Abstract
Cancer patients with advanced disease display signs of immune suppression, which constitute a major obstacle for effective immunotherapy. Both T cells and NK cells are affected by a multitude of mechanisms of which the generation of reactive oxygen species is of major importance. Therefore, we hypothesized that two weeks of high-dose treatment with the anti-oxidant vitamin E may enhance NK cell function in cancer patients by protecting from oxidative stress. Seven patients with colorectal cancer (Dukes stage C and D) received a daily dose of 750 mg of vitamin E during a period of two weeks and the function, phenotype and receptor expression of NK cells were analyzed. The short-term vitamin E treatment significantly improved NK cell cytolytic activity in six out of the seven patients analyzed. The increased NK cell activity in patients’ PBMC was not due to increased numbers of NK cells or an increase in the proportion of the CD56dim NK cell subpopulation. Furthermore, neither an increased perforin expression nor an enhanced ability of NK cells to produce IFN-γ was observed as a result of vitamin E treatment. Finally, vitamin E treatment was associated with a minor, but consistent, induction of NKG2D expression in all patients analyzed. In conclusion, this pilot study demonstrates that vitamin E may boost NK cell function in patients with colorectal cancer. Further studies are warranted to explore the potential of vitamin E as an adjuvant for immunotherapy against cancer and to determine the underlying mechanism(s) behind vitamin E induced NK cell activation.
Keywords: Immune suppression, Colorectal cancer, Vitamin E, Natural killer cells, Tumor immunobiology
Introduction
The immune system of patients and experimental animals with advanced cancer is often severely compromised, constituting a barrier to efficient immunotherapy, and contributing to the spread of the disease and to opportunistic infections [37]. This can be accounted for by a multitude of factors, including production of PGE2 and gangliosides by the tumor cells, Fas–FasL interaction inducing T cell apoptosis, and production of arginase I by myeloid suppressor cells [20, 30, 42, 43, 55]. An important role for reactive oxygen species (ROS) was demonstrated in tumor-induced immune suppression (reviewed in Refs. [28, 37]). ROS produced by macrophages isolated from metastatic lymph nodes of melanoma patients [29] or from mice with experimental tumors [2] were able to inhibit immune effector functions and down-regulate CD3-ζ levels in autologous T cells. A variety of myeloid cell types were responsible for ROS production in tumor-bearing individuals, including tumor-derived monocytes/macrophages, myeloid suppressor cells, and activated granulocytes (reviewed in Refs. [17, 50]). This may result both in local immune suppression in tumor infiltrating lymphocytes and systemic immune suppression often observed in cancer patients.
Tumor induced immune suppression is not limited to the adaptive T cell system, and defects in dendritic cell (DC) and NK cell functions have been observed in humans and mice with advanced cancer [17, 24]. NK cells are of importance both as effector cells and as regulators of the adaptive immune system [40], and may be involved in the inhibition of the metastatic spread of MHC class I deficient human and mouse tumors [13, 53]. It was previously shown that NK cell mediated cytotoxicity could be suppressed by H2O2, secreted by monocytes recovered from human PBMC, and that this effect could be prevented by catalase or by histamine [23, 29, 47]. The increased levels of ROS produced in cancer patients and in tumor-bearing mice may therefore be an important mechanism accounting for the reduced NK activity in tumor bearing individuals.
The clinical effect of immunotherapy in patients with advanced cancer has been limited [44]. This may be related to a multitude of factors, such as immunological selection of tumor cells with reduced or lost expression of MHC class I expression [48] or of tumor antigenic epitopes [27]. As immunotherapy often is applied to patients with a large tumor burden, a plausible explanation for its limited clinical effect could be the tumor-induced immune suppression in the treated patients. This has motivated efforts to enhance the immune functions in cancer patients by combining immunotherapy with drugs that stimulate the immune system. One example of a potential drug to be used in combination with immunotherapy is vitamin E, which is a lipid-soluble anti-oxidant located in cellular membranes, where it protects the cell from oxidative stress and increases membrane stability (reviewed in [49]). We have previously published results from a phase I trial where 13 patients with advanced colorectal cancer received a short-term (2 weeks) dietary supplementation of 750 mg vitamin E [36]. The rational for carrying out this trial was to evaluate the effect of vitamin E on the functions of the T cells from cancer patient.
NK cells are particularly sensitive to oxidative stress [22], and studies by others in mice and in vitro-systems using human NK cells have shown that vitamin E can enhance NK activity [4, 15]. This motivated us to investigate if NK functions were affected by vitamin E substitution also in patients with advanced cancer. Our results from a small pilot study in the same cohort of patients as our earlier study demonstrated enhanced NK activity in the majority of the treated patients following short-term dietary Vitamin E substitution. This justifies further larger clinical studies investigating if vitamin E should be used as an adjuvant with T cell or NK based immune therapies.
Patients, materials and methods
Patients and treatment plan
Thirteen patients were treated with vitamin E from the end of 1999 to September 2000 (for details on diagnosis, eligibility, and exclusion criteria, see Ref. [36]). The study was approved by the Institutional ethics committee (Karolinska Institutet, approval number 99-247) with informed consent given from all patients. In brief, patients received a daily dose of 750 mg of vitamin E (recommended dietary allowance (RDA), 8–10 mg), 60 μg selenium, and 90 mg vitamin C for 2 weeks. This dose was distributed as follows: Vitamin E (100 mg/tablet; ACO AB, Sollentuna, Sweden) three tablets in the morning and four in the evening, OxiGard’ (50 mg vitamin E, 60 μg selenium, and 90 mg vitamin C; ACO AB) one tablet every morning. Vitamin C and selenium were included at doses of RDA for their capacity to recycle vitamin E, allowing vitamin E to function optimally [6, 21, 33, 38]. One patient, patient 12, after inclusion in the study, developed ileus and was therefore not eligible for the study. The regimen of vitamin E was well tolerated, and no side effects were reported. The treatment also significantly increased the plasma levels of α-tocopherol [36]. The initial analysis of this clinical trial evaluated the effect of vitamin E on the phenotype and function of T cells [36]. However, sufficient material from 7 out of the 12 patients still remained, thus giving us the opportunity to evaluate the effect of vitamin E treatment on NK cell function.
Cells
Peripheral blood mononuclear cells (PBMC) from patients were isolated from peripheral blood taken before and after 15 days of vitamin E treatment by Ficoll Paque (Pharmacia Biotech AB, Uppsala, Sweden) gradient centrifugation (upper layer blood mixed 1:3 in PBS, lower layer 100% Ficoll Paque) for 20 min at 20°C. The PBMC were then frozen in fetal calf serum (FCS) (Gibco, Grand Is., NY, USA)/10% dimethylsulfoxide (Sigma–Aldrich, St Louis, MO, USA) and stored in liquid nitrogen for further analyzes. At the same time, plasma from patients was collected after the gradient centrifugation and stored initially at −20°C and transferred to −80°C for long-time storage. The patient samples acquired before and after vitamin E treatment were analyzed in parallel in all experiments. The erythroleukemia cell line, K562 was cultured in complete RPMI 1640 medium, containing 100 μg/ml l-glutamine, 10% heat-inactivated FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Gibco, Grand Is., NY, USA).
Cytotoxicity assay
The ability of NK cells to lyse K562 cells was measured in a standard 4-h 51Cr release assay. PBMC from patients taken before and after vitamin E treatment were thawed and washed once and resuspended complete RPMI medium and diluted to the appropriate cell concentration. K562 cells were collected and labeled with 51Cr (Amersham Biosciences, Uppsala, Sweden) 1 h at 37°C. The cells were then washed and resuspended in complete RPMI medium and diluted to the appropriate cell concentration. In a 96-well V-bottom plate, 5,000 cells per well was added, followed by PBMC at different effector to target (E:T) ratios. The experiments were carried out in triplicates. The release of 51Cr was measured 4 h later by quantification of gamma radiation in the supernatant by a gamma counter. Specific lysis was calculated according to the formula: % specific lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Degranulation assay
Freshly thawed PBMCs were mixed with K562 cells at a ratio 10:1 in a final volume of 200 μl and co-incubated in round-bottom 96-well plates at 37°C and 5% CO2 for 6 h. Anti-CD107a mAb and the corresponding IgG1 isotype control were added at the start of the assay. After 1 h of incubation, GolgiSTOP was added. Surface antigen staining was performed by incubation with anti-CD3 and anti-CD56 mAbs on ice for 15 min. The cells were washed, resuspended in CellFix and analyzed by flow cytometry.
Lymphocyte activation
PBMCs were thawed and washed, and the lymphocyte concentration was adjusted to 1 × 106 cells/ml. The lymphocytes were stimulated for 4 h at 37°C with PMA and Ionomycin (Sigma–Aldrich) in the presence of 10 μg/ml Brefeldin (Sigma–Aldrich).
Antibodies and reagents
The following antibodies and reagents from Becton Dickinson (Franklin Lakes, NJ, USA) were used: anti-CD3-APC, or anti-CD56-PE, anti-perforin-FITC, anti-IFN-γ-PE, anti-CD226-FITC, anti-NKG2D-APC, anti-CD112-PE (Necin-2), anti-mouse IgG1-APC, IgG1-APC, Cytofix/Cytoperm, CellFix and GolgiSTOP (Monensin). Anti-CD3-Pacific blue was purchased from DAKO Cytomation (Glostrup, Denmark). The following antibodies from Beckman Coulter (Fullerton, CA, USA) were used: anti-CD56-PE-Cy5, anti-NKp30-PE, anti-NKp44-PE, anti-NKp46-PE, anti-CD155 (poliovirus receptor, PVR), and IgG1-PE. Anti-HLA-E was kindly provided by Dr D. Geraghty (Fred Hutchinson Cancer Center, Seattle, WA, USA). Anti-CD48-RPE was purchased from Biosource (Camarillo, California, USA), and IgG1 from BioLegend (San Diego, CA, USA). The anti-ULBP1, anti-ULBP2, anti-ULBP3, anti-MIC-A, and anti-MIC-B antibodies were from R&D system (Minneapolis, MN, USA), and the anti-HLA-ABC-FITC antibody was purchased from Serotec (Oxford, UK). PMA, Ionomycin, and Brefeldin were purchased from Sigma–Aldrich (St. Louis, MO, USA). Human regulatory T cell Staining kit was purchased from eBioscience containing a cocktail of anti-human CD4-FITC and anti-human CD25-APC, anti-human foxp3-PE, Rat IgG2a-PE isotype control, normal rat serum, flow cytometry staining buffer and fixation/permeabilization buffer.
Flowcytometry
PBMC (1 × 105/well) were stained in a V-bottom 96 well plate according to standard FACS staining protocols. Intracellular staining, permeabilization and fixation was done according to manufactures protocol in Cytofix/Cytoperm. K562 cells (2 × 105/well) were stained in a V-bottom 96 well plate according to standard FACS staining protocols. Cells were analyzed using a nine-color (CyAn-ADP, DakoCytomation) or a four-color (FACSCalibur, Becton Dickinson) FACS machine. The data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR, USA) or CellQuest Pro (Becton Dickinson).
Detection of cytokines in plasma
Plasma was collected after the gradient centrifugation as described above and was frozen at −20°C and thereafter transferred to −80°C for long-term storage until analysis. The human cytokine 13 Plex kit (Linco Research, Inc., St. Charles, MO, USA) was used to measure 13 different cytokines [IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IFN-γ, granulocyte-monocyte colony stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α)] in patients’ plasma before and after vitamin E treatment according to the manufacturer’s protocol. All samples were run in duplicate on a Luminex 100 instrument equipped with BioPlex manager software 3.0.
Statistical analysis
The statistical analysis was done using Wilcoxon’s two-tailed matched pairs test. Brackets in figures indicate which groups that are compared and asterisk indicates P value <0.05 between groups.
Results
Enhanced cytolytic activity of NK cells after vitamin E treatment
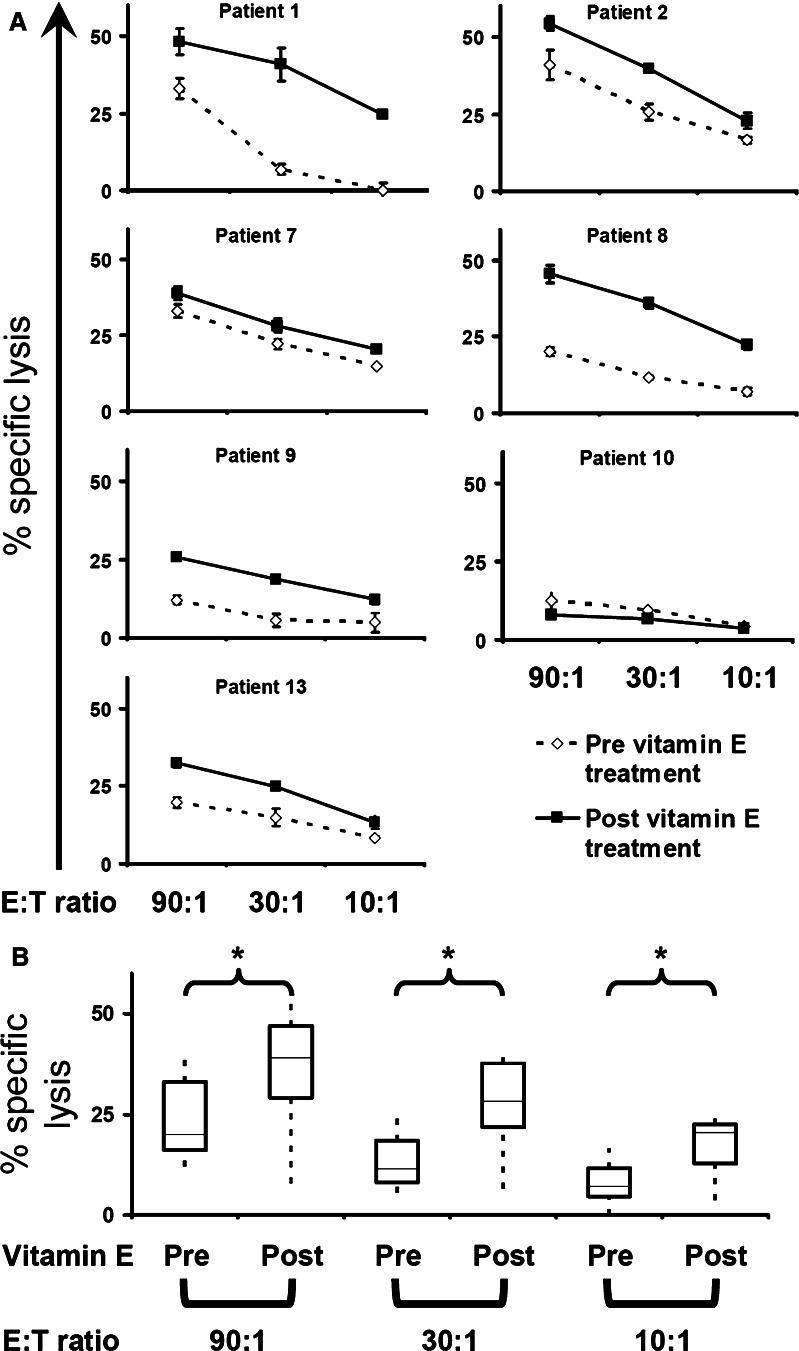
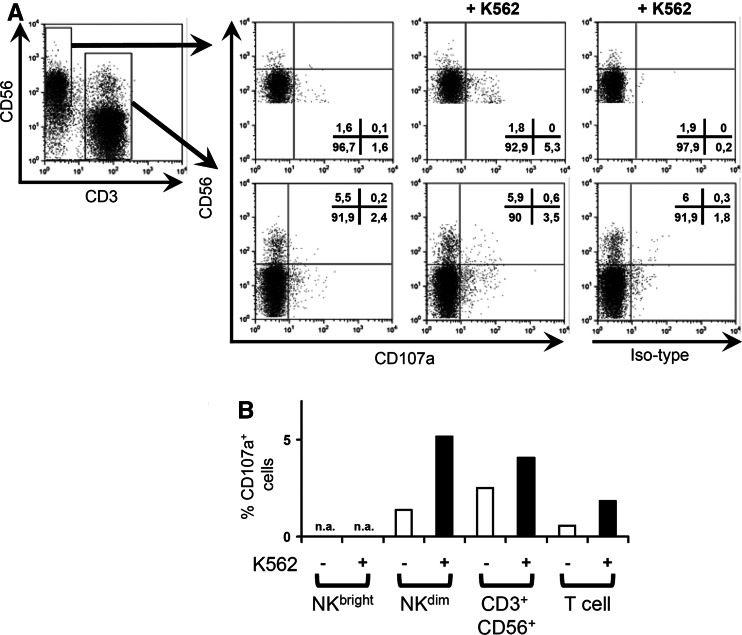
To evaluate the cytolytic activity of NK cells in PBMC samples from patients pre- and post- vitamin E substitution, 51Cr release assays against the K562 target cell were carried out. PBMC from six out of seven patients showed an increase in the ability to lyse K562 (Fig. 1a). One patient, patient 10, showed a marginal decrease in cytotoxicity post vitamin E. The statistical analysis of all seven patients showed a significant difference in the cytolytic capacity of PBMC (Fig. 1b). To confirm the phenotype of the NK cell that was responsible for the lysis of K562 cells, we used a recently described method to monitor surface expression of CD107a on NK cells upon contact with target cells [1]. Surface expression of CD107a correlates with the degranulation of cytolytic granules and the release of perforin in T cells [7]. As shown in Fig. 2a and b, CD56dim NK cells were the main cell type that degranulated following incubation with K562. In conclusion, we show that vitamin E treatment leads to a significantly enhanced NK cell cytotoxicity.
Fig. 1.
Increased NK cell activity in PBMC after vitamin E treatment. Patients’ PBMC were co-cultured with 51Cr labeled K562 and the specific lysis was measured in a standard 4 h 51Cr-release assay. a The specific lysis of K562 by individual patients’ PBMC at 90:1, 30:1 10:1 E:T ratio, before and after vitamin E treatment, is shown. b The box plots show a group-wise comparison between specific lysis of K562 by patients’ PBMC before (pre) and after (post) vitamin E treatment at different E:T ratios. The line within the box shows the median and the upper and lower boundaries of the box shows the upper and lower quartiles around the median. The dotted line connects the nearest observations within 1.5 IQRs (inter-quartile ranges) of the lower and upper quartiles. The brackets indicates the statistical comparison between groups, * P < 0.05
Fig. 2.
CD56dim NK cells are the cells that predominantly degranulate upon co-culture with K562 and PBMC. PBMCs from patient 1 were co-cultured with or without K562 cells in the presence of either anti-CD107a mAb or isotype mAb for 6 h. After one hour of incubation, GolgiSTOP was added to minimize endocytosis. Surface antigen staining was performed using anti-CD56 and anti-CD3 mAbs. a One representative CD107a assay (patient 1 post vitamin E treatment) out of four experiments is shown. Cells were gated for either CD3 or CD56 expression (first panel). The binding of anti-CD107a-mAb to CD56bright CD3− (NKbright), CD56dim CD3− (NKdim), CD56+ CD3+, and CD3+ CD56− (T cells) was quantified without (second panel) or with (third panel) K562. Panel four shows background staining of isotype control when PBMC is co-cultured with K562. B, Bar graph showing % CD107a binding after isotype subtraction of patient 1 post vitamin E treatment for the different cell subsets with or without co-culture of K562. NA not applicable due to few cells
Phenotypic analysis of NK cell compartments after vitamin E treatment
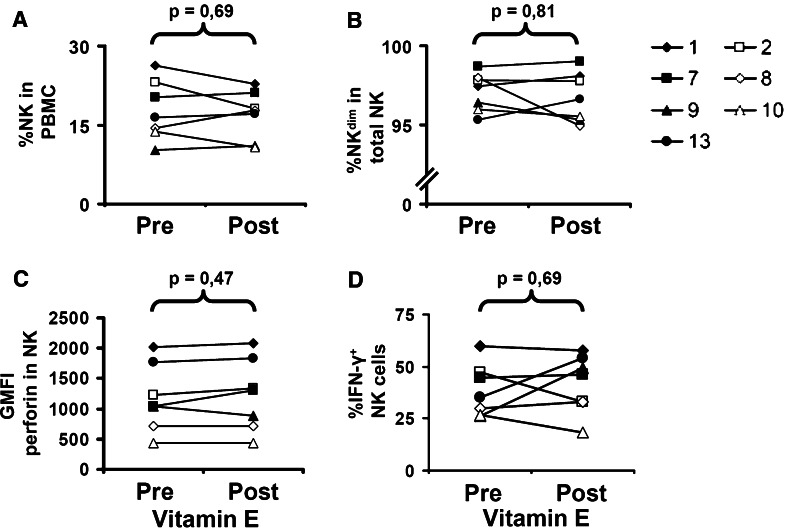
To determine if the increased capacity of PBMC to lyse K562 following vitamin E substitution was due to an increased percentage of NK cells, PBMC were stained for CD3 and CD56 expression. The percentage of NK cells of all patients ranged from 10.3 to 26.4 before treatment and from 10.8 to 22.8 after treatment (Fig. 3a). A pair-wise statistical analysis revealed no significant difference in the percentage of NK cells in PBMC before and after treatment. Thus, the increased cytolytic capability of PBMC after vitamin E treatment was not due to an expanded NK cell compartment. In our previous analysis of the effect of vitamin E on the T cells, we observed a recovery of the altered CD4/CD8 subset ratios associated with colorectal cancer. In the NK cell population, two NK cell subsets can be identified based on their CD56 expression [11]. As the CD56dim NK cell subset is known to be more cytotoxic, we speculated that our observed increase in NK cell cytotoxicity associated with vitamin E treatment could be a consequence of altered CD56 dim and bright subset distribution. However, as shown in Fig. 3b, vitamin E did not significantly alter the percentage of CD56dim NK cells.
Fig. 3.
Analysis of shifts in NK cell population in PBMC, phenotype shift of NK cells, and altered expression of perforin and IFN-γ in NK cells. a, b PBMC from all patients, before and after vitamin E treatment, were stained with anti-CD56 and anti-CD3 mAb. The % CD56+ CD3− (NK) cells (a) and % CD56dim CD3− (NKdim) (b) is shown for each patients. c PBMC from all patients, before and after vitamin E treatment, were stained with anti-CD56 and anti-CD3 mAb, followed by fixation and permeabilization, and intracellular staining with anti-perforin mAb. The geomean fluorescence intensity (GMFI) of perforin staining of CD56+ CD3− is shown. d PBMC from all patients, before and after vitamin E treatment, were activated by PMA/Ionomycin and further stained with anti-CD56 and anti-CD3 mAb, followed by fixation and permeabilization, and intracellular staining with anti-IFN-γ mAb. The % IFN-γ+ CD56+ CD3− cells/total CD56+ CD3− cells is shown. The brackets indicate the statistical comparison and p value between groups
Constant expression of perforin by NK cells during vitamin E treatment
NK cells kill target cells predominantly via perforin-mediated cytotoxicity [54]. Thus, we hypothesized that the increased cytolytic capacity of NK cells after vitamin E treatment was due to increased expression of perforin. To test this, we stained PBMC from patients for CD3, CD56, and intracellular perforin expression. However, CD3− CD56+ NK cells did not display an increased expression of perforin after vitamin E treatment (Fig. 3c). Therefore, we could conclude that the increased cytolytic activity of NK cells was independent of their perforin content.
Cytokine (IFN-γ) production by NK cells following vitamin E treatment
Our previous results have demonstrated that vitamin E substitution enhanced the capacity of T cells to produce the Th 1 cytokines IFN-γ and IL-2 upon anti-CD3 cross linking or PMA/Ionomycin [36]. We therefore tested if also NK cells produced more IFN-γ following vitamin E administration. The experiment showed that two patients (9 and 13) had an increased IFN-γ production, three patients had no change in IFN-γ production, and two patients (2 and 10) showed a decreased IFN-γ production by NK cells after vitamin E treatment. Thus, there was no clear pattern regarding the PMA/Ionomycin activated IFN-γ production among the CD3− CD56+ NK cells (Fig. 3d), indicating no enhanced capability of NK cells to produce cytokines after vitamin E treatment.
% CD4+ CD25high Foxp3+ cells in total CD4+ cells after vitamin E treatment
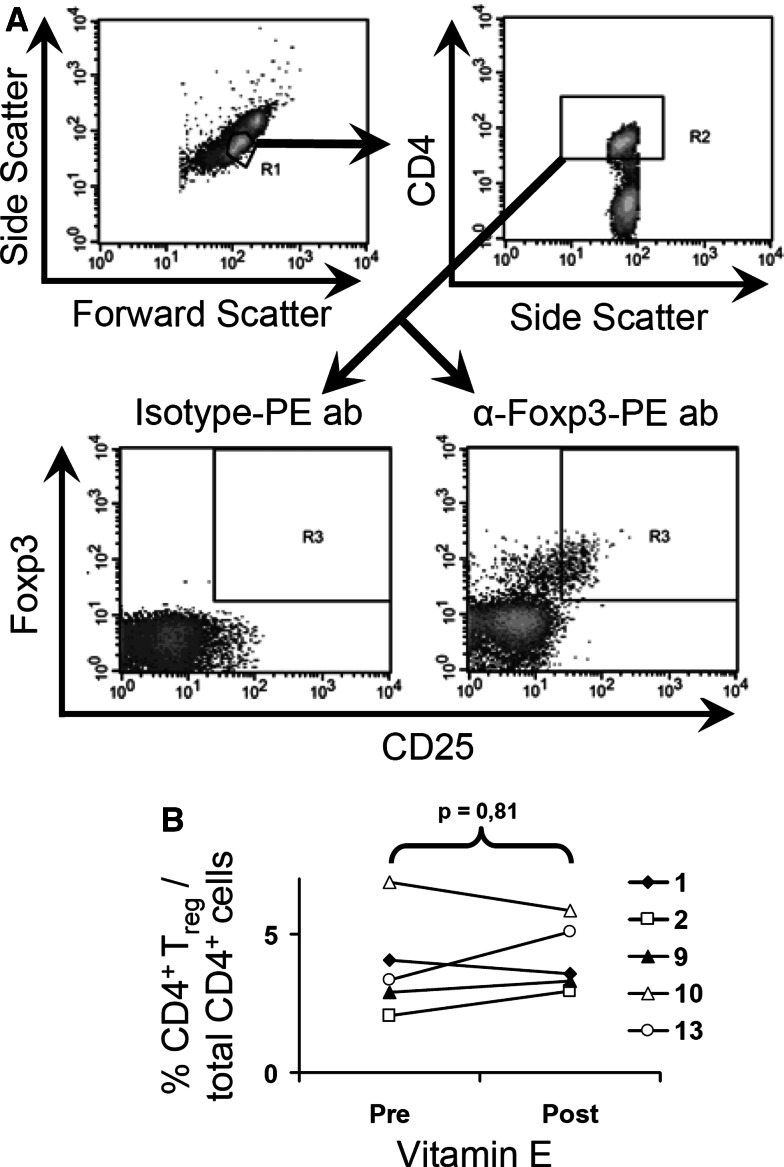
Recently, Ghiringhelli et al. [19] have shown that NK cell activity is inhibited in co-culture experiments with CD4+ Treg in a TGF-β-dependent manner. Since the presence of Treg in our cytotoxicity assays therefore may influence the NK activity, we stained for CD4, CD25, and Foxp3 expression as markers for Treg cells [16]. However, when we tested the presence of Treg in patient’s blood, the % CD4+ CD25high Foxp3+ cells in total CD4+ cells was not altered after vitamin E treatment (Fig. 4a, b). Consequently, we could conclude that there was no decrease in the proportion of Treg after vitamin E treatment, indicating that Treg is not involved in the inhibition of NK cells prior vitamin E treatment.
Fig. 4.
Levels of CD4+ Treg in total CD4+ T cell population in PBMC before and after vitamin E treatment. PBMC from five patients were stained with anti-CD4 and anti-CD25 mAb, followed by fixation and permeabilization, and intracellular staining with anti-Foxp3 Ab. a A representive analysis (patient 9) is shown. b The % Foxp3+ CD4+ CD25high cells/total CD4+ cells for five patients is shown. The brackets indicate the statistical comparison and P value between groups
Serum levels of cytokines after vitamin E treatment
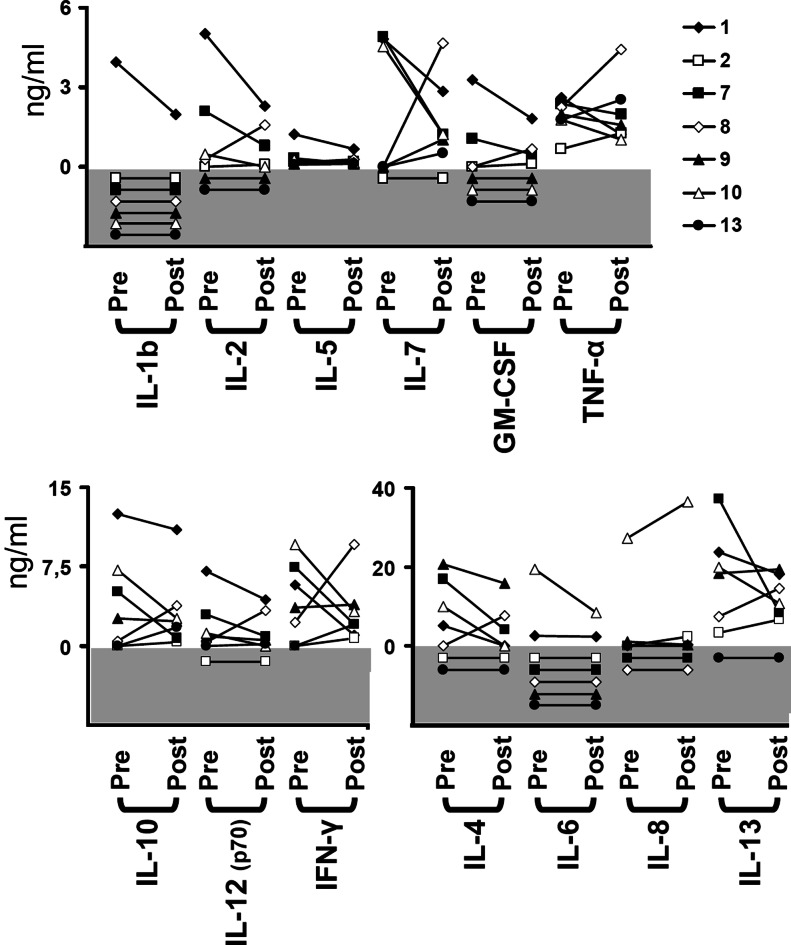
To further investigate the reason for the increased cytotoxicity of NK cells after vitamin E treatment, we examined the cytokine profile in the patients’ serum using a multiplex bead array method, which made it possible for us to simultaneously measure the levels of 13 different cytokines in plasma of the patients (Fig. 5). However, no obvious patterns could be determined when analyzing the immune-modulating cytokines. Noteworthy, IL-4 levels in plasma decreased in four patients, was below detection limit in two patients, and was increased in one patient, but the difference did not reach statistical difference (P = 0.31).
Fig. 5.
Analysis of cytokine levels in plasma before and after vitamin E treatment. Plasma from all patients were tested for 13 different cytokines, before and after vitamin E treatment, using a bead-based multiplex flow cytometry test. The gray area of the graph should be regarded as 0 ng/ml and is displayed for visualization of individual samples below detection limit
Modulation of NK cell receptor expression by vitamin E treatment
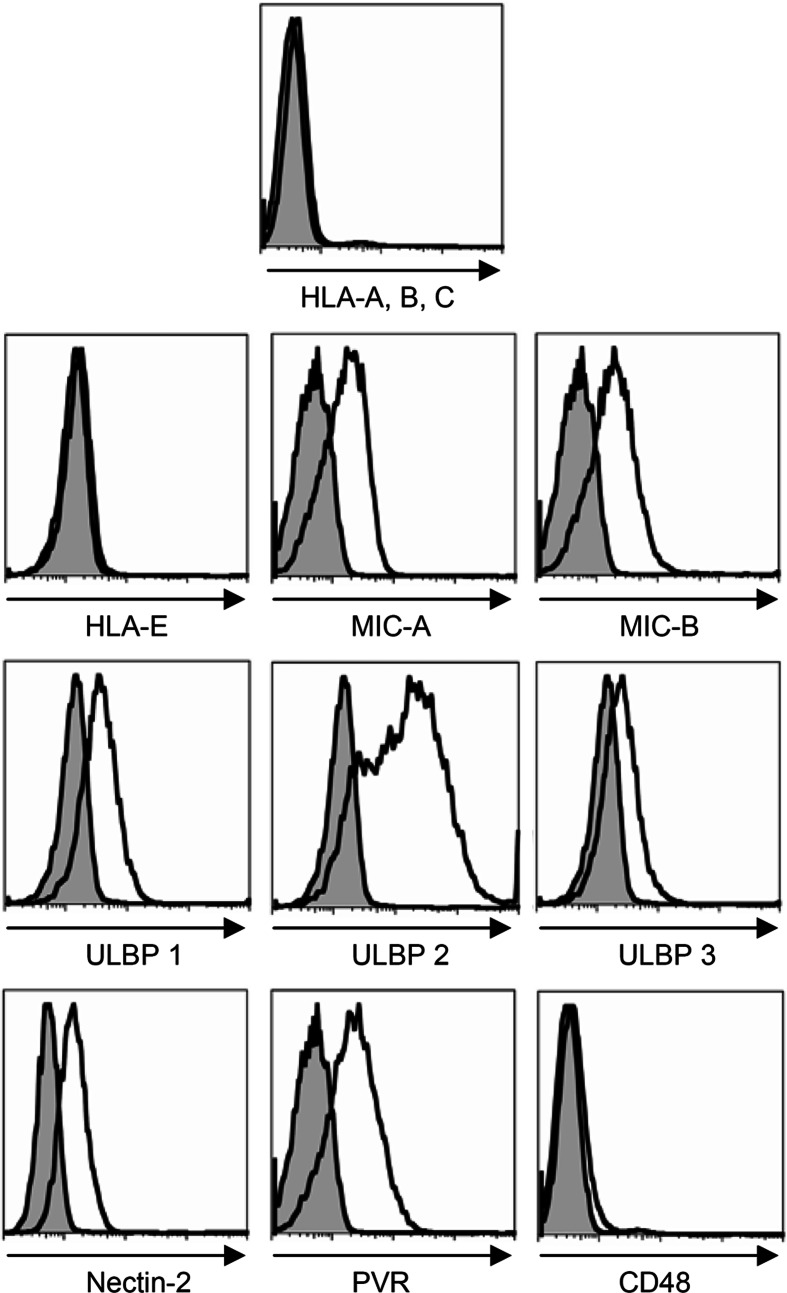
NK cell activation is dependent on, and fine tuned by, signaling through a wide array of activating and co-stimulatory receptors including NKG2D, DNAX-accessory molecule-1 (DNAM-1), 2B4, and the natural cytotoxicity receptors (NCR) (NKp30, NKp44, NKp46) [32]. Since we detected a difference in NK cell activity against the “gold standard” NK cell target K562 following administration of vitamin E (Fig. 1b), we first confirmed the expression of several ligands for activating receptors on K562 (Fig. 6). K562 expressed MICA/B, UBLP 1 through 3 (ligands for NKG2D), Nectin-2, and PVR (ligands for DNAM-1). In agreement with the literature, K562 did not express CD48 or the classical (HLA-A, B, C) and non-classical (HLA-E) HLA class I molecules (Fig. 6). Next, we evaluated the expression of the corresponding NK cell receptors on the NK cell from patients treated with vitamin E. Included in the analysis were the NCRs, which ligands remain unknown, but may be expressed on K562. The expression of NCR on NK cells is important for NK cell cytotoxicity as mAb mediated masking of the NCRs inhibits NK cell killing of various targets [39].
Fig. 6.
NK cell receptor ligands on K562. K562 was single stained with anti-HLA-class I-APC, anti-HLA E, anti-MIC A, anti-MIC B, anti-ULBP 1, anti-ULBP 2, anti-ULBP 3, anti-Nectin-2-PE, anti-PVR, and anti-CD48-PE mAb, followed by secondary anti-mouse IgG1-APC mAb if needed. The figure shows the histogram of each ligand and the proper isotype control
Vitamin E did not induce any coordinated changes in the expression of activating receptors on NK cells (Table 1). NKp44 was not expressed at all (data not shown), which is in agreement with that we were analyzing resting NK cells [32]. However, a discrete but consistent increase in the expression of activating NKG2D-receptors was seen in all patients analyzed (P = 0.0625) (Table 1). It is possible that this minor induction of an important activating NK cell receptor contributed to the enhanced cytotoxicity by NK cells following vitamin E treatment.
Table 1.
Levels of NK cell receptors expressed by NKdim cells before and after vitamin E treatment
| Patient | NKG2D MFI* | DNAM-1 (CD226) MF1* | NKp30 MFI* | NKp46 MFI* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | % Change (Post/Pre) −100% | Pre | Post | % Change (Post/Pre) −100% | Pre | Post | % Change (Post/Pre) −100% | Pre | Post | % Change (Post/Pre) −100% | |
| 1 | 122 | 137 | 12 | 16.1 | 15.7 | −2 | 4.6 | 4.5 | −2 | 30.2 | 27.5 | −9 |
| 2 | 139 | 145 | 4 | 10.6 | 10.1 | −5 | 6.5 | 6.4 | −2 | 72.8 | 76.0 | 4 |
| 9 | 132 | 139 | 5 | 29.4 | 33.3 | 13 | 18.0 | 20.6 | 14 | 26.0 | 38.0 | 46 |
| 10 | 119 | 126 | 6 | 30.1 | 28.5 | −5 | 33.8 | 33.0 | −2 | 40.4 | 43.2 | 7 |
| 13 | 103 | 116 | 13 | 37.9 | 39.2 | 3 | 25.1 | 25.2 | 0 | 31.3 | 36.5 | 17 |
| P = 0.0625† | P = 1.0† | P = 1.0† | P = 0.125† | |||||||||
*Mean fluorescence intensity of NKdim cell population
† P value between pre and post vitamin E treatment
Discussion
This study was motivated by our earlier findings where T cells from patients with advanced colorectal cancer, as a result of a two weeks treatment with high doses of vitamin E, demonstrated enhanced production of the Th1 cytokines IL-2 and IFN-γ and increased their CD4/CD8 ratios [36]. Natural killer cells have been reported to be susceptible to oxidative stress [22], and several lines of evidence suggest that patients with colorectal cancer have increased levels of oxidative stress. These include demonstrations that the levels of malondialdehyde and 4-HNE, which both indicates extent of lipid peroxidation by oxygen radical production, were significantly increased in colorectal cancer tissue with clinical staging of the disease [51]. Furthermore, others have shown increased levels of 8-oxo-2′-deoxyguanosine (8-OxoGua), indicating oxidative stress-induced DNA-damage, in DNA from lymphocytes of colorectal cancer patients, compared to healthy individuals [18]. These studies also show decreased levels of vitamin E in plasma of colon cancer patients compared to control [18, 51].
In this study, we have shown that by restoring the antioxidant status in colorectal cancer patients through supplementation of vitamin E, one may improve the function of NK cells. The treatment plan also included vitamin C and selenium at daily doses of recommended dietary allowance. Studies have shown that vitamin E treatment in combination with low doses of vitamin C and selenium increases the biological effect of vitamin E [6, 21, 33, 38]. As the doses of vitamin C and selenium are very low, compared to the very high dose of vitamin E, we argue that the increased NK cytolytic activity is due to vitamin E treatment, rather than the very low vitamin C and selenium supplement. We have chosen the 51Cr release assays against the K562 target as the method to investigate NK activity in the PBMC samples from the vitamin E treated patients, as this assay is widely accepted as the “gold standard” for human NK activity [25]. Six of the seven analyzed patients demonstrated a higher NK activity against K562 after vitamin E treatment, which showed that this anti-oxidant markedly enhanced NK cytolytic activity. We confirmed [31] in a degranulation assay that the CD3− CD56dim NK cell population from the PBMCs of was responsible for the lysis of K562 cells.
The increased cytolytic capability of PBMC after vitamin E treatment was not due to an increase in the proportion of NK cells in the PBMC, as enumerated by the frequency of CD3− CD56+ NK cells. Neither could this effect be explained by an increased viability of NK cells after vitamin E treatment, as there were no differences in staining by the viability markers Annexin-V and 7-AAD of NK cells in four patients before and after treatment (data not shown). CD56dim NK cells have higher capability to lyse target cells compared to the CD56bright NK cells [31], while the inverse relationship was described for the capacity of NK cells to produce cytokines [12]. We therefore considered a shift in the “CD56 profile” as a possible explanation to increased NK activity. As there was no difference in the percentage of CD56dim NK cells in the NK cell population before and after vitamin E administration, also this possibility was dismissed.
Several other possibilities were considered to explain the difference between NK activity before and after vitamin E treatment, including effects on levels of perforin expression by NK cells or on the ability of NK cells to produce IFN-γ. When examining each of these possibilities, however, none was able to explain the observed effects of vitamin E on NK activity. Furthermore, levels of 13 different cytokines in plasma of the patients were also examined. No consistent difference of cytokine levels in plasma before and after vitamin E treatment was found (Fig. 5). Further, as it has been shown that CD4+ T regulatory (Treg) cells can inhibit NK cells [19] we analyzed the % CD4+ Treg (CD4+, CD25high, Foxp3+) in PBMC before and after vitamin E treatment. However, no differences in the frequencies of CD4+ Treg in PBMC before and after vitamin E treatment were found (Fig. 4a, b).
NK cell function is determined by a complex balance between triggering of activating and inhibitory NK receptors [32]. Therefore, we evaluated the influence of vitamin E on the expression of several activating NK receptors. Although discrete shifts were noted in individual patients, our data revealed no consistent differences in the expression of most activating receptors before and after vitamin E treatment. The activating NKG2D-receptor was slightly induced in all patients that were analyzed, which could possibly contribute to an enhanced NK cell function. This difference alone could however not explain the effect of vitamin E as also patient 10, which did not respond to treatment by enhanced NK cell activity, displayed a somewhat higher expression of NKG2D. In two patients (patient 9 and 13), the NK cells had a higher expression of NKG2D, DNAM-1, NKp30 and NKp46. Although all changes were very small it is possible that the recently described synergies between NKG2D and NKp46, as well as between DNAM-1 and NKp46 [8], could lead to a substantially improved NK cell activation and partly explain the increased NK cell function observed.
Vitamin E is a major lipophilic antioxidant, located in cellular membrane, with radical scavenging properties, which protects cells from oxidation of lipids and proteins and DNA-damage caused by reactive oxygen and nitrogen species [41, 49]. Furthermore, vitamin E enhances NK activity both in a mouse model and in vitro using human cells [4, 15]. In co-culture experiments, vitamin E has shown to protect lymphocytes from DNA-damage from reactive oxygen species derived from monocytes stimulated with PMA [14]. Additionally, a clinical trial, which enrolled 96 healthy elderly individuals, concluded that supplementation of vitamins and trace elements enhanced the NK cell activity [9]. Although the mechanism of vitamin E in this study cannot be determined, one may speculate that vitamin E may decrease the oxidative stress in two ways. Firstly, NK cells may incorporate vitamin E directly into the cell membrane allowing a barrier of protection from oxidative stress. Secondly, the overall anti-oxidative stress in peripheral blood may be decreased as the level of vitamin E in serum is increased [36]. This in turn will decrease the oxidative stress that NK cells encounter.
The mechanism by which oxidative stress can induce lymphocyte hypo-responsiveness is not known, but has been reproduced in vitro in our laboratory by exposing T cells to low-levels of H2O2 [35, 52]. This results in a suppressed signal transduction, a block in NF-κB activation and decreased cytokine production in response to non-specific and antigen-specific stimulation [35]. This effect of H2O2 does not act indiscriminately on lymphocytes, as we have recently shown that in particular the effector memory T cells subset (TEM) (CCR7− CD45RA−) is highly sensitive to low-doses of oxidative stress, while CD45RA+ T cells are more resistant [52]. However, the block in NF-κB activation described by us has been contradicted by other studies, demonstrating an increased activity of NF-κB in T cells exposed to oxidative stress (reviewed in [10, 26]). In our study we employed freshly isolated PBMC while studies showing activation of NF-κB by oxidative stress have been based mainly on the Jurkat T cell line [5, 45, 46]. Thus, the effect of H2O2 on NF-κB in T cells, in terms of activation or inhibition, remains controversial and we speculate that the discrepancies in the results may be cell type related and depend on differences between freshly isolated human T cells and the Jurkat cell line.
The concept of “conditioning” patients who are undergoing immunotherapy by protecting T cells and NK cells from ROS has been tried by others. Histamine inhibits phagocyte-derived production of reactive oxygen species and improves the anti-tumor efficiency of interleukin-2 (IL-2) and interferon-alpha (IFN-alpha) in patients with metastatic melanoma [34]. Asemissen et al. [3] treated melanoma patients with liver metastases with IL-2 in combination with histamine dihydrochloride (HDC). They demonstrated that treatment with HDC in combination with IL-2 increased type 1 T cell responses, which the authors speculate may promote the induction of melanoma-specific T cells. Our findings demonstrate that also vitamin E has the ability to increase production of the Th 1 cytokines IL-2 and IFN-γ and as shown here also to increase NK activity by a mechanism which most likely is different from the one of histamine. The ability of vitamin E to increase NK activity in cancer patients is novel and warrants larger clinical trials investigating in depth vitamin E’s effect on NK cells of cancer patients. Further, studies combining regiments of vitamin-E treatment with non-specific immunotherapy such as IL-2 treatment or specific tumor vaccine protocols should also be considered.
Acknowledgments
This work was supported by grants to R.K. from the Swedish Cancer Society, the Cancer Society of Stockholm, the European Union, the Karolinska Institutet, “ALF-project”-grant from the Stockholm City Council and National Institutes of Health (Grant CA102280). K-J.M. was supported by grants from the Cancer Society of Stockholm, the Swedish Society for Medical Research and the Swedish Children’s Cancer Foundation. G.M. was supported by grants from NORDFORSK-NCEV network (Grant 040226), the Cancer Society of Stockholm, and the King Gustaf V Jubilee Fund.
References
- 1.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995;181:1881–1886. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asemissen AM, Scheibenbogen C, Letsch A, Hellstrand K, Thoren F, Gehlsen K, Schmittel A, Thiel E, Keilholz U. Addition of histamine to interleukin 2 treatment augments type 1 T-cell responses in patients with melanoma in vivo: immunologic results from a randomized clinical trial of interleukin 2 with or without histamine (MP 104) Clin Cancer Res. 2005;11:290–297. [PubMed] [Google Scholar]
- 4.Ashfaq MK, Zuberi HS, Anwar Waqar M. Vitamin E and beta-carotene affect natural killer cell function. Int J Food Sci Nutr. 2000;51(Suppl):S13–S20. doi: 10.1080/096374800750049530. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 6.Beharka A, Redican S, Leka L, Meydani SN. Vitamin E status and immune function. Methods Enzymol. 1997;282:247–263. doi: 10.1016/s0076-6879(97)82112-x. [DOI] [PubMed] [Google Scholar]
- 7.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 8.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra RK. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet. 1992;340:1124–1127. doi: 10.1016/0140-6736(92)93151-C. [DOI] [PubMed] [Google Scholar]
- 10.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 13.Ericsson C, Seregard S, Bartolazzi A, Levitskaya E, Ferrone S, Kiessling R, Larsson O. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:2153–2156. [PubMed] [Google Scholar]
- 14.Fabiani R, De Bartolomeo A, Rosignoli P, Morozzi G. Antioxidants prevent the lymphocyte DNA damage induced by PMA-stimulated monocytes. Nutr Cancer. 2001;39:284–291. doi: 10.1207/S15327914nc392_19. [DOI] [PubMed] [Google Scholar]
- 15.Ferrandez MD, Correa R, Del Rio M, De la Fuente M. Effects in vitro of several antioxidants on the natural killer function of aging mice. Exp Gerontol. 1999;34:675–685. doi: 10.1016/S0531-5565(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 18.Gackowski D, Banaszkiewicz Z, Rozalski R, Jawien A, Olinski R. Persistent oxidative stress in colorectal carcinoma patients. Int J Cancer. 2002;101:395–397. doi: 10.1002/ijc.10610. [DOI] [PubMed] [Google Scholar]
- 19.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 21.Halpner AD, Handelman GJ, Harris JM, Belmont CA, Blumberg JB. Protection by vitamin C of loss of vitamin E in cultured rat hepatocytes. Arch Biochem Biophys. 1998;359:305–309. doi: 10.1006/abbi.1998.0914. [DOI] [PubMed] [Google Scholar]
- 22.Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J Immunol. 1996;156:42–47. [PubMed] [Google Scholar]
- 23.Hellstrand K, Asea A, Dahlgren C, Hermodsson S. Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J Immunol. 1994;153:4940–4947. [PubMed] [Google Scholar]
- 24.Horiguchi S, Petersson M, Nakazawa T, Kanda M, Zea AH, Ochoa AC, Kiessling R. Primary chemically induced tumors induce profound immunosuppression concomitant with apoptosis and alterations in signal transduction in T cells and NK cells. Cancer Res. 1999;59:2950–2956. [PubMed] [Google Scholar]
- 25.Jondal M, Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975;15:596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- 26.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 27.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 30.Ladisch S, Becker H, Ulsh L. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of T cell responses. Biochim Biophys Acta. 1992;1125:180–188. doi: 10.1016/0005-2760(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 31.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–4486. [PubMed] [Google Scholar]
- 32.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Hill KE, Burk RF, May JM. Selenium spares ascorbate and alpha-tocopherol in cultured liver cell lines under oxidant stress. FEBS Lett. 2001;508:489–492. doi: 10.1016/S0014-5793(01)03129-5. [DOI] [PubMed] [Google Scholar]
- 34.Lindner P, Rizell M, Mattsson J, Hellstrand K, Naredi P. Combined treatment with histamine dihydrochloride, interleukin-2 and interferon-alpha in patients with metastatic melanoma. Anticancer Res. 2004;24:1837–1842. [PubMed] [Google Scholar]
- 35.Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, Lenkei R, Masucci G, Pettersson S, Kiessling R. Inhibition of activated/memory (CD45RO(+)) T cells by oxidative stress associated with block of NF-kappaB activation. J Immunol. 2001;167:2595–2601. doi: 10.4049/jimmunol.167.5.2595. [DOI] [PubMed] [Google Scholar]
- 36.Malmberg KJ, Lenkei R, Petersson M, Ohlum T, Ichihara F, Glimelius B, Frodin JE, Masucci G, Kiessling R. A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clin Cancer Res. 2002;8:1772–1778. [PubMed] [Google Scholar]
- 37.Malmberg KJ, Ljunggren HG. Escape from immune- and nonimmune-mediated tumor surveillance. Semin Cancer Biol. 2006;16:16–31. doi: 10.1016/j.semcancer.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 38.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 39.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 40.Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- 41.Niki E, Noguchi N. Dynamics of antioxidant action of vitamin E. Acc Chem Res. 2004;37:45–51. doi: 10.1021/ar030069m. [DOI] [PubMed] [Google Scholar]
- 42.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL. Lymphocyte apoptosis induced by Fas ligand- expressing ovarian carcinoma cells. Implications for altered expression of T cell receptor in tumor-associated lymphocytes. J Clin Invest. 1998;101:2579–88. doi: 10.1172/JCI1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. Identification of hydrogen peroxide as the relevant messenger in the activation pathway of transcription factor NF-kappaB. Adv Exp Med Biol. 1996;387:63–68. doi: 10.1007/978-1-4757-9480-9_9. [DOI] [PubMed] [Google Scholar]
- 46.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seaman WE, Gindhart TD, Blackman MA, Dalal B, Talal N, Werb Z. Suppression of natural killing in vitro by monocytes and polymorphonuclear leukocytes: requirement for reactive metabolites of oxygen. J Clin Invest. 1982;69:876–888. doi: 10.1172/JCI110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seliger B, Cabrera T, Garrido F, Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol. 2002;12:3–13. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 49.Serafini M. Dietary vitamin E and T cell-mediated function in the elderly: effectiveness and mechanism of action. Int J Dev Neurosci. 2000;18:401–410. doi: 10.1016/S0736-5748(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 50.Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–406. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi A, Hanson MG, Norell HR, Havelka AM, Kono K, Malmberg KJ, Kiessling RV. Preferential cell death of CD8+ effector memory (CCR7-CD45RA-) T cells by hydrogen peroxide-induced oxidative stress. J Immunol. 2005;174:6080–6087. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- 53.Taniguchi K, Petersson M, Hoglund P, Kiessling R, Klein G, Karre K. Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci USA. 1987;84:3405–3409. doi: 10.1073/pnas.84.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 55.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]