Abstract
The human epidermal growth factor receptor 2 (HER2) has been targeted as a breast cancer-associated antigen by immunotherapeutical approaches based on HER2-directed monoclonal antibodies and cancer vaccines. We describe the adoptive transfer of autologous HER2-specific T-lymphocyte clones to a patient with metastatic HER2-overexpressing breast cancer. The HLA/multimer-based monitoring of the transferred T lymphocytes revealed that the T cells rapidly disappeared from the peripheral blood. The imaging studies indicated that the T cells accumulated in the bone marrow (BM) and migrated to the liver, but were unable to penetrate into the solid metastases. The disseminated tumor cells in the BM disappeared after the completion of adoptive T-cell therapy. This study suggests the therapeutic potential for HER2-specific T cells for eliminating disseminated HER2-positive tumor cells and proposes the combination of T cell-based therapies with strategies targeting the tumor stroma to improve T-cell infiltration into solid tumors.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0355-7) contains supplementary material, which is available to authorized users.
Keywords: Tumor immunity, Human, Cytotoxic T cells, Antigens/peptides/epitopes, MHC
Introduction
It has been a matter of debate whether an endogenous T-cell response can mediate regressions of spontaneous tumors [1], because tumor progression is often observed even in the presence of high levels of blood-circulating or tumor-infiltrating T cells [2–4]. Only recently, the identification of various subtypes of tumor-infiltrating lymphocytes has facilitated the detailed analyses and it becomes evident that the composition of the T-cell infiltrate is crucial. There is now growing evidence of an association between the presence of intratumoral T lymphocytes and a favorable prognosis of primary tumors, such as ovarian and colorectal cancer, if the lymphocyte infiltrate is dominated by cytokine-secreting effector memory CD3+CD8+ T cells but not by suppressing CD4+ regulatory T cells [5]. During tumor progression the paradoxical expansion of non-responsive T cells may be caused by the prevalence of antigen presentation and tolerance induction both mediated by the growing tumor.
The rationale for adoptive T-cell transfer is based on the attempt to circumvent tolerance by taking out the potentially tumor-reactive T cells from the tumor-bearing host and stimulating the T cells ex vivo [6, 7]. Indeed, it has recently been shown that the transfer of ex vivo activated EBV-reactive T cells can promote objective responses and control disease progression in patients with EBV+ stage IV Hodgkin’s disease resistant to conventional treatments [8]. In addition, several groups have shown that the adoptive transfer of autologous T-cell populations directed against the melanoma-associated antigens results in tumor regressions in some melanoma patients [9–11].
In this study, we describe the isolation and expansion of cytotoxic T lymphocytes (CTLs) specific for the human epidermal growth factor receptor 2 (HER2) molecule known to be overexpressed in breast cancer and to be immunogenic in patients [12]. We report for the first time the adoptive transfer of autologous HER2-specific T-cell clones to a patient with HER2-overexpressing breast cancer. The accompanying clinical and immunological monitoring revealed that the transferred T cells accumulated into the patient’s bone marrow (BM), which correlated with the disappearance of BM-residing disseminated tumor cells. In contrast, the adoptively transferred T cells were unable to penetrate into solid tumor masses, which was associated with tumor progression. Our data support the hypothesis that cytotoxic T cells alone can eliminate single tumor cells, but need additional help to reduce solid tumors due to the inhibiting effects of the surrounding tumor stroma [13].
Materials and methods
Patients and treatment schedule
The study was approved by the Institutional Ethics Committee. Eligibility was dependent upon subjects (a) being diagnosed with stage IV breast cancer, (b) being refractory to standard regimens including surgery, radiotherapy, chemotherapy, trastuzumab therapy, or combined modalities, (c) showing HER2 protein overexpression in the primary tumor or metastasis (DAKO Score 3+), (d) being HLA-A*0201 positive, (e) being off immunosuppressive drugs and chemotherapy for at least 30 days before T-cell transfer, (f) having an Eastern Cooperative Oncology Group performance status of 0–1. All of the patients gave written informed consent to participate in the study. The enrollment of five patients was planned. From three patients, the autologous T cells specific for HER2 could be successfully cloned. Patient #1 had liver metastases and disseminated tumor cells in the BM; patient #2 had metastases in the liver, lung, lymph nodes, bone and brain; patient #3 had metastases in the bone and liver. All of the three patients had metastatic disease refractory to multiple therapy regimens including the treatment with trastuzumab. Of note, the actual treatment (chemotherapy and/or trastuzumab therapy) was not interrupted for the generation of autologous T-cell clones.
Quality controls of the expanded CTL clones included the documentation of the peptide specificity (HER2369–377), the restriction element (HLA-A*0201) and the tumor recognition efficiency (lysis) prior to T-cell transfer. Peptide specificity and HLA restriction were documented in vitro. For one of the three patients (patient #1), the tumor-reactivity of the established HER2-specific T cells could be documented in vitro and, subsequently, these T-cell clones were used for the adoptive transfer herein described in detail. In two cases (patient #2 and #3), the established peptide-specific T-cell clones did not display any tumor-lytic activity in vitro and, therefore, were not adoptively transferred to the patients. The enrollment of patients was stopped due to the difficulty to establish HER2-specific, tumor-reactive T-cell clones based on the isolation technique used in this study.
Generation of human HER2-specific CTL clones
The number of HER2369–377-specific peripheral blood T lymphocytes was enhanced by stimulating PBMCs with peptide-loaded dendritic cells (DCs). In detail, monocyte-derived DCs were incubated with 10 μg/ml peptide for 2 h at room temperature and then cocultured with autologous PBMCs in 200 μl/well RPMI 1640 medium (Gibco BRL, Karlsruhe, Germany) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine and 5% autologous serum in 96-well round bottom plates. Supplemented medium for growing T cells is further referred to as T-cell medium. Recombinant human IL-2 (IL-2; Chiron Behring, Marburg, Germany) (20 U/ml) was added on day 4. Responding T cells were restimulated with peptide-pulsed DCs at weekly intervals in the presence of IL-2. The stimulator to responder cell ratio was 1:20 for priming and 1:40 for restimulation. Following repetitive stimulations, proliferating T cells were evaluated for the presence of peptide-specific CTLs by staining with A2/HER2369–377 multimers. Synthesis of PE-labeled HLA-A2/peptide multimer complexes was performed as previously described [14]. For sorting a Moflo cell sorter (Cytomation, Fort Collins, CO, USA) was used with 25,000 events/s and max. 1 psi. For cloning, sorted T cells were plated at limiting dilution leading to one T cell per well [15]. T cells were cultured with 200 μl/well T-cell medium in the presence of 5 × 104 allogeneic irradiated (30 Gy) PBMCs and 105 irradiated (100 Gy) lymphoblastoid cell lines (LCLs). At day 14 after plating, the growing T-cell clones were screened for lytic activity (for details see cytotoxicity assay). HER2369–377-specific CTLs were transferred from 96-well round bottom plates to culture flasks and further expanded. For expansion of bulk cultures, the cloned T cells were transferred to culture flasks (T30; Greiner, Solingen, Germany) and further cultured with T-cell medium, 30 ng/ml anti-CD3 mAb (Okt-3; Janssen-CILAG, Neuss, Germany), and 50 U/ml IL-2. As feeder cells, 2.5 × 107 allogeneic irradiated (30 Gy) PBMCs and 5 × 106 irradiated (100 Gy) B LCLs from healthy donors were added per flask.
Cytotoxicity assay
Cytolytic activity was analyzed in a standard 4 h chromium release assay as described [15]. In brief, tumor cell lines (5 × 105 cells in 100 μl FCS) were incubated with 100 μCi 51Cr (ICN Biochemicals, Irvine, CA, USA) for 1 h at 37°C, washed and then used as target cells. Peptide-loaded T2 cells (provided by Peter Cresswell, Yale University, New Haven, CT, USA), were first labeled with 51Cr for 1.5 h at 37°C and then loaded with 10 μg/ml HER2369–377 peptide for an additional 1 h at room temperature. As negative control, 51Cr-labled T2 cells were loaded with the HLA-A0201-restricted peptide motif HIVpol476–484 (ILKEPVHGV) [16]. 51Cr-labeled target cells were cultured with T cells in 200 μl/well RPMI with 10% FCS in V-bottom 96-well tissue culture plates (Greiner, Solingen, Germany). For evaluating the efficacy of CTL-mediated lysis, T cells were serially diluted and then co-cultured with a fixed amount of target cells, resulting in graded effector cell to target cell (E:T) ratios. For testing the functional avidity of CTL clones, a fixed E:T ratio of 30:1 was used while the peptide concentration was titrated. For screening the CTL clones, half of the T cells growing in each well were harvested and equally subdivided for evaluation of peptide-specific killing. Of note, the HER2+ HLA-A2− cell line SKOV3, the HER2+ HLA-A2-transfected cell line SKOV3tA2 (provided by M. L. Disis, University of Washington, Seattle, WA, USA), and the HER2− HLA-A2-transfected cell line K562 (provided by T. Wölfel, Johannes Gutenberg-Universität, Mainz, Germany) were first treated with 100 U/ml IFN-γ for 48 h and then used as target cells. After 4 h of co-culturing effector and target cells at 37°C, 100 μl of supernatant were collected and radioactivity was measured in a gamma counter. CTL killing was calculated as the percentage of specific 51Cr release using the following equation: % specific lysis = [(sample release − spontaneous release):(maximal release − spontaneous release)] × 100. Background lysis was generally less than 15%. The data in the figures refer to the mean of two replicates. The standard deviation was below 5% of the mean.
Flow cytometry of T cells
For evaluating the circulating CD8+ HER2369–377-specific T cells, the patient’s PBMCs were prepared and visualized with the relevant multimer HLA-A2/HER2369–377 as previously described [14]. As negative control, an irrelevant HLA-A2 multimer folded with the HLA-A2-binding peptide HIVpol476–484 was used. Propidium iodide-negative T cells were gated and the frequencies of multimer+ CD8+ T cells were determined by using Coulter Epics XL (Beckmann-Coulter, Hialeah, IL, USA) and documented with FlowJo software (Treestar, San Carlos, CA, USA). All histograms and dot plots are shown for propidium iodide-negative cells. For phenotyping of the CTL clones the T cells were stained for surface molecules with the following monoclonal antibodies: rat-anti-mouse-IgM-FITC, anti-CD8-FITC, anti-CD25-PE (Caltag Laboratories, Burlingame, CA, USA); anti-HLA-DR-PE, anti-CD45RO-FITC, anti-CD45RA-FITC, anti-CD62L-PE, anti-CD28-PE, anti-CCR7, anti-IgG1-FITC, anti-IgG1-PE, anti-IgM-purified (BD Biosciences, San Diego, CA, USA); anti-CD69-PC5, anti-CD3-PC5, anti-CD127-PE, anti-CD27-PE, anti-IgG1-PC5 (Immunotech, Marseille, France).
Imaging studies
For labeling, 7.1 × 108 T cells were re-suspended in 1 ml of indium-111 (111In) oxine (Amersham, Braunschweig, Germany) with a total radioactivity of 32 MBq. Following a 30-min incubation at room temperature, the radiolabeled cells were washed twice in PBS and re-suspended in 50 ml of 5% human serum albumin and a total of 20 MBq of labeled cells were injected intravenously. Whole body analyses as well as the single photon emission computed tomography (SPECT) of the liver were obtained with dual head gamma camera (ADAC Vertex, Milpitas, CA, USA) at various time points post injection. In order to quantitatively assess the distribution of lymphocytes, the regions of interest were placed around liver, spleen, lungs and the largest liver metastasis. The decay corrected mean radioactivity counts in these regions were divided by the total counts in a whole body scan 4 h post injection to estimate the percentage of cells in these sites.
Bone marrow preparation and immunocytochemical staining
The aspirations were performed at the spina iliaca posterior superior. The preparation of mononuclear cells (MNCs) from BM aspirates was performed as previously described [17]. In brief, the MNCs were isolated from each BM sample and the total number of MNCs was calculated for each BM sample. An aliquot of the MNCs was centrifuged onto ten glass slides and then stained using a monoclonal antibody directed against a panel of cytokeratins including CK 8, 18, and 19 (mAb clone A45-B/B3; Micromet, Munich, Germany). The specific antibody reaction was developed with the alkaline phosphatase/anti-alkaline phosphatase technique. The cytokeratin-positive tumor cells of the ten glass slides were counted and then the amount of tumor cells per 106 MNCs was calculated. The HER2 expression of the disseminated tumor cells was determined by using a polyclonal anti-HER2 serum (serum A0485, HercepTestR; DAKO).
Results and discussion
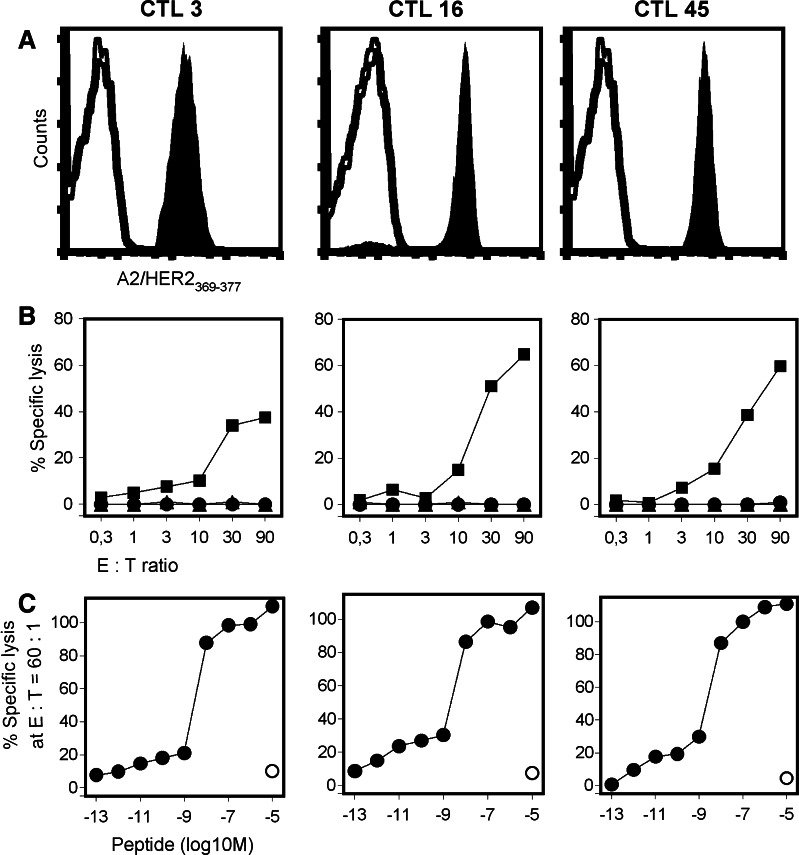
As a first approach, we sought to isolate T cells directed against the HER2-derived peptide p369–377 that is naturally processed and serves as an epitope for HLA-A2-restricted T cells [18]. Given that HER2-reactive T cells are only present in low numbers in the peripheral blood, PBLs from HLA-A2+ breast cancer patients were stimulated with autologous DCs loaded with the HLA-A2-binding peptide HER2369–377. Following repetitive in vitro stimulations the amount of peptide-specific T cells could be increased to numbers that allowed visualization and sorting by HLA/peptide multimers. Using this technique we succeeded to isolate, clone, and expand HER2369–377-specific T cells from one patient with HER2-overexpressing, metastatic breast cancer (patient #1). The procedure of stimulating, sorting, cloning and expanding the HER2-specific T cells took 10 weeks. During that period, patient #1 received further treatment with trastuzumab. The established CTL clones were stored at −180°C, and the CTLs were thawed for adoptive transfer as soon as the patient had progressive disease. Three CD8+ T-cell clones with the highest lytic activity and best proliferative capacity were selected for further expansion (Fig. 1). The T-cell clones CTL3, CTL16 and CTL45 retained their antigen specificity during upscale (Fig. 1a). Upon antigen stimulation the T-cell clones specifically released IFNγ and TNFα, but not IL-4 and IL-10, corresponding to a cytokine pattern typical for a Tc1 phenotype (unpublished data). The expanded CTL clones were able to kill HLA-A2-positive HER2-overexpressing tumor cells, but not HLA-A2-negative HER2-negative tumor cells indicating the HER2-dependent killing of tumor cells that endogenously process and present HER2369–377 with HLA-A2 (Fig. 1b). The tumor recognition efficiency of the established CTL clones was low and variable. We have observed this unevenness of the tumor-lytic potential also with CTL clones with other antigen-specificities (e.g. NY-ESO-1, Melan-A). This observation may be due to the variability of the long-term culture conditions, which include the ill-defined support by so-called feeder cells (irradiated PBMCs and LCLs). The CTL clones may have still down-regulated the TCR and/or CD8 expression after the expansion period and, therefore, may not display the maximum of their tumor-lytic capacity.
Fig. 1.
Specificity, avidity and function of HLA-A2-restricted, HER2-specific CTL clones. a Following expansion, the antigen-specificity of three CTL clones (CTL 3, 16, 45) was documented by A2/HER2369–377 tetramer staining (filled histogram). Non-binding A2/Melan-A26–35A27L multimer was used as negative control (open histogram). b The tumor recognition by CTL clones (second expansion) was documented by using the ovarian cancer cell line SKOV3tA2 (filled square) as target cell line. The HLA-A2+ HER2− K562tA2 cell line (filled triangle) and the HLA-A2− HER2+ SKOV3 cell line (filled circle) were used as negative controls. c Peptide avidity of CTL clones (third expansion) was determined by lysis of T2 cells pulsed with graded amounts of HER2369–377 peptide (filled circle) at a fixed E:T ratio of 60:1. T2 cells loaded with HIVpol476–484 peptide (open circle) were used as negative control. Half (50%) maximal target lysis occurred between 1 nM (10−9 M) and 10 nM (10−8 M) of peptide HER2369–377
The functional TCR avidity was determined by the recognition of T2 cells loaded with serially diluted amounts of the peptide HER2369–377 (Fig. 1c). The CTL clones displayed a half maximum lysis of peptide-loaded T2 cells at a peptide concentration between 10−9 M (1 nM) and HER2369–377, which is a considerably high peptide concentration compared to 10−10 M (0.1 nM) of CMV-derived peptides being sufficient for the half maximum lysis mediated by CMV-specific T cells [19]. This may be due to the fact that HER2 is a self-antigen and that T cells with high avidity TCRs against HER2 may be partly deleted in the thymus. However, the TCR avidity, as measured by peptide titration assays, does not necessarily reflect the T cell’s ability of recognizing tumor cells. It has been shown that T cells with a ‘low avidity’ TCR against various antigenic peptides, including HER2369–377, can recognize the tumor cells expressing the cognate peptide in context with the HLA-molecule [20]. Using the herein described technology of peptide-stimulation, we were able to isolate and expand HLA-A2-restricted T-cell clones specific for HER2369–377 from two additional HLA-A2+ patients (patient #2 and #3) with advanced HER2-overexpressing breast cancer (Fig. S1). However, these established peptide-specific T-cell clones were unable to recognize A2+ HER2+ tumor cells and, therefore, were not used for the treatment of those patients. This is in accordance with the observation that immunizing patients with the peptide HER2369–377 in combination with Freund’s incomplete adjuvant led to the induction of T cells that were HER2369–377-specific, but lacked the ability to recognize HER2-expressing tumor cells [21]. There may be many factors being responsible for the differential tumor recognition by HER2369–377-specific CTLs. Firstly, the tumor lysis by HER2369–377-specific CTLs may depend on the ability of HER2-overexpressing tumor cells to endogenously process the peptide [22]. Secondly, the divergent ability of HER2369–377-specific T cells to lyse tumor cells may also depend on the co-expression of HER3 and HER4 due to the potential cross-specificity of HER2-reactive CTLs with the homologous amino acid sequences of HER3 and HER4 (Conrad et al., submitted). Thirdly, the naturally processed HER2369–377 epitope may be glycosylated and, therefore, CTLs stimulated with synthetic peptides might not be able to bind the naturally processed, glycosylated peptide with the same avidity. The efficacy of generating tumor-lytic T cells against this or other HER2 immunodominant epitopes may be enhanced by using altered or cross-reactive peptides for stimulating T cells in vitro [23–25]. Alternative antigen delivery modes based on antigen processing, e.g. the viral or non-viral transfer of genes encoding the HER2 antigen may facilitate both the selection of peptide-specific and tumor-reactive T cells [15, 26].
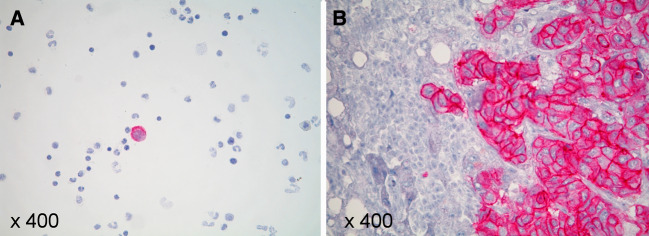
The adoptive transfer of the autologous, HER2-specific T-cell clones CTL3, CTL16 and CTL45 was performed for breast cancer patient #1, who had undergone multiple previous therapies for metastatic disease including chemotherapy and antibody therapy with trastuzumab. At the time of the adoptive T-cell transfer she had refractory liver metastases as determined by magnetic resonance tomography (MRI). In addition, disseminated tumor cells were documented in the BM by aspiration. The immunohistochemical analysis of a tumor biopsy demonstrated the overexpression of HER2 in the biopsied liver metastasis and the immunocytological analysis of the BM aspirate documented the abundant HER2 expression by the disseminated tumor cells (Fig. 2).
Fig. 2.
HER2 expression of liver metastases and disseminated tumor cells in the BM. a The mononuclear cells from the BM of patient #1 revealed disseminated tumor cells that also overexpressed HER2 as documented by immunocytochemical analysis (A085-HercepTestR). b The liver metastasis of patient #1 overexpressed HER2 (Score 3±) as determined by immunohistochemical analysis (A085-HercepTestR)
The patient received the HER2-specific T-cell clones as infusions. The patient received the last trastuzumab administration 6 weeks prior to the first transfer of autologous HER2369–377-reactive T-cell clones. In total, five adoptive transfers were performed at 2-week intervals. Each transfusion consisted of one singular CTL clone. The CTL clones were transfused in the following amount and order: 1.2 × 108 of CTL3, 2.6 × 108 of CTL3, 8.5 × 108 of CTL16, 7.1 × 108 of CTL45, and 7.1 × 108 of CTL45. To improve the in vivo persistence of transferred CD8+ CTL clones [9], low doses of IL-2 (0.5 MU IL-2/m2) were administered subcutaneously, once daily, starting 1 week prior to the first T-cell transfer and ending 1 week after the fifth transfer. The T-cell infusions were generally well tolerated. Side effects were not observed with the exception of chills and low-grade fever up to 38°C that occurred within 6–8 h after the third and fourth infusion and resolved with paracetamol. There were no signs of heart failure as determined by clinical examination and echocardiography.
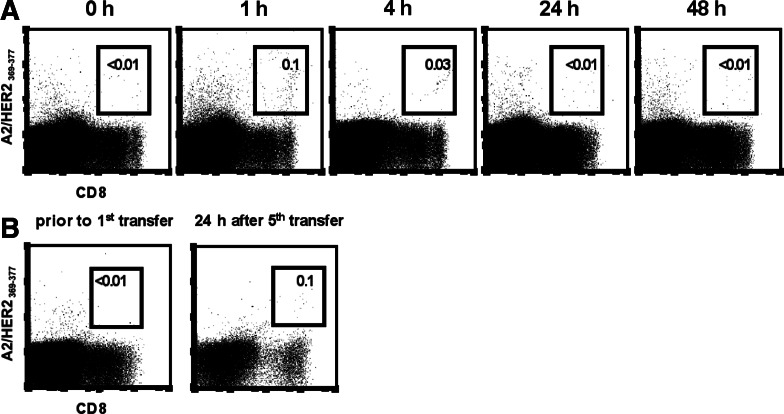
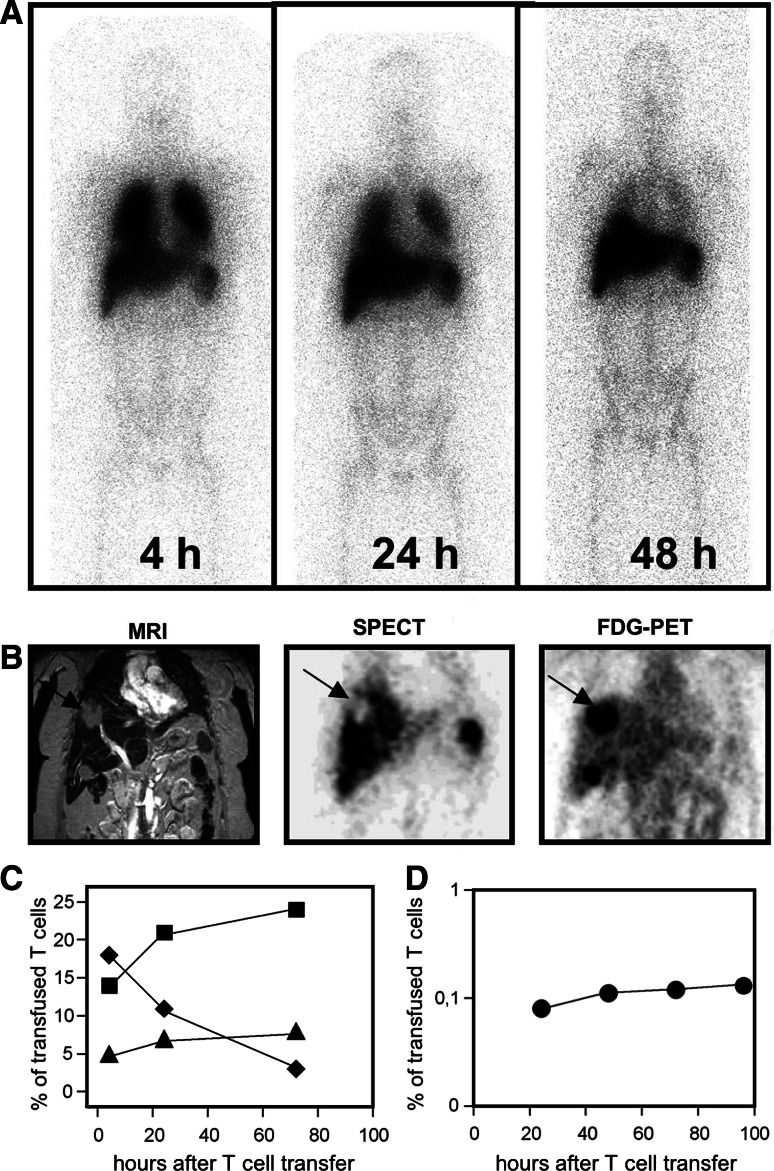
To evaluate the circulation of transferred T cells in the peripheral blood, PBLs were prepared from the patient’s blood samples drawn prior to and after T-cell transfer, and the frequency of HER2-specific T cells was determined by A2/HER2 multimer staining (Fig. 3a). The monitoring revealed that the frequency of HER2-specific T cells circulating in the peripheral blood increased from non-measurable amounts prior to transfer to a maximum of 0.1% of all CD8+ T cells at 1 h after transfer. The transferred HER2-specific T cells quickly disappeared from the peripheral blood and were not detectable any more after 24 h. Repetitive A2/HER2369–377 frequency analyses from PBMC samples collected from the same time points revealed that the amount of HER2369–377-specific T cells was 0.1% of CD8+ T cells at 1 h and 0.05% at 4 h, whereas the percentage of non-specific binding with the A2/HIV476–484 multimer remained at 0.02% of CD8+ T cells (data not shown). The peak frequency and the time course of circulating HER2-specific T cells were similar after each transfer. In order to follow the migration of adoptively transferred T cells in the whole body we performed radioimaging studies to visualize the trafficking and tumor localization of 111In-labeled HER2-specific T cells in vivo (Fig. 4a). At 4 h after injection of the radiolabeled T-cell clones, the gamma camera images showed a predominant localization of radioactivity in the lung, which cleared during 72 h after infusion (Fig. 4a, c). In contrast, the uptake of 111In-labeled CTLs by the liver and the spleen increased over time reaching a maximum at 24 h and remaining stable for as long as 72 h (Fig. 4a, c). To answer the key question of whether the HER2-specific T cells migrated into the HER2+ liver metastases, sections derived from 111In SPECT, MRI, and [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) were analyzed (Fig. 4b). Comparable sections revealed that the 111In-labeled T cells were not able to accumulate in big solid tumor masses, as demonstrated for the metastasis in liver segment S8 displaying a maximum diameter of 3 × 5 cm in bidimensional measurement (Fig. 4b). The quantification of radioactivity over time revealed that only exceedingly low numbers of radiolabeled CTLs were detectable in this liver metastasis (Fig. 4d). The MRI-based restaging of the liver metastases revealed progressive disease at 4 weeks after the T-cell therapy had been completed.
Fig. 3.
Frequency analyses of HER2-specific T cells in the peripheral blood and the BM prior to and after adoptive transfer. a PBMCs of the patient were isolated before and at indicated time points after adoptive T-cell transfer and then stained with A2/HER2369–377 multimers and anti-CD8 antibody in the presence of PI at 4°C. Frequencies of A2/HER2369–377-specific T cells were documented by analyzing 1,782,715 events prior to transfer; 1,051,196 events at 1 h; 811,238 events at 4 h; 1,718,357 events at 24 h; and 1,652,305 events at 48 h after transfer. Indicated frequencies refer to CD8+ T cells. b Frequency of HER2-specific T cells in the BM of the patient was measured prior to the first T-cell transfer and 24 h after the fifth transfer using A2/HER2369–377 multimers. Staining was performed in the presence of anti-CD8 antibody and PI at 4°C. Dot plots are gated on PI-negative cells. The frequencies of A2/HER2369–377-specific T cells were analyzed by counting 889,608 events prior to transfer and 1,961,744 events after the last transfer. Indicated frequencies refer to CD8+ T cells
Fig. 4.
Systemic distribution of adoptively transferred HER2-specific T cells. Prior to transfer, the T cells of clone 45 were labeled with 111In in order to monitor the migration in vivo. a Whole body camera images were obtained from the patient at 4, 24 and 48 h after infusion. At 4 h, the uptake of labeled T cells was detected in the lung, spleen, liver and BM. After 24–48 h, the lung activity cleared whereas activity in the spleen, liver and BM remained stable. b Comparable sections of MRI, 111In single photon emission computed tomography and [18F] fluorodeoxyglucose positron emission tomography. The arrow points to the 3 × 5 cm measuring liver metastases located in segment S8. The metastasis displays high FDG-uptake indicating viable tumor tissue. However, accumulation of 111In-labeled T cells is lower than in surrounding normal liver tissue (c). Migration kinetics of the 111In-labeled T cells to the lung (filled diamond), the liver (filled square) and the spleen (filled triangle). d Over a time period of 96 h about 0.1% of the infused CTLs was detected in the metastases located in the liver segment S8
The lack of T-cell infiltration into solid tumor masses observed in our study is in accordance with findings derived from a transgenic mouse model of spontaneously developing tumors that display an intrinsic resistance to lymphocyte infiltration and effector function [27]. This tumor barrier is most likely due to the fact that solid tumors are not only composed of malignant cells but also of different nonmalignant cells, referred to as tumor stroma. Stromal cells, including endothelial cells, fibroblasts and inflammatory cells, can inhibit T-cell activity and support tumor growth. The abnormal tumor vasculature, including increased expression of vascular endothelial factor and impaired blood/lymphatic flow can contribute to the failure of T cells to leave the vessels and penetrate into the tumor tissue [5, 28]. Consequently, effective immunotherapeutical strategies must also target the stromal cells to hit the tumor cells. It has been shown in several murine models that the T cell-mediated rejection of established tumors critically requires the modulation of stromal cells, including endothelial cells and fibroblasts [13]. Targeting the tumor barrier will be an effective strategy for promoting the extravasation of T cells from tumor vessels to the tumor cells, thereby enhancing the efficacy of adoptive T-cell transfer. Indeed, the attraction of adoptively transferred T cells into solid tumors, e.g. by irradiation or CpG-containing deoxynucleotides, subsequently leads to tumor rejection in mice [27, 29]. Normalizing the tumor vasculature with the novel antiangiogenic agents, such as the anti-VEGF mAb bevacizumab, may also normalize the extravasation of T lymphocytes and merits further investigation in clinical trials.
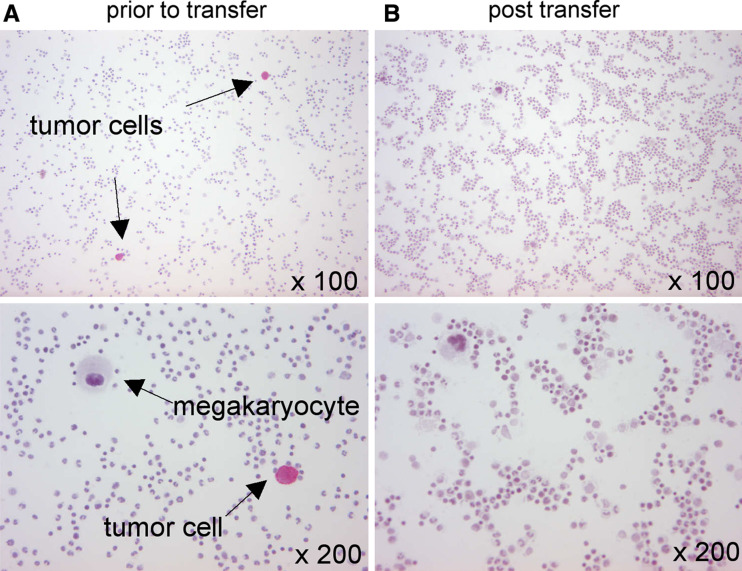
It has been shown previously that naturally occurring HER2-specific T cells can home to the BM [30]. Based on these findings, we searched for HER2-specific T cells in the BM. In our patient, the natural frequency of BM-residing, HER2-specific T cells was below the detection level of the HLA/peptide multimer technique. Following T-cell transfer, an increased frequency of HER2-specific T cells was found in the patient’s BM by A2/HER2 multimer staining (Fig. 3b). In addition, the uptake of tracer following infusion of 111In-labeled CTLs could be detected in the vertebral column, the pelvis, and the humeral and femoral bones, indicating the accumulation of adoptively transferred T cells in the BM (Fig. 4a). In parallel, we followed the kinetics of HER2-positive breast cancer cells that were spread out in the patient’s BM (Fig. 5). Disseminated tumor cells present at high frequencies in the BM prior to T-cell transfer (256/106 MNCs) were not detectable any more after the last transfer of HER2-specific T cells (0/106 MNCs). The inverse relationship of T-cell accumulation and tumor-cell disappearance may imply the ability of T cells to eradicate single tumor cells in the absence of surrounding tumor stroma. However, it cannot be ruled out that secondary events, e.g. the recruitment of NK cells [31], may be responsible for the tumor cell disappearance. It has been recently shown by the Cancer and Leukemia Group B 9661 that the administration of low-dose IL-2 together with trastuzumab to breast cancer patients led to the ex vivo expansion of natural killer cells [32]. Future studies need to direct this issue, because the mere correlation between tumor regressions and ex vivo expansion and/or tumor infiltration of the transferred T cells [8–11] does not preclude the possibility that secondary events may be responsible for the final hit.
Fig. 5.
Evaluation of disseminated tumor cells in the BM prior to and after transfer of HER2-specific T cells. BM-derived MNCs were stained with a cytokeratin-directed antibody. a Prior to T-cell transfer cytokeratin-positive tumor cells were detected at a frequency of 256 in 106 MNCs. b Disseminated tumor cells were not visible 24 h after the fifth T-cell transfer (0/106 MNCs)
Future immunotherapeutical approaches should include the prospective monitoring of micrometastatic cells by aspirating BM at two different sites, because the presence of micrometastases in the BM at the time of diagnosis of early breast cancer is associated with a poor prognosis [17]. In particular, the incidence of HER2-positive tumor cells characterizes a subset of patients with poor prognosis. The relative resistance of HER2-positive disseminated tumor cells to adjuvant chemotherapy might be successfully overcome by HER2-targeted immunotherapeutical approaches that are independent of the proliferative status of the target cells. Indeed, adjuvant treatment with trastuzumab, a monoclonal antibody against HER2, leads to an improved outcome among women with surgically removed HER2-positive breast cancer. For the HER2 antigen, our group has shown that the cytolytic activity of human HER2-specific CD8+ CTLs is augmented by anti-HER2 monoclonal antibody trastuzumab [33] encouraging the development of combinatorial immunotherapeutical regimens directed against HER2.
In this study, the HER2-stimulated T cells were cloned, which had the advantage of investigating a homogeneous T-cell population with a defined specificity, avidity and effector function. As the cloning procedure required a long culture period, the resulting T-cell clones displayed a marker profile that is consistent with a terminally differentiated effector T-cell phenotype: CD3+, CD8+, CD25+, CD69+, HLA-DR+, CD45RO+, CD45RA−, CD62L−, CD28−, CD27−, CCR7−, CD127− (data not shown). The magnitude and persistence of the transferred HER2-specific T-cell clones may have been limited by this cloning approach, because the acquisition of full effector function in vitro is inversely correlated with the in vivo survival and anti-tumor efficacy of adoptively transferred CTLs as described in various murine models [34]. This is consistent with our finding that the herein described human HER2-specific CTL clones did not express markers, e.g. IL-7R, which reflect the capacity of transferred T cells to proliferate and persist in vivo [35]. The T-cell phenotype may partly explain the clinical success reported for the adoptive transfer of tumor-infiltrating lymphocytes for melanoma patients, because prior to transfer those TILs had been cultured for only a short period of time and, therefore, still expressed crucial survival markers, such as IL-7R [10, 36–38]. The efficacy of transferred T cells—regardless of the original source and the antigen specificity—strongly depends on their ability to retain function and survive long term in the host.
In addition, strategies are being developed that prepare the recipient’s immune system for the T cells to be transferred in order to enhance both their proliferation and survival in vivo [39]. In melanoma patients, the conditioning with lymphodepleting chemotherapy led to the clonal expansion and persistence of the transferred T cells, which correlated with durable tumor remissions [10]. Using the conditioning approach, a small number of tumor-reactive T cells, including vaccine-induced T cells [40], may be sufficient for the repopulation in vivo and, therefore, the in vitro expansion of T cells prior to transfer may not be necessary for mediating tumor regressions. The underlying mechanism for lymphopenia-induced homeostatic proliferation is complex and includes the removal of suppressive cells such as T regulatory cells [40]. Further studies on the host-tumor interactions in particular the tumor-stroma-immune cell contacts will thus be of prime importance, both to gain insights into disease progression and to develop more precise and effective immunotherapies against cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank the patients for taking part in this clinical trial; Burkhard Schmidt, Evelyn Schulz and Matthias Schiemann for excellent technical assistance; Peter Schmidkonz for expert clinical care; and Wendy Batten for helpful discussion and critical reading of the manuscript. This work was supported by the Research Council of Germany grant SFB 456 (to H.B. and D.H.B.) and the Wilhelm Sander-Stiftung grant 2000.017.3 (to H.B.).
Abbreviations
- HER2
Human epidermal growth factor receptor 2
- 111In
Indium-111
- SPECT
Single photon emission computed tomography
- MRI
Magnetic resonance tomography
- FDG-PET
[18F] Fluorodeoxyglucose positron emission tomography
- MNC
Mononuclear cell
Footnotes
This manuscript is published with a commentary by Vy Phan and Mary L. Disis entitled “Tumor stromal barriers to the success of adoptive T cell therapy”.
References
- 1.Qin Z, Blankenstein T. A cancer immunosurveillance controversy. Nat Immunol. 2004;5:3–4. doi: 10.1038/ni0104-3. [DOI] [PubMed] [Google Scholar]
- 2.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 3.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungblut A, Jäger D, Arand M, Ritter G, Cerundolo V, Dupont B, Chen Y-T, Old LJ, Knuth A. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Öhlén C, Kalos M, Hong DJ, Shur AC, Greenberg PD. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J Immunol. 2001;166:2863–2870. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- 7.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan S, Sutton SE, Cooke MP, Öhlén C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 8.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, Sixbey J, Gresik MV, Carrum G, Hudson M, Dilloo D, Gee A, Brenner MK, Rooney CM, Heslop HE. Cytotoxic T lymphocyte therapy for Epstein–Barr virus Hodgkin’s disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. PNAS. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meidenbauer N, Marienhagen J, Laumer M, Vogl S, Heymann J, Andreesen R, Mackensen A. Survival and tumor localization of adoptively transferred melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161–2169. doi: 10.4049/jimmunol.170.4.2161. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 13.Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol. 2005;17:180–186. doi: 10.1016/j.coi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B, Bernhard H, Wagner H, Busch DH. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002;8:631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 15.Meyer zum Büschenfelde C, Metzger J, Hermann C, Nicklisch N, Peschel C, Bernhard H. The generation of both T killer and T helper cell clones specific for the tumor-associated antigen HER2 using retrovirally transduced dendritic cells. J Immunol. 2001;167:1712–1719. doi: 10.4049/jimmunol.167.3.1712. [DOI] [PubMed] [Google Scholar]
- 16.Walker BD, Flexner C, Birch-Limberger K, Fisher L, Paradis TJ, Aldovini A, Young R, Moss B, Schooley RT. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes C, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga J-Y, Marth C, Oruzio D, Wiedswang G, Solomayer E-F, Kundt G, Strobl B, Fehm T, Wong GYC, Bliss J, Vincent-Salomon A, Pantel K. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 18.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, Schmidt T, Germeroth L, Wagner H, Peschel C, Busch DH, Bernhard H. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320:119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Rongcun Y, Salazar-Onfray F, Charo J, Malmberg K-J, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiesling R. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol. 1999;163:1037–1044. [PubMed] [Google Scholar]
- 21.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–4908. [PubMed] [Google Scholar]
- 22.Herrmann F, Lehr H-A, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;64:215–220. doi: 10.1158/0008-5472.CAN-2522-2. [DOI] [PubMed] [Google Scholar]
- 23.Castilleja A, Carter D, Efferson CL, Ward NE, Kawano K, Fisk B, Kudelka AP, Gershenson DM, Muarray JL, O’Brian CA, Ioannides CG. Induction of tumor-reactive CTL by c-side chain variants of the CTL epitope HER-2/neu protooncogene (369–377) selected by molecular modeling of the peptide: HLA-A2 complex. J Immunol. 2002;169:3545–3554. doi: 10.4049/jimmunol.169.7.3545. [DOI] [PubMed] [Google Scholar]
- 24.Vertuani S, Sette A, Sidney J, Southwood S, Fikes J, Keogh E, Lindencrona JA, Ishioka G, Levitskaya J, Kiessling R. Improved immunogenicity of an immunodominant epitope of the Her-2/neu protooncogene by alterations of MHC contact residues. J Immunol. 2004;172:3501–3508. doi: 10.4049/jimmunol.172.6.3501. [DOI] [PubMed] [Google Scholar]
- 25.Lustgarten J, Dominguez AL, Pinilla C. Identification of cross-reactive peptides using combinatorial libraries circumvents tolerance against Her-2/neu-immunodominant epitope. J Immunol. 2006;176:1796–1805. doi: 10.4049/jimmunol.176.3.1796. [DOI] [PubMed] [Google Scholar]
- 26.Müller MR, Grünebach F, Nencioni A, Brossart P. Transfection of dendritic cells with RNA induces CD4- and CD8-mediated T cell immunity against breast carcinomas and reveals the immunodominance of presented T cell epitopes. J Immunol. 2003;170:5892–5896. doi: 10.4049/jimmunol.170.12.5892. [DOI] [PubMed] [Google Scholar]
- 27.Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 28.Padera TP, Stoll BR, Tooredman JB, Capen D, diTomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 29.Garbi N, Arnold B, Gordon S, Hämmerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004;172:5861–5869. doi: 10.4049/jimmunol.172.10.5861. [DOI] [PubMed] [Google Scholar]
- 30.Feuerer M, Beckhove P, Bai L, Solomayer E-F, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nature Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 31.Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 32.Fleming GF, Meropol NJ, Rosner GL, Hollis DR, Carson WE, Caligiuri M, Mortimer J, Tkaczuk K, Parihar R, Schilsky RL, Ratain MJ. A phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin 2: report of Cancer and Leukemia Group B 9661. Clin Cancer Res. 2002;8:3718–3727. [PubMed] [Google Scholar]
- 33.Meyer zum Büschenfelde C, Hermann C, Schmidt B, Peschel C, Bernhard H. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Research. 2002;62:2244–2247. [PubMed] [Google Scholar]
- 34.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theroret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. PNAS. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Rosenberg SA. Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J Immunol. 2004;173:7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger C, Huang M-L, Gough M, Greenberg PD, Riddell SR, Kiem H-P. Nonmyeloablative immunosuppressive regimen prolongs in vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J Virol. 2000;75:799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.