Abstract
IL-21, and to a lesser extent IL-15, inhibits differentiation of antigen-primed CD8 T cells and promotes their homeostasis and anti-tumour activity. Here, we investigated molecular mechanisms behind tumour-specific responses of primary murine T lymphocytes engineered to express a TCR directed against human gp100/HLA-A2 following short-term exposure to IL-15 and/or IL-21. We demonstrated that IL-15 + IL-21, and to a lesser extent IL-21, enhanced antigen-specific T-cell cytotoxicity, which was related to enhanced expression of granzymes A and B, and perforin 1. Furthermore, IL-15 + IL-21 synergistically enhanced release levels and kinetics of T-cell IFNγ and IL-2, but not IL-10. Enhanced secretion of IFNγ was accompanied by increased gene expression and cytosolic protein content, and was restricted to effector memory T cells. To summarize, we show that IL-15 + IL-21 improves antigen-specific responses of TCR-transduced effector T cells at multiple levels, which provides a rationale to treat T cells with a combination of these cytokines prior to their use in adoptive TCR gene therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0818-0) contains supplementary material, which is available to authorized users.
Keywords: IL-15, IL-21, Cytokines, T lymphocyte, Cytotoxicity
Introduction
TCR gene transfer represents an attractive therapeutic strategy to provide patients with tumour-specific T-cell immunity [1–5]. The clinical feasibility of this experimental therapy has recently been demonstrated in trials with TCR-gene modified T cells to treat melanoma patients who were pre-conditioned with lympho-ablative chemotherapy [6, 7]. Objective response rates in these trials ranged from 12% to 30%, and although impressive, still lagged behind the 50% objective response rates observed in earlier trials with non-gene modified T cells [8, 9].
One way to enhance the efficacy of TCR gene therapy, other than choosing high-affinity or genetically improved TCR transgenes [6, 10], is to expose T cells to common-γ cytokines other than interleukin-2 (IL-2) prior to their adoptive transfer. Notably, antigen priming of CD8 + T cells in the presence of IL-21, and to a lesser extent IL-15, suppresses differentiation of naive T cells into effector T cells, which in turn enhances in vivo persistence of T cells and antigen-specific effectiveness [11, 12]. In fact, clinical anti-tumour efficacy of T cells irrespective of gene-modification appears to be directly related to T-cell persistence [13–15], which in turn is reported to be associated with differentiation state and replicative history of transferred T cells [16].
IL-2, IL-15 and IL-21 all act as co-mitogens for antigen-activated effector CD8 T cells [17, 18]. In contrast to IL-2, however, IL-15 enhances survival of CD8 effector T cells [19] and IL-21 does not induce proliferation of regulatory T cells [20]. Next to their effects on CD8 T-cell homeostasis, IL-15 and IL-21 are able to enhance the in vivo anti-tumour effects of CD8 T cells [12, 21–25]. For example, adoptively transferred T cells resulted in potentiated tumour regression when pre-treated with IL-15 or IL-21 [12, 21, 23]. It is of interest that IL-21 turns out to be superior to IL-15 in inducing anti-tumour responses and achieving long-term tumour control by endogenous as well as adoptively transferred CD8 T cells [12, 18]. Moreover, combined in vivo treatment with IL-15 and IL-21 enhanced CD8 T-cell expansion and anti-melanoma responses relative to the administration of either cytokine separately [26].
Here, we investigated molecular mechanisms behind functional responses of primary murine T lymphocytes engineered to express a TCR directed against human gp100/HLA-A2 and treated with IL-15 and/or IL-21. Unexpectedly, we observed that a combination of IL-15 and IL-21 promotes antigen-specific T-cell responses at multiple levels. First, IL-15 + IL-21 enhanced T-cell cytotoxicity in response to hgp100-positive tumour cells, which paralleled up-regulated expression of granzymes A, B and perforin-1. Second, these cytokines showed a synergistic effect in enhancing levels and kinetics of secreted IFNγ and IL-2, but not IL-10, in an antigen-specific manner. The enhanced IFNγ protein production, the most significant response noted upon co-treatment with IL-15 and IL-21, was due to enhanced IFNγ mRNA levels and was restricted to the subset of CD62L−/CD44+ effector memory CD8 T cells.
Results
IL-15 decreases the number of TCR transgene-positive T cells, whereas IL-21 increases the expression levels of TCR transgenes per cell
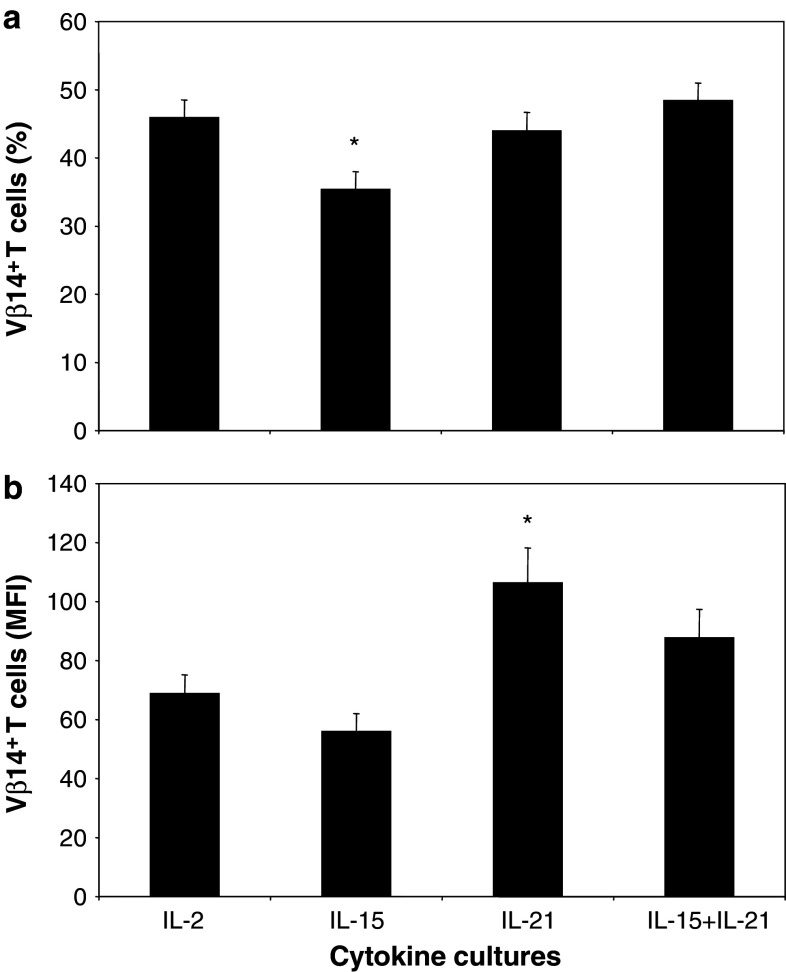
Primary murine T cells were retrovirally transduced with TCRαβ directed against human gp100280–288/HLA-A2 (gp100/A2) and subsequently treated either with IL-2, IL-15, IL-21 or the combination of IL-15 and IL-21 (i.e., IL-15 + IL-21). Analysis of TCR-Vβ14 transgene expression revealed that differences among T-cell cultures were small, yet significant when analysing large series of TCR transductants (n = 18). Percentages of TCR-Vβ14 + T cells following exposure to IL-15 were decreased compared to T-cell cultures exposed to IL-2 (Fig. 1a: 46, 35, 44 and 48% TCR-Vβ14 + cells for IL-2, IL-15, IL-21 and IL-15 + IL-21 T-cell cultures, respectively, IL-15 vs. IL-2 p < 0.05). Although percentages of TCR-Vβ14 + T cells in IL-21 T-cell cultures did not differ from those in IL-2 T-cell cultures, the mean fluorescence intensity (MFI) of TCR-Vβ14 + T cells was increased in IL-21 T-cell cultures relative to that of IL-2 T-cell cultures (Fig. 1b: MFI of 69, 56, 106 and 88 for IL-2, IL-15, IL-21 and IL15 + IL-21 T-cell cultures, respectively, IL-21 vs. IL-2 p < 0.05). Percentages as well as MFI of TCR-Vβ14 + T cells following exposure to IL-15 + IL-21 were not significantly different from IL-2 T-cell cultures. Mock-transduced T cells consisted of <5% TCR-Vβ14 + T cells (data not shown).
Fig. 1.
IL-15 decreases the number of TCR transgene-positive T cells, whereas IL-21 increases the expression levels of TCR transgenes per cell. Primary murine T cells were retrovirally transduced with TCRαβ directed against human gp100280–288/HLA-A2 (gp100/A2) and subsequently treated either with IL-2 (100 U/ml), IL-15 (50 ng/ml), IL-21 (50 ng/ml) or IL-15 + IL-21 (50 ng/ml each). T cells were analysed by flow cytometry for expression of TCR-Vβ14 within the FSC/SSC lymphocyte gate. Mock-transduced T cells were used as negative controls and showed <5% TCR-Vβ14 staining (data not shown). Transduction efficiencies at day 5 after start of culture (i.e., 3 days after addition of various cytokines) are indicated as percentages (a) or mean fluorescence intensities (MFI) (b) of T cells expressing TCR-Vβ14 (mean ± SEM, n = 18, *p < 0.01 compared to IL-2)
T cells treated with a combination of IL-15 and IL-21 show enhanced antigen-specific cytotoxicity
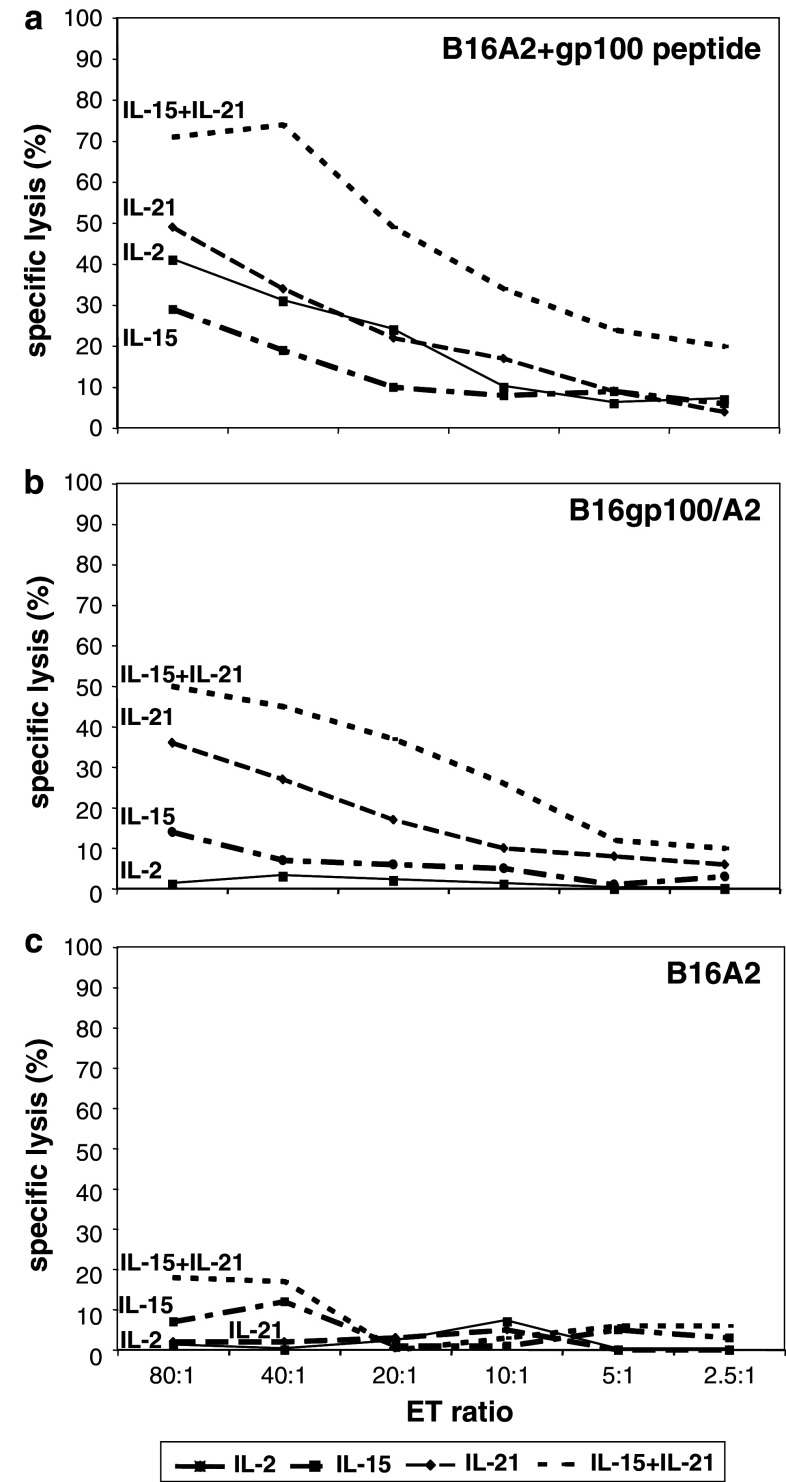
Chromium release assays demonstrated that T cells were able to recognize human gp100280–288 peptide-loaded B16 melanoma target cells expressing human HLA-A2 (B16A2), irrespective of the cytokine added to the T-cell cultures (Fig. 2a). B16gp100/A2 cells, which present endogenously processed human gp100 in the context of HLA-A2 were not, or only to a low extent, killed by T cells that had been cultured in the presence of IL-2 or IL-15 (Fig. 2b). However, B16gp100/A2 cells were clearly lysed by IL-21 cultured T cells, an effect that was even more pronounced when T cells were cultured in the presence of IL-15 + IL-21 (Fig. 2b). Cytotoxic responses towards peptide loaded B16A2 and B16gp100/A2 cells were qualitatively similar in that IL-15 + IL-21 cultured T cells showed the highest cytotoxicity, IL-2 cultured T cells the lowest cytotoxicity and IL-15 or IL-21 cultured T cells an intermediate cytotoxicity. Mock-transduced T cells showed no background activity, except for some background activity (up to 20% at E:T > 40:1) of IL-15 + IL-21 T cells towards B16gp100/A2 (not towards B16A2 with or without gp100 peptide).
Fig. 2.
T cells treated with a combination of IL-15 and IL-21 show enhanced antigen-specific cytotoxicity. Murine splenocytes were gp100/A2 TCR-transduced and cultured with IL-2, IL-15, IL-21 or IL-15 + IL-21 as described in legend to Fig. 1. Cytokine-stimulated T cells were co-cultured for 4 h with 51Cr-labelled target cells. Target cells included B16A2 cells loaded with human gp100 peptide (a), B16gp100/A2 cells (b) or B16A2 cells (c). Effector to target ratios and percentages of specific 51Cr-release are indicated in the figures. Mock-transduced T cells served as negative controls. Only in case of mock-transduced T cells cultured with IL-15 + IL-21 there was some background activity towards B16gp100/A2 (not towards B16A2 with or without gp100 peptide) up to 20% of 51Cr-release at E:T ratio’s of >40:1. At the moment of cytotoxicity assay, 2 weeks after start of culture, TCR-Vβ14 surface expression was around 40% (data not shown). Results are from a representative experiment out of 6 experiments with similar results
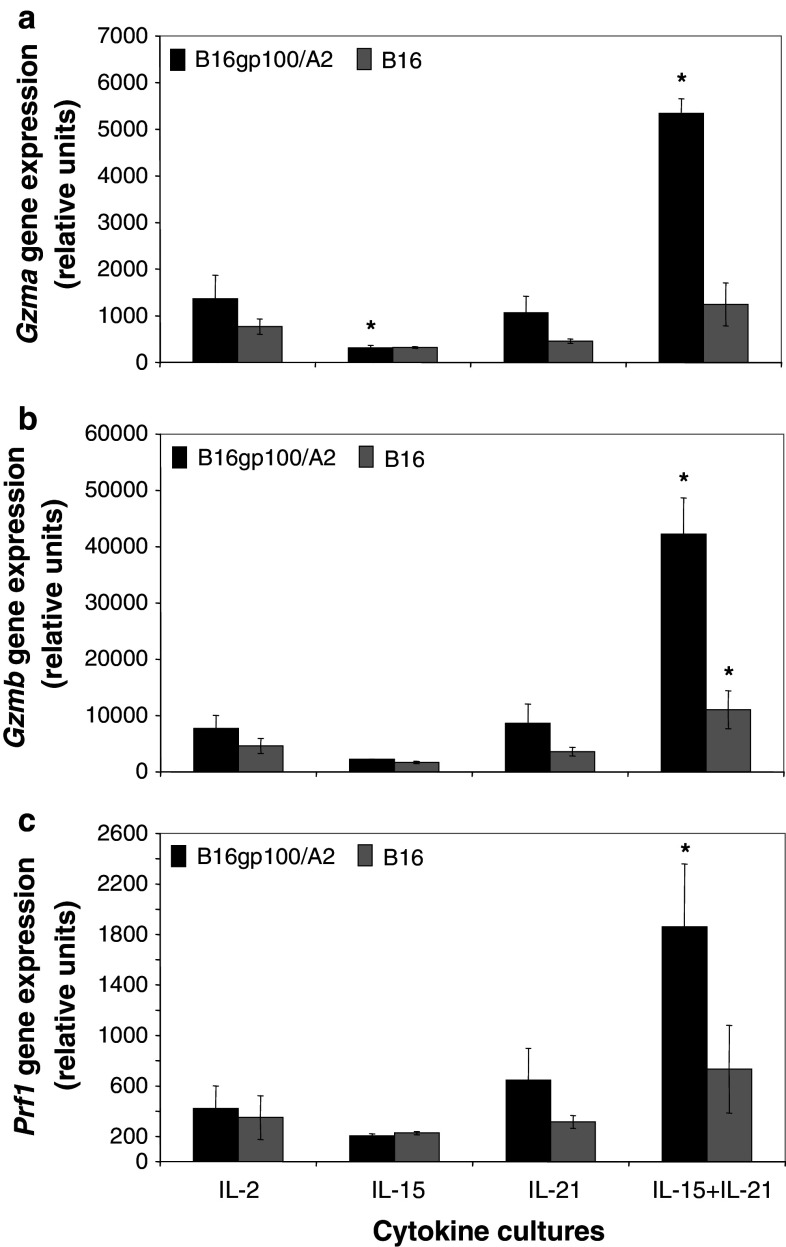
The abilities of T cells to kill tumour cells paralleled gene expression levels of effector molecules. Figure 3 shows that gene expression of granzyme A (Gzma, Fig. 3a), granzyme B (Gzmb, Fig. 3b) and perforin 1 (Prf1, Fig. 3c) were most profoundly increased in IL-15 + IL-21 treated T cells following antigen stimulation (4- to 5-fold increase for Gzma, Gzmb and Prf1, p < 0.05 when compared to IL-2 T cells). Interestingly, IL-15 reduced gene expression of Gzma, but not of the other lytic effector molecules (Fig. 3a; p < 0.05 when compared to IL-2 T cells). Gene expression levels of Gzmb, but not Gzma or Prf1, were slightly but significantly up-regulated in T cells cultured with IL-15 + IL-21 following stimulation with antigen-negative B16 cells (Fig. 3b).
Fig. 3.
A combination of IL-15 and IL-21 enhances antigen-specific Gzma, Gzmb and Prf1 gene expression in T cells. Murine splenocytes were gp100/A2 TCR-transduced and cultured with IL-2, IL-15, IL-21 or IL-15 + IL-21 as described in legend to Fig. 1. Five days after start of culture, T cells were stimulated with B16gp100/A2 or B16 cells for 20 h. Stimulated T cells were analysed by micro-array for gene expression of Granzyme A (Gzma, a), Granzyme B (Gzmb, b) or Perforin 1 (Prf1, c). See “Materials and methods” for details about micro-array procedures. Y-axes show relative gene expression for cytokine cultures compared to a common reference. Two independent experiments (starting from T-cell cultures) gave similar results (mean ± SD, n = 3, *p < 0.05 compared to IL-2)
Antigen-specific secretion of IFNγ is enhanced and shows accelerated kinetics upon combined treatment with IL-15 and IL-21
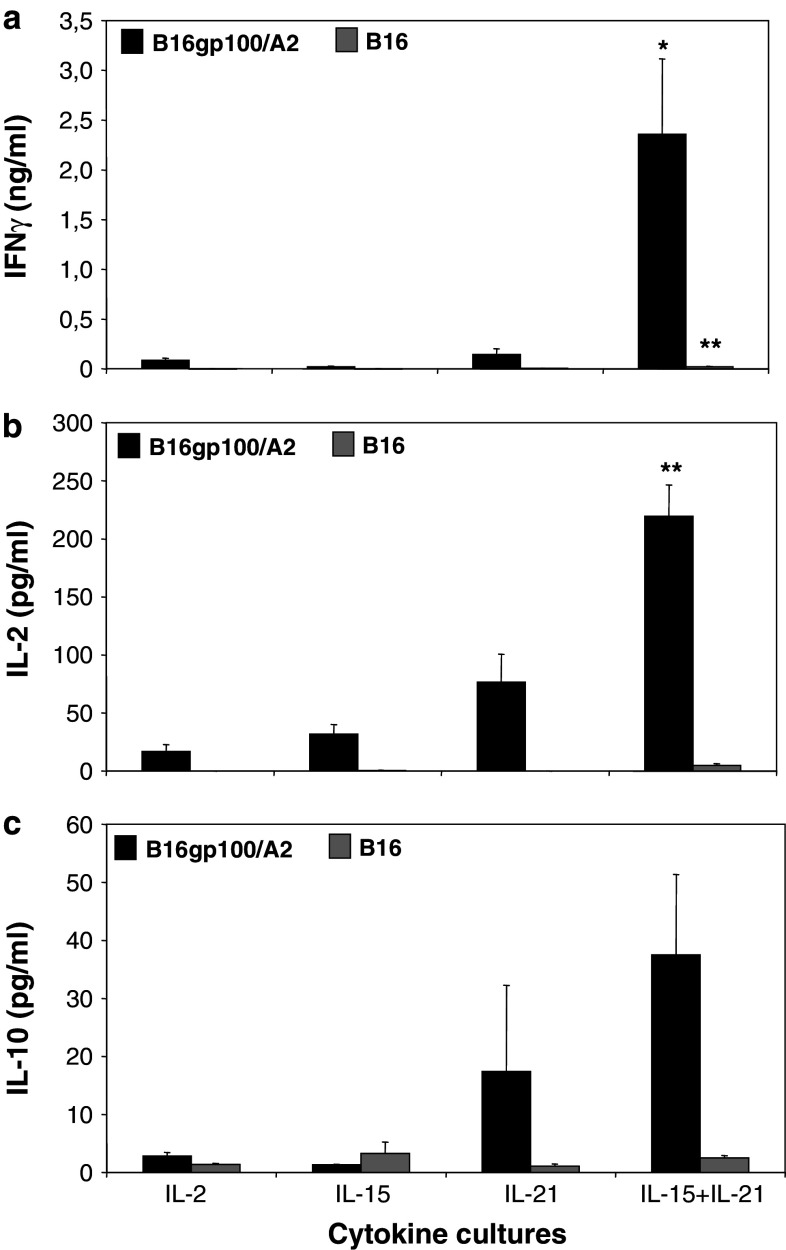
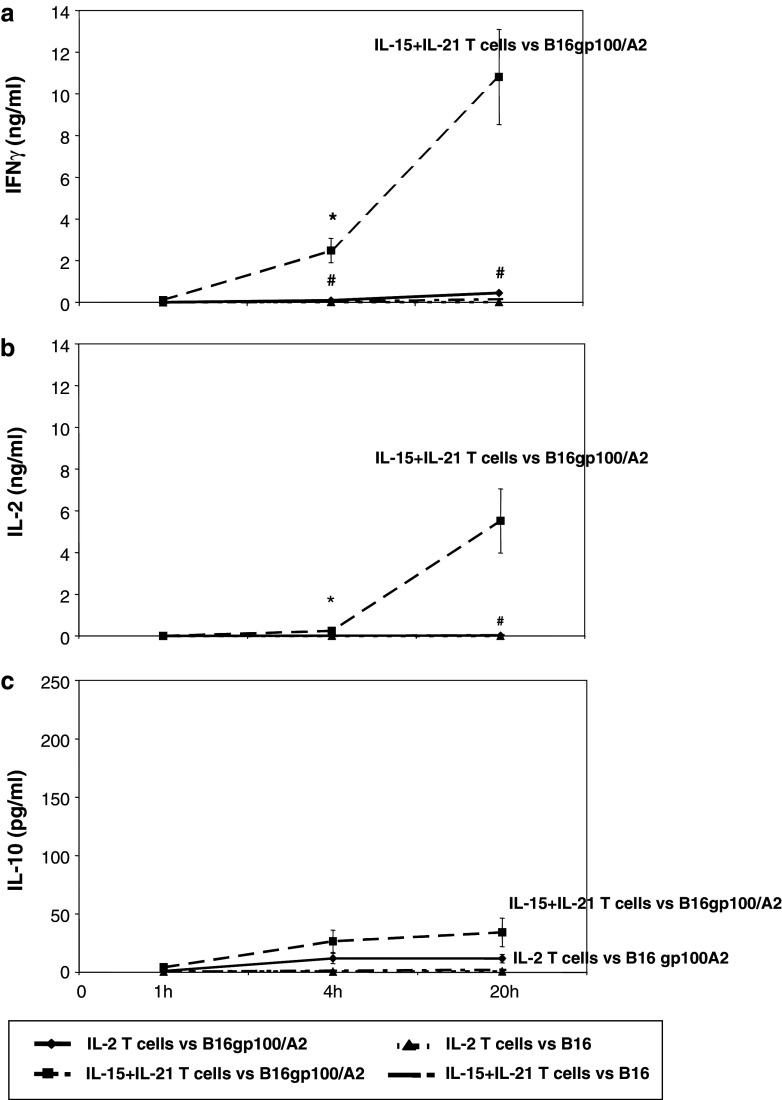
In cytokine release assays, IL-2 and IL-15 T-cell cultures showed low levels of IFNγ (Fig. 4a), IL-2 (Fig. 4b) or IL-10 (Fig. 4c) secretion in response to B16gp100/A2 target cells. IL-21 cultured T cells secreted higher levels of IL-2 and IL-10, but not IFNγ, whereas the highest levels of secretion for all three cytokines were observed for IL-15 + IL-21 cultured T cells (Fig. 4). Secreted levels of IFNγ and IL-2 by IL-15 + IL-21 T cells reached statistical significance when compared to IL-2 T cells. IL-10 levels were generally low and not statistically different among the different cytokine cultures. Notably, IL-15 + IL-21 cultured T cells secreted 27-fold more IFNγ than IL-2 cultured T cells (Fig. 4a: 2,400 and 90 pg/ml, respectively), whereas IL-15 or IL-21 alone hardly induced secretion of IFNγ (Fig. 4a: 22 and 143 pg/ml, respectively). This observed synergy between IL-15 and IL-21 for IFNγ secretion was also evident, although to a lesser extent, for IL-2 and IL-10 secretion, with a 13-fold increase both in IL-2 and in IL-10 secretion for IL-15 + IL-21 T cells compared to IL-2 T cells (Fig. 4b, c, with IL-15 and IL-21 alone resulting in only a 2- to 5-fold increase in IL-2 or IL-10 secretion). Stimulation with antigen-negative B16 cells resulted in low levels of secreted IFNγ and negligible levels of secreted IL-2 or IL-10 for IL-15 + IL-21 T cells (Fig. 4). Mock-transduced T cells showed no cytokine secretion in any condition tested (data not shown).
Fig. 4.
Antigen-specific secretion of IFNγ is enhanced upon combined treatment with IL-15 and IL-21. Murine splenocytes were gp100/A2 TCR-transduced, cultured with cytokines and stimulated with B16 target cells as described in legend to Fig. 3. Stimulated T cells were analysed by commercial ELISA for the secretion of IFNγ (a), IL-2 (b) and IL-10 (c). During the stimulation assay, no exogenous cytokines were added to the T-cell:target cell co-cultures. T cells cultured with medium or Con A served as negative and positive controls, respectively. Mock-transduced T cells showed no cytokine secretion in any condition tested (data not shown). The different T-cell cultures and the levels of cytokines that were present in supernatants are indicated on the X- and Y-axis, respectively (mean ± SEM, n = 4 (IL-2 and IL-10) or n = 10 (IFNγ), *p < 0.05 and **p < 0.005 compared to IL-2)
Next, we investigated the kinetics of antigen-specific cytokine secretion, and observed that IL-15 + IL-21 cultured T cells secreted IFNγ at enhanced levels already at 4 h after stimulation (Fig. 5a, p < 0.05 when compared to IL-2 T cells). Kinetics of IL-2 secretion by IL-15 + IL-21 T cells were similar but somewhat delayed when compared to IFNγ secretion (Fig. 5a, b: 4% of maximum IL-2 level as measured at t = 20 h was secreted after 4 h compared to 23% for IFNγ), whereas kinetics of IL-10 secretion were accelerated when compared to IFNγ or IL-2 (Fig. 5c: 78% of maximum IL-10 level was secreted after 4 h of stimulation).
Fig. 5.
Antigen-specific secretion of IFNγ and IL-2, but not IL-10, shows accelerated kinetics upon combined treatment with IL-15 and IL-21. Murine splenocytes were gp100/A2 TCR-transduced and cultured with cytokines as described in legend to Fig. 1. Cytokine cultured T cells were stimulated with B16gp100/A2 and B16 cells for 1, 4 or 20 h, and analysed by commercial ELISA for the secretion of IFNγ (a), IL-2 (b) or IL-10 (c). Mock-transduced T cells showed no cytokine secretion at any time-point tested (data not shown). Time points following target cell stimulations are indicated at the X-axes. Absolute levels of cytokines present in supernatants are indicated at the Y-axes (mean ± SEM, n = 3, *,# p < 0.05 compared to IL-2 for B16gp100/A2 and B16, respectively)
The combination of IL-15 and IL-21 enhances antigen-specific Ifng gene expression in T cells
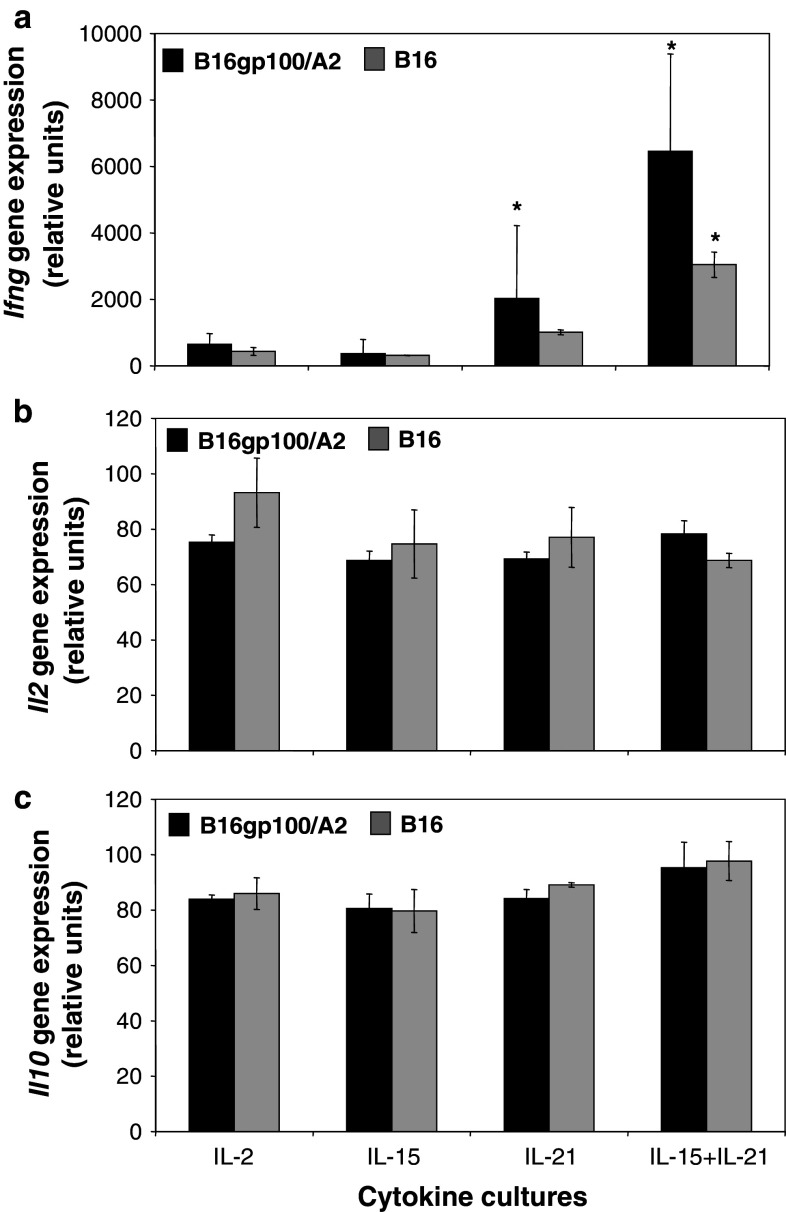
Studying mRNA expression, we observed that IL-2 and IL-15 cultured T cells show low levels of Ifng gene expression in response to B16gp100/A2 target cell stimulation (Fig. 6a). IL-21 cultured T cells expressed significantly higher levels of Ifng (Fig. 6a: 2031 vs. 650 relative gene expression units for IL-21 and IL-2 cultured T cells, respectively, p < 0.05). In line with IFNγ protein levels, highest Ifng gene expression levels were observed for IL-15 + IL-21 T cells (Fig. 6a: 6461 relative gene expression units, p < 0.05 when compared to IL-2). No differences were found in Il2 and Il10 gene expression levels for T cells cultured in the presence of different common-γ cytokines (Fig. 6b, c).
Fig. 6.
The combination of IL-15 and IL-21 enhances antigen-specific Ifng gene expression in T cells. Murine splenocytes were gp100/A2 TCR-transduced, cultured with cytokines as described in legend to Fig. 1. Five days after start of culture, T cells were stimulated with B16gp100/A2 or B16 cells for 20 h. Stimulated T cells were analysed by micro-array for gene expression of IFNγ (a), IL-2 (b) or IL-10 (c). See “Materials and methods” for details about micro-array procedures. Y-axes show relative gene expression for cytokine cultures compared to a common reference. Two independent experiments (starting from T-cell cultures) gave similar results (mean ± SD, *p < 0.05 compared to IL-2)
Effector memory T cells are the major source of enhanced antigen-specific IFNγ protein production upon combined treatment with IL-15 and IL-21
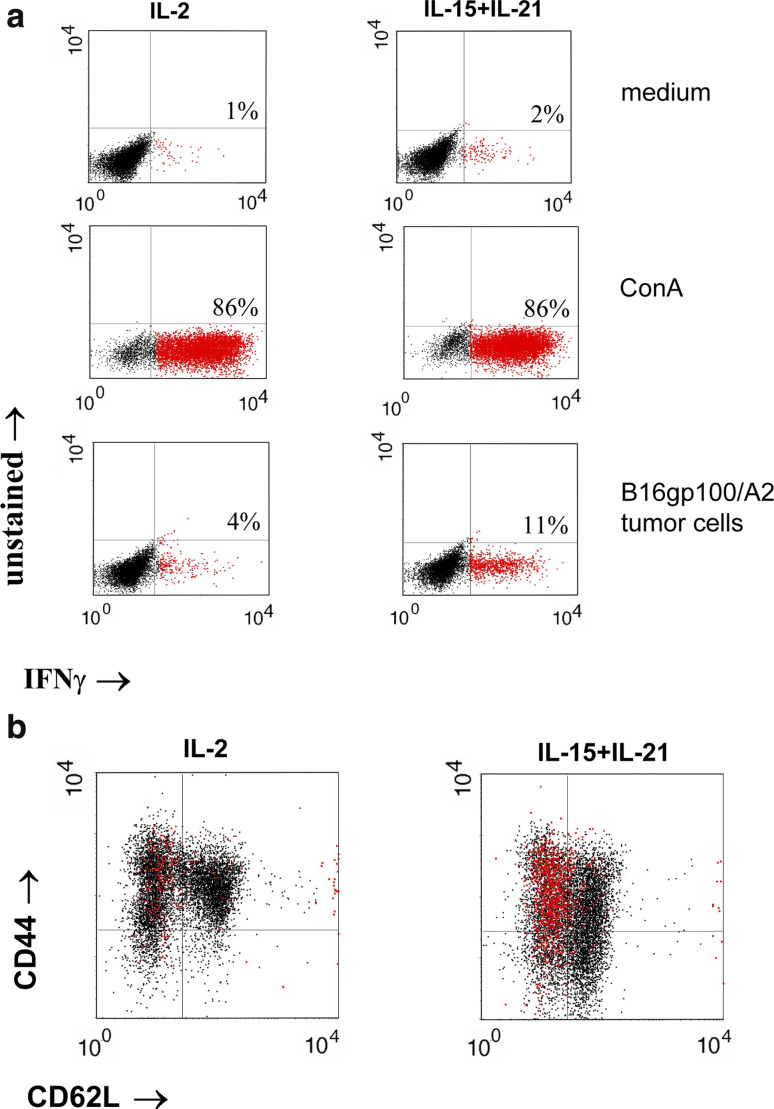
To determine the cell type responsible for IFNγ production, especially relevant in the context of IL-15 and IL-21’s reported inhibitory actions on T-cell differentiation, we performed intracellular cytokine stainings in combination with stainings for T-cell differentiation markers. The number of IFNγ + T cells after stimulation with B16gp100/A2 target cells was enhanced in IL-21 T-cell cultures, although not to a statistically significant extent, and highest in IL-15 + IL-21 T-cell cultures, which corroborated the secretion data (see Supplementary Fig S1a, p < 0.005 when compared to IL-2 T-cell cultures). See Fig. 7a for a representative dot plot example. No significant differences could be found with respect to IL-2 or IL-10 + T cells after stimulation with B16gp100/A2 target cells (see Supplementary Fig S1b and S1c). CD62L/CD44/IFNγ triple stainings revealed that CD62L−/CD44+ effector memory T cells were the major source of antigen-induced IFNγ (Fig. 7b). Antigen-negative B16 stimulation resulted in low numbers of intracellular cytokine-positive T cells (see Supplementary Fig S1), and mock-transduced T cells showed no cytokine production in any condition tested (data not shown).
Fig. 7.
IL-15 + IL-21-induced IFNγ production is restricted to CD62L+/CD44− effector memory T cells. Murine splenocytes were gp100/A2 TCR-transduced and cultured with cytokines as described in legend to Fig. 1. Five days after start of culture, T cells were stimulated with medium, Con A (10 ng/ml) or B16gp100/A2 cells, and analysed by flow cytometry for the expression of IFNγ (a). In (b) IFNγ staining was combined with the T-cell differentiation markers CD62L and CD44. In the latter case, T cells were first stained with anti-CD62L mAbFITC and anti-CD44 mAbAPC, followed by intracellular staining with anti-IFNγ mAbPE. Cells were FSC/SSC gated on viable lymphocytes and percentages of IFNγ+ T cells (in red) were determined. The results for IL-2 and IL-15 + IL-21 cultures are shown. Results are from a representative experiment out of 3 independent experiments with similar results
Discussion
In the present paper we studied molecular mechanisms behind tumour-specific responses of primary murine T cells treated with IL-2, IL-15, IL-21 or a combination of IL-15 and IL-21 in the setting of TCR gene transfer. Combined treatment with IL-15 and IL-21 of primary TCR-engineered T cells resulted in enhanced tumour-specific responsiveness at the following levels. First, cytotoxicity and the expression of lytic effector molecules were increased. Second, levels of secreted IL-2 and IFNγ were enhanced, likely regulated at the post-transcriptional and transcriptional level, respectively. Intriguingly, IL-15 and IL-21 inhibit T-cell differentiation, yet they acted most profoundly on IFNγ production by effector memory T cells.
Analysis of TCR transgene expression in TCR-engineered T cells revealed that differences upon short-term culture with various cytokines were small, yet IL-15 decreased percentages and IL-21 increased MFIs of TCR transgene-positive T cells (Fig. 1). Currently, we cannot rule out whether IL-15 and IL-21 as single cytokines act directly on TCR surface expression or indirectly via their effects on T-cell growth (IL-15 enhances T-cell growth, whereas IL-21 does not support T-cell growth when compared to IL-2, data not shown). Importantly, IL-15 + IL-21, a combination of cytokines with significant consequences for TCR-mediated functions (see below), did not alter transduction efficiencies. This indicates that the effects of IL-15 + IL-21 on T-cell function must be related to T-cell properties rather than TCR surface expression levels. Moreover, IL-15 + IL-21 results in similar T-cell numbers at the day of phenotypical and functional analyses when compared to IL-2 (data not shown). It is of interest to note that the potentially beneficial effect of IL-21 on T-cell differentiation [12], in particular the enrichment of T cells with a CD62L+/CD44− naive T-cell phenotype, is independent of prior exposure to IL-2 (inherent to our transduction protocol) and genetic introduction of TCR transgenes (see Supplementary Fig S2). The same holds true for IL-15 + IL-21 treatment of T cells, which results in an enrichment of CD62L+/CD44− T cells, although to a lesser extent when compared to IL-21, that is not compromised following TCR gene transfer (Fig. 7b, and described in more detail in Pouw et al., manuscript submitted).
T cells cultured in the presence of IL-21, but not IL-2 or IL-15, clearly lysed hgp100/HLA-A2-expressing B16 melanoma cells (Fig. 2). Antigen-specific cytotoxicity observed for IL-21 T-cell cultures is in line with findings by Casey et al. [27] showing that IL-21, in contrast to IL-2, enhances T-cell cytotoxicity. In addition, we noted that IL-21 up-regulated perforin 1 gene expression (Fig. 3c: p < 0.05 B16gp100/A2 when compared to B16), which is in agreement with a report by Ebert et al. [28] demonstrating up-regulated perforin-mediated cytotoxic activity of human intra-epithelial lymphocytes after exposure to IL-21. The enhanced cytotoxicity was even more pronounced when T cells were cultured in the presence of IL-15 + IL-21 (Fig. 2). Enhanced cytotoxic killing of IL-15 + IL-21 T cells coincided with significantly increased gene expression of the effector molecules granzymes A, B and perforin 1 (Fig. 3). Both IL-15 and IL-21 have been shown to up-regulate intracellular granzyme B and perforin expression [12, 28–31]. Zeng et al. [26] demonstrated that granzyme B gene expression was higher in freshly isolated human T cells cultured in IL-15 plus IL-21, than in T cells cultured with either cytokine alone. To our knowledge, our report is the first to demonstrate up-regulated gene expression of three cytotoxic molecules (granzyme A, granzyme B and perforin 1) after antigen-specific stimulation of T cells cultured in IL-15 plus IL-21.
In addition, T cells cultured in the presence of both IL-15 and IL-21 showed the highest levels and accelerated kinetics of secreted IFNγ in response to B16gp100/A2 melanoma cells (Figs. 4a, 5a). The effect of IL-15 + IL-21 on IFNγ secretion is of a synergistic nature and is most likely explained by enhanced Ifng gene expression (Fig. 6a). Interestingly, T cells cultured in the presence of IL-21 only, also showed increased Ifng gene expression, yet the gene expression level appeared to have been insufficient to result in significantly enhanced IFNγ protein production or release (see Supplementary Fig S1a and Fig. 4a, respectively). Since IL-15 and IL-21 affect T-cell differentiation, we studied the effect of IL-15 + IL-21 on IFNγ production via triple flow cytometry stainings, and identified CD62L−/CD44+ effector memory T cells as the major source for IFNγ (Fig. 7b). Currently, we cannot exclude the contribution of NK and NKT cells towards the observed IFNγ production. In fact, IL-15 increased percentages of NK1.1 cells and CD8 T cells and decreased those of CD4 T and CD19 B cells, whereas IL-21 minimally but significantly decreased percentages of CD3 T cells (see Supplementary Fig S3, and in agreement with [32–37]). The IL-15-induced increase in NK1.1+ cells was found both in the CD3− and in the CD3+ subsets, indicating an increase both in NK and in NKT cells. Notably, both these cell types express CD44, and IL-15 has been shown to up-regulate expression and activation of CD44 on NK cells, which in turn regulates the expression of IFNγ [38].
With respect to IL-2 and IL-10 production, the IL-21-induced IL-2 secretion that we observed is in line with previous reports related to IL-21’s suppressive effect on T-cell differentiation [11, 12, 39]. IL-15 + IL-21 also increased levels and accelerated kinetics of T-cell secreted IL-2, but not IL-10, following tumour cell stimulation (Figs. 4, 5). Enhanced antigen-specific IL-2 secretion did not correlate with increased levels of IL-2 mRNA and cytosolic protein, suggesting that enhanced secretion was not caused by enhanced gene transcription. Antigen-specific IL-10 gene expression, intracellular protein and secretion were not differentially regulated in the different cytokine cultures (Figs. 4, 5, 6).
IL-15 and IL-21, when combined, resulted in predominantly CD8 T cells (see Supplementary Fig S3) with potent T-cell effector functions. The cooperative effects of IL-15 and IL-21 with respect to cytotoxicity, IFNγ and IL-2 secretion may be explained as follows. First and with respect to cytokine production, activation of distinct downstream mediators of IL-15 and IL-21 may amplify production of IL-2 and IFNγ. For example, IL-21 signals predominantly through Jak3, STAT1 and STAT3, whereas IL-15 signals predominantly through STAT5 and ERK1/2 [40]. In fact, regulatory sites in the IFNγ promoter are responsive to both STAT- and ERK-dependent transcription factors [41, 42]. Second and with respect to tumour cell killing, expression of perforin in memory CD8 + T cells may be induced by IL-21, whereas degranulation of CD8 + T cells and release of perforin is triggered by IL-15 (Fig. 3c, and reference [31]).
We postulate that enhanced IL-2 production of T cells cultured with IL-15 + IL-21 may support in vivo T-cell expansion, and that enhanced IFNγ production and cytotoxicity may contribute to in vivo anti-melanoma activities. This would provide a molecular basis to explain the findings by Zeng et al. [26], who reported enhanced CD8 + T-cell expansion and anti-melanoma responses upon in vivo administration of both IL-15 and IL-21. Although differences between murine and human IL-15 and IL-21 cannot be excluded, we argue that our findings may be well translated to the human setting. First, the combination of IL-15 and IL-21 has been reported to improve the proliferation of human effector T cells [44], which may be of importance to adoptive T-cell therapy trials. In fact, this combination of cytokines may promote proliferation of human T cells better than murine T cells since we observed no beneficial effect of IL-15 + IL-21 on murine T-cell growth when compared to IL-2 (data not shown). Second, IL-15 combined with IL-21 reverses IL-15-induced down-regulation of CD28 and results in enhanced IL-2 and IFNγ production upon TCR and CD28 triggering [43]. Moreover, a combination of IL-15 and IL-21 has recently been shown to enhance cytotoxicity and IFNγ production by PBMC of melanoma patients [44], which is in strict accordance with our findings using mouse TCR-engineered T cells.
Taken together, we conclude that a combination of IL-15 and IL-21 provides T cells with two distinct properties, both having a potential advantage in clinical T-cell therapy. On the one hand, the combination of IL-15 and IL-21 results in enrichment of less differentiated T cells [11, 12], which may improve persistence and anti-tumour activities of adoptively transferred T cells [13, 15, 16, 45]. And on the other hand, this combination of cytokines results in functional potentiation of effector T cells, which enables T cells to directly act against a tumour. Our findings argue for the combined ex vivo treatment of TCR-engineered T cells prior to adoptive therapy with IL-15 and IL-21.
Materials and methods
Cells and reagents
The packaging cell lines 293T and Phoenix-amp, and the melanoma cell lines B16 wild-type (B16), B16 transfected with AAD DNA (human HLA-A2 containing the α3 loop of murine H2-Kd, termed B16A2) and B16 transfected with HLA-A2 and hgp100 DNA’s (B16gp100/A2) (kindly provided by dr. G. Adema, Nijmegen, The Netherlands) were cultured as described elsewhere [46]. B16A2 was grown under selection of 1 mg/ml G418, and B16gp100/A2 was grown under selection of 1 mg/ml G418 and 0.5 mg/ml Hygromycin B. Monoclonal Abs used in this study were: PerCP-conjugated anti-CD3ε (145-2C11), FITC-conjugated anti-CD4 (RM4-5), Allophycocyanin (APC)-conjugated anti-CD8α (53-6.7), APC-conjugated anti-CD11c (HL3), PE-conjugated anti-CD19 (1D3), PE-conjugated anti-NK1.1 (PK136), PE- or biotin-conjugated anti-CD27 (LG.3A10), APC-conjugated anti-CD44 (IM7), FITC- or PE-conjugated anti-CD62L (MEL-14), PE-conjugated anti-IFNγ (XMG1.2), APC-conjugated anti-IL-2 (JES6-5H4), APC-conjugated anti-IL-10 (JES5-16E3) (all from BD Biosciences, Breda, the Netherlands), PE- or APC-conjugated anti-CCR7 (4B12, eBiosciences, San Diego, CA), and PE-conjugated anti-human TCR-Vβ14 (CAS1.1.3, Beckman Coulter, Marseille, France). Other reagents used in this study were human gp100 wild-type peptide (YLEPGPVTA) (4), Streptavidin (SaV)-FITC conjugate (BD Biosciences), Concanavalin A (Con A, Sigma, St. Louis, MS), human rIL-2 (Proleukin, Chiron, Amsterdam, The Netherlands), human rIL-15 (Peprotech, Rocky Hill, NY) and murine rIL-21 (R&D Systems).
TCR gene transfer and cytokine stimulation
Single cell suspensions of C57BL/6 mouse spleens (Erasmus MC animal housing facility, Rotterdam, The Netherlands) were obtained in compliance with a national animal license. T cells were genetically modified with TCRαβ genes prior to cytokine exposure as described elsewhere [42]. In short, T cells were stimulated for 24 h with 2.5 μg/ml Con A in the presence of 100 U/ml IL-2 (which is considered the start of culture, t = day 0). Subsequently, T cells were retrovirally transduced via retronectin-mediated virus supernatant centrifugations with transgenes encoding a human TCRαβ specific for gp100280–288/HLA-A2 (gp100/A2) that had previously been murinized for TCR-Cα and Cβ. Mock-transduced T cells were exposed to the same procedure except for the use of virus-free instead of virus-containing supernatant, and were used as experimental controls for TCR-transduced T cells. Following T-cell activation and gene transfer (at t = day 2), T cells were cultured at 0.15–1 × 106 cells/ml in 24-well plates in medium [46] supplemented with 100 U/ml rIL-2, 50 ng/ml IL-15, 50 ng/ml IL-21 or 50 ng/ml IL-21 + 50 ng/ml IL-15. Concentrations of the different cytokines were pre-determined by titration experiments (ranging from 10–100 ng/ml, data not shown). T-cell exposure times to cytokines are indicated in the various figures. Cytokines were refreshed every 2–3 days.
Cytotoxicity and cytokine secretion
Cytotoxic activity of TCR-transduced T cells was measured in a standard 4–6 h 51Cr-release assay, principally as described [46, 47]. B16F10, B16gp100/A2 and B16A2 cells were used as target cells, and in some experiments B16A2 cells were pulsed with human gp100 peptide (final concentration: 10 μM) for 15 min at 37°C and 5% CO2 prior to co-cultivation with effector T cells. Cytokine secretion in response to the above-mentioned B16 target cells, and Con A and medium as controls, was measured in supernatants from 20 h co-cultivations (6 × 104 T cells and 2 × 104 target cells per well in 96-well plates) by mouse IFNγ ELISA (U-CyTech, Utrecht, The Netherlands), mouse IL-2 ELISA (Endogen, Pierce Biotechnology, Rockford, IL) and mouse IL-10 ELISA (U-CyTech). During target cell stimulations no exogenous cytokines were added to the T-cell cultures.
Flow cytometry and FACSort
Immune monitoring comprised detection of leucocyte markers, T-cell differentiation markers, TCR transgene expression and intracellular cytokines by flow cytometry using a FACSCalibur (BD Biosciences). To analyse surface expression of leucocyte or T-cell markers as well as the introduced human gp100/A2 TCRαβ, 0.1–0.5 × 106 T cells were washed with PBS, incubated for 30 min at 4°C (or 37°C for CCR7 detection) with 10 μl of mAb (or 5 μl of the TCR-Vβ14 mAb) added to a cell pellet, washed again, and fixed with 1% paraformaldehyde prior to flow cytometry. For intracellular detection of IFNγ, IL-2 and IL-10, T cells were stimulated with target cells in the presence of brefeldin A (1 mg/ml) for 20 h and subsequently stained using the Cytofix/Cytoperm kit (BD Biosciences) in 96-well plates according to the manufacturer’s protocol. Medium and Con A (10 μg/ml) were used as negative and positive stimulation controls, respectively. Conjugated and matched isotype control mAbs were used to determine non-specific intracellular stainings. In some experiments, T cells were incubated with CD62L and CD44 mAbs for 30 min at 4°C prior to intracellular IFNγ staining. Flow cytometry analysis was performed using CellQuest Pro software 5.2.1. Data are presented either as absolute numbers of cells present in a fixed volume that were positive for a certain cytokine (see Supplementary Figure S1) or as dot plots of IFNγ-stained cells with quadrants set at IL-2 T cells that were stimulated with medium (Fig. 7).
Micro array analysis
TCR-transduced T cells were cultured in the presence of IL-2, IL-15, IL-21 or IL-15 + IL-21 for 3 days, washed and co-cultured with B16 or B16gp100/A2 cells (2 × 106 T cells and 0.5 × 106 target cells in 2 ml medium) for another 18 h without exogenous cytokines. Cellular RNA was extracted by the Rneasy Mini kit following RNase-Free DNase treatment (Qiagen, Valencia, CA). Concentration was measured on a nanodrop ND-100 (NanoDrop Technologies Inc., Wilmington, DE), and quality was checked (RNA integrity number >7) on an Agilent 2100 BioAnalyzer (Agilent Technologies, Palo Alto, CA). A 1 μg of RNA was labelled with cyanine 3 (test samples) and cyanine 5 (common reference) by the Low NA input Linear Amplification kit (Agilent). A common reference was generated by labelling 1 μg portions of a pool comprising 1 μg of each test RNA, and mixing the labelled portions afterwards. Mouse Genome 4 × 44 K micro arrays were hybridized overnight at 65°C, scanned with a DNA Micro Array Scanner, and analysed with Feature Extraction software 9.5.1 (Agilent). To process data, outlier removed median signals were used in the R 2.5.0 and Bioconductor 2.0 MAANOVA packages. All slides were subjected to a set of quality control checks and, after log2 transformations, data were normalized by a spacial Lowess smoothing procedure and analysed using a two-stage mixed linear model [48, 49]. Analyses were performed using full factorial design with a group-means-parameterized linear model quantifying Array (random), Slide (random) and Batch (random) effects in addition to the reference and the experimental effects. For each analysis the mixed model was re-parameterized with three different contrast matrices to test the hypotheses of interest. For hypothesis testing an Empirical Bayes Fs test was used and its null distribution was estimated from a mixed distribution based on pooling the statistics from a limited number (40) of permutations [50, 51].
Statistical analyses
Statistical analyses of T-cell phenotype and function were performed with two-tailored the Student’s t tests for paired samples. p < 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Authors would like to thank Cindy Vermeulen and Debby Schipper (Laboratory of Experimental and Surgical Oncology, Dept. Surgery, Erasmus MC, Rotterdam, the Netherlands) for providing and preparing spleens from C57BL/6 mice. Zsolt Sebestyen and Andre Bijkerk are thanked for their assistance with flow cytometric stainings and analyses. This study was financed in part by the Dutch Cancer Society (grant number 1999-1865), the European Union Framework Program 6 Adoptive engineered T cell Targeting to Activate Cancer Killing (EUFP6 ATTACK) project (number 018914) and the European Union Framework 6 CANCERIMMUNOTHERAPY (EUFP6 CIT) project (number 518234).
Conflict of interest statement
The authors declare no conflict of interests.
Abbreviations
- Gzm
Granzyme
- MFI
Mean fluorescence intensity
- NKT
Natural killer T cell
- Prf1
Perforin 1
References
- 1.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- 2.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74:8207–8212. doi: 10.1128/JVI.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orentas RJ, Roskopf SJ, Nolan GP, Nishimura MI. Retroviral transduction of a T cell receptor specific for an Epstein-Barr virus-encoded peptide. Clin Immunol. 2001;98:220–228. doi: 10.1006/clim.2000.4977. [DOI] [PubMed] [Google Scholar]
- 4.Schaft N, Willemsen RA, de Vries J, Lankiewicz B, Essers BW, Gratama JW, Figdor CG, Bolhuis RL, Debets R, Adema GJ. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J Immunol. 2003;170:2186–2194. doi: 10.4049/jimmunol.170.4.2186. [DOI] [PubMed] [Google Scholar]
- 5.Willemsen RA, Weijtens ME, Ronteltap C, Eshhar Z, Gratama JW, Chames P, Bolhuis RL. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 2000;7:1369–1377. doi: 10.1038/sj.gt.3301253. [DOI] [PubMed] [Google Scholar]
- 6.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciceri F, Bonini C, Marktel S, Zappone E, Servida P, Bernardi M, Pescarollo A, Bondanza A, Peccatori J, Rossini S, Magnani Z, Salomoni M, Benati C, Ponzoni M, Callegaro L, Corradini P, Bregni M, Traversari C, Bordignon C. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 14.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 18.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175:7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 21.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 23.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sondergaard H, Frederiksen KS, Thygesen P, Galsgaard ED, Skak K, Kristjansen PE, Odum N, Kragh M. Interleukin 21 therapy increases the density of tumor infiltrating CD8+ T cells and inhibits the growth of syngeneic tumors. Cancer Immunol Immunother. 2007;56:1417–1428. doi: 10.1007/s00262-007-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 26.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 28.Ebert EC. Interleukin 21 up-regulates perforin-mediated cytotoxic activity of human intra-epithelial lymphocytes. Immunology. 2009;127:206–215. doi: 10.1111/j.1365-2567.2008.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 30.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, Petri A, Hansen LT, McArthur GA, Davis ID, Skak K. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, Pahwa RN, Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill N, Paltser G, Ashkar AA. Interleukin-15 expression affects homeostasis and function of B cells through NK cell-derived interferon-gamma. Cell Immunol. 2009;258:59–64. doi: 10.1016/j.cellimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima S, Hida S, Taki S. IL-15 inhibits pre-B cell proliferation by selectively expanding Mac-1+B220+ NK cells. Biochem Biophys Res Commun. 2008;369:1139–1143. doi: 10.1016/j.bbrc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 36.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 38.Sague SL, Tato C, Pure E, Hunter CA. The regulation and activation of CD44 by natural killer (NK) cells and its role in the production of IFN-gamma. J Interferon Cytokine Res. 2004;24:301–309. doi: 10.1089/107999004323065093. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 40.de Totero D, Meazza R, Capaia M, Fabbi M, Azzarone B, Balleari E, Gobbi M, Cutrona G, Ferrarini M, Ferrini S. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111:517–524. doi: 10.1182/blood-2007-04-087882. [DOI] [PubMed] [Google Scholar]
- 41.Egerton M, Fitzpatrick DR, Kelso A. Activation of the extracellular signal-regulated kinase pathway is differentially required for TCR-stimulated production of six cytokines in primary T lymphocytes. Int Immunol. 1998;10:223–229. doi: 10.1093/intimm/10.2.223. [DOI] [PubMed] [Google Scholar]
- 42.Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 43.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huarte E, Fisher J, Turk MJ, Mellinger D, Foster C, Wolf B, Meehan KR, Fadul CE, Ernstoff MS. Ex vivo expansion of tumor specific lymphocytes with IL-15 and IL-21 for adoptive immunotherapy in melanoma. Cancer Lett. 2009;285:80–88. doi: 10.1016/j.canlet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 46.Pouw NM, Westerlaken EJ, Willemsen RA, Debets R. Gene transfer of human TCR in primary murine T cells is improved by pseudo-typing with amphotropic and ecotropic envelopes. J Gene Med. 2007;9:561–570. doi: 10.1002/jgm.1047. [DOI] [PubMed] [Google Scholar]
- 47.Weijtens ME, Willemsen RA, Hart EH, Bolhuis RL. A retroviral vector system ‘STITCH’ in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther. 1998;5:1195–1203. doi: 10.1038/sj.gt.3300696. [DOI] [PubMed] [Google Scholar]
- 48.Kerr MK, Martin M, Churchill GA. Analysis of variance for gene expression microarray data. J Comput Biol. 2000;7:819–837. doi: 10.1089/10665270050514954. [DOI] [PubMed] [Google Scholar]
- 49.Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS. Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol. 2001;8:625–637. doi: 10.1089/106652701753307520. [DOI] [PubMed] [Google Scholar]
- 50.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Churchill G. Estimating p-values in small microarray experiments. Bioinformatics. 2007;23:38–43. doi: 10.1093/bioinformatics/btl548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.