Abstract
We tested the efficacy of CD8+ T cells lacking the Cbl-b gene against a panel of mammary tumor lines with different intrinsic sensitivities to T cells. Mice bearing established tumors expressing an ovalbumin-tagged version of HER-2/neu underwent adoptive transfer with Cbl-b-replete or -null CD8+ T cells from OT-I T cell receptor transgenic donor mice. In general, Cbl-b-null OT-I cells showed enhanced expansion, persistence, and capacity for tumor infiltration. This resulted in markedly enhanced efficacy against two tumor lines that normally demonstrate complete (NOP21) or partial (NOP23) regression. Moreover, a third tumor line (NOP6) that normally demonstrates progressive disease underwent complete regression in response to Cbl-b-null OT-I cells. However, a fourth tumor line (NOP18) was resistant to Cbl-b-null OT-I cells owing to a profound barrier to lymphocyte infiltration. Thus, Cbl-b-null CD8+ T cells are generally more efficacious but are nonetheless unable to mediate curative responses against all tumor phenotypes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-009-0698-3) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Animal models for tumor immunology, Adoptive T cell therapy
Introduction
Adoptive immunotherapy is a promising approach for the treatment of cancer [8, 9, 16, 27]. The strategy involves the isolation and expansion of autologous, tumor-reactive T cells, which are then infused into the patient with the expectation they will recognize cognate tumor antigen and elicit tumor-specific cytotoxicity. While objective clinical responses have been reported in over 50% of patients with advanced melanoma [8, 9], responses are often mixed, wherein some tumor nodules regress while others do not [29]. One barrier to success has been the limited persistence of adoptively transferred T cells in vivo [28, 31, 38, 42]. In addition, barriers in the tumor microenvironment can impede T cell trafficking, infiltration, and effector function at a local level, which likely accounts for the mixed responses seen in many patients [20].
To investigate barriers to successful adoptive immunotherapy, we have developed a novel transgenic mouse model of breast cancer. Briefly, CD4+ and CD8+ T cell epitopes from the model antigen ovalbumin (OVA) were linked to the C terminus of rat neu to generate a fusion protein designated NEUOT-I/OT-II. The OVA epitopes are recognized in the context of MHC class I and II by the TCR transgenic mouse strains OT-I (CD8+) and OT-II (CD4+), respectively [2, 14]. Expression of Neu OT-I/OT-II as a transgene in the mammary epithelium of C57BL/6 mice, together with a dominant negative version of p53, leads to the spontaneous development of mammary adenocarcinomas when mice are 6–10 months of age [32]. Remarkably, adoptive transfer of OT-I and OT-II T cells induces complete regression of approximately 37% of tumors with the remaining tumors showing partial/stable responses (40%) or progressive disease (23%) [32]. Thus, OT-I and OT-II T cells are effective against some but not all tumors in this model, similar to the mixed responses seen in clinical immunotherapy trials, as discussed above. This provides a unique experimental system to evaluate strategies to improve the efficacy of adoptive T cell therapy.
The E3-ubiquitin ligase CBL-B plays a negative regulatory role in T cell activation by marking TCR-related signaling molecules for ubiquitin-mediated degradation. Molecules affected by Cbl-b-mediated regulation include VAV-1, LFA-1, TCR, CRK-L, and the p85 regulatory subunit of PI3-kinase [1, 5, 6, 10, 11, 33, 41]. Accordingly, Cbl-b-null T cells show enhanced activity of signaling mediators such as AKT, PLC-γ1, ERK, PKC-θ, and NF-κB [23, 24, 26, 30, 33, 40]. Such T cells also show increased TCR clustering and lipid raft aggregation [17], as well as enhanced clustering of LFA-1 and adhesion to ICAM-1 [41]. Upon CD28 costimulation, CBL-B itself is ubiquitinated and degraded, thereby reversing CBL-B-mediated inhibition and facilitating T cell activation [19, 39]. In terms of functional properties, Cbl-b-null CD4+ and CD8+ T cells have a reduced requirement for CD28 co-stimulation with respect to proliferation and production of IL-2 and IFN-γ [1, 4, 5]. Moreover, Cbl-b-null T cells are resistant to anergy [15], inhibition by TGF-β [4, 36, 37], suppression by regulatory T cells (Tregs) [21, 36], and functional exhaustion [25]. As might be expected, Cbl-b-null mice are prone to experimental and spontaneous autoimmune disease [1, 5, 15].
Since lack of costimulatory molecules in the tumor microenvironment can be a barrier to T cell activity, the use of Cbl-b-null T cells has been explored in several tumor models. With respect to immune surveillance, Cbl-b-null mice have been shown to develop fewer tumors in the settings of UVB irradiation and ataxia telangiectasia-mutated (ATM) deficiency [4, 21]. Of greater clinical relevance, adoptively transferred Cbl-b-null CD8+ T cells showed enhanced efficacy against E.G7 lymphomas and TC-1 fibroblast tumors [4, 21] with a reduced dependence on CD4+ T cell help [21], suggesting this may represent an attractive treatment strategy for cancer. While these results are promising, it remains to be determined whether the enhanced efficacy of Cbl-b-null T cells applies to other tumor types, particularly those displaying the phenotypic diversity evident in human cancer. Given our previous results showing that spontaneous mammary tumors differ widely in their inherent sensitivity to T cells, we investigated whether Cbl-b-null T cells would show universally enhanced efficacy in this model system.
Materials and methods
Mice
This study followed Canadian Council for Animal Care guidelines and was approved by the University of Victoria Animal Care Committee. All mice were C57BL/6 (H-2b). Mice expressing the Neu OT-I/OT-II transgene in mammary epithelium under the control of the MMTV promoter have been described [32], as have TCR transgenic OT-I mice that recognize OVA residues 257-264 on MHC class I [14] (Jackson Labs). Cbl-b +/− mice kindly provided by J. Penninger were backcrossed onto the C57BL/6 background for at least ten generations and then crossed with OT-I mice. The resulting Cbl-b +/− OT-I mice were then crossed with Cbl-b +/− mice to generate Cbl-b −/− OT-I mice. To discriminate donor cells and endogenous cells, we bred Neu OT-I/OT-II transgenic mice onto a homozygous CD45.1 (Ly5.1) background, whereas donor OT-I T cells expressed CD45.2 (Ly5.2). OT-I mice were also bred with CD90.1 (Thy1.1) congenic mice to allow T cells from these animals to be tracked in CD90.2 (Thy1.2) and CD45.1 host mice. All genotyping was PCR-based [32], and transgenic TCR expression was confirmed by flow cytometry.
Tumor cell lines
The mammary tumor cell lines NOP6, NOP18, NOP21, and NOP23 were derived from spontaneous tumors in transgenic mice expressing NEUOT-I/OT-II and a dominant negative version of p53 in mammary epithelium [32] (Martin et al., in preparation). Cell lines were assessed by flow cytometry for expression of H-2 kb/H-2Db (Cat. #553575 Pharmingen, Mississauga, ON), I-Ab (Cat. #06261D, Pharmingen), c-NEU (Ab-4, Oncogene Research, San Diego, CA), and SIINFEKL/MHC class I (Clone 25-D1.15, a generous gift from Dr. Jonathan Bramson). Tumor cells were injected subcutaneously into the mammary fat pad of 6- to 8-week-old female Neu OT-I/OT-II transgenic mice, which lead to the development of 5–70 mm2 tumors after 3–6 weeks. Typically, two tumors were implanted in each host animal, contralateral to one another, which provided a further measure of the reproducibility of tumor responses. Note that Neu OT-I/OT-II transgenic mice were used as hosts, as WT mice reject NOP tumors due to the immunogenicity of the OT-I and OT-II T cell epitopes [32].
Adoptive transfer and flow cytometry
Single-cell lymphocyte suspensions were prepared from TCR transgenic OT-I donor mice and introduced into host mice via tail vein injection. Unless otherwise indicated, a dose of 15 × 106 splenocytes from OT-I transgenic mice was used for adoptive transfer; this contained approximately 4.5 × 106 CD8+ OT-I cells. As NOP21 tumors completely regress after adoptive transfer of this dose of WT OT-I cells, a sub-curative (threefold lower) dose was used to compare the efficacy of WT and CBL-B-deficient OT-I cells in mice bearing NOP21 tumors.
To isolate tumor-infiltrating lymphocytes, tumors were pressed through a 40 μm membrane, and lymphocytes in the supernatant were stained with fluorescently labeled antibodies to CD8a (Cat. #553034, Pharmingen), CD45.2 (Ly5.2, Cat. #553772, Pharmingen), and CD90.1 (Thy1.1, Cat. #11-0900-85, eBioscience, San Diego, CA). After staining for cell surface markers, intracellular staining was performed for Ki67 expression (Clone SP6, Labvision, Fremont, CA) by fixing cells in fresh 1% paraformaldehyde and permeabilizing with 0.5% Tween 20 in 1% paraformaldehyde. Data were collected using a BD FACSCalibur flow cytometer and analyzed using FlowJo software. Statistical analysis was performed using the Mann Whitney t test.
Measurement of tumor responses
Vernier calipers were used to measure tumor size (length × width). Responses were classified as CR (complete response; no measurable tumor), PR (partial response; >50% tumor reduction), SD (stable disease; <50% reduction or <25% increase), or PD (progressive disease; >25% increase) [32].
Tissue analysis
Tumor tissue was processed following standard methods and either stained with hematoxylin and eosin (H&E) or subjected to immunohistochemistry (IHC) with antibodies to NEU (polyclonal antibody, catalogue # 2242, Cell Signaling, Danvers, MA), CD3 (clone SP7, Labvision), Granzyme B (catalogue #ab4059, Abcam, Cambridge, MA) or FOXP3 (clone FJK-16s, eBioscience). Briefly, tissue was fixed in 10% neutral buffered formalin for a minimum of 24 h. The tissue was then processed using a standard overnight protocol on a Thermo Shandon Pathcentre tissue processor, embedded in Paraplast Plus (McCormick Scientific, St Louis, MO), and sectioned at 5 μm onto Superfrost Plus slides (Fisher Scientific, Ottawa, ON). Following deparaffinization, slides were placed in a Ventana Discovery XT autostainer (Ventana, Tucson, AZ) for IHC. Ventana’s standard CC1 protocol was used for antigen retrieval. After incubation with biotinylated secondary antibody (Jackson Immunoresearch, West Grove, PA), bound antibodies were detected using the DABMap kit (Ventana) and counterstained with hematoxylin (Ventana). Slides were visualized using a Zeiss Axioskop 2 Plus microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) and images captured using a PAXcam ARC digital microscope camera and PAX-it imaging software Version 6.9 (MIS Inc, Villa Park, IL).
Immune cells were scored using a Chaulkley 49-point grid. Briefly, each immunostained tumor was reviewed under a 40× objective with a 49 cross hair grid overlaid on the image. Under a 40× objective magnification, this grid defines an area of 0.56 mm2. Five epithelial and five stromal fields were evaluated in each case. The proportion of the field occupied by tumor epithelium was estimated using grid cross hairs, as was the total number of positive immune cells within the area of the grid. The number of grid points that coincided with positively staining immune cells within both epithelial and stromal areas was then determined.
Results
A panel of mammary tumor lines with differing sensitivities to CD8+ T cells
We have previously shown that female mice co-expressing the Neu OT-I/OT-II transgene in mammary epithelium, together with a dominant negative version of p53 (DNp53), spontaneously develop mammary adenocarcinomas at 6–10 months of age [32]. We generated a panel of cell lines from such tumors (referred to as “NOP” cell lines, in reference to the components NEU, OVA, and P53). Each cell line was implanted in at least three recipient mice and empirically tested for sensitivity to adoptively transferred OT-I and OT-II cells, as described [32]. While all untreated tumors invariably showed progressive growth, we identified four tumor cell lines with distinct, reproducible responses to adoptively transferred OT-I and OT-II cells. Specifically, NOP21 reproducibly demonstrated a complete response (CR); NOP23 underwent a partial response (PR); and NOP6 and NOP18 demonstrated progressive disease (PD) (Supplementary Fig. 1) [32]. By flow cytometric analysis, all four lines expressed NEUOT-I/OT-II, MHC class I, and the OVA SIINFEKL epitope/MHC class I complex (Supplementary Fig. 2). Thus, the cell lines NOP21, NOP23, NOP6, and NOP18 all express the OT-I and OT-II epitopes, yet differ in the extent of tumor regression after adoptive transfer of OT-I and OT-II cells. This provided a unique model system to evaluate whether Cbl-b deficiency can enhance the efficacy of adoptive immunotherapy against phenotypically diverse tumors.
Cbl-b-null CD8+ T cells show enhanced efficacy against a partially responsive tumor
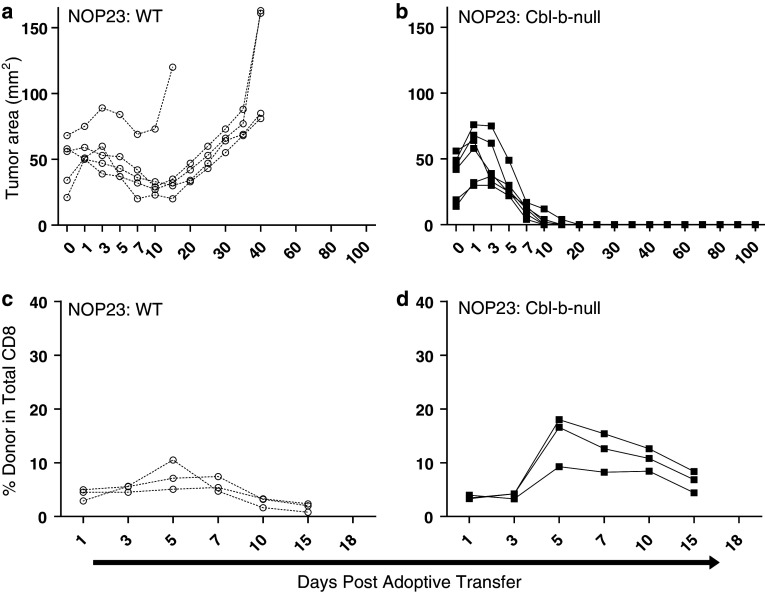
To determine whether Cbl-b deficiency could enhance CD8+ T cell responses in the NEUOT-I/OT-II mammary tumor model, we generated OT-I cells with homozygous deletion of the Cbl-b gene. The anti-tumor activity of Cbl-b-null OT-I cells was initially tested against NOP23 tumors, as we reasoned that any enhanced efficacy would be most obvious in the setting of a partial response. Furthermore, adoptive transfers were performed with OT-I cells alone (i.e., without co-transfer of OT-II cells) to create a more challenging experimental setting such that any advantages of Cbl-b deficiency would be more pronounced. Mice bearing established NOP23 tumors (≤80 mm2) received ~4.5 × 106 naïve Cbl-b-null OT-I cells or, as a control, an equivalent dose of wild type (WT) OT-I cells. As expected, WT OT-I cells induced partial regression of NOP23 tumors within 7–10 days of adoptive transfer, and tumors began to re-grow by day 20 (Fig. 1a). In contrast, Cbl-b-null OT-I cells induced complete regression of NOP23 tumors. In 3/3 mice monitored long term, no tumor recurrences were observed for the duration of the experiment (100 days; Fig. 1b). Thus, adoptive transfer of Cbl-b-null OT-I cells can induce complete regression of tumors that otherwise demonstrate partial regression.
Fig. 1.
Cbl-b-null CD8+ cells demonstrate increased in vivo expansion and enhanced efficacy against a partially responsive tumor. Mice bearing NOP23 mammary tumors underwent adoptive transfer of either WT (a, c) or Cbl-b-null (b, d) OT-I cells. Tumor size (a, b) and donor cell number in peripheral blood (c, d) were measured at serial time points after adoptive transfer. For flow cytometry, lymphocytes were gated by forward and side scatter, and CD8+ CD45.2+ OT-I cells were enumerated relative to total CD8+ T cells. X axes are not linear
Cbl-b-null CD8+ T cells demonstrate increased expansion in vivo
In time course experiments involving NOP23 tumors, both Cbl-b-null and WT OT-I cells underwent clonal expansion between days 3 and 5 (Fig. 1c, d). However, Cbl-b-null OT-I cells expanded at a greater rate than WT OT-I cells, as evidenced by the slope of the respective growth curves (5.4 vs. 1.2% per day, respectively; Fig. 1c, d). Consequently, Cbl-b-null OT-I cells achieved an approximately twofold higher level in peripheral blood, constituting from 8 to 22% of circulating CD8+ cells on day 7 compared to 5–9% for WT OT-I cells (mean 13.5 vs. 7.0, P = 0.0362). Cbl-b-null OT-I cells also persisted at higher levels than WT OT-I cells (Fig. 1c, d). A subset of mice was sacrificed 7 days after adoptive transfer, and the proliferative status of adoptively transferred cells was determined by flow cytometry for Ki67 expression. When T cells from peripheral blood or lymph node were analyzed, Cbl-b-null and WT OT-I cells showed a similar proliferative index (Fig. 2a). Surprisingly, when tumor-infiltrating T cells were analyzed, WT OT-I cells showed a markedly higher proliferative index than Cbl-b-null OT-I cells (Fig. 2a), despite being less numerous in peripheral blood.
Fig. 2.
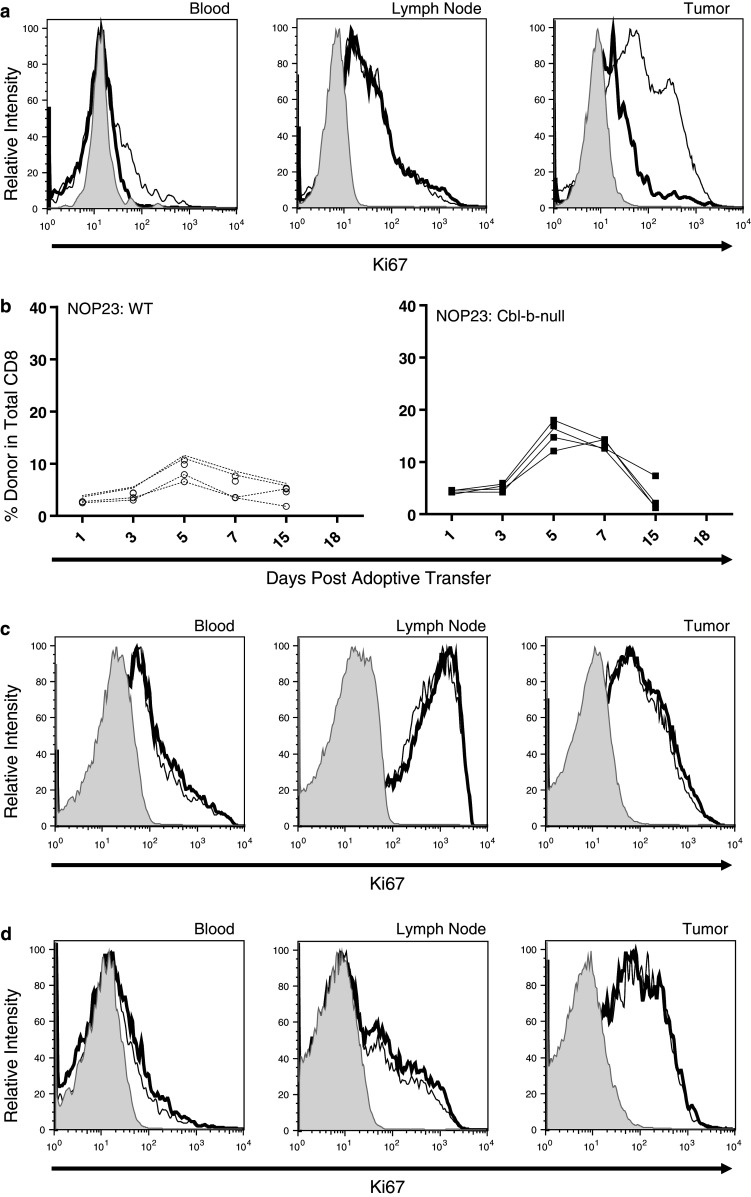
Proliferation of adoptively transferred WT and Cbl-b-null OT-I cells in mice bearing NOP23 mammary tumors. a Expression of Ki67 by adoptively transferred OT-I cells in different tissue compartments. Cbl-b-null and WT OT-I cells were transferred into separate host mice, and tissues were harvested 7 days later. Lymphocytes were gated by forward and side scatter, and analyzed for expression of CD8, CD45.2 (which marks all donor cells), and Ki67, a marker of proliferation, compared to cells stained with secondary antibody alone (shaded). The proliferative index of WT (thin line) and Cbl-b-null (heavy line) OT-I cells was similar in blood and lymph node, whereas tumor-infiltrating WT OT-I cells had a higher proliferative index. b-d Proliferation of Cbl-b-null and WT OT-I cells co-transferred into the same host mice. Lymphocytes were gated by forward and side scatter, and analyzed for expression of CD8a, CD45.2 (which marks all donor cells), and CD90.1 (which distinguishes WT from Cbl-b-null OT-I cells). b Expansion of OT-I cells in peripheral blood relative to total CD8+ T cells. c, d Expression of Ki67 by WT and Cbl-b-null OT-I cells in different tissue compartments on day 5 (c) and day 7 (d). Control cells were stained with secondary antibody alone (shaded). X-axes are not linear
The above results were potentially confounded by the fact that NOP23 tumors regressed at different rates and to different extents in response to Cbl-b-null versus WT OT-I cells. To make a more direct comparison, we co-transferred Cbl-b-null and WT OT-I cells into mice bearing NOP23 tumors. Cbl-b-null and WT OT-I cells were distinguished by expression of the Thy-1 congenic marker. As before, Cbl-b-null OT-I cells expanded at a greater rate than WT OT-I cells (5.1 vs. 2.5% per day, respectively) and achieved an approximately twofold higher level in peripheral blood (15.3 vs 8.4% of total CD8+ T cells, respectively on day 5, and 13.8 vs. 5.8% on day 7; P < 0.0001) (Fig. 2b). When tumor-infiltrating T cells were analyzed, Cbl-b-null and WT cells were present in approximately equal numbers on day 5, constituting 55 and 45% of donor CD8+ T cells, respectively (data not shown). By day 7, Cbl-b-null OT-I cells were twice as abundant as WT OT-I cells, constituting 66% of donor CD8+ T cells (data not shown). Thus, when compared within the same animal, Cbl-b-null OT-I cells achieved approximately twofold higher levels in blood and tumor compared to WT OT-I cells. Despite this difference in abundance, on both days 5 and 7 Cbl-b-null and WT OT-I cells showed a nearly identical proliferative index (Ki67) in blood, lymph node, and tumor (Fig. 2c, d). This suggests that the difference in abundance seen between Cbl-b-null and WT OT-I cells arises from factors other than proliferation rate, such as different apoptotic rates or trafficking patterns.
Cbl-b-null OT-I cells mediate curative responses against some but not all mammary tumors
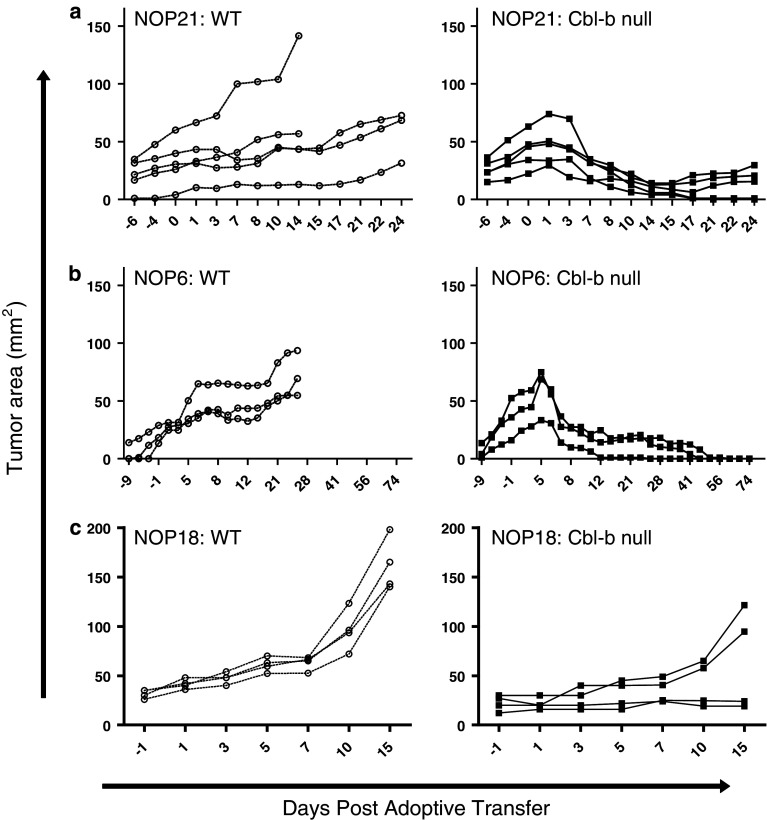
We next investigated whether the enhanced expansion and effector activity shown by Cbl-b-null OT-I cells against NOP23 tumors applied to mammary tumors with different response characteristics. The NOP21 tumor line normally undergoes complete regression after adoptive transfer of WT OT-I cells. To test whether Cbl-b-null OT-I cells show enhanced efficacy against NOP21, mice were given a subcurative (threefold lower) dose of WT or Cbl-b-null OT-I cells. As expected, this dose of WT OT-I cells was insufficient to induce NOP21 tumor regression, and instead tumors grew slowly after adoptive transfer (Fig. 3a). In contrast, the same dose of Cbl-b-null cells induced complete, permanent regression of 2/5 tumors, and partial regression of 3/5 tumors (Fig. 3a). This enhanced efficacy was accompanied by a modest increase in T cell expansion, with Cbl-b-null OT-I cells reaching 1.3–16.2% of circulating CD8+ cells on day 10, compared to 0.9–1.5% for WT OT-I cells (data not shown).
Fig. 3.
Cbl-b-null CD8+ cells demonstrate enhanced efficacy against some but not all mammary tumors. Mice bearing NOP21 (a), NOP6 (b), or NOP18 (c) mammary tumors underwent adoptive transfer of either WT (left panels) or Cbl-b-null (right panels) OT-I cells. Tumor size was measured at serial time points after adoptive transfer. Mice bearing tumors demonstrating progressive growth despite adoptive transfer of OT-I cells were sacrificed 2–4 weeks post-adoptive transfer, depending on tumor growth rates. X axes are not linear
The NOP6 tumor line normally shows progressive growth after adoptive transfer of WT OT-I cells; mice were therefore given a standard dose (~4.5 × 106) of cbl-b-null or WT OT-I cells. As expected, WT OT-I cells failed to induce NOP6 tumor regression (Fig. 3b). In contrast, the same dose of Cbl-b-null OT-I cells induced complete regression of 3/3 tumors (Fig. 3b). This was accompanied by increased clonal expansion of OT-I cells in peripheral blood: on day 7, the mean frequency of Cbl-b-null OT-I cells was 10.2% of CD8+ cells, while the frequency of WT OT-I cells was 2.5% (P = 0.0286; data not shown). In a long-term experiment involving three mice, NOP6 tumors underwent complete regression after treatment with Cbl-b-null OT-I cells and did not recur for the duration of the experiment (6 months; data not shown). Thus, Cbl-b-null cells can mediate a curative response against a tumor that otherwise demonstrates progressive growth.
Finally, we evaluated the NOP18 tumor line, which also shows progressive growth after adoptive transfer of WT OT-I cells. As expected, after adoptive transfer of a standard dose (~4.5 × 106) of WT OT-I cells, 4/4 tumors demonstrated PD (Fig. 3c). In contrast, an equivalent dose of Cbl-b-null OT-I cells mediated a modest anti-tumor response (Fig. 3c). Specifically, 1/4 tumors demonstrated SD, and 3/4 tumors demonstrated PD. This was reflected by different tumor growth rates between the WT and Cbl-b-null groups (8.0 vs. 2.9 mm2/day, respectively from days 1–15). Cbl-b-null OT-I cells also showed increased expansion in peripheral blood: on day 7, the mean frequency of Cbl-b-null OT-I cells was 10.8% of CD8+ cells, while the frequency of WT OT-I cells was 3.1% (P = 0.0286; data not shown). In a separate experiment, NOP18 tumors failed to regress even after adoptive transfer of a mixture of ~4.5 × 106 Cbl-b-null OT-I cells and ~4.5 × 106 WT OT-I cells, double the standard dose (data not shown). Thus, Cbl-b-null OT-I cells were unable to mediate a curative response against NOP18 tumors.
Tumor infiltration by Cbl-b-null OT-I cells
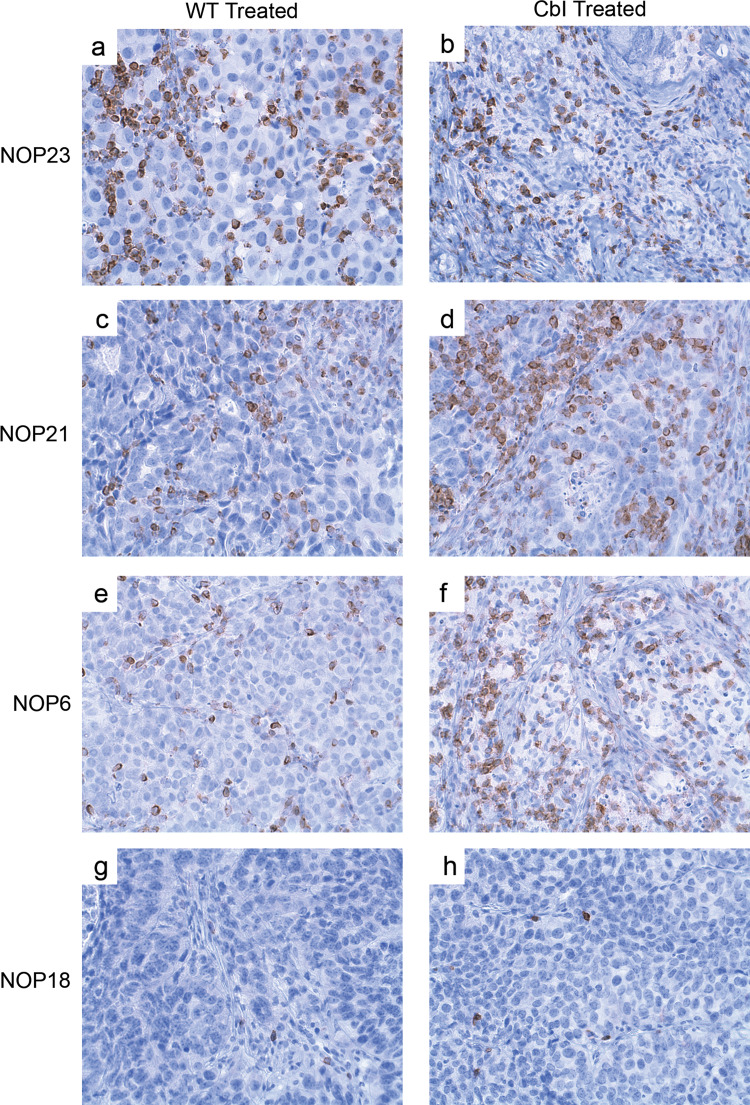
We have previously reported that a significant fraction of NEUOT-I/OT-II-induced tumors are inherently resistant to T cell infiltration and accordingly demonstrate a PD phenotype [32]. To evaluate whether Cbl-b-null OT-I cells can overcome such infiltration barriers, we assessed by IHC and Chaukley grid analysis the density of intratumoral CD3+ T cells on day 7 in a subset of mice from the above adoptive transfer experiments. None of the four tumor lines showed significant CD3+ T cell infiltrates prior to adoptive transfer (data not shown). As expected, in mice receiving WT OT-I cells, NOP21 and NOP23 tumors showed the densest T cell infiltration, followed in order by NOP6 and NOP18 tumors (Fig. 4; Table 1). In the case of NOP21, NOP23, and NOP6 tumors, T cells infiltrated both the stromal and epithelial tumor regions (Table 1), and flow cytometry for the CD45 congenic marker revealed that the vast majority were of donor origin (data not shown). In contrast, with NOP18 tumors, infiltrating T cells were few in number, primarily restricted to stroma (Fig. 4; Table 1), and mostly of host origin (i.e., 60% host vs. 40% donor; data not shown).
Fig. 4.
Cbl-b-null CD8+ cells demonstrate enhanced infiltration of some but not all mammary tumors. Immunohistochemical analysis of CD3+ T cells 7 days after adoptive transfer of WT (left panels) or Cbl-b-null (right panels) OT-I cells into mice bearing a, b NOP23 tumors, c, d NOP21 tumors, e, f NOP6 tumors, or g, h NOP18 tumors
Table 1.
Frequency of CD3+ and FOXP3+ tumor infiltrating T cells in mice after adoptive transfer of WT or Cbl-b-null cells
| NOP21 | P value | NOP23 | P value | NOP6 | P value | NOP18 | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Intraepithelial | |||||||||
| CD3+ cells | |||||||||
| WT | 46.5 | ns | 82.7 | ns | 27.4 | 0.0286 | 3.1 | ns | |
| Cbl-b-null | 67.8 | 65.7 | 75.6 | 3.5 | |||||
| FOXP3+ cells | |||||||||
| WT | 4.9 | ns | 6.2 | 0.0294 | 6.5 | ns | 0.1 | ns | |
| Cbl-b-null | 5.5 | 2.2 | 6.2 | 0.1 | |||||
| %FOXP3/CD3 | |||||||||
| WT | 10.9 | ns | 7.4 | ns | 24.3 | ns | 4.2 | ns | |
| Cbl-b-null | 8.4 | 3.3 | 8.4 | 3.6 | |||||
| Intrastromal | |||||||||
| CD3+ cells | |||||||||
| WT | 64.3 | ns | 72.1 | ns | 52.8 | ns | 30.0 | ns | |
| Cbl-b-null | 69.3 | 56.5 | 53.8 | 33.9 | |||||
| FOXP3+ cells | |||||||||
| WT | 9.4 | ns | 1.5 | ns | 11.5 | 0.0286 | 4.6 | ns | |
| Cbl-b-null | 12.6 | 1.7 | 3.4 | 5.3 | |||||
| %FOXP3/CD3 | |||||||||
| WT | 14.7 | ns | 2.1 | ns | 22.0 | 0.0286 | 16.0 | ns | |
| Cbl-b-null | 18.1 | 2.6 | 6.2 | 15.5 | |||||
ns not significant
Compared to WT OT-I cells, adoptive transfer of Cbl-b-null OT-I cells resulted in enhanced T cell infiltration of NOP21 and NOP6 tumor epithelium (i.e., 1.5-fold increase for NOP21, and 2.8-fold increase for NOP6), whereas infiltration of tumor stroma was unchanged for both NOP21 and NOP6 (Table 1). For NOP23 tumors, there was actually a 20% decrease in infiltration of tumor epithelium with Cbl-b-null OT-I cells compared to WT OT-I cells, probably because the tumors had largely regressed by day 7 in the former case (Table 1). For NOP18 tumors, Cbl-b-null OT-I cells failed to enhance the overall infiltration of tumor epithelium or stroma (Table 1), and the relative proportion of host and donor T cells was essentially unchanged. Thus, Cbl-b-null OT-I cells demonstrated enhanced infiltration of some but not all mammary tumors.
Finally, tissue sections from the above studies were stained with antibodies to GRANZYME B and FOXP3, which are markers of cytolytic and regulatory T cells, respectively. In general, tumors contained very few GRANZYME B+ cells, and there was no obvious difference between the WT and Cbl-b-null groups (data not shown). In contrast, FOXP3+ cells were sufficiently numerous to be quantified by Chaukley grid analysis, which allowed their abundance relative to CD3+ T cells to be estimated. For tumors treated with WT OT-I cells, FOXP3+ cells represented anywhere from 4.2 to 24.3% of CD3+ cells in the tumor epithelium depending on the tumor cell line (Table 1). In contrast, after adoptive transfer of Cbl-b-null OT-I cells, FOXP3+ cells were proportionately less numerous in all tumors except NOP18, although these trends did not reach statistical significance possibly due to low sample size (Table 1). The effect was most striking for NOP6, in which 24.3% of intraepithelial CD3+ cells were FoxP3+ after adoptive transfer of WT OT-I cells, versus 8.4% with Cbl-b-null OT-I cells (Table 1). Thus, adoptive transfer of Cbl-b-null OT-I cells may trigger proportionately less infiltration of the tumor epithelium by FoxP3+ cells in some tumors.
Discussion
We have evaluated the efficacy of Cbl-b-null CD8+ T cells against a panel of phenotypically diverse mammary tumors. In general, Cbl-b-null OT-I cells showed enhanced expansion and persistence in peripheral blood compared to WT OT-I cells (Fig. 1). For three of four tumor lines, this was accompanied by enhanced infiltration of tumor epithelium (Fig. 4; Table 1) and an improved anti-tumor response (Figs. 1, 3). The most striking results were seen with NOP6 tumors, which showed progressive growth in the face of WT OT-I cells yet completely regressed when challenged with Cbl-b-null OT-I cells. By contrast, Cbl-b-null OT-I cells were unable to eliminate a second PD tumor line (NOP18) due to a profound infiltration barrier that restricted T cells to tumor stroma (Fig. 4; Table 1). Thus, not all tumor phenotypes are permissive to infiltration and anti-tumor activity by Cbl-b-null T cells.
In time course experiments with NOP23 tumors, Cbl-b-null OT-I cells expanded to an approximately two-fold higher level in peripheral blood compared to WT OT-I cells (Figs. 1c, d, 2b). Similarly, Cbl-b-null CD8+ T cells have shown modestly enhanced primary expansion in other models, including peptide immunization [15] and LCMV infection [12], whereas primary expansion was similar to WT CD8+ T cells in two other LCMV studies [25, 30]. In another study, when CD4+ Cbl-b-null T cells were stimulated by peptide in vivo, they proliferated at about the same rate as WT cells, but continued proliferating for an extra day, resulting in approximately one extra cell division [40]. Thus, Cbl-b-null T cells appear to have only a modest proliferative advantage over WT T cells in vivo. In the present study, despite undergoing a two-fold difference in overall expansion, co-transferred Cbl-b-null and WT OT-I cells showed a nearly identical proliferative index (as measured by Ki67 staining) in blood, lymph node, and tumor on days 5 and 7 (Fig. 2c, d). There are several possible explanations for this apparent discrepancy. First, a two-fold difference in expansion reflects a difference of only one cell division between Cbl-b-null and WT OT-I cells, which may be difficult to detect by Ki67 staining. Second, Cbl-b-null OT-I cells may have had a higher proliferative index at a time point earlier than day 5. Third, WT and Cbl-b-null OT-I cells may have a similar proliferative rate but different apoptotic rates. While a formal possibility, it is noteworthy that studies in an LCMV model showed similar Annexin V expression by WT and Cbl-b-null CD8+ T cells [30]. Finally, WT OT-I cells might have preferentially trafficked to tissue compartments other than those analyzed (lymph node, blood, and tumor), therefore seeming to be less abundant than Cbl-b-null OT-I cells. Irrespective of the underlying mechanism, the modest difference in cell number between WT and Cbl-b-null T cells suggests that other mechanisms likely contribute to the enhanced efficacy of Cbl-b–null OT-I cells.
Compared to WT OT-I cells, Cbl-b-null cells displayed dramatically enhanced infiltration of the epithelial regions of NOP21 and NOP6 tumors (Fig. 4; Table 1). We suspect that Cbl-b-null OT-I cells also infiltrated NOP23 tumors more extensively, given the striking tumor regression that occurred by day 7. Improved tumor infiltration by adoptively transferred Cbl-b-null cells has also been observed in E.G7 lymphomas [4], and might be attributable to the increased adhesion of Cbl-b-null T cells to ICAM-1 [41]. Similarly, Cbl-b-null macrophages show increased activation of VAV1 and LFA-1, which results in enhanced adhesion to endothelial cells and greater tissue infiltration in models of diabetes and peritonitis [6, 13, 35]. Importantly, however, CBL-B deficiency is not sufficient to overcome all infiltration barriers, as OT-I cells remained restricted to stromal regions in NOP18 tumors, unable to penetrate the malignant epithelium. We are currently investigating the infiltration barrier that distinguishes NOP18 tumors and leads to this exceptionally immune-resistant phenotype.
Adoptive transfer of either Cbl-b-null or WT OT-I cells induced marked infiltration of tumors by FOXP3+ cells (Table 1). We analyzed a subset of tumors and found that FOXP3+ cells were exclusively of host origin, as manifested by expression of host congenic markers (data not shown). In the case of NOP23 and NOP6 tumors, the FOXP3+ cell infiltrate was less dense after adoptive transfer of Cbl-b-null OT-I cells compared to WT OT-I cells, suggesting a more favorable effector:Treg ratio in the former case. In NOP23 tumors, this difference appeared to arise from a reduced absolute number of FOXP3+ cells in the tumor bed of animals treated with Cbl-b-null OT-I cells (Table 1). Recruitment of FOXP3+ cells to sites of infection, inflammation, or malignancy is mediated largely by chemokines [34]. Furthermore, conventional CD4+ T cells can be converted to a FOXP3+ regulatory phenotype by factors such as TGF-β [3]. One can speculate that such factors may be less abundant in the inflammatory milieu created by Cbl-b-null OT-I cells compared to WT cells. One can further speculate that the reduced proportion of FOXP3+ cells could contribute to the increased efficacy of Cbl-b-null OT-I cells. This effect may be further enhanced by the intrinsic resistance of Cbl-b-null T cells to suppression by FOXP3+ T cells [21, 36] and the immunosuppressive cytokine TGF-β [4, 21, 36, 37].
A major implication of this study is that Cbl-b-null T cells, while showing enhanced efficacy against some tumors, are unable to mediate curative responses against all tumor phenotypes, especially those with strong infiltration barriers as exemplified by NOP18. When considering clinical translation of this strategy, one must consider whether the potential advantages of using Cbl-b-null T cells outweigh the technical challenges and potential risks associated with the genetic modification of T cells. Our results show that the enhanced efficacy of Cbl-b-null T cells is associated with relatively modest increases in cell proliferation and tumor infiltration and decreased regulatory T cell recruitment. Equivalent results could potentially be achieved by combining adoptive immunotherapy with standard treatments. For example, in human breast and ovarian cancer, taxane-based chemotherapy can enhance tumor infiltration by CD3+ and CD8+ T cells to a similar or greater extent as seen in the present study [7, 18, 22]. We are currently evaluating whether chemotherapy can enhance the anti-tumor activity of OT-I cells to a similar extent as seen with Cbl-b deficiency, thereby providing a clinically feasible alternative to T cell engineering.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Mammary tumors show reprodu ible responses to adoptively transferred OT-I and OT-II cells. Tumor size before and after adoptive transfer of 15 × 106 splenocytes from each of OT-I and OT-II transgenic mice into mice bearing (a) NOP21, (b) NOP23, (c) NOP6, or (d) NOP18 tumors. (EPS 22 kb)
Supplementary Figure 2. Antigen expression and presentation by the mammary tumor cell lines NOP21, NOP23, NOP6, and NOP18. Each tumor cell line was grown in the presence (heavy line) or absence (thin line) of 100 units/mL IFN-γ for 48 hours and analyzed by flow cytometry for expression of (a) Neu, (b) MHC class I, or (c) the SIINFEKL epitope from OVA in the context of MHC class I. Shaded histograms represent cells stained with secondary antibody alone. (EPS 22 kb)
Acknowledgments
This study was supported by grants from the US Department of Defense (BC990655), US National Cancer Institute (CA845359), Canadian Institutes for Health Research (MOP-173868), Canadian Breast Cancer Foundation, Michael Smith Foundation for Health Research, and British Columbia Cancer Foundation.
Footnotes
M. L. Martin and J. S. Nielsen have contributed equally to this study.
References
- 1.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 2.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25- naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang JY, Jang IK, Hodes R, Gu H. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J Clin Invest. 2007;117:1029–1036. doi: 10.1172/JCI29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 6.Choi EY, Orlova VV, Fagerholm SC, Nurmi SM, Zhang L, Ballantyne CM, Gahmberg CG, Chavakis T. Regulation of LFA-1-dependent inflammatory cell recruitment by Cbl-b and 14–3-3 proteins. Blood. 2008;111:3607–3614. doi: 10.1182/blood-2007-07-103077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang D, Liu YC. Proteolysis-independent regulation of PI3 K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 11.Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem. 2001;276:4872–4878. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- 12.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, Gallimore A, Gascoigne NR, Ohashi PS. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 13.Hirasaka K, Kohno S, Goto J, Furochi H, Mawatari K, Harada N, Hosaka T, Nakaya Y, Ishidoh K, Obata T, Ebina Y, Gu H, Takeda S, Kishi K, Nikawa T. Deficiency of Cbl-b gene enhances infiltration and activation of macrophages in adipose tissue and causes peripheral insulin resistance in mice. Diabetes. 2007;56:2511–2522. doi: 10.2337/db06-1768. [DOI] [PubMed] [Google Scholar]
- 14.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 15.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Kawaoka T, Oka M, Takashima M, Ueno T, Yamamoto K, Yahara N, Yoshino S, Hazama S. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20:155–163. [PubMed] [Google Scholar]
- 17.Krawczyk C, Bachmaier K, Sasaki T, Jones RG, Snapper SB, Bouchard D, Kozieradzki I, Ohashi PS, Alt FW, Penninger JM. Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity. 2000;13:463–473. doi: 10.1016/S1074-7613(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 18.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Gal I, Vermes C, Alegre ML, Chong AS, Chen L, Shao Q, Adarichev V, Xu X, Koreny T, Mikecz K, Finnegan A, Glant TT, Zhang J. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J Immunol. 2004;173:7135–7139. doi: 10.4049/jimmunol.173.12.7135. [DOI] [PubMed] [Google Scholar]
- 20.Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 21.Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, Beissert S, Melief CJ, Penninger JM. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med. 2007;204:879–891. doi: 10.1084/jem.20061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne K, Barnes RO, Girardin A, Mawer MA, Nesslinger NJ, Ng A, Nielsen JS, Sahota R, Tran E, Webb JR, Wong MQ, Wick DA, Wray A, McMurtrie E, Kobel M, Kalloger SE, Gilks CB, Watson PH, Nelson BH. Tumor-infiltrating T cells correlate with NY-ESO-1-specific autoantibodies in ovarian cancer. PLoS ONE. 2008;3:e3409. doi: 10.1371/journal.pone.0003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, Langdon WY, Bowtell DD. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 25.Ou R, Zhang M, Huang L, Moskophidis D. Control of virus-specific CD8+ T-cell exhaustion and immune-mediated pathology by E3 ubiquitin ligase Cbl-b during chronic viral infection. J Virol. 2008;82:3353–3368. doi: 10.1128/JVI.01350-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintarelli C, Dotti G, De Angelis B, Hoyos V, Mims M, Luciano L, Heslop HE, Rooney CM, Pane F, Savoldo B. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112:1876–1885. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamim M, Nanjappa SG, Singh A, Plisch EH, LeBlanc SE, Walent J, Svaren J, Seroogy C, Suresh M. Cbl-b regulates antigen-induced TCR down-regulation and IFN-gamma production by effector CD8 T cells without affecting functional avidity. J Immunol. 2007;179:7233–7243. doi: 10.4049/jimmunol.179.11.7233. [DOI] [PubMed] [Google Scholar]
- 31.Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ, Jr, Rosenberg SA, Hodes RJ. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall EM, Milne K, Martin ML, Watson PH, Theiss P, Nelson BH. Spontaneous mammary tumors differ widely in their inherent sensitivity to adoptively transferred T cells. Cancer Res. 2007;67:6442–6450. doi: 10.1158/0008-5472.CAN-07-0622. [DOI] [PubMed] [Google Scholar]
- 33.Wang HY, Altman Y, Fang D, Elly C, Dai Y, Shao Y, Liu YC. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J Biol Chem. 2001;276:26004–26011. doi: 10.1074/jbc.M010738200. [DOI] [PubMed] [Google Scholar]
- 34.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells CM, Bhavsar PJ, Evans IR, Vigorito E, Turner M, Tybulewicz V, Ridley AJ. Vav1 and Vav2 play different roles in macrophage migration and cytoskeletal organization. Exp Cell Res. 2005;310:303–310. doi: 10.1016/j.yexcr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+ CD25+ regulatory T cells and TGF-beta in Cbl-b-/- mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 37.Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 38.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, Zhang N, Mueller DL. Casitas B-lineage lymphoma b inhibits antigen recognition and slows cell cycle progression at late times during CD4+ T cell clonal expansion. J Immunol. 2008;181:5331–5339. doi: 10.4049/jimmunol.181.8.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Shao Y, Fang D, Huang J, Jeon MS, Liu YC. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. J Biol Chem. 2003;278:23978–23983. doi: 10.1074/jbc.M212671200. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Mammary tumors show reprodu ible responses to adoptively transferred OT-I and OT-II cells. Tumor size before and after adoptive transfer of 15 × 106 splenocytes from each of OT-I and OT-II transgenic mice into mice bearing (a) NOP21, (b) NOP23, (c) NOP6, or (d) NOP18 tumors. (EPS 22 kb)
Supplementary Figure 2. Antigen expression and presentation by the mammary tumor cell lines NOP21, NOP23, NOP6, and NOP18. Each tumor cell line was grown in the presence (heavy line) or absence (thin line) of 100 units/mL IFN-γ for 48 hours and analyzed by flow cytometry for expression of (a) Neu, (b) MHC class I, or (c) the SIINFEKL epitope from OVA in the context of MHC class I. Shaded histograms represent cells stained with secondary antibody alone. (EPS 22 kb)