Abstract
Thymic function decreases in line with tumor progression in patients with cancer, resulting in immunodeficiency and a poor prognosis. In the present study, we attempted to restore thymic function by BALB/c (H-2d) syngeneic (Syn), or B6 (H-2b) allogeneic (Allo) bone marrow transplantation (BMT) using intra-bone marrow–bone marrow transplantation (IBM–BMT) plus Syn-, Allo- or C3H (H-2k) 3rd-party fetal thymus transplantation (TT). Although the BALB/c mice with advanced tumors (Meth-A sarcoma; H-2d, >4 cm2) treated with either Syn- or Allo-BMT alone showed a slight improvement in survival compared with non-treated controls, the mice treated with BMT + TT showed a longer survival. The mice treated with Allo-BMT + Allo-TT or 3rd-party TT showed the longest survival. Interestingly, although there was no difference in main tumor size among the BMT groups, lung metastasis was significantly inhibited by Allo-BMT + Allo-TT or 3rd-party TT. Numbers of CD4+ and CD8+ T cells, Con A response, and IFN-γ production increased significantly, whereas number of Gr-1+/CD11b+ myeloid suppressor cells and the percentage of FoxP3+ cells in CD4+ T cells significantly decreased in these mice. Furthermore, there was a positive correlation between survival days and the number of T cells or T cell function, while there was a negative correlation between survival days and lung metastasis, the number of Gr-1+/CD11b+ cells, or the percentage of FoxP3+ cells. These results suggest that BMT + TT, particularly Allo-BMT + Allo-TT or 3rd-party TT, is most effective in prolonging survival as a result of the restoration of T cell function in hosts with advanced tumors.
Keywords: Advanced cancer, Bone marrow transplantation, Thymus transplantation, Metastasis, Regulatory T cell, Myeloid suppressor cell
Introduction
Patients with malignant tumors show reduced immune function, which strongly influences prognosis [1–3]. A number of mechanisms for the reduction of immune function have been postulated. One is that the thymus is, as a result of the tumor progression, involved in the maturation block of thymocytes, which leads to a reduction in the number and function of T cells [4–7]. The other is that the number of Gr-1+ CD11b+ myeloid suppressor cells increases [8], which results in inhibited T cell signaling with tumor growth factor (TGF)-β and IL-13 [9, 10]. In addition, recent studies have shown that regulatory T cells, which express CD4+FoxP3+ and inhibit T cell function, play a crucial role in the development of autoimmune disease and graft-versus-host disease (GVHD) [11, 12]. The number of regulatory T cells increases in patients with cancer, and the cells inhibit antitumor immunity [13, 14]. These cellular factors have been suggested to play important roles in the immune suppression of patients with cancer.
In humans, auto or allogeneic BMT or peripheral blood stem cell transplantation (PBSCT) are used to treat malignant tumors. Auto BMT or PBSCT is applied to recover hematopoiesis after intensive chemo- or irradiation therapy [15], whereas Allo BMT or PBSCT are used to replace host cells with donor cells to induce the graft-versus-tumor (GVT) effect, although the very harmful GVHD is elicited if the effect is too strong [16, 17]. We have recently developed a new BMT method, intra-bone marrow–bone marrow transplantation (IBM–BMT), in which BMCs are injected directly into the bone marrow cavity [18]. IBM–BMT results in a reduced incidence of GVHD and greater engraftment of donor cells, including mesenchymal stem cells (MSC), than the conventional intravenous (iv) method [19, 20].
Very recently, we have developed a BMT method in conjunction with thymus transplantation (TT). The combination of BMT plus TT is effective in restoring donor-derived T cell function even in aged mice, chimeric-resistant mice, tumor-bearing mice, supralethally irradiated mice, and low-dose irradiated mice, and in mice injected with low numbers of BMCs [21–25]. However, TT has only been applied clinically in patients with DiGeorge syndrome or HIV infection who show hypoplasia of the thymus [26, 27]. The effects of BMT plus TT have not been examined in cases of advanced cancer in relation to the involution of the thymus.
In the present study, we examined Syn or Allo IBM–BMT plus fetal TT (IBM–BMT + TT) in mice with advanced malignant tumors. We also used 3rd-party TT, in which the major histocompatibility complex (MHC) type of the thymus was different from the MHC types of the donor BMCs and of the recipient (microenvironment) [28]. We did this because it is difficult to obtain such immature thymus from the BMC donor. From the results of this study, we propose that IBM–BMT + TT could become a powerful strategy for the treatment of patients with advanced tumors.
Materials and methods
Mice
Eight-week-old female BALB/c (H-2d) and C57BL/6 (B6) (H-2b) mice and fetal (day-16) BALB/c, B6, and C3H (H-2k) mice were purchased from Shimizu Experimental Animal Laboratory (Shizuoka, Japan), and maintained until use in our animal facilities under specific pathogen-free conditions. All animal research was reviewed and approved by the Animal Experimentation Committee of Kansai Medical University.
Cell lines
Meth-A cells (H-2d) were derived from methylcholanthrene-induced sarcoma in BALB/c mice, as previously used [23]. The cells were kindly provided by Dr. Junko Yoshida of Kanazawa Medical School (Kanazawa, Japan) from the Cell Research Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan). The cells were maintained in RPMI1640 medium supplemented with 10% fetal calf serum with antibiotics.
Inoculation of tumor cells
One day before the transplantation of tumor cells, the recipients (BALB/c mice) underwent total body irradiation (3 Gy) using a 137Cs irradiator (Gammacell 40 Exactor; MDS Nordion International, Ottawa, ON, Canada). The next day, 2 × 106 Meth-A cells were inoculated subcutaneously into the right flank of the mice.
IBM–BMT and TT
When the tumor had reached >4 cm2 in size (about 3 weeks after transplanting the cells), the tumor-bearing BALB/c mice were lethally irradiated (7 Gy) using a 137Cs irradiator (Gammacell 40 Exactor; MDS Nordion International) 1 day before IBM–BMT. BMCs were flushed from the shafts of donor femora and tibiae, and single-cell suspensions were prepared. Next, 1 × 107 BMCs were injected directly into the bone marrow cavity of the recipient’s tibia, as described previously for the IBM–BMT method [18]. Briefly, the knee was flexed to 90° and the proximal side of the tibia was drawn to the anterior. A 26-gauge needle was inserted into the joint surface of the tibia through the patellar tendon and then inserted into the bone cavity. Simultaneously, a fetal day-16 thymus was grafted under the renal capsule of the left kidney in some mice. We also treated tumor-bearing mice with irradiation only.
Experimental groups
Group 1 consisted of BALB/c mice with advanced tumors (>4 cm2) without treatment as controls (Non-treated) (Table 1). Group 2 consisted of lethally-irradiated BALB/c mice with advanced tumors transplanted with BMCs from syngeneic BALB/c mice by IBM–BMT (Syn-BMT). Groups 3 and 4 consisted of mice from Group 1 plus TT from syngeneic BALB/c or allogeneic B6 mice (Syn-BMT + Syn-TT and Syn-BMT + Allo-TT, respectively). The lethally irradiated BALB/c mice with advanced tumors transplanted with BMCs from allogeneic B6 by IBM–BMT mice comprised Group 5 (Allo-BMT). The mice in Groups 6 and 7 consisted of Group 5 plus TT from allogeneic B6 or 3rd-party C3H mice (Allo-BMT + Allo-TT and Allo-BMT + 3rd-party TT, respectively).
Table 1.
Experimental groups and their chimerism
| Groups | Description | n | Transplantation | Chimerisma | |||
|---|---|---|---|---|---|---|---|
| BMCs | Thymus | H-2Kb | H-2Kd | H-2Kk | |||
| 1 | Non-treated | 8 | (–) | (–) | 0.1 ± 0.1 | 99.8 ± 0.2 | ND |
| 2 | Syn-BMT | 11 | BALB/c | (–) | 0.2 ± 0.1 | 97.8 ± 0.2 | ND |
| 3 | Syn-BMT + Syn-TT | 11 | BALB/c | BALB/c | 0.7 ± 0.3 | 98.2 ± 0.3 | ND |
| 4 | Syn-BMT + Allo-TT | 13 | BALB/c | B6 | 0.5 ± 0.5 | 99.5 ± 0.2 | ND |
| 5 | Allo-BMT | 13 | B6 | (–) | 98.8 ± 0.2 | 0.2 ± 0.2 | ND |
| 6 | Allo-BMT + Allo-TT | 12 | B6 | B6 | 99.6 ± 0.5 | 0.2 ± 0.3 | ND |
| 7 | Allo-BMT + 3rd party-TT | 12 | B6 | C3H | 96.7 ± 0.7 | 0.7 ± 0.5 | 0.9 ± 0.7 |
The categorizing of each group is described in “Materials and methods”
aChimerism was determined in spleen cells of the mice in the non-treatment group (group 1, n = 4) 2–3 weeks after the tumor reached >4 cm2 in size and in the mice in the BMT groups (groups 2–7, n = 4–6) 4–5 weeks after transplantation
Histological studies
Several organs, including the small intestine, lung, liver, kidney, and transplanted thymus, were removed from the chimeric mice, fixed in 10% formalin for 48 h, and embedded in paraffin according to standard procedures. Sections of 4 μm thickness were stained using hematoxylin and eosin (HE). The average numbers of metastatic nodules (100× magnification) were calculated from ten blind fields from every five sections of both left and right lungs.
Analyses of mitogen responses
To analyze lymphocyte function, mitogen responses were examined as described previously [28]. The stimulation index (SI) was calculated as the average 3H-TdR incorporation of triplicate samples of responding cells with mitogen/3H-TdR incorporation of responding cells in medium alone.
Analyses of surface markers and intracellular FoxP3 expression and cytokine production by flow cytometry
Surface markers on lymphocytes in the spleen were analyzed under three-color fluorescence staining using a FACScan system (BD Pharmingen, Franklin Lakes, NJ, USA). Fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE) anti-H-2Kb, anti-H-2Kd, and anti-H-2Kk antibodies (BD Pharmingen) were used to determine chimerism. FITC, PE or biotin-conjugated CD4, CD8, B220 (BD Pharmingen), were used to analyze lymphocyte subsets. Avidin-PE-Cy5 (Dako, Kyoto, Japan) was used as the third color in the avidin/biotin system. Intracytoplasmic FoxP3 staining was performed using an FITC-anti mouse/rat FoxP3 staining set (eBioscience, San Diego, CA, USA). The procedure was performed in accordance with the manufacturer’s instructions. Intracellular cytokines (IL-2, IL-4, IL-10, IFN-γ and TNF) were detected using an Intracellular Cytokine Staining Kit (BD Pharmingen) in accordance with the manufacturer’s instructions.
Statistical analyses
Non-parametric analyses (Mann–Whitney U test and log-rank test) and simple regression were performed using StatView software (Abacus Concepts, Berkley, CA, USA). Values of P < 0.05 were considered statistically significant.
Results
Chimerism and survival rates
To examine the effects of thymic function on hosts with advanced tumors, we performed Syn- or Allo-BMT with or without Syn-, Allo-, or 3rd-party TT in mice with Meth-A sarcomas measuring >4 cm2 (Table 1). In humans, due to the difficulty in performing the combination of Allo-BMT + Allo-TT from the same donor, we also carried out the Allo-BMT + 3rd-party TT, as we described previously [28].
H-2 typing showed full donor chimerism in all groups treated with BMT even in the mice treated with Allo-BMT + 3rd-party TT (Table 1), suggesting that BMT was successfully carried out. With regard to survival, all of the non-treated control mice died within 20 days due to the growth of tumors, and all the mice treated with irradiation alone died within 12 days as a result of hematopoietic failure (Fig. 1a). The mice treated with Syn-BMT or Allo-BMT showed a slight improvement in survival rates, compared with the non-treated control, although the mice treated with Allo-BMT fared far better than the mice treated with Syn-BMT. Interestingly, the mice treated with BMT (Syn or Allo) + TT (Syn or Allo) showed significantly prolonged survival rates in comparison with the mice treated with Syn- or Allo-BMT alone. In these combinations, the mice treated with Allo-BMT + Allo-TT showed significantly longer survival than those treated with Syn-BMT + Syn-TT or Syn-BMT + Allo-TT. The survival rate of the mice treated with Syn-BMT + Allo-TT was comparable to the mice treated with Syn-BMT + Syn-TT. Notably, the mice treated with Allo-BMT + 3rd-party TT also showed a comparable survival rate to the mice treated with Allo-BMT + Allo-TT (Fig. 1a). At autopsy, GVHD was seen only minimally in the mice from all groups (data not shown).
Fig. 1.
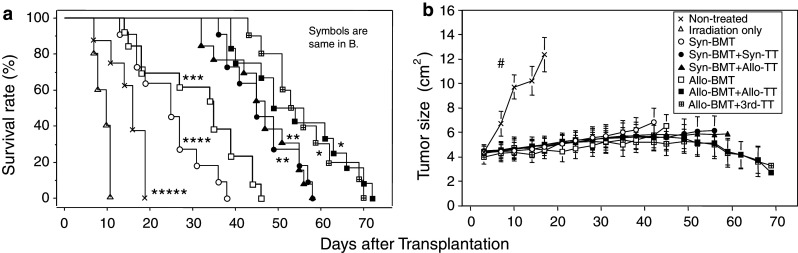
Survival rate and tumor size in mice with advanced tumors treated with BMT + TT. Survival rate (a) and tumor size (b) in the mice with advanced tumors are shown. The treatments for the mice are described in Table 1. *P < 0.05 compared with the non-treated controls, the mice treated with irradiation only, Syn-BMT, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, and Allo-BMT. **P < 0.01 compared with the non-treated controls, the mice treated with irradiation only, Syn-BMT, and Allo-BMT. ***P < 0.05 compared with the non-treated controls, the mice treated with irradiation only, and Syn-BMT. ****P < 0.005 compared with the non-treated controls and the mice treated with irradiation only. *****P < 0.005 compared with the mice treated with irradiation only. # P < 0.0001 compared with mice treated with irradiation only, Syn-BMT, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT, Allo-BMT + Allo-TT, and Allo-BMT + 3rd-party TT. Non-treated (n = 8), Irradiation only (n = 5), Syn-BMT (n = 11), Syn-BMT + Syn-TT (n = 11), Syn-BMT + Allo-TT (n = 13), Allo-BMT (n = 13), Allo-BMT + Allo-TT (n = 12), and Allo-BMT + 3rd-party TT (n = 10)
Primary tumor size and lung metastasis
Mice in the non-treated control group showed significantly greater tumor growth than mice in the other seven groups (Fig. 1b). The tumor grew very slowly in all the other seven groups, and there were no significant differences between the groups.
Next, we examined lung metastasis of the tumors. We confirmed that there was no lung metastasis in the mice with tumors before BMT. The mice in the non-treated control group showed many metastatic tumor nodules 2–3 weeks after reaching a tumor size of >4 cm2 (Fig. 2a, b). Although the day of analysis (4–5 weeks after transplantation) was different from the mice in the non-treated control group because of their early death, the mice treated with Syn-BMT showed the greatest numbers of metastatic tumor nodules in all the groups. This strong metastasis was due to the immunosuppressive effect of irradiation. The mice treated with Syn-BMT + Syn-TT and Syn-BMT + Allo-TT showed no significant difference in the number of metastatic tumors compared with those in the non-treated control group. However, the mice treated with Allo-BMT showed a significant inhibition of metastasis compared with non-treated controls. Furthermore, the mice treated with Allo-BMT + Allo-TT or Allo-BMT + 3rd-party TT showed the lowest rates of metastasis.
Fig. 2.
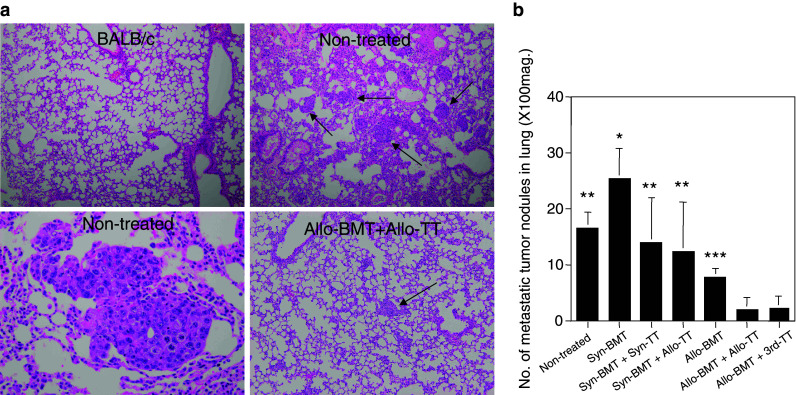
Lung metastasis in mice with advanced tumors treated with BMT + TT. Lung metastasis in the mice with advanced tumors is shown. Autopsy and analysis for the metastasis were performed in the non-treated mice 2–3 weeks after the tumor reached a size of >4 cm2 because of early death and in Syn-BMT, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT, Allo-BMT + Allo-TT, and Allo-BMT + 3rd-party TT groups 4–5 weeks after BMT. Representative histological findings of lung metastasis in mice with advanced tumors are shown (a). The non-treated controls showed a number of metastatic tumor nodules (arrows) (upper right ×100 and lower left ×400), whereas no tumor was found in the BALB/c mice (upper left ×100). Those treated with Allo-BMT + Allo-TT showed only a few nodules (arrow) (lower right ×100). The results are summarized in b. *P < 0.02 compared with non-treated controls, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT, Allo-BMT + Allo-TT, and Allo-BMT + 3rd-party TT. **P < 0.02 compared with Allo-BMT + Allo-TT, and Allo-BMT + 3rd-party TT. ***P < 0.02 compared with non-treated controls and Syn-BMT. Non-treated (n = 5), Syn-BMT (n = 4), Syn-BMT + Syn-TT (n = 4), Syn-BMT + Allo-TT (n = 4), Allo-BMT (n = 5), Allo-BMT + Allo-TT (n = 4), and Allo-BMT + 3rd-party TT (n = 6). Data are shown as mean ± SD
Host thymus and implanted thymus
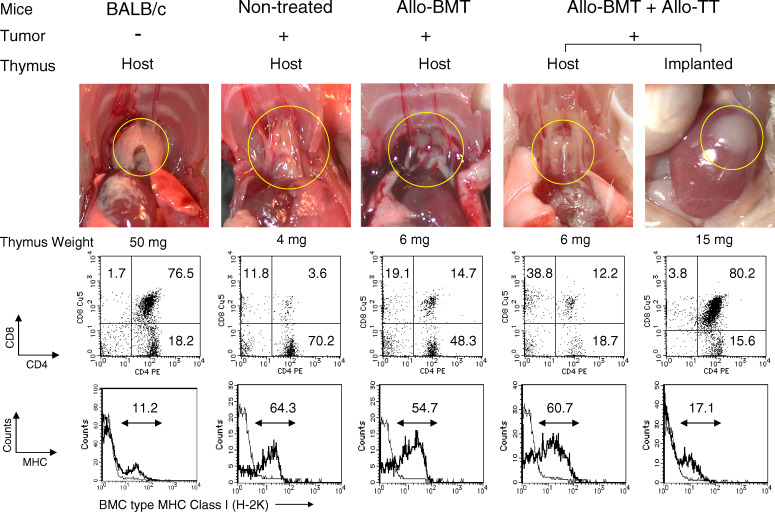
We next examined the host and implanted thymus in mice with advanced tumors. The host thymus in mice in the non-treated control group showed marked involution (reduction in size), compared with the thymus from BALB/c mice without tumors (Fig. 3). In addition, the subsets of thymocytes were abnormally regulated in the mice with tumors: the percentage of CD4+CD8+ double-positive thymocytes markedly decreased, whereas the percentages of CD4+CD8−, CD4−CD8+, and CD4−CD8− thymocytes increased. The donor-type MHC class I expression of H-2K region in the thymocytes of the mice with tumors increased due to relative maturation of thymocytes, compared with the BALB/c mice without tumors. Although the day of analysis was different from the mice in the non-treated control group because of their early death, the mice treated with either Syn-BMT (data not shown) or Allo-BMT alone showed similar results. Interestingly, although the host thymus showed involution in the mice treated with Allo-BMT + Allo-TT, the transplanted thymus grew and engrafted well. The thymocyte subsets of the transplanted thymus were similar to those of normal control mice. The mice treated with Allo-BMT + 3rd-party TT showed results comparable to those of the mice treated with Allo-BMT + Allo-TT (data not shown).
Fig. 3.
Findings related to host and transplanted thymus, CD4/CD8 subsets, and MHC expression in thymocytes from mice with advanced tumors treated with BMT + TT. The macroscopic findings (upper panel thymus in yellow circle), FACS profile (middle panel), and MHC class I (H-2K) expression (lower panel) in thymocytes from the host and transplanted thymus in BALB/c mice, non-treated controls with advanced tumors, and those treated with Allo-BMT and Allo-BMT + TT are shown. Autopsy and analysis were performed at the same time as those in Fig. 2. H-2K expression thick line, BMC type (H-2Kd in BALB/c and non-treated controls, H-2Kb in Allo-BMT and Allo-BMT + Allo-TT), thin line, negative control or host type (H-2Kb in BALB/c and non-treated controls, H-2Kd in Allo-BMT and Allo-BMT + Allo-TT). Representative data of 3 or 4 experiments are shown
Lymphocyte subsets
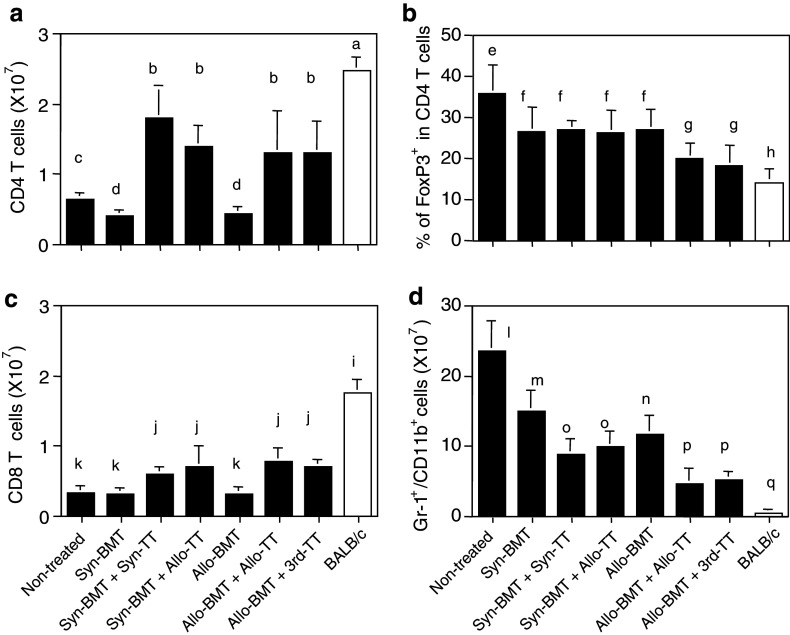
We next investigated lymphocyte subsets in the spleens from mice with advanced tumors. The number of CD4+ T cells in the mice in the non-treated control group was significantly reduced, compared with BALB/c mice 2–3 weeks after reaching a tumor size of >4 cm2 (Fig. 4a). Although the day of analysis was different from the mice in the non-treated control group because of their early death, BALB/c mice treated with Syn-BMT or Allo-BMT showed further reductions in the number of CD4+ T cells, compared with the non-treated control. However, the numbers were significantly elevated in mice treated with Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT + Allo-TT and Allo-BMT + 3rd-party TT, compared with the mice treated with Syn-BMT or Allo-BMT alone or non-treated controls. However, the number of CD4+ T cells was still lower than that in BALB/c mice.
Fig. 4.
The numbers of the cells in spleen from the mice with advanced tumors treated with BMT + TT. The numbers of CD4+ T cells (a), % of FoxP3+ cells in CD4+ T cells (b), the numbers of CD8+ T cells (c) and Gr-1/CD11b cells (d) in the spleen were evaluated in each group. Analyses were performed at the same time as those in Fig. 2. a versus b, c, and d: P < 0.03; b versus c and d: P < 0.02; c versus d: P < 0.02; e versus f, g, and h: P < 0.02; f versus g and h: P < 0.03; g versus h: P < 0.03; i versus j and k: P < 0.02; j versus k: P < 0.03; l versus m, n, o, p and q: P < 0.02; m versus o, p, and q: P < 0.03; n or o versus p and q: P < 0.03; p versus q: P < 0.03. Non-treated (n = 5), Syn-BMT (n = 4), Syn-BMT + Syn-TT (n = 4), Syn-BMT + Allo-TT (n = 4), Allo-BMT (n = 5), Allo-BMT + Allo-TT (n = 4), and Allo-BMT + 3rd-party TT (n = 4). Data are shown as mean ± SD
We next investigated CD4+FoxP3+Treg cells (Fig. 4b). The highest percentage of FoxP3+Treg cells in CD4+ T cells was observed in the non-treated control group, followed by the mice treated with Syn-BMT, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, and Allo-BMT, and the lowest percentages were observed in the mice treated with Allo-BMT + Allo-TT or Allo-BMT + 3rd-party TT. However, the percentages in all groups were significantly higher than that in BALB/c mice. The results regarding CD8+ T cells were similar to those of CD4+ T cells (Fig. 4c). The numbers of B cells in the mice treated with Syn-BMT or Allo-BMT were lowest (data not shown). The number of Gr-1+/CD11b+ myeloid suppressor cells, which increases with tumor progression, was highest in the non-treated control group (Fig. 4d). Interestingly, the mice treated with Allo-BMT + Allo-TT or Allo-BMT + 3rd-party TT showed the lowest numbers of these cells. The cell numbers were lower in the mice treated with Syn-BMT + Syn-TT, Syn-BMT + Allo-TT or Allo-BMT than in the mice treated with Syn-BMT alone. However, the levels were higher in all of these groups than in normal BALB/c mice.
Mitogen responses and cytokine production
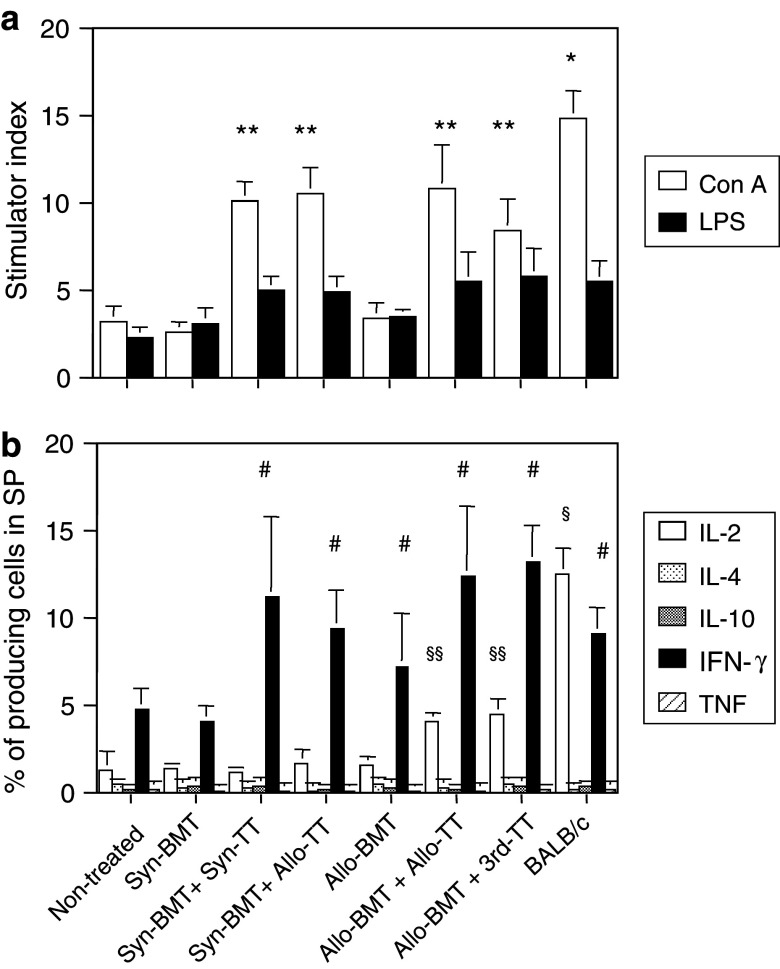
We next analyzed the lymphocyte function of the spleen cells. Con A responsiveness was low in the non-treated control group and in the mice treated with either Syn-BMT or Allo-BMT alone (Fig. 5a). However, it increased significantly in mice treated with either BMT (Syn or Allo) + TT (Syn, Allo, or 3rd-party), although the level did not reach that of the BALB/c mice. LPS responsiveness showed no significant differences between any of the groups.
Fig. 5.
The mitogen responses and percentage of cytokine-producing cells in the spleens from mice with advanced tumors treated with BMT + TT. Mitogen responses: Con A and LPS (a) and percentage of cytokine-producing cells (b) in the spleen were evaluated in each group. Analyses were performed at the same time as those in Fig. 2. *P < 0.03 compared with the non-treated controls, Syn-BMT, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT, Allo-BMT + Allo-TT, and Allo-BMT + 3rd-party TT. **P < 0.03 compared with the non-treated controls, Syn-BMT, and, Allo-BMT. # P < 0.03 compared with the non-treated controls and Syn-BMT. § P < 0.03 compared with the non-treated controls, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT, Allo-BMT + Allo-TT, and Allo-BMT + 3rd-party TT. §§ P < 0.03 compared with the non-treated controls, Syn-BMT, Syn-BMT + Syn-TT, Syn-BMT + Allo-TT and Allo-BMT. Non-treated (n = 4), Syn-BMT (n = 4), Syn-BMT + Syn-TT (n = 4), Syn-BMT + Allo-TT (n = 4), Allo-BMT (n = 4), Allo-BMT + Allo-TT (n = 4), Allo-BMT + 3rd-party TT (n = 4), and BALB/c mice (n = 4). Data are shown as mean ± SD
In the analysis of cytokine production (Fig. 5b), the level of IL-2 was significantly elevated in the mice treated with Allo-BMT + Allo-TT or Allo-BMT + 3rd-party TT, although the level was still lower than that of BALB/c mice. In contrast, IFN-γ was significantly elevated in the mice treated with Syn-BMT + Syn-TT, Syn-BMT + Allo-TT, Allo-BMT, Allo-BMT + Allo-TT or Allo-BMT + 3rd-party TT compared with non-treated control group or those treated with Syn-BMT. The levels were the same as those in BALB/c mice. The levels of the other cytokines (IL-4, IL-10, and TNF) were very low in all the groups in this experiment.
Factors correlated to survival
Finally, we examined the correlations between the above factors and survival rates in all the groups (Table 2). There were positive correlations between the median survival days and the numbers of CD4+ and CD8+ T cells, the Con A response, or the IFN-γ production, whereas there were negative correlations between the survival days and the lung metastasis, the numbers of Gr-1+/CD11b+ cells, or the percentage of FoxP3+ cells in CD4+ T cells. These factors were also correlated with each other (data not shown).
Table 2.
Analysis of correlations with survival period in mice with advanced cancer
| Factors | P value* |
|---|---|
| Main tumor | NS |
| Lung metastasis | 0.048# |
| CD4+ T cells | 0.045 |
| CD8+ T cells | 0.004 |
| B220+ B cells | NS |
| Gr-1 +/CD11b + cells | 0.004# |
| % of FoxP3/CD4 T cells | 0.018# |
| Con A | 0.010 |
| LPS | NS |
| IL-2 | NS |
| IL-4 | NS |
| IL-10 | NS |
| IFN-γ | 0.011 |
| TNF | NS |
NS not significant
* P values were calculated for median survival period by simple regression in seven groups
# A negative correlation was observed
There was no correlation between survival rates and the size of the main tumor, the numbers of B220+ B cells, the LPS response, and the IL-2-, IL-4-, IL-10-, or TNF-production.
Discussion
In the present study, we examined the effects of BMT (Syn or Allo) + TT (Syn, Allo or 3rd-party) in mice with advanced tumors. The mice treated with BMT + TT showed better survival rates than the mice treated with BMT only. Interestingly, the mice treated with Allo-BMT + Allo-TT or Allo-BMT + 3rd-party TT showed the longest survival rates. In addition, lung metastasis was significantly inhibited in these mice. T cell number, T cell function, and IFN-γ production significantly increased, whereas the number of Gr-1+/CD11b+ cells and the percentage of FoxP3+ cells in CD4+ T cells significantly decreased in these mice, compared with the other groups. These results suggest that BMT + TT, and particularly “Allo-BMT + Allo-TT” or “Allo-BMT + 3rd-party TT”, is most effective for hosts with advanced tumors in prolonging survival by restoring T cell function.
First, we have shown that TT plays an important role in BMT for the long-term survival of hosts with advanced cancer (Fig. 1a). A significant inhibition of lung metastasis was observed by Allo-BMT + Allo-TT or 3rd-party TT; there was a negative correlation between metastasis and survival (Fig. 2a, b; Table 2). In contrast, the gradual growth of the main tumor may be the result of the irradiation, because there was no significant difference in size between the mice treated with BMT and those treated with irradiation only, although it should be noted that the duration of observation in the latter was cut short by the hematopoietic failure. These findings suggest that the inhibition of metastasis is one of the mechanisms promoting survival.
In addition, it should be noted that GVHD was not particularly obvious in the “Allo-BMT + Allo-TT” or “Allo-BMT + 3rd-party TT” mice. This may be due to the relatively high proportion of FoxP3+ regulatory T cells, which inhibit GVHD but preserve the GVT effect [29], and/or the induction of tolerance by BMC-derived and transplanted thymus-derived thymic DCs [28]. Furthermore, the IBM–BMT method itself suppresses GVHD [20]. Thus, the contribution of GVHD to survival may be minimal.
Next, we showed that the thymus in mice with advanced tumors shows marked involution with or without BMT (Fig. 2). In the thymocyte subsets, the number of CD4+CD8+ thymocytes decreased, whereas that of CD4−CD8−, CD4+CD8−, or CD4−CD8+ thymocytes increased, as previously described [4, 5]. In contrast, the transplanted thymus grew large, showing almost normal subsets in donor-derived thymocytes, although the host thymus showed involution. The high expression of class I of H-2K region, which is low in most normal thymocytes [30], also supports the hypothesis that there is a dysregulation of thymocytes with the relative maturation of those thymocytes. These findings indicate that the transplanted thymus (but not the host thymus) can grow even in the presence of advanced tumors. Although the mechanism is unknown, the high proliferative activity of the transplanted thymus seems to overcome the immunosuppressed status.
We next examined lymphocyte subsets and T cell function in the spleens of mice with advanced tumors. The numbers of both CD4+ T and CD8+ T cells were significantly higher in the mice treated with either “Allo-BMT + TT” or “Syn-BMT + TT” than in the non-treated controls or in the mice treated with either Allo-BMT or Syn-BMT alone, although these numbers were still lower than those of normal BALB/c mice (Fig. 4a, c). The elevated T cell counts were probably from the result of the TT [23], and there was a positive correlation with survival (Table 2), suggesting that T cells may play a significant role in prolonging survival by preventing infection and tumor growth.
We further investigated the FoxP3+CD4+ regulatory T cells. The percentage of FoxP3+ cells among CD4+ T cells, which reflects the suppressor activity directly by the ratio of regulatory/effector T cells, was highest in the non-treated control group and lowest in the mice treated with Allo-BMT + Allo-TT or 3rd-party TT (Fig. 4b). Since a reduction in the percentage of FoxP3+ cells in CD4+ regulatory T cells enhances GVT activity [31, 32], the strong inhibition of metastasis in these mice may reflect this. It should be noted that the percentage of FoxP3+ cells among CD4+ T cells in these mice was still higher than that in normal BALB/c mice, suggesting that apparent GVHD could not be elicited by these cells, since GVHD is evident with a lower percentage than normal of these cells [23].
The mice treated with “Allo-BMT + Allo-TT” or “Allo-BMT + 3rd-party TT” showed the lowest number of Gr-1+/CD11b+ myeloid suppressor cells (Fig. 4d), indicating that the reduction of these cells may also facilitate GVT activity. As the myeloid suppressor cells can be induced by tumor exosomes [33], the inhibition of metastasis by GVT seems to be due to the reduction in the number of these cells. There was a negative correlation between the survival and not only the number of myeloid suppressor cells but also the percentage of FoxP3+ in CD4+ T cells (Table 2). In addition, there was a very strong correlation between the numbers of myeloid cells and the percentage of FoxP3+ cells among CD4+ T cells (P = 0.0032). In this respect, Gr-1+/CD11b+ myeloid suppressor cells may induce FoxP3+CD4+ regulatory T cells, as previously reported [34, 35].
We have also found that the mice treated with BMT + TT show a significantly higher Con A response than the mice treated with BMT alone, although the response was still lower than that in normal BALB/c mice (Fig. 5). In the analyses of cytokine production, there was a positive correlation between the survival and the level of IFN-γ (but not IL-2 production), suggesting that the level of IFN-γ may be more related to prolonged survival than that of IL-2.
Based on these results, it is evident that the elevated number of T cells and improved function resulting from TT play a crucial role in prolonging the survival of hosts with advanced cancers. Although T cell number and function did not reach normal levels, several other factors, such as regulatory T cells and myeloid suppressor cells, were synchronously suppressed.
Although, for both technical and ethical reasons (including donor age), it may be clinically difficult to obtain adequate thymic tissue, it is conceivable that grafts could be obtained from patients with congenital heart diseases or from aborted fetuses, as described previously [26, 27]. The combinations of Syn-BMT + Allo-TT or Allo-BMT + 3rd-party TT might then be practical in a clinical setting. Here, the allo-BMT + 3rd-party TT have shown tolerance to all three MHC determinants [28], suggesting a benefit for transplantation of other organs from the two MHC-disparate donors. Alternatively, a method of regenerating the thymus has also been developed [36, 37]. Thus, regenerated thymus could be expected to use all combinations in future.
In summary, we have shown that BMT + TT can prolong survival in hosts with advanced tumors by restoring T cell function. Although our model may be different from the varied immunogenic cancers in humans, the elevation of T cell function itself will be effective for the many complications induced by cancer. We think that BMT + TT could become a viable strategy for the treatment of advanced cancer in humans.
Acknowledgments
This work was supported by a grant from Haiteku Research Center of the Ministry of Education, a grant from the Millennium Program of the Ministry of Education, Culture, Sports, Science, and Technology, a grant from the Science Frontier Program of the Ministry of Education, Culture, Sports, Science, and Technology, a grant from The 21st Century Center of Excellence (COE) Program of the Ministry of Education, Culture, Sports, Science, and Technology, a Research Grant B from Kansai Medical University, Health and Labor Sciences research grants (Research on Human Genome, Tissue Engineering Food Biotechnology), a grant from the Department of Transplantation for Regeneration Therapy (sponsored by Otsuka Pharmaceutical Co., Ltd.), a grant from the Molecular Medical Science Institute (Otsuka Pharmaceutical Co., Ltd.), and a grant from Japan Immunoresearch Laboratories Co., Ltd. (JIMRO). We wish to thank Ms. Y. Tokuyama, and Ms. A. Kitajima for their technical assistance, and Mr. Hilary Eastwick-Field, and Ms. K. Ando for their help in the preparation of the manuscript.
Conflict of interest statement
We have no conflict of interest in this manuscript.
Abbreviations
- BMT
Bone marrow transplantation
- IBM–BMT
Intra-bone marrow–bone marrow transplantation
- TT
Thymus transplantation
- BMC
Bone marrow cell
- MHC
Major histocompatibility complex
Footnotes
N. Hosaka and W. Cui contributed equally to this study.
References
- 1.Toge T, Oride M, Yanagawa E, et al. Prognostic significance of lymphocyte proliferative responses to mitogens in gastric cancer patients. Jpn J Surg. 1982;12:424–428. doi: 10.1007/BF02469832. [DOI] [PubMed] [Google Scholar]
- 2.Das SN, Khanna NN, Khanna S. A multiparametric observation of immune competence in breast cancer and its correlation with tumour load and prognosis. Ann Acad Med Singapore. 1985;14:374–381. [PubMed] [Google Scholar]
- 3.Adler A, Stein JA, Ben-Efraim S, Immunocompetence, immunosuppression, human breast cancer. III Prognostic significance of initial level of immunocompetence in early and advanced disease. Cancer. 1980;45:2074–2083. doi: 10.1002/1097-0142(19800415)45:8<2074::AID-CNCR2820450814>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Adkins B, Charyulu V, Sun QL, Lobo D, Lopez DM. Early block in maturation is associated with thymic involution in mammary tumor-bearing mice. J Immunol. 2000;164:5635–5640. doi: 10.4049/jimmunol.164.11.5635. [DOI] [PubMed] [Google Scholar]
- 5.Mandal D, Bhattacharyya A, Lahiry L, Choudhuri T, Sa G, Das T. Failure in peripheral immuno-surveillance due to thymic atrophy: importance of thymocyte maturation and apoptosis in adult tumor-bearer. Life Sci. 2005;77:2703–2716. doi: 10.1016/j.lfs.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 6.Carrio R, Lopez DM. Impaired thymopoiesis occurring during the thymic involution of tumor-bearing mice is associated with a down-regulation of the antiapoptotic proteins Bcl-XL and A1. Int J Mol Med. 2009;23:89–98. [PubMed] [Google Scholar]
- 7.Mandal D, Lahiry L, Bhattacharyya A, Bhattacharyya S, Sa G, Das T. Tumor-induced thymic involution via inhibition of IL-7R alpha and its JAK-STAT signaling pathway: protection by black tea. Int Immunopharmacol. 2006;6:433–444. doi: 10.1016/j.intimp.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 9.Kusmartsev SA, Li Y, Chen SH. Gr-1 + myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 10.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;98:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann P, Edinger M. CD4+CD25+ regulatory T cells and graft-versus-host disease. Semin Hematol. 2006;43:62–69. doi: 10.1053/j.seminhematol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Kono K, Kawaida H, Takahashi A, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 15.Verdeguer A, Muñoz A, Cañete A, et al. Long-term results of high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma patients: a report of the Spanish working party for BMT in children (Getmon) Pediatr Hematol Oncol. 2004;21:495–504. doi: 10.1080/08880010490477284. [DOI] [PubMed] [Google Scholar]
- 16.Fefer A, Sullivan KM, Weiden P, et al. Graft versus leukemia effect in man: the relapse rate of acute leukemia is lower after allogeneic than after syngeneic marrow transplantation. Prog Clin Biol Res. 1987;244:401–408. [PubMed] [Google Scholar]
- 17.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol. 2005;174:3051–3058. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- 18.Kushida T, Inaba M, Hisha H, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–3299. doi: 10.1182/blood.V97.10.3292. [DOI] [PubMed] [Google Scholar]
- 19.Ikehara S. A novel method of bone marrow transplantation (BMT) for intractable autoimmune diseases. J Autoimmun. 2008;30:108–113. doi: 10.1016/j.jaut.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Inaba M, Sugiura K, et al. Enhancement of allogeneic hematopoietic stem cell engraftment and prevention of graft-versus-host diseases (GvHD) by intra-bone marrow–bone marrow transplantation plus donor lymphocyte infusion. Stem Cells. 2004;22:125–134. doi: 10.1634/stemcells.22-2-125. [DOI] [PubMed] [Google Scholar]
- 21.Hosaka N, Nose M, Kyogoku M, Nagata N, et al. Thymus transplantation, a critical factor for correction of autoimmune disease in aging MRL/+mice. Proc Natl Acad Sci USA. 1996;93:8558–8562. doi: 10.1073/pnas.93.16.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosaka N, Ryu T, Miyake T, et al. Treatment of autoimmune diseases in MRL/lpr mice by allogeneic bone marrow transplantation plus adult thymus transplantation. Clin Exp Immunol. 2007;147:555–663. doi: 10.1111/j.1365-2249.2006.03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyake T, Hosaka N, Cui W, et al. Adult thymus transplantation with allogeneic intra-bone marrow–bone marrow transplantation from same donor induces high thymopoiesis, mild graft-versus-host reaction and strong graft-versus-tumour effects. Immunology. 2009;126:552–564. doi: 10.1111/j.1365-2567.2008.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu T, Hosaka N, Miyake T, et al. Transplantation of newborn thymus plus hematopoietic stem cells can rescue supralethal irradiated mice. Bone Marrow Transplant. 2008;41:659–666. doi: 10.1038/sj.bmt.1705957. [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Hosaka N, Takaki T, et al. Allogeneic intra-BM–BMT plus adult thymus transplantation from same donor has benefits for long-term survival even after sublethal irradiation or low-dose BM cell injection. Bone Marrow Transplant. 2009;43:829–837. doi: 10.1038/bmt.2008.396. [DOI] [PubMed] [Google Scholar]
- 26.Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 27.Markert ML, Hicks CB, Bartlett JA, Harmon JL. Effect of highly active antiretroviral therapy and thymic transplantation on immunoreconstitution in HIV infection. AIDS Res Hum Retroviruses. 2000;16:403–413. doi: 10.1089/088922200309061. [DOI] [PubMed] [Google Scholar]
- 28.Cui W, Hosaka N, Miyake T, et al. Analysis of tolerance induction using triple chimeric mice: MHC-disparate thymus, hemopoietic cells and microenvironment. Transplantation. 2008;85:1151–1158. doi: 10.1097/TP.0b013e31816a8f1f. [DOI] [PubMed] [Google Scholar]
- 29.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 30.Scollay R, Jacobs S, Jerabek L, Butcher E, Weissman I. T cell maturation: thymocyte and thymus migrant subpopulations defined with monoclonal antibodies to MHC region antigens. J Immunol. 1980;124:2845–2853. [PubMed] [Google Scholar]
- 31.Lin YC, Chang LY, Huang CT, et al. Effector/memory but not naive regulatory T cells are responsible for the loss of concomitant tumor immunity. J Immunol 182:6095-6104 [DOI] [PubMed]
- 32.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 33.Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Sun L, Zhao Y. Thymic epithelial progenitor cells and thymus regeneration: an update. Cell Res. 2007;17:50–55. doi: 10.1038/sj.cr.7310114. [DOI] [PubMed] [Google Scholar]
- 37.Takaki T, Hosaka N, Miyake T, et al. Presence of donor-derived thymic epithelial cells in [B6–>MRL/lpr] mice after allogeneic intra-bone marrow–bone marrow transplantation (IBM–BMT) J Autoimmun. 2008;31:408–415. doi: 10.1016/j.jaut.2008.09.003. [DOI] [PubMed] [Google Scholar]