medina CA, Nathanson KL, O’Dwyer PJ, Flaherty KT (2007) Updated results of a randomized phase II study comparing two schedules of temozolomide in combination with sorafenib in patients with advanced melanoma. Proc Am Assoc Cancer Res 25:Abstract 8527
- 3.Andersen MH, Becker JC, Thor Straten P. The antiapoptotic member of the Bcl-2 family Mcl-1 is a CTL target in cancer patients. Leukemia. 2005;19:484–485. doi: 10.1038/sj.leu.2403621. [DOI] [PubMed] [Google Scholar]
- 4.Andersen MH, Jurgen CB, Thor Straten P. Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev. 2005;4:399–409. doi: 10.1038/nrd1717. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MH, Reker S, Kvistborg P, Becker JC, Thor Straten P. Spontaneous immunity against Bcl-xL in cancer patients. J Immunol. 2005;175:2709–2714. doi: 10.4049/jimmunol.175.4.2709. [DOI] [PubMed] [Google Scholar]
- 6.Andersen MH, Reker S, Becker JC, Thor Straten P. The melanoma inhibitor of apoptosis protein: a target for spontaneous cytotoxic T cell responses. J Invest Dermatol. 2004;122:392–399. doi: 10.1046/j.0022-202X.2004.22242.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen MH, Thor Straten P. Survivin—a universal tumor antigen. Histol Histopathol. 2002;17:669–675. doi: 10.14670/HH-17.669. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 10.Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 11.Baltzis D, Pluquet O, Papadakis AI, Kasemi S, Qu LK, Koromilas AE. The EIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of P53. J Biol Chem. 2007;282:31675–31687. doi: 10.1074/jbc.M704491200. [DOI] [PubMed] [Google Scholar]
- 12.Barbara JAJ, Van Ostade X, Lopez AF. Tumour necrosis factor-alpha (TNF-α): the good, the bad and potentially very effective. Immunol Cell Biol. 1996;74:434–443. doi: 10.1038/icb.1996.73. [DOI] [PubMed] [Google Scholar]
- 13.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 14.Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007;19:339–347. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Brahimi-Horn MC, Pouyssegur J. Harnessing the hypoxia-inducible factor in cancer and ischemic disease. Biochem Pharmacol. 2007;73:450–457. doi: 10.1016/j.bcp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 17.Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, McMahon M. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 21.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 22.Cossu F, Mastrangelo E, Milani M, Sorrentino G, Lecis D, Delia D, Manzoni L, Seneci P, Scolastico C, Bolognesi M. Designing Smac-mimetics as antagonists of XIAP, cIAP1, and cIAP2. Biochem Biophys Res Commun. 2009;378:162–167. doi: 10.1016/j.bbrc.2008.10.139. [DOI] [PubMed] [Google Scholar]
- 23.De Milito A, Iessi E, Logozzi M, Lozupone F, Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini L, Nilsson A, Fais S. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007;67:5408–5417. doi: 10.1158/0008-5472.CAN-06-4095. [DOI] [PubMed] [Google Scholar]
- 24.Dummer R, Robert C, Chapman PB, Sosman JA, Middleton M, Bastholt L, Kemsley K, Cantarini MV, Morris C, Kirkwood JM (2008) AZD6244 (ARRY-142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: an open-label, randomized, multicenter, phase II study. J Clin Oncol ASCO Annual Meeting May 20 Suppl, Abstract 9033
- 25.Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003;22:3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- 26.El-Deiry WS. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 2001;8:1066–1075. doi: 10.1038/sj.cdd.4400943. [DOI] [PubMed] [Google Scholar]
- 27.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Fang J, Nakamura H, Iyer AK. Tumor-targeted induction of oxystress for cancer therapy. J Drug Target. 2007;15:475–486. doi: 10.1080/10611860701498286. [DOI] [PubMed] [Google Scholar]
- 29.Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007;25:1606–1620. doi: 10.1200/JCO.2006.06.0442. [DOI] [PubMed] [Google Scholar]
- 30.Feldman D, Koong AC. Irestatin, a potent inhibitor of IRE1α and the unfolded protein response, is a hypoxia-selective cytotoxin and impairs tumor growth. 2007 ASCO annual meeting proceedings (post-meeting edition) J Clin Oncol. 2007;25:3514. [Google Scholar]
- 31.Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12:2366s–2370s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- 32.Fouladi M, Nicholson HS, Zhou T, Laningham F, Helton KJ, Holmes E, Cohen K, Speights RA, Wright J, Pollack IF, Children’s Oncology Group A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children’s Oncology Group study. Cancer. 2007;110:2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 33.Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, Nguyen T, Hersey P. The Role of NF-κB in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol. 2001;166:5337–5345. doi: 10.4049/jimmunol.166.9.5337. [DOI] [PubMed] [Google Scholar]
- 34.Frew AJ, Lindemann RK, Martin BP, Clarke CJ, Sharkey J, Anthony DA, Banks KM, Haynes NM, Gangatirkar P, Stanley K, Bolden JE, Takeda K, Yagita H, Secrist JP, Smyth MJ, Johnstone RW. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci USA. 2008;105:11317–11322. doi: 10.1073/pnas.0801868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 36.Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, Murali R, Lu Y, Mills GB, Kundra V, Shu HK, Peng Q, Durden DL. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie S, Zhang XD, Hersey P. Variable expression of protein kinase Cε in human melanoma cells regulates sensitivity to TRAIL-induced apoptosis. Mol Cancer Ther. 2005;4:668–676. doi: 10.1158/1535-7163.MCT-04-0332. [DOI] [PubMed] [Google Scholar]
- 38.Goda AE, Yoshida T, Horinaka M, Yasuda T, Shiraishi T, Wakada M, Sakai T. Mechanisms of enhancement of TRAIL tumoricidal activity against human cancer cells of different origin by dipyridamole. Oncogene. 2008;27:3435–3445. doi: 10.1038/sj.onc.1211008. [DOI] [PubMed] [Google Scholar]
- 39.Green DR. At the gates of death. Cancer Cell. 2006;9:328–330. doi: 10.1016/j.ccr.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Green DR, Chipuk JE. p53 and metabolism: inside the TIGAR. Cell. 2006;126:30–32. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 42.Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, Heim D, Larson RA, Branford S, Muller MC, Agarwal P, Gollerkeri A, Talpaz M. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 43.Gyrd-Hansen M, Darding M, Miasari M, Santoro MM, Zender L, Xue W, Tenev T, da Fonseca PC, Zvelebil M, Bujnicki JM, Lowe S, Silke J, Meier P. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat Cell Biol. 2008;10:1309–1317. doi: 10.1038/ncb1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahnel PS, Thaler S, Antunes E, Huber C, Theobald M, Schuler M. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res. 2008;68:3899–3906. doi: 10.1158/0008-5472.CAN-07-6286. [DOI] [PubMed] [Google Scholar]
- 45.Haluska P, Dy GK, Adjei AA. Farnesyl transferase inhibitors as anticancer agents. Eur J Cancer. 2002;38:1685–1700. doi: 10.1016/s0959-8049(02)00166-1. [DOI] [PubMed] [Google Scholar]
- 46.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, Eggermot A, Grabbe S, Gonzalez R, Gille J, Peschel C, Schadendorf D, Garbe C, O’Day S, Daud A, White M, Xia C, Patel K, Korkwood J, Keilholz U (2009) Results of a phase III randomized, placebo-controlled study of Sorafenib in combination with Carboplatin and Paclitaxel in second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. doi:10.1200/JCO.2007.15.7636 [DOI] [PubMed]
- 47.Hersey P. Advances in the non-surgical treatment of melanoma. Expert Opin Investig Drugs. 2002;11:75–85. doi: 10.1517/13543784.11.1.75. [DOI] [PubMed] [Google Scholar]
- 48.Hersey P, Zhang XD. How melanoma cells evade TRAIL-induced apoptosis. Nat Rev Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 49.Hersey P, Zhang XD. Immunotherapy of melanoma: principles. In: Thompson JF, Morton DL, Kroon BBR, editors. Textbook of melanoma. London: Martin Dunits/Taylor and Francis; 2004. pp. 559–572. [Google Scholar]
- 50.Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res. 2008;21:358–367. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 51.Hetz CA. ER stress signaling and the BCL-2 family of proteins: from adaptation to irreversible cellular damage. Antioxid Redox Signal. 2007;9:2345–2355. doi: 10.1089/ars.2007.1793. [DOI] [PubMed] [Google Scholar]
- 52.Hipp MM, Hilf N, Walter S, Werth D, Kanz L, Weinschenk T, Singh H, Brossart P (2007) Sorafenib but not sunitinib affects the induction of immune responses. ASCO annual meeting proceedings part 1. J Clin Oncol 25:Abstract 3504
- 53.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofmann UB, Voigt H, Andersen MH, Straten PT, Becker JC, Eggert AO. Identification and characterization of surviving-derived H-2Kb-restricted CTL epitopes. Eur J Immunol. 2009;39:1419–1424. doi: 10.1002/eji.200839098. [DOI] [PubMed] [Google Scholar]
- 55.Holmstrom TH, Shmitz I, Soderstrom TS, Poukkula M, Johnson VL, Chow SC, Krammer PH, Eriksson JE. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 2000;19:5418–5428. doi: 10.1093/emboj/19.20.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, Sato E, Hirohashi Y, Masumori N, Tsukamoto T, Sato N (2009) Phase I clinical study of anti-apoptosis protein surviving-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother (epub ahead of print) [DOI] [PMC free article] [PubMed]
- 57.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- 58.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 59.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res. 2008;314:1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanov VN, Hei TK. Sodium arsenite accelerates TRAIL-mediated apoptosis in melanoma cells through upregulation of TRAIL-R1/R2 surface levels and downregulation of cFLIP expression. Exp Cell Res. 2006;312:4120–4138. doi: 10.1016/j.yexcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwasa T, Okamoto I, Suzuki M, Nakahara T, Yamanaka K, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res. 2008;14:6496–6504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- 63.Jiang CC, Chen LH, Gillespie S, Wang YF, Kiejda KA, Zhang XD, Hersey P. Inhibition of MEK sensitizes human melanoma cells to endoplasmic reticulum stress-induced apoptosis. Cancer Res. 2007;67:9750–9761. doi: 10.1158/0008-5472.CAN-07-2047. [DOI] [PubMed] [Google Scholar]
- 64.Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, de Bock CE, Thorne RF, Allen J, Hersey P, Zhang XD. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon ER stress. Cancer Res. 2008;68:6708–6717. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 65.Jin Z, McDonald ER, 3rd, Dicker DT, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem. 2004;279:35829–35839. doi: 10.1074/jbc.M405538200. [DOI] [PubMed] [Google Scholar]
- 66.Jung EM, Park JW, Choi KS, Park JW, Lee HI, Lee KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–2017. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- 67.Kefford R, Millward M, Hersey P, Brady B, Graham M, Johson RG, Hannah AL. Phase II trial of tanespimycin (KOS-953), a heat shock protein-90 (Hsp90) inhibitor in patients with metastatic melanoma (Abstract 8558) Proc Am Soc Clin Oncol. 2007;25:486. [Google Scholar]
- 68.Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H, Friess H, Stremmel W, Krammer PH, Schirmacher P, Muller M. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–7066. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- 69.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 70.Kim S-H, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 71.Kono M, Dunn IS, Durda PJ, Butera D, Rose LB, Haggerty TJ, Benson EM, Kurnick JT. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res. 2006;4:779–792. doi: 10.1158/1541-7786.MCR-06-0077. [DOI] [PubMed] [Google Scholar]
- 72.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 73.Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007;67:5814–5820. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lickliter JD, Cox J, McCarron J, Martinez NR, Schmidt CW, Lin H, Nieda M, Nicol AJ. Small-molecule Bcl-2 inhibitors sensitise tumour cells to immune-mediated destruction. Br J Cancer. 2007;96:600–608. doi: 10.1038/sj.bjc.6603599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim JH, Lee ES, You HJ, Lee JW, Park JW, Chun YS. Ras-dependent induction of HIF-1alpha785 via the Raf/MEK/ERK pathway: a novel mechanism of Ras-mediated tumor promotion. Oncogene. 2004;23:9427–9431. doi: 10.1038/sj.onc.1208003. [DOI] [PubMed] [Google Scholar]
- 76.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem. 2005;280:72–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 77.Lopiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the P13K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, Meagher JL, Stuckey JA, Wang S. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F, Federici C, Iessi E, Parmiani G, Arancia G, Belardelli F, Fais S. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 80.Luster TA, Carrell JA, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther. 2009;8:292–302. doi: 10.1158/1535-7163.MCT-08-0918. [DOI] [PubMed] [Google Scholar]
- 81.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 82.MacVicar GR, Kuzel TM, Curti BD, Poiesz B, Somer B, Greco FA, Gressler V, Brill K, Leopold L. An open-label, multicenter, phase I/II study of AT-101 in combination with docetaxel (D) and prednisone (P) in men with hormone refractory prostate cancer (HRPC). ASCO annual meeting, May 20 Suppl, Abstract 16043. J Clin Oncol. 2008;26:2008. [Google Scholar]
- 83.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55:1410–1419. doi: 10.1227/01.neu.0000143034.62913.59. [DOI] [PubMed] [Google Scholar]
- 85.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 86.McDermott DF, Sosman JA, Hodi FS, Gonzalez R, Linette G, Richards J, Jakub JK, Beeram M, Patel K, Cranmer L. Randomized phase II study of dacarbazine with or without sorafenib in patients with advanced melanoma. Proc Am Soc Clin Oncol. 2007;25:474s. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 87.Meslin F, Thiery J, Richon C, Jalil A, Chouaib S. Granzyme B-induced cell death involves induction of p53 tumor suppressor gene and its activation in tumor target cells. J Biol Chem. 2007;282:32991–32999. doi: 10.1074/jbc.M705290200. [DOI] [PubMed] [Google Scholar]
- 88.Mita MM, Britten CD, Poplin E, Tap WD, Carmona A, Yonemoto L, Wages DS, Bedrosian CL, Rubin EH, Tolcher AW. Deforolimus trial 106—a phase I trial evaluating 7 regimens of oral Deforolimus (AP23573, MK-8669) J Clin Oncol. 2008;26:361–367. [Google Scholar]
- 89.Natali PG, Nicotra MR, Di Renzo MF, Prat M, Bigotti A, Cavaliere R, Comoglio PM. Expression of the c-Met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993;68:746–750. doi: 10.1038/bjc.1993.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, Verhaegen M, Varambally S, Chinnaiyan AM, Jakubowiak AJ, Soengas MS. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci USA. 2007;104:19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oliveira JB, Bidere N, Niemela JE, Zheng L, Sakai K, Nix CP, Danner RL, Barb J, Munson PJ, Puck JM, Dale J, Straus SE, Fleisher TA, Lenardo MJ. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci USA. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan JG, Mak TW (2007) Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE 2007:Issue 381, pe14 [DOI] [PubMed]
- 93.Panka DJ, Mano T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- 94.Papadopoulos KP, Markman B, Tabernero J, Patnaik A, Heath EI, DeCillis A, Laird D, Aggarwal SK, Nguyen L, LoRusso PM (2008) A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of a novel PI3K inhibitor, XL76, administered orally to patients (pts) with advanced solid tumors. J Clin Oncol 26:May 20 Supplement, Abstract 3510
- 95.Piesche M, Hildebrandt Y, Zetti F, Chapuy B, Schmitz M, Wulf G, Trumper L, Schroers R. Identification of a promiscuous HLA DR-restricted T-cell epitope derived from the inhibitor of apoptosis is protein surviving. Hum Immunol. 2007;68:572–576. doi: 10.1016/j.humimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol. 2007;19:301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pitter K, Bernal F, Labelle J, Walensky LD. Dissection of the BCL-2 family signaling network with stabilized alpha-helices of BCL-2 domains. Methods Enzymol. 2008;446:387–408. doi: 10.1016/S0076-6879(08)01623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis is in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007;6:2591–2599. doi: 10.1158/1535-7163.MCT-07-0001. [DOI] [PubMed] [Google Scholar]
- 99.Puri N, Ahmed S, Janamanchi V, Tretiakova M, Zumba O, Krausz T, Jagadeeswaran R, Salgia R. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res. 2007;13:2246–2253. doi: 10.1158/1078-0432.CCR-06-0776. [DOI] [PubMed] [Google Scholar]
- 100.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 101.Qu L, Huang S, Baltzis D, Rivas-Estilla A-M, Pluquet O, Hatzoglou M, Koumenis C, Taya Y, Yoshimura A, Koromilas AE. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev. 2004;18:261–277. doi: 10.1101/gad.1165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, de Haven BA, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 103.Rosenthal R, Viehl CT, Guller U, Weber WP, Adamina M, Spagnoli GC, Heberer M, Zuber M. Active specific immunotherapy phase III trials for malignant melanoma: systematic analysis and critical appraisal. J Am Coll Surg. 2008;207:95–105. doi: 10.1016/j.jamcollsurg.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 104.Rottmann S, Wang Y, Nasoff M, Deveraux QL, Quon KC. A TRAIL receptor-dependent synthetic lethal relationship between MYC activation and GSK3beta/FBW7 loss of function. Proc Natl Acad Sci USA. 2005;102:15195–15200. doi: 10.1073/pnas.0505114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, Yee K, Ravandi F, Giles F, Schuh A, Gupta V, Andreeff M, Koller C, Chang H, Kamel-Reid S, Berger M, Viallet J, Borthakur G. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 106.Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Shiraishi T, Yoshida T, Nakata S, Horinaka M, Wakada M, Mizutani Y, Miki T, Sakai T. Tunicamycin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human prostate cancer cells. Cancer Res. 2005;65:6364–6370. doi: 10.1158/0008-5472.CAN-05-0312. [DOI] [PubMed] [Google Scholar]
- 109.Siddiquee KA, Gunning PT, Glenn M, Katt WP, Zhang S, Schroeck C, Sebti SM, Jove R, Hamilton AD, Turkson J. An oxazole-based small-molecule Stat3 inhibitor modulates Stat3 stability and processing and induces antitumor cell effects. ACS Chem Biol. 2007;2:787–798. doi: 10.1021/cb7001973. [DOI] [PubMed] [Google Scholar]
- 110.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 111.Smalley KSM, Contractor R, Haass NK, Kulp AN, Atilla-Gokcumen GE, Williams DS, Bregman H, Flaherty KT, Soengas MS, Meggers E, Herlyn M. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:209–217. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 112.Smalley KSM, Flaherty KT. Integrating BRAF/MEK inhibitors into combination therapy for melanoma. Br J Cancer. 2009;100:431–435. doi: 10.1038/sj.bjc.6604891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 115.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KSM, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim S-H, Schlessinger J, Zhang KYJ, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 117.Tsuruma T, Iwayama Y, Ohmura T, Katsuramaki T, Hata F, Furuhata T, Yamaguchi K, Kimura Y, Torigoe T, Toyota N, Yagihashi A, Hirohashi Y, Asanuma H, Shimozawa K, Okazaki M, Mizushima Y, Nomura N, Sato N, Hirata K. Clinical and immunological evaluation of anti-apoptosis protein, surviving-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med. 2008;6:24. doi: 10.1186/1479-5876-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer Res. 2009;69:1985–1994. doi: 10.1158/0008-5472.CAN-08-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2006;13:23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Vilimanovich U, Bumbasirevic V. TRAIL induces proliferation of human glioma cells by c-FLIPL-mediated activation of ERK1/2. Cell Mol Life Sci. 2008;65:814–826. doi: 10.1007/s00018-008-7513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nature. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 124.Wahl ML, Owen JA, Burd R, Herlands RA, Nogai SS, Rodeck U, Berd D, Leeper DB, Owen CS. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol Cancer Ther. 2002;1:617–628. [PubMed] [Google Scholar]
- 125.Walden P. Therapeutic vaccination for the treatment of malignant melanoma. Recent Results Cancer Res. 2007;176:219–227. doi: 10.1007/978-3-540-46091-6_19. [DOI] [PubMed] [Google Scholar]
- 126.Wang JY, Wilcoxen KM, Nomoto K, Wu S. Recent advances of MEK inhibitors and their clinical progress. Curr Top Med Chem. 2007;7:1364–1378. doi: 10.2174/156802607781696837. [DOI] [PubMed] [Google Scholar]
- 127.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 128.Wang X, Chen W, Zeng W, Bai L, Tesfaigzi Y, Belinsky SA, Lin Y. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther. 2008;7:1156–1163. doi: 10.1158/1535-7163.MCT-07-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 130.Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang S, Sarkar FH, Mohammad RM. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and induces apoptosis in pancreatic cancer: involvement of Notch-1 signaling pathway. Cancer Res. 2009;69:2757–2765. doi: 10.1158/0008-5472.CAN-08-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Waterhouse NJ, Sedelies KA, Trapani JA. Role of Bid-induced mitochondrial outer membrane permeabilization in granzyme B-induced apoptosis. Immunol Cell Biol. 2006;84:72–78. doi: 10.1111/j.1440-1711.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- 132.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DCS. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-XL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu JW, Shi Y. FLIP and the death effector domain family. Oncogene. 2008;27:6216–6227. doi: 10.1038/onc.2008.299. [DOI] [PubMed] [Google Scholar]
- 135.Zhang B, Zhang J, Tian Z. Comparison in the effects of IL-2, IL-12, IL-15 and IFNα on gene regulation of granzymes of human NK cell line NK-92. Int Immunopharmacol. 2008;8:989–996. doi: 10.1016/j.intimp.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 136.Zhang M, Fang X, Liu H, Guo R, Wu X, Li B, Zhu F, Ling Y, Griffith BN, Wang S, Yang D. Bioinformatics-based discovery and characterization of an AKT-selective inhibitor 9-chloro-2-methylellipticinium acetate (CMEP) in breast cancer cells. Cancer Lett. 2007;252:244–258. doi: 10.1016/j.canlet.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 137.Zhang XD, Borrow JM, Zhang XY, Nguyen T, Hersey P. Activation of ERK1/2 protects melanoma cells from TRAIL-induced apoptosis by inhibiting Smac/DIABLO release from mitochondria. Oncogene. 2003;22:2869–2881. doi: 10.1038/sj.onc.1206427. [DOI] [PubMed] [Google Scholar]
- 138.Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–2753. [PubMed] [Google Scholar]
- 139.Zhang XD, Gillespie SK, Borrow JM, Hersey P. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther. 2004;3:425–435. [PubMed] [Google Scholar]
- 140.Zhang XD, Zhang XY, Gray CP, Nguyen T, Hersey P. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of human melanoma is regulated by Smac/DIABLO release from mitochondria. Cancer Res. 2001;61:7339–7348. [PubMed] [Google Scholar]
- 141.Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Palmer AA, Zhang XD, Thompson JF, Bron LP, Hersey P. Activation of the extracellular-signal related kinase (ERK) pathway in human melanoma. J Clin Pathol. 2005;58:1163–1169. doi: 10.1136/jcp.2005.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Zhang XD, Thompson JF, Hersey P. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol. 2007;20:416–426. doi: 10.1038/modpathol.3800750. [DOI] [PubMed] [Google Scholar]
- 143.Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Zhang XD, Thompson JF, Screaton G, Hersey P. Progression in melanoma is associated with decreased expression of death receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Hum Pathol. 2006;37:1286–1294. doi: 10.1016/j.humpath.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 144.Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJA, Okawa DC, Flygare JA, Vucic D, Fairbrother WJDK. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol. 2006;1:525–533. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 145.Zou W, Chen S, Liu X, Yue P, Sporn MB, Khuri FR, Sun S-Y. c-FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid Methyl-2-Cyano-3, 12-Dioxooleana-1, 9-Dien-28-Oate (CDDO-Me) in human lung cancer cells. Cancer Biol Ther. 2007;6:1614–1620. doi: 10.4161/cbt.6.10.4763. [DOI] [PubMed] [Google Scholar]