Abstract
Purpose: A phase I/II study was conducted to investigate the safety, tolerability and clinical response to vaccination with a combination of telomerase peptides GV1001 (hTERT: 611–626) and HR2822 (hTERT: 540–548) in patients with non-small cell lung cancer. Experimental design: Twenty-six patients with non-small cell lung cancer received intradermal administrations of either 60 nmole (112 μg) or 300 nmole (560 μg) GV1001 in combination with 60 nM (68.4 μg) HR2822 and granulocyte macrophage-colony stimulating factor. The treatment period was 10 weeks. Booster vaccinations with 300 nM GV1001 were offered as follow-up. Monitoring of blood samples, clinical examination and radiological staging were performed regularly. Immune responses were measured as delayed-type hypersensitivity skin reaction and in vitro T cell proliferation. Bone marrow function was monitored in long time survivors. Results: The treatment was well tolerated with minor side effects. No bone marrow toxicities were observed in long time survivors with immune responses. Immune responses against GV1001 were detected in 11 of 24 evaluable patients during the primary regimen and in additional two patients following booster injections. Two patients responded to HR2822. Cloned GV1001-specific CD4+ T cells displayed a Th1 cytokine profile and recognized autologous antigen presenting cells pulsed with recombinant telomerase protein. A complete tumor response was observed in one patient who developed GV1001-specific cytotoxic T cells that could be cloned from peripheral blood. Conclusion: The results demonstrate that GV1001 and HR2822 are immunogenic and safe to use in patients with NSCLC. Induction of GV1001-specific immune responses may result in objective tumor responses. Based on these initial encouraging results, further clinical studies of GV1001 in NSCLC patients are warranted.
Keywords: Clinical trial, hTERT vaccine, Promiscuous Th epitope, NSCLC
Introduction
Lung cancer is the leading cause of cancer death both in men and women in the western world. Non-small cell lung cancer (NSCLC) accounts for 75–80% of all lung cancers. The most common subgroups in North America are adenocarcinoma (35% of patients), squamous (30%) and large cell carcinomas (10–15%) [1]. About 10% are classified as poorly differentiated NSCLC. Patients with advanced NSCLC, i.e. stages IIIB and IV, have a poor prognosis with a median survival time of less than 12 months. Both radiotherapy and chemotherapy have good palliative effects but often of short duration [2]. New treatment options for NSCLC are urgently needed. Although NSCLC is often considered to be minimally- or non-immunogenic and may induce CD4+ regulatory T cells that suppress generation of cytotoxic lymphocytes (CTL) [3], many studies employing immunotherapy are being evaluated for treatment of lung cancer. Induction of immune responses as well as tumor regression in late stage disease has been demonstrated using anti-tumor vaccination, and several strategies are moving into late stage clinical development [4]. Telomerase is an attractive, perhaps universal target for cancer therapy and anti-cancer vaccination [5], and is highly expressed in NSCLC, where it is also used diagnostically [6].
It is well established that T cells of the human immune system can recognize telomerase [7–15]. Since telomerase is also expressed in some normal cells, such as bone marrow stem cells [16] and epithelial cells in colonic crypts [17], safety is consequently a particularly important issue related to the development of telomerase-targeted therapies.
Based on our earlier experience with anti-cancer vaccination with mutant RAS peptides [18], a protocol for telomerase peptide vaccination of patients with NSCLC was developed. Two telomerase peptides were used in the protocol, GV10011 and HR2822. GV1001 is a unique peptide corresponding to a sequence of hTERT derived from its active site. It contains the sequence hTERT (611–626) and is capable of binding to molecules encoded by multiple alleles of all three loci of HLA class II. GV 1001 can also be further processed into CTL epitopes; HR2822 (hTERT: 540–548) is a high-affinity CTL epitope identical to the previously identified HLA-A2-restricted I540 peptide [7]. In a recent clinical trial with this peptide, no serious adverse effects were observed [19]. By the current approach a combined form of CD4+ and CD8+ T cell responses may be elicited. CD4+ T lymphocytes are pivotal in generating optimal activation of CD8+ T cells and in the maintenance of immune memory, and therefore their activation is critical for cancer vaccine efficacy (reviewed in [20]). The primary objectives of the trial were to investigate safety and tolerability, and characterize any immunological response following GV1001 and HR2822 administration. The secondary objective was objective tumor response. We, here, report that T cell immune responses were observed in more than half of the patients after intradermal injection of the telomerase peptide vaccine in combination with GM-CSF. The vaccine was well tolerated and no serious side effects related to vaccination were observed. We also describe in detail data from a single patient exhibiting a specific immunological and clinical response.
Materials and methods
Patient selection
During the time period October 2001–June 2003 a total of 26 eligible patients with NSCLC fulfilling the inclusion criteria (Table 1) were enrolled in the study. Patient characteristics are described in Table 2. Patients that had received radiotherapy had to wait at least six 6 weeks after the last irradiation treatment before vaccination was started. The performance status at baseline was considered good since the majority of the patients scored as Eastern Cooperative Oncology Group (ECOG) grade 1. The Tumor Node Metastasis (TNM) criteria of the American Joint Committee on Cancer (AJCC) were used for staging the patients’ tumors [21]. Twelve patients were included in the low-dose and 14 patients in the high-dose group. Twenty-four patients qualified for immune response evaluation (i.e. completed visit six (week 4) and received at least six vaccinations). Only 14 patients finished the eight vaccination regimen according to protocol (nine and five in the low-dose and high-dose regimen, respectively). Seven patients were withdrawn due to brain metastasis during the 8 week period. Most of the patients included in the present study had stage IIIB and IV tumors and were admitted to our hospital after primary treatment or for inclusion in experimental trials after having progressed on standard chemotherapy.
Table 1.
Criteria for inclusion and exclusion of patients
| Inclusion criteria | Exclusion criteria |
|---|---|
| Histological confirmed diagnosis of non-small cell lung cancer | Chemotherapy or other potentially immunosuppressive therapy administered within 4 weeks prior to the start of treatment |
| Previously treated or untreated patients with advanced non-small cell lung cancer | Clinical signs of brain metastases |
| Measurable or evaluable tumour | Severe cardiac insufficiency (NYHA III or IV) with uncontrolled and/or unstable cardiac or coronary artery disease |
| If previous chemotherapy, 4 weeks must pass before start of vaccination | Severe active infections such as HIV or hepatitis B |
| Age ≥18 and ≤75 years | Require concomitant treatment with a non-permitted medication including: |
| Performance status ECOG-WHO 0.1 and 2 | Medication for severe intercurrent disease which might affect immunocompetence (e.g. immunosuppressants, systemic corticosteroids) |
| Written informed consent | Chronic use of systemic antihistamines or chronic high-dose NSAIDs |
| Adequate bone marrow liver, heart and renal function: | Pregnancy, breast-feeding or absence of adequate contraception for fertile patients |
| WBC count >3.0×109/l and platelets count >100×109/l | Simultaneous participation in other clinical studies |
| ASAT, ALAT <2× upper normal laboratory value | Any reason why, in the opinion of the investigator, the patient should not participate |
| Serum creatinine <2× upper normal laboratory value | Use of alternative/complementary medicines not compatible with the study drug |
Table 2.
Characteristics of included patients and summary of responses and survival for the individual patients
| Treatment, week 1–12 | Follow-up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No | Age | Sex | Previous treatment | Tumor histology | Stage | No. of injects | DTH/T cells | Compl. Study | Disease status w12 | No. of booster injects | DTH T cells | Survival months from start of treatment | |
| Low dose | 701 | 60 | M | Dc/Rad | Nsclc | IIIB | 8 | −/− | Yes | SD | 11 | ||
| 702 | 69 | M | Dc/P | Adeno | IV | 6 | W | No | PD | 4 | |||
| 703 | 68 | F | Rad | Adeno | IV | 8 | +/− | Yes | PD | 15 | |||
| 704 | 60 | F | Opr/Rad | Adeno | IV | 8 | +/+ | Yes | SD | 18 | |||
| 706 | 60 | M | Dc | nsclc | IV | 8 | +/− | Yes | PD | 3 | |||
| 707 | 59 | M | P | adeno | IV | 6 | −/− | No | PD | 2.5 | |||
| 708 | 39 | M | Dc/P | squam | IV | 8 | +/+ | Yes | SD | 7 | |||
| 709 | 63 | F | Dc/P/Rad | squam | IV | 6 | W | No | PD | 3 | |||
| 710 | 55 | M | P/Rad | adeno | IIIA | 8 | −/− | Yes | SD | 13 | −/+ | 46a | |
| 711 | 48 | M | Dc | squam | IV | 8 | +/+ | Yes | PD | 25 | |||
| 712 | 49 | F | Opr/Dc/P | adeno | IV | 8 | +/+ | Yes | SD | 8 | −/+ | 44a | |
| 713 | 76 | F | Rad | squam | IIIB | 8 | +/+ | Yes | PD | 19 | |||
| High dose | 714 | 68 | M | Rad | squam | IV | 8 | +/− | Yes | PD | 32 | ||
| 715 | 45 | F | P/Rad/Dc | adeno | IV | 4 | W | No | PD | 5 | |||
| 716 | 72 | M | Rad | nsclc | IV | 6 | W | No | PD | 1.5 | |||
| 717 | 56 | M | P/NP/Dc | squam | IV | 7 | W | No | PD | 2.5 | |||
| 718 | 57 | F | None | adeno | IV | 6 | W | No | PD | 4 | |||
| 719 | 44 | M | P/Rad | adeno | IV | 7 | W | No | PD | 3.5 | |||
| 720 | 57 | F | Rad | largecell | IV | 4 | W | No | NE | 8.5 | |||
| 721 | 63 | F | P/Dc/P | nsclc | IV | 7 | +/+ | No | PD | 5.5 | |||
| 722 | 60 | F | Rad/P | adeno | IV | 6 | W | No | PD | 4 | |||
| 723 | 53 | M | Opr/Rad/Dc | squam | IV | 8 | −/+ | Yes | PD | 16.5 | |||
| 724 | 60 | F | None | bronch | IV | 8 | −/− | Yes | PD | 10 | |||
| 725 | 55 | F | Opr | adeno | IIB | 8 | +/− | Yes | SD | 3 | +/+ | 30a | |
| 726 | 49 | M | P | adeno | IV | 7 | −/− | No | PD | 3.5 | |||
| 727 | 57 | M | Opr/Dc/Rad | squam | IIIB | 8 | −/− | Yes | SD | 6 | −/+ | 29a | |
Dc docetaxel, P platinum, NP non-platinum, Rad radiation, Opr operation, W withdrawn
aAlive at time of reporting
In the first version of the protocol, the patients were not screened for presence of brain metastasis prior to inclusion in the study. Subsequently, two amendments dated January and April 2003 modified the inclusion criteria to exclude patients with brain metastasis detected after screening (prior to inclusion) and to allow inclusion of patients subject to prior operation (from patient no. 724). Permission was also granted to give patients radiotherapy during the vaccination period, if the radiation field did not include a lesion that was used to assess the objective effect of the vaccines.
The trial was approved by the Norwegian Medicines Agency, the Committee for Medical Research Ethics, Region South, the Hospital Review Board and performed in compliance with the Helsinki declaration. Written informed consent was obtained from all patients. All patients participating in bone marrow examinations also signed a separate informed consent.
Vaccine and control peptides
GemVax AS, Porsgrunn, Norway, provided the telomerase peptide vaccines GV1001 and HR2822. The vaccine GV1001 consists of a synthetic peptide corresponding to the 16 amino acid residue 611–626 fragment (EARPALLTSRLRFIPK) of the hTERT protein. The vaccine HR2822 consists of a synthetic peptide corresponding to the 9 amino acid residue 540–548 fragment (ILAKFLHWL) of the hTERT protein. HR2822 is a well-known HLA-A2 epitope [7], but predictions (http://www.syfpeithi.bmi-heidelberg.com) show that this peptide also may be presented by many other HLA class I molecules. Both peptides were produced by Avecia Biotechnology (Cheshire, England). GV1001 and HR 2822 were manufactured by Isopharma AS (Kjeller, Norway), supplied in vials as freeze-dried products, and released for clinical use by GemVax AS (Porsgrunn, Norway). Manufacturing was in compliance with GMP. Two peptides, one derived from telomerase, Pep 544 (hTERT 548–566), and one derived from mutant p21 RAS, Pep 508; (KRAS 52–70, Q61H), served as negative controls in the T cell proliferation assays used to monitor T cell responses in blood samples from the vaccinated patients. The control peptides were supplied as lyophilized powder by The Corporate Research Centre Norsk Hydro (Porsgrunn, Norway).
Recombinant hTERT
Recombinant hTERT (563–735) was cloned in frame with the N-terminal 6× His tag in Escherichia coli expression vector pET28b (+) (Novagen, Darmstadt, Germany). The protein was produced in E. coli BL21 Codon Plus (DE3)-RIPL (Stratagene, La Jolla, CA, USA), purified by using NiNTA chromatography under denaturing conditions (Qiagen, Hilden, Germany) and tested by Western blot analysis with anti-His antibodies (Qiagen) and Rabbit Anti-Mouse Ig HRP (DakoCytomation, Glostrup, Denmark). The fraction of interest was dialyzed in Slide-A-Lyzer® Dialysis Cassette 10000 MWCO (Pierce, Rockford, IL, USA) against MQ-water and sterile filtered before use.
Treatment protocol
Prior to vaccination (0–2 weeks) the following baseline screenings were performed: Physical examination (including medical history), assessment of ECOG performance status and haematological testing for hemoglobin, hematocrit, leucocyte and platelet counts, C-reactive protein, as well as blood chemistry panel. CT scans of the thorax and upper abdomen were obtained prior to inclusion. Blood was also taken for testing of general immuno-competence (reactivity with purified protein derivative of BCG (PPD) and Staphylococcal Enterotoxin C (SEC) and baseline T cell reactivity against the vaccine peptides. All patients responded to SEC, the response to PPD was variable. Bone marrow was harvested from the iliac crest for assessing haematopoietic stem cell activity.
The vaccine was administered by intra-dermal (i.d.) injection in the right para-umbilical area. The vaccination schedule included three injections in week 1 (Mon, Tue, Fri) and one weekly injection in weeks 2, 3, 4, 6 and 10. The patients were consecutively divided into two groups, each receiving either a low or high dose of GV1001. The low-dose group patients (patients 701–713) received 60 nmole (112 μg) of peptide in 0.10 ml saline; the high-dose group patients (patients 714–727) received 300 nmole (560 μg) of peptide in 0.20 ml saline. In addition all patients were administered a low dose of HR2822, 60 nmole (68.4 μg) at the same site. The dose levels were selected based on previous experience with the same vaccine in patients with pancreatic cancer (Bernhard et al., unpublished results). Between 5 and 15 min before each vaccine injection, 30 μg GM-CSF (patients 701–713) or 75 μg (patients 714–727) Leucomax® (Schering-Plough, Cork, Ireland) in 0.10 ml saline was injected i.d. at the vaccination site. At each vaccination visit, a comprehensive assessment of adverse drug reactions, blood screening, physical examination and assessment of ECOG performance status was performed. At the completion of the study a thorough clinical and immunological screening similar to the baseline screening was performed.
Four patients (patient nos. 710, 712, 725, 727) with demonstrable immune responses to GV1001 and/or stable disease were offered booster vaccinations as a follow-up treatment. Only the high dose of GV1001 was administered in the follow-up. Due to supply limitations Leucomax was replaced by Leukine (Sargramostim, Berlex, Montville, NJ, USA) during the booster regimen.
Delayed-type hypersensitivity
Delayed-type hypersensitivity (DTH) skin test was performed at visit one (baseline) and at all the following weekly vaccination visits from week 2 and onwards. As DTH test 60 nmole of each vaccine peptide (GV1001; 112 μg, HR2822; 68.4 μg) in 0.10 ml saline was injected i.d. at separate sites in the left para-umbilical area. A positive DTH test was defined as an erythema/induration ≥25 mm2 (average diameter ≥5 mm) 48 h after the administration. The patients were instructed to measure the erythema/induration and record the result in a diary card.
Monitoring of T cell responses
Prior to vaccination and at week 6 and 10, 50 ml of ACD-blood was drawn to assess in vitro proliferative T cell responses against GV1001 and HR2822. PBMCs were isolated from peripheral blood using density centrifugation over Ficoll-Hypaque (Lymphoprep; Nycomed, Oslo, Norway), washed and frozen in RPMI-1640 (Gibco, Paisley, UK) with 20% FCS (PAA Laboratories GmbH, Paching, Austria) and 10% DMSO and stored in liquid N2. Following the inclusion of patient number 711, FCS was substituted by 100 mg/ml (10%) human albumin (HA) due to the generation of high background proliferative responses against antigens present in FCS. In vitro T cell responses were assayed in parallel in thawed PBMC from samples harvested before and after vaccination. All assays were done on thawn, frozen samples and pre- and post-vaccine samples were analyzed in parallel. The assay was performed after two cycles of antigen stimulation (25 μM GV1001 and 10 μM HR2822), essentially as described [22]. Very similar results were obtained using one cycle of antigen stimulation in a serum-free medium (DC medium, CellGenix, Freiburg, Germany), and this procedure was used during long-term follow up of patients receiving multiple booster injections. Values are shown as mean counts per minute (cpm) from triplicate wells. Background responses (without antigen) were usually below 1,000 cpm with SD <10%. An antigen-specific response was considered positive when the stimulatory index (SI; response with antigen divided by response without antigen) was above 2. Peptide-specific T cells from responding patients were cloned by limiting dilution as previously described [22]. Cytotoxicity assays were performed essentially as described [22]; using peptide pulsed, 51Cr-labeled autologous EBV transformed B cells as targets. Specific cytotoxicity was calculated according to the formula: (experimental release−spontaneous release)/(maximum release−spontaneous release)×100. Cytokine production by T cell clones was measured in supernatants taken from T cell assays 24 after peptide stimulation, using a human 17-plex cytokine kit and the Bio-Plex instrument (BioRad Laboratories Inc., USA), as described by the manufacturer. As a measure of general immunocompetence, the T cell response to purified protein derivative (PPD) of tuberculin and Staphylococcal enterotoxin C (SEC) was measured in blood samples taken at inclusion. All patients demonstrated responses within the normal range, and there was no correlation between the response to these antigens and the subsequent response to the vaccine peptides.
Toxicity and safety
Adverse events were evaluated at each visit and graded according to the National Cancer Institute of Canada Clinical Trial Group Common Toxicity Criteria Scale (NCIC CTG CTC version 2.0). The clinical investigator graded the events as probable, suspected, unlikely or not related to treatment. Adverse events were considered related to the treatment if the relationship was reported as probable or suspected. Treatment-related adverse events are reported here with the term “toxicities”.
Bone marrow aspiration
Measure of 10–20 ml of bone marrow was aspirated from the posterior iliac crest immediately before vaccination and 3–6 months following the treatment. Additional bone marrow samples were collected from patients who were given booster injections. Following LymphoprepTM (AXIS-SHIELD AS, Oslo, Norway) separation the mononuclear bone marrow cells were collected, washed with RPMI medium, counted and frozen in RPMI with 20% FCS and 10% DMSO in a control rate freezer. If not otherwise stated, all experiments were done on thawed samples.
Haematopoietic progenitor cell assays
Colony-forming unit cells (CFU-c) were assessed by a standardized clonogenic assay obtained from Stem Cell Technologies (Vancouver, Canada). Cultures were plated in two different concentrations in triplicate. Plates were incubated in a humidified atmosphere of 5% CO2 at 37°C for 14 days. Colonies were scored according to standard criteria [23].
Assay for long-term culture-initiating cells (LTC-IC)
Long-term culture-initiating cells define primitive human haematopoietic cells that are multipotent as evidenced by their generation of erythroid as well as granulopoietic progeny [24, 25]. The cultures were established and maintained according to procedures previously described [26]. Briefly, fresh or thawed mononuclear bone marrow samples were washed and resuspended in long-term cell (LTC) media (alpha medium supplemented with 40 mg/l inositol, 10 mg/l folic acid, 400 mg/l glutamine, 12.5% horse serum, 12.5% FCS, and 106 mol/l hydrocortisone sodium succinate). One million bone marrow cells were thereafter transferred to 35 mm Corning tissue culture dishes (Corning Glassworks, Corning, NY, USA) on pre-established, irradiated, normal marrow feeder cells. In all experiments feeder cells from only one donor were used. The cells were cultured for the first 3–4 days at 37°C and thereafter at 33°C, in 5% CO2. At weekly intervals, half of the non-adherent cells were removed and substituted with fresh medium. After 5 weeks of culture aliquots of both trypsinized adherent and non-adherent cells were seeded in triplicate and in different concentrations, employing the standardized CFU-c assay as described before. After 14 days of incubation the colonies were scored. The total number of clonogenic cells in each well was used as an estimate of the number of LTC-ICs initially present in the test cell populations.
Clinical response
Tumor response was assessed by CT/MRI scans and conventional radiography at week 12 of the study following 8 cycles of vaccinations. Results were compared with baseline tumor measurements. Tumor response was evaluated according to accepted criteria (WHO).
Data from patients who received at least one vaccination were included in the safety analysis. All patients who completed week 4 of the trial and complied with the inclusion criteria were included in the analysis of immune responses (evaluable patients).
Results
Safety of hTERT vaccination
GV1001 and HR2822 were well tolerated in all the 26 patients that received the vaccine. A total of 214 vaccine doses were administered (4–21 per patient). No sign of toxicity or clinically severe adverse events related to the treatment was observed in any of the vaccinated patients. Ten adverse events were reported (nine patients), three from the low-dose and seven from the high-dose group. The reported side effects were mostly flu-like symptoms, mild episodes of fever and chills.
Induction of immune responses
The patients in this study were unselected, with advanced/metastatic disease and had been heavily pre-treated (Table 2). Twenty-four patients were eligible for immune response evaluation according to the preset definitions (completed vaccination week 4), 12 in each GV1001 dose group. Of these 24 patients, 11 (46%) patients demonstrated an immune response) against GV1001 and only 2 (8%) against HR2822 (patient numbers 708 and 714). Seven of the patients that responded were in the GV1001 low-dose group and four in the high-dose group (Table 2). Two of the four patients given booster vaccinations were non-immune responders at the end of the standard vaccine regimen (8 vaccine injections). These two patients were converted to immune responders during the follow-up treatment (Table 2). Thus a total of 13 patients (54%) responded to the vaccination.
The withdrawal ratio was very high in the GV1001 high-dose group where 7 of 14 patients were withdrawn, mainly due to development of brain metastasis, before receiving all eight scheduled vaccinations (i.e. before 9 weeks of vaccination). This reflects the advanced stage of the patients and is believed to have had a significant negative impact on the overall induction of immune response in this group. Due to the high number of withdrawals in the high-dose group, it was not possible to evaluate the impact of the dose on the number of immune responders.
Only 14 patients completed the treatment according to the protocol. Twelve of these patients (85%) demonstrated an immune response either during the basic regimen or after booster vaccination. It is possible that there will be a delay in detectable immune response in some patients that have been pre-treated with chemotherapy or radiation. Based on the weekly DTH monitoring the immune response was induced after 3–9 weeks of vaccination.
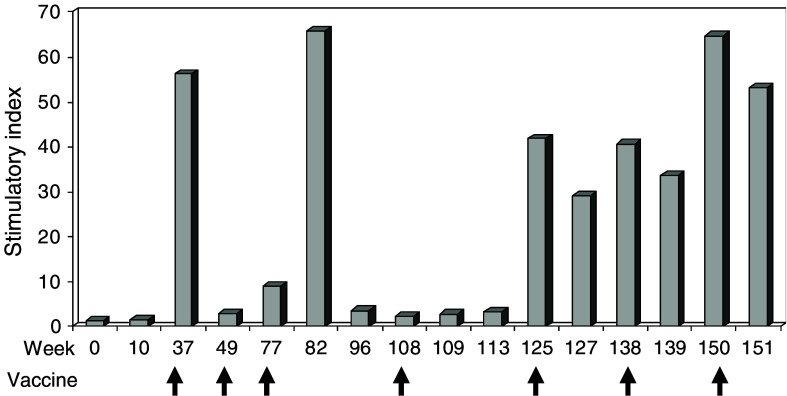
The T cell responses in patient 710 are illustrated in Fig. 1. This patient, who had been treated with the low-dose regimen, mounted a strong in vitro response to the first booster vaccination with the increased peptide dose (week 29) as measured in week 32. The patient received booster injections for the next 28 months and all blood samples taken during this period were positive, with marked fluctuations in the magnitude of the response recorded. From week 125 onward, a stable very high level of memory was established. Based on limiting dilution, the frequency of responding cells in the sample taken at week 32 was calculated to be approximately 1/2,000 T cells, whereas the frequency was below the detection limit in a pre-vaccine sample (results not shown).
Fig. 1.
In vitro T cell response against GV1001 in patient 710. T cell proliferation of PBMC is given as SI at baseline, following one complete cycle of vaccination with low peptide dose (Week 10) and following booster vaccinations with high peptide dose. SI is defined as response with antigen divided by response without antigen. Arrows indicate booster vaccinations
To further characterize the immune responses resulting from vaccination, T cells from in vitro-stimulated bulk cultures from several of the patients were cloned and used to characterize the HLA molecules involved in vaccine peptide presentation and to perform functional studies.
Processing of Telomerase: recognition of protein pulsed APCs
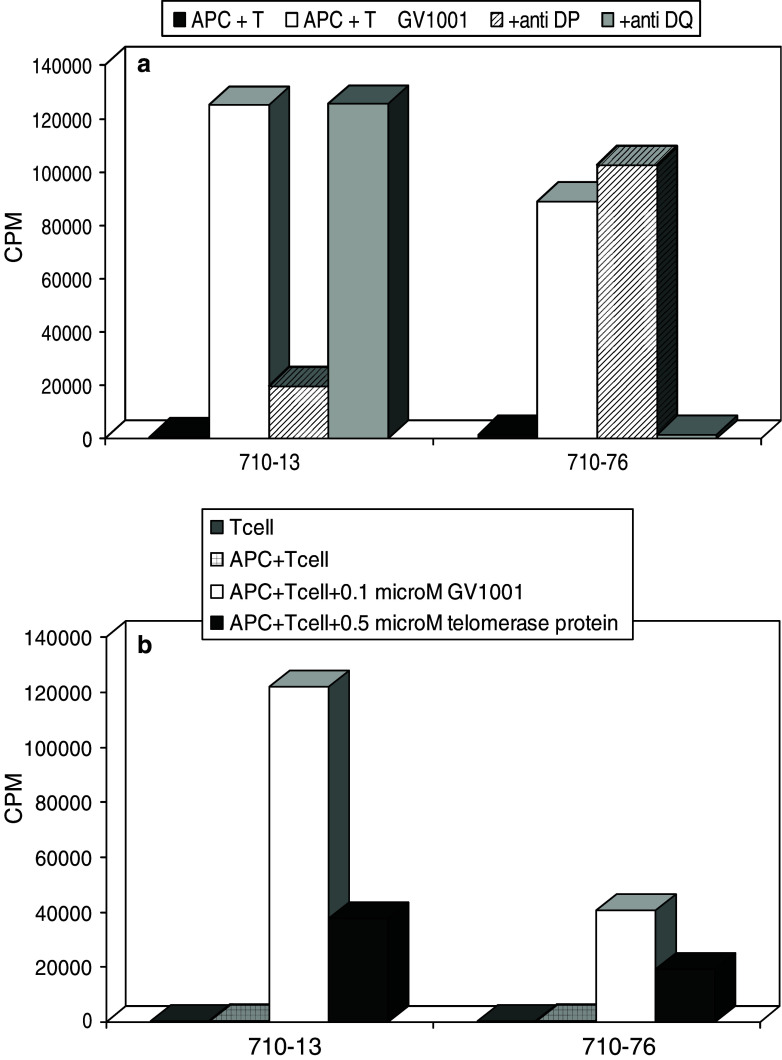
An important issue in peptide vaccination is whether the responding T cells are capable of recognizing epitopes resulting from natural processing of the corresponding protein. In the present study, the specific T cells from several of the responding patients were cloned. More than 100 GV1001-specific T cell clones were obtained from patients 710 and 725. These T cell clones proliferated vigorously and specifically against GV1001-pulsed antigen presenting cells, as demonstrated in Fig. 2a for two T cell clones derived from patient 710. The clone 710–13 was blocked by an anti-DP antibody and the clone 710–76 was blocked by an anti-DQ antibody. Subsequent studies with a panel of HLA homozygous EBV transformed B cells as APCs demonstrated that the presenting alleles were, respectively, HLA-DPB*0401/0402 and −DQB*04 (Data not shown). Blocking experiments with many different T cell clones demonstrated that the vaccine was presented on HLA-DR, -DQ and -DP molecules (Fig. 2a and data not shown). The use of a broad array of presenting HLA molecules explains why as many as 54 % of the patients responded to the vaccine. Since hTERT may be processed to peptides in many different ways, and the binding motifs of different class II molecules are different, it was important to investigate if different T cell clones were also capable of recognizing hTERT fragments generated by feeding recombinant hTERT to APCs. The HLA-DQB*04 and the HLA-DPB*0401/0402 restricted T cell clones both recognized autologous PBMC that were pulsed with recombinant telomerase protein (Fig. 2b). The same was found to hold true for all 16 T cell clones that were tested from the two patients, strongly suggesting that natural processing of hTERT may give rise to many different peptide fragments, fitting into multiple HLA class II molecules.
Fig. 2.
Recognition of GV1001 and recombinant hTERT by two CD4 + T cell clones derived from patient 710 after booster vaccination. HLA-class II restriction was determined by antibody blocking (Fig. 2a). Fifty thousand T cells were cultured with 25,000 irradiated autologous PBMC as APC and 1 μM GV1001 for 3 days in the presence or absence of blocking antibody as indicated. The antibodies used were SPVL3 (anti DQ) and B7/21 (anti DP). Final concentration of antibody was 10 μg/ml. In a parallel experiment (Fig. 2b), the same T cell clones were tested with recombinant hTERT (0.5 μM) as an antigen under the same conditions. GV1001 (0.1 μM) and medium alone were used as controls. Results are recorded as mean cpm of triplicate wells
GV1001-specific CD4+ T cells kill peptide pulsed target cells
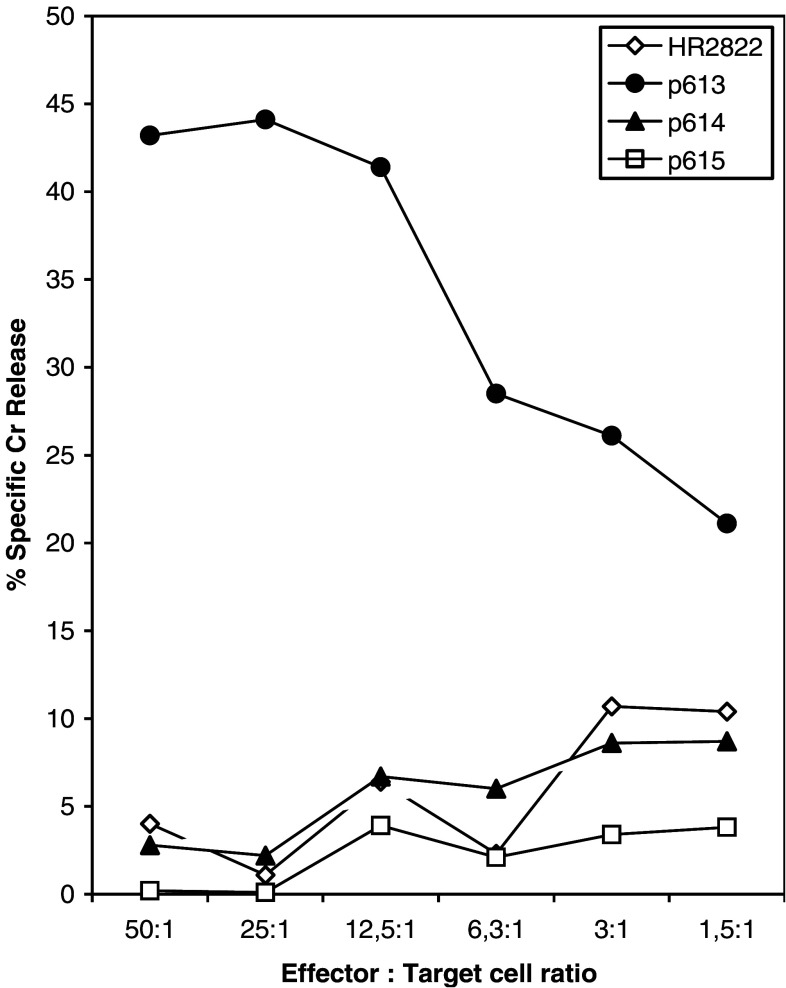
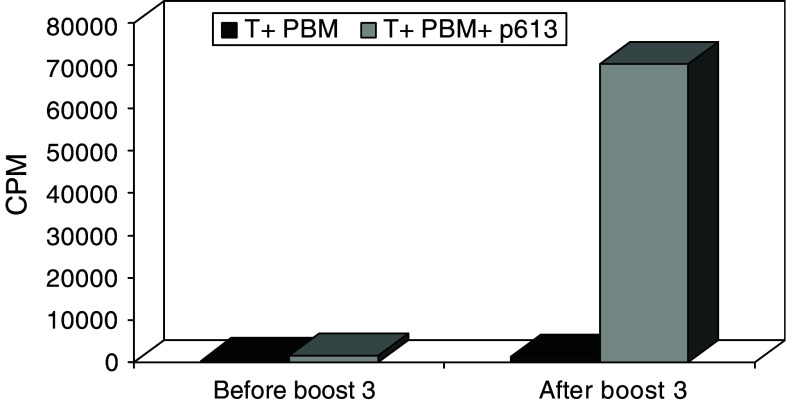
The T cell clones from patient 710 were initially also screened for cytotoxicity in a classical 51-Cr release assay, using autologous target cells pulsed with a pool of 9-mer peptides derived from GV1001. Data presented in Fig. 3 together with results shown in Fig. 2 demonstrate that the CD4+ clone 710–76 is an HLA-class II-restricted CTL capable of killing autologous target cells pulsed with a 9-mer peptide (p613, TRLRFIPK) derived from the C-terminal part of GV1001, in a specific manner. No killing was observed when the target cells were pulsed with the hTERT peptide 540–548 or with two other 9-mer peptides derived from GV1001, p614 and p615 (Fig. 3). Interestingly, the T cell reactivity against the GV1001 fragment p613 was greatly enhanced after the third booster vaccination (Fig. 4).
Fig. 3.
Specific killing of autologus Epstein–Barr virus transformed blasts pulsed with peptide p613 by the HLA-DQB*04 restricted GV1001 specific CD4+ T clone 710–76. Target cells were labeled with 51Cr for 1 h and 2,000 cells were added to each well before addition of peptide (1 μM) and effector cells at the ratios indicated. Supernatants containing released 51Cr were harvested after 6 h and counted. The peptides p613 (TSRLRFIPK), p614 (LTSRLRFIP) and p615 (LLTSRLRFI) are all nine mere fragments from the C-terminal part of GV1001. The vaccine peptide HR2822 was used as a negative control. Results are expressed as percentage of specific killing and represent mean of triplicate wells
Fig. 4.
In vitro proliferative T cell response of PBMC against the N-terminal 9-mere fragment of GV1001 in patient 710. T cell proliferation is given as mean cpm of triplicate wells. Responses shown are from samples taken 1 h before booster vaccine 3 and 12 days after booster vaccination 3
GV1001 vaccination generates an inflammatory immune response
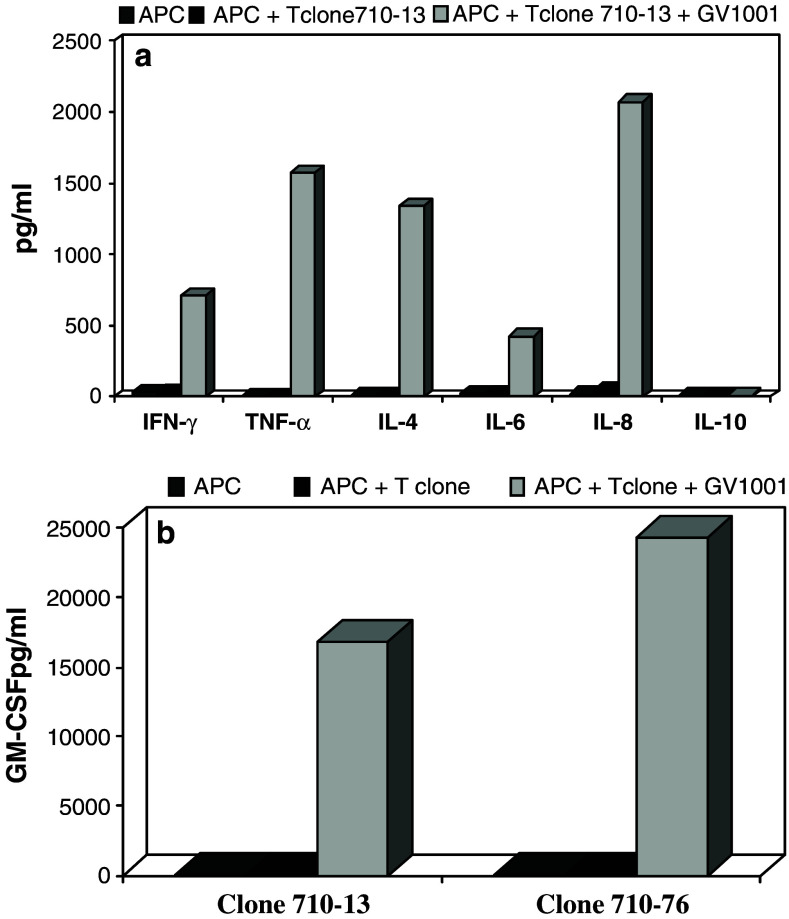
To characterize the T cell response further, we also investigated the cytokine production by the T cell clones. Figure 5 presents some representative results from two T cell clones from patient 710. Similar results were obtained using T cell clones from other patients. Although clones 710–76 and 710–13 were found to produce substantial amounts of IL-4, they displayed a predominantly Th1 cytokine profile with high amounts of IFN-γ and TNF-α as well as the proinflammatory chemokine IL-8 (granulocyte chemotactic protein 1). They also produced very high amounts of GM-CSF upon recognition of GV1001 (Fig. 5). The same profile was obtained when PBMC were stimulated with GV1001 (data not shown).
Fig. 5.
a Cytokine profile of the HLA-DPB*0401/0402 restricted CD4+ clone 710–13 following activation by GV1001. Supernatants were harvested 24 h after stimulation of 50,000 T cells with 25,000 APC and GV1001 (1 μM) in serum-free medium. Seventy-five microliters was harvested from duplicate wells and analyzed using a Bio-rad Bio-Plex instrument. Results are expressed as mean concentration of cytokines (pg/ml) in the supernatants based on calculation from standard curves established with recombinant cytokines. b GM-CSF production by two CD4+ clones from patient no. 710. The GM-CSF production by 50,000 T cells from the cytotoxic clone 710–76 corresponds to 500 ng/106 T cells/24 h. Results were obtained as described in a
The hTERT-specific immune responses are non-toxic to bone marrow stem cells
Telomerase activity is also expressed in bone marrow stem cells, and therefore bone marrow toxicity due to autoimmune destruction by hTERT-specific effector cells could not be ruled out. To detect changes in bone marrow clonogenicity, CFU-C and LTIC, bone marrow samples from patients mounting an immune response to the vaccine were examined. For patients receiving booster vaccinations over an extended period of time, bone marrow aspirates were taken every 6 months. The results presented in Tables 3, 4 show that no significant differences were seen in the number of BFU-E and CFU-GM before and after the vaccination treatment. These results are in accordance with the findings in peripheral blood, where no changes in cell counts were observed, even in patients with persistent strong immune responses for more than 2 years (data not shown).
Table 3.
Clonogenic growth of haematopoietic progenitor cells before and after 8 vaccinations
| Patient no. | Before vaccination | After vaccination | ||||
|---|---|---|---|---|---|---|
| BFU-E/1000 MNC/BM | CFU-GM/1000 MNC/BM | LTC-IC/10000 MNC/BM | BFU-E/1000 MNC/BM | CFU-GM/1000 MNC/BM | LTC-IC/10000 MNC/BM | |
| 704 | 3.5 | 4.0 | 4.8 | 4.2 | 3.5 | 4.6 |
| 708 | 0.8* | 3.9* | 4.3 | 0.5* | 1.6* | 3.4 |
| 711 | 0.5* | 2.1* | 0.6 | 0.8* | 2.0* | 0.6 |
| 712 | 0.9 | 2.6 | 5.1 | 1.0 | 1.9 | 6.3 |
*Fresh bone marrow was used in the experiments
Table 4.
Clonogenic growth of haematopoietic progenitor cells before and after 8 vaccinations and booster vaccinations
| Patient no. | Before vaccination | After vaccination 4–5:7–12:21 months |
||||
|---|---|---|---|---|---|---|
| BFU-E/1000 MNC/BM | CFU-GM/1000 MNC/BM | LTC-IC/10000 MNC/BM | BFU-E/1000 MNC/BM | CFU-GM/1000 MNC/BM | LTC-IC/10000 MNC/BM | |
| 710 | 0.7 | 1.2 | 2.6 | 1.5:2.0:1.2 | 1.7:1.6:1.2 | 5.4:1.6:3.6 |
| 727 | 4.4 | 3.5 | 18.6 | 3.1:5.7:na | 2.2:3.9:na | 9.6:28.8:na |
| 725 | 1.8 | 1.9 | 3.0 | 1.4:2.4:na | 1.2:2.4:na | 3.4:2.6:na |
na not applicable
There is considerable evidence to indicate that the LTC-IC assay measures a very primitive human progenitor cell in the bone marrow and that eradication of such cells most likely predicts a serious toxic effect on bone marrow function [24–26]. As it can be seen (Tables 3, 4), the numbers of LTC-IC were essentially unaltered in all patients monitored. These findings did also hold true for the three patients who had received booster vaccinations over a period of 7–21 months (Tables 3, 4).
Tumor response and patient survival
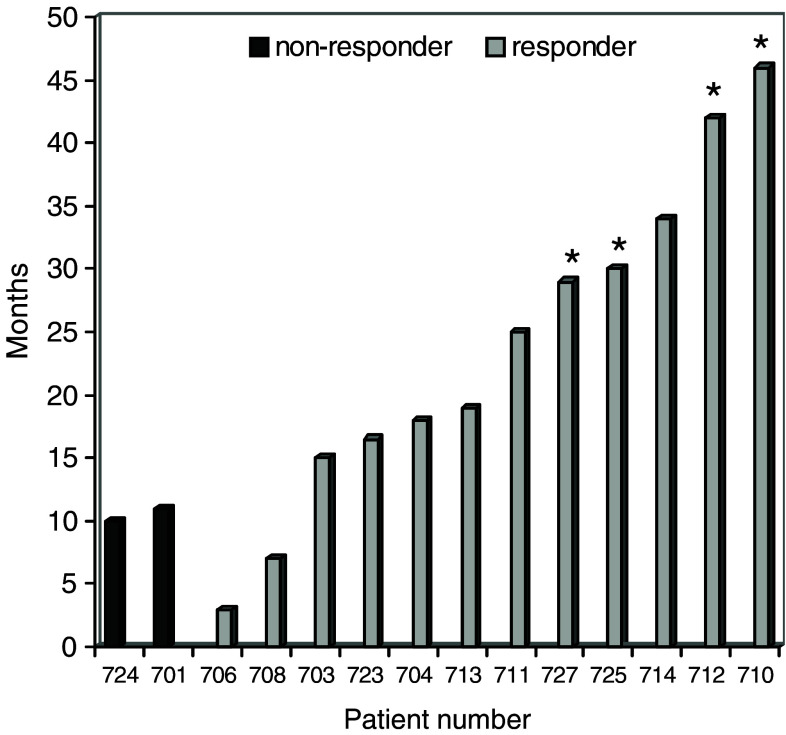
As assessed by CT or MRI, 18 patients (72%) had progressive disease (PD) and seven (28%) had stable disease (SD) at week 12 of the study (Table 2). One patient was withdrawn due to infection and could therefore not be evaluated. Twelve patients were withdrawn before they received all vaccines (week 10). Eight of these patients had developed brain metastases. For the 26 patients who entered the study, the median survival time was 8.5 months. Eight and six patients were alive at 12 and 18 months, respectively. The survival for all patients who completed the treatment (10 weeks vaccination) is recorded in Fig. 6. Of the four patients still alive, one has CR, three SD and one PD (Table 2). Two of these patients still receive booster vaccinations every 3–6 months and have developed stable (>6 months) high levels of immunity against GV1001.
Fig. 6.
Survival for patients that completed 10 weeks of vaccination. Patient 725 is stage IIB, patient 710 stage IIIA, patients 701,713 and 727 are stage IIIB. The remaining patients are all stage IV. Patients marked asterisk are still alive
Complete response in an immune responding patient
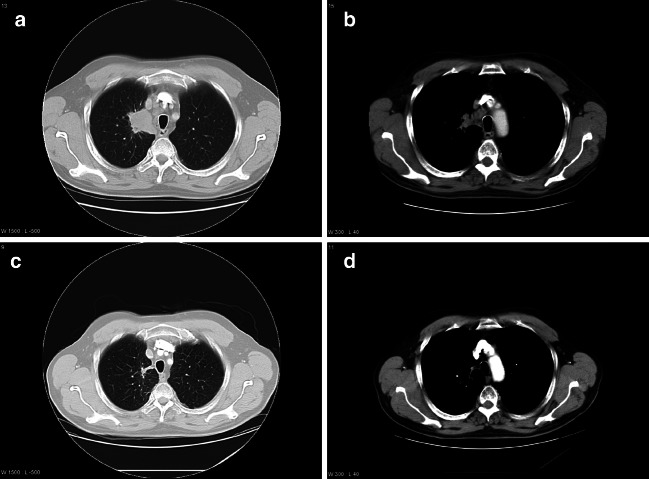
Prior to inclusion in the trial, patient no. 710 was diagnosed with a locally advanced adenocarcinoma of stage IIIB with mediastinal extension and mediastinal glands. The patient was treated with five courses of platinum-containing chemotherapy with last infusion 9 weeks before and radiotherapy 2.5 weeks before vaccination was started. At the time of inclusion in this study the patient was restaged to IIIA and the tumor in his right lung was measured at 32 mm×32 mm (Fig. 7). The patient continued with booster vaccinations and the last two CT scans taken, respectively, 17 and 22 months after entering the vaccination trial, show a CR without any visible tumor (Fig. 6c, d). A CT scan recently taken after 29 months (not shown) confirmed this, as only post-irradiation changes were visible. The immune response in this patient was only detected after he had received his first booster vaccination. At this stage, high affinity, GV1001-specific CD4+ T cells could be isolated from peripheral blood. The cloned T cells produced large amounts of inflammatory cytokines and were capable of killing target cells pulsed with the shorter peptide fragment (HR2822) of the vaccine (Figs. 5, 3 ).
Fig. 7.
a At inclusion in the vaccination trial patient no. 710 had a 32 mm×32 mm tumor in right upper lobe. b CT scan of the patient’s mediastinal glands at inclusion in the trial. c, d right upper lobe and mediastinal glands 22 months after start of vaccination, respectively
Discussion
In this study we report the first clinical testing of an anti-cancer vaccine based on a combination of a general HLA class II telomerase T-helper cell peptide epitope (GV1001) and a HLA-A2 restricted telomerase CTL epitope (HR2822).
Our results demonstrate that vaccination with the telomerase peptides is well tolerated by patients with NSCLC. The observed adverse events related to the treatment were not of a serious nature. Indurations and erythema at the injection site as well as chills and fever were interpreted as symptoms of an effective activation of the immune system rather than unwanted side effects. All the patients were closely monitored by blood testing during the vaccination period and repeated bone marrow examinations were undertaken for seven patients without detection of any significant changes in blood cell counts or haematopoietic progenitor cells. The finding that repeated bone marrow examinations over a long period of time in seven immune responding patients, including patient no. 710 with a complete tumor response (Fig. 4), did not reveal any bone marrow toxicities, was particularly important. These results demonstrate that hTERT-specific Th cells do not harm haematopoietic stem cells and their progenitors. Similar observations have recently been published from a clinical trial with the hTERT I540 peptide [27]. This peptide is the same as the HR2822 peptide used in this trial. Together, these findings suggest that also induction of tumor-lytic hTERT-specific T cells in vivo by vaccination does not result in a detectable decline in the hematopoietic potential of cancer patients.
The overall induction of immune responses in those patients that completed the 10-week basic treatment was 12/14(86%). Ten of these patients demonstrated an immune response during the primary treatment and two additional during the follow-up booster vaccination regimen. Most of the patients had been heavily pre-treated with CT or RT (or both) and insufficient time for the immune system to recover from those treatments before starting with the vaccination, may be an explanation for the delayed induction of immune response in some of the responders. The chemotherapy consisted mostly of platinum-containing regimens or docetaxel. Some patients had been treated with radiotherapy and low-dose docetaxel as a radiosensitizer. Progression of disease and detection of brain metastasis, and subsequent start of treatment with immunosuppressive corticosteroids were the main reasons for patient withdrawal from the study. The frequency of brain metastases in patients with advanced NSCLC is high [28]. It has been reported that tolerance to self-proteins is dominated by high-affinity immuno-dominant peptide epitopes and that the tolerance to such epitopes is very difficult to overcome [29, 30]. Clearly our results indicate that tolerance for GV1001 is not a major obstacle, and corroborate our findings in a parallel study (S. Bernhardt et al. unpublished), demonstrating that GV1001 is a broadly recognized vaccine that can be used for vaccination of cancer patients without inclusion based on prior HLA typing.
We, here, demonstrate that GV1001 reactive cells are capable of recognizing APCs pulsed with recombinant hTERT (Fig. 2). Of <50 individual T cell clones, restricted by a broad array of HLA-DR, -DQ and -DP molecules, none have so far failed to recognize hTERT (unpublished results). These observations suggest that the main mechanism of action in vivo by GV1001-specific Th cells will be recognition of APCs that have engulfed dead tumor cells in situ in the tumor or in draining lymph nodes. GV1001 does also comprise embedded CTL epitopes, and we were able to show that CD4+ clones killed target cells pulsed with the peptide p613, which represents a fragment of GV1001. In patient 710 who had a CR, we were able to demonstrate that the frequency of cells specific for p613 increased after booster vaccination and thus may be involved in the observed tumor regression.
Furthermore, GV1001-specific clones from this patient produced inflammatory cytokines and very high levels of GM-CSF, which could participate in the process of tumor regression by locally enhancing the immune response against not only hTERT but also other lung cancer antigens taken up by APCs. It is thus highly interesting that the concentration of GM-CSF produced by T cell clones from patient 710 (500 ng/106 T cells/24 h) is >2× that of a tumor cell line genetically modified to express high amounts of GM-CSF (205 ng/106 T cells/24 h) has been used in clinical trial [31].
Only two (14%) of the patients mounted an immune response after vaccination with HR2822. Tolerance to HR2822, which is classified as a high-affinity epitope, could be an explanation for this observation. However, our results are in contrast with results obtained in two recent trials. Vonderheide et al [19] reported that 4/7 (57%) patients with advanced breast or prostate cancer vaccinated with peptide pulsed dendritic cells (DC) responded to this peptide. Similarly Parkhurst et al [31] observed that 7/14 (50%) patients with mainly renal cancer responded to the same peptide, following vaccination with 1 mg of peptide emulsified in adjuvant (Montanide—ISA51), indicating that also here tolerance is not a major issue. One obvious explanation may be that the frequency of HLA-A2 positive patients in our trial is lower than in the other trials, since these had positive HLA-A2 as an inclusion criterion. Thus the frequency of HLA-A2 may have been around 50% rather than 100%. The lower frequency of immune responders in our study may also reflect differences in vaccination method (GM-CSF as adjuvant vs. DC pulsing or Montanide ISA51), patient group or detection method.
Objective tumor response is used as a surrogate endpoint to measure efficacy in phase I/II studies. The clinical response to the telomerase vaccine is rather difficult to evaluate. Although a number of patients (especially patient 710) demonstrated remarkable responses to vaccination, these responses combined with survival data are difficult to compare with other studies. Recently two vaccine studies with a similar group of patients with advanced disease have been published [32, 33]. These studies utilized different approaches, either vaccinating with allogeneic adenocarcinoma cell lines or with dendritic cells loaded with apoptotic allogeneic NSCLC cells. In both studies modest clinical responses were observed. Although the total number of patients included are low and the data preliminary, these results taken together with the results from the present study indicate that cancer vaccines may be developed into a novel treatment modality in patients with NSCLC. It is conceivable that better effects would have been obtained in patients with less advanced disease, as indicated by recent data from a vaccine trial presented at ESMO 2004 [32], where clinically meaningful survival advantage was observed in patients with late stage NSCLC treated with a Muc-1 vaccine. In accordance with this, a new clinical trial involving patients with inoperable NSCLC stage IIIA/IIB is under planning. Here, the patients will be treated with chemo-radiotherapy followed by telomerase vaccination.
Acknowledgements
We thank Kari Lislerud for excellent technical assistance, and Mericon AS for monitoring of the study. This work has been supported by grants from The Norwegian Cancer Society (IS), the Norwegian Research Council and was supported by GemVax AS.
Footnotes
In the study protocol GV1001 is referred to as HR2802
The clinical trial was supported by the Norwegian Research Council, Oslo, Norway and by GemVax AS, Oslo, Norway.
The study has been presented in part: ASCO, abstract no 666, Chicago May 2003. ASCO, abstract no 2580, Orlando May 2005.
References
- 1.Shepherd F, Carney D (2000) In: Hansen H (ed) Treatment of NSCLC in Textbook of lung cancer. Martin Dunitz
- 2.Pfister D, Johnson D, Azzoli C, et al. American Society of clinical oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. JCO. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 3.Woo Ey, Yeh H, Chu S, et al. Cutting edge; regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 4.Hege K, Carbone D. Lung cancer vaccines and gene therapy. Lung Cancer. 2003;41:S103–S113. doi: 10.1016/S0169-5002(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 5.Autexier C. Telomerase as a possible target for anticancer therapy. Chem Biol. 1999;6:R299–R303. doi: 10.1016/S1074-5521(99)80122-7. [DOI] [PubMed] [Google Scholar]
- 6.Fujita Y, Fujikane T, Fujiuchi, et al. The diagnostic and prognostic relevance of Human Telomerase reverse transcriptase mRNA expression detected in situ in patients with nonsmall cell lung carcinoma. Cancer. 2003;298:1008–1013. doi: 10.1002/cncr.11611. [DOI] [PubMed] [Google Scholar]
- 7.Vonderheide RH, Hahn WC, Schultze JL, et al. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 8.Minev B, Hipp J, Firat H, et al. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci USA. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonderheide RH, Anderson KS, Hahn WC, et al. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res. 2001;7:3343–3348. [PubMed] [Google Scholar]
- 10.Arai J, Yasukawa M, Ohminami H, et al. Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood. 2001;97:2903–2907. doi: 10.1182/blood.V97.9.2903. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez J, Garcia-Pons F, Lone YC, et al. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc Natl Acad Sci USA. 2002;99:12275–12280. doi: 10.1073/pnas.182418399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scardino A, Gross DA, Alves P, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 13.Gross DA, Graff-Dubois S, Opolon P, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–433. doi: 10.1172/JCI200419418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroers R, Huang XF, Hammer J, et al. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
- 15.Schroers R, Shen L, Rollins L, et al. Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res. 2003;9:4743–4755. [PubMed] [Google Scholar]
- 16.Uchida N, Otsuka T, Shigematsu H, et al. Differential gene expression of human telomerase-associated protein hTERT and TEP1 in human hematopoietic cells. Leuk Res. 1999;23:1127–1132. doi: 10.1016/S0145-2126(99)00149-6. [DOI] [PubMed] [Google Scholar]
- 17.Tahara H, Yasui W, Tahara E, et al. Immuno-histochemical detection of human telomerase catalytic component, hTERT, in human colorectal tumor and non-tumor tissue sections. Oncogene. 1999;18:1561–1567. doi: 10.1038/sj.onc.1202458. [DOI] [PubMed] [Google Scholar]
- 18.Gjertsen MK, Buanes T, Rossland AR, et al. Intradermal RAS peptide vaccination with granulocyte-macrophage colony-stimulation factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92:441–450. doi: 10.1002/ijc.1205. [DOI] [PubMed] [Google Scholar]
- 19.Vonderheide RH, Domchek SM, Schultze JL, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.CCR-0620-3. [DOI] [PubMed] [Google Scholar]
- 20.Ostrand-Rosenberg S. CD4+ lymphocytes: a critical component of antitumor immunity. Cancer Invest. 2005;23:413–419. doi: 10.1081/CNV-67428. [DOI] [PubMed] [Google Scholar]
- 21.Mountain C, Dresler C. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 22.Gjertsen MK, Saeterdal I, Saeboe-Larssen S, Gaudernack G. HLA-A3 restricted mutant ras specific cytotoxic T-lymphocytes induced by vaccination with T-helper epitopes. J Mol Med. 2003;81:43–50. doi: 10.1007/s00109-002-0390-y. [DOI] [PubMed] [Google Scholar]
- 23.Testa NG, Molineux G (1993) Haematopoiesis: A practical approach, Volume 1, and First Edition. Oxford University Press, Oxford
- 24.Sutherland HJ, Eaves CJ, et al. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991;3:666–672. [PubMed] [Google Scholar]
- 25.Pettengell R, Luft T, et al. Direct comparison by limiting dilution analysis of long-term culture initiating cells in human bone marrow, umbilical cord blood, and blood stem cells. Blood. 1994;11:3653–3859. [PubMed] [Google Scholar]
- 26.Rusten LS, Jacobsen SE, et al. Functional differences between CD38- and DR-subfractions of CD34+ bone marrow cells. Blood. 1994;84:1473–1481. [PubMed] [Google Scholar]
- 27.Danet-Desnoyers GA, Luongo JL, Bonnet DA, Domcheck SM, Vonderheide RH. Telomerase vaccination has no detectable effect on SCID-repopulating and colony-forming activities in the bone marrow of cancer patients. Exp Hematol. 2005;33:1275–1280. doi: 10.1016/j.exphem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen JB, Hansen HH, Hansen M, et al. Brain metastasis in adenocarcinoma of the lung; frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1479–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 29.Cibotti R, Kanellopoulos JM, Cabaniols J-P, et al. Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Nati Acad Sci USA. 1992;89:416–420. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross DA, Graff-Dubois S, Opolon P, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–433. doi: 10.1172/JCI200419418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhurst M, Riley J, et al. Immunization of patients with hTERT: 540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing Telomerase. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abstr. # 3IN http://www.ex2.excerptamedica.com/ciw-04esmo/
- 33.Hirschowitz E, Foody T, Yanelli J, et al. Autologous dendritic cell vaccines for non-small cell lung cancer. JCO. 2004;22:2808–2815. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]