Abstract
Immunization of mice with dendritic cells transfected ex vivo with tumor-associated antigen (TAA)-encoding mRNA primes cytotoxic T lymphocytes (CTL) that mediate tumor rejection. Here we investigated whether direct injection of TAA mRNA, encapsulated in cationic liposomes, could function similarly as cancer immunotherapy. Intradermal and intravenous injection of ovalbumin (OVA) mRNA generated specific CTL activity and inhibited the growth of OVA-expressing tumors. Vaccination studies with DNA have demonstrated that co-administration of antigen (Ag)- and cytokine-encoding plasmids potentiate the T cell response; in analogous fashion, the inclusion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mRNA enhanced OVA-specific cytotoxicity. The ability of this GM-CSF-augmented mRNA vaccine to treat an established spontaneous tumor was evaluated in the Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) mouse, using the SV40 large T Ag (TAg) as a model tumor/self Ag. Repeated vaccination elicited vigorous TAg-specific CTL activity in nontransgenic mice, but tumor-bearing TRAMP mice remained tolerant. Adoptive transfer of naïve splenocytes into TRAMP mice prior to the first vaccination restored TAg reactivity, and slowed tumor progression. The data from this study suggests that vaccination with TAA mRNA is a simple and effective means of priming antitumor CTL, and that immunogenicity of the vaccine can be augmented by co-delivery of GM-CSF mRNA. Nonetheless, limitations of such vaccines in overcoming tolerance to tumor/self Ag may mandate prior or simultaneous reconstitution of the autoreactive T cell repertoire for this form of immunization to be effective.
Keywords: CTL, mRNA vaccine, GM-CSF, SV40 large T antigen, Tolerance
Introduction
In constructing vaccines for priming antitumor cytotoxic T lymphocyte (CTL) responses, nucleic acids have advantages over other forms of antigen (Ag), as polynucleotides are easily replicated and purified, encode multiple class I and class II epitopes unrestricted by haplotype, and provide long-lasting Ag expression, which can be critical for efficient activation and expansion of T cells [1, 2]. Although plasmid DNA (pDNA) immunization is most commonly used, previous work from our laboratory has shown that mRNA encoding model and native tumor-associated Ags (TAAs), transfected into dendritic cells (DCs) ex vivo, can induce tumor-protective CTL activity [3, 4]. Immunizing mice directly with in vitro transcribed (IVT) or tumor-extracted mRNA may also be a feasible strategy, but studies of RNA vaccination are limited. Martinon et al. [5] first demonstrated that injection of liposome-encapsulated, influenza virus nucleoprotein mRNA could prime CTL responses; similar findings were reported in a study using protamine-protected mRNA encoding β-galactosidase [6]. Antitumor responses in mice vaccinated with single TAA-encoding mRNAs have been reported by a few investigators using self-replicating mRNA (i.e., transcribed from alphavirus-based vectors) [7–11].
The intrinsic immunogenicity of pDNA vaccines is due largely to unmethylated CpG dinucleotides, while the mechanism of T cell priming by mRNA vaccines has not been established. For self-replicating mRNA, immunogenicity is associated with replicase-induced apoptosis and Ag uptake by DCs [8]. The immunogenicity of nonreplicating mRNA may depend on the adjuvant effects of encapsulating liposomes [12, 13], but CTL responses can be elicited by naked mRNA as well [6]. The native immunogenicity of pDNA vaccines can be enhanced by the inclusion of adjuvant molecules encoded as DNA. For example, co-injection of DNA vectors encoding viral or tumor Ags and the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) strengthens T cell responses [14–18]. The possibility of augmenting the immunogenicity of an mRNA vaccine in an analogous fashion, by adding cytokine mRNA, has not been previously investigated. We found that vaccinating with liposome-encapsulated, nonreplicating IVT mRNA encoding the model TAA ovalbumin (OVA) mRNA primed vigorous CTL responses that inhibited the growth of OVA-expressing tumors. The efficacy of vaccination depended on the cationic liposome and the route of administration. With optimal parameters, a single injection of 1 μg mRNA primed CTL. Co-immunization with OVA and murine GM-CSF mRNAs potentiated cytotoxic responses.
While induction of CTL responses with a strong immunogen demonstrates proof-of-principle, the ability to prime tumor-specific CD8+ T cells in a spontaneous mouse model provides a more stringent assessment of the utility of mRNA vaccination for cancer therapy. Tumor-bearing hosts may have acquired functional defects in Ag-presenting cells (APCs) [19, 20] or T cells [21–23] that can inhibit CTL responses. Further, most cancer Ags that have been identified so far are nonmutated, differentiation proteins—so-called tumor/self Ags—for which some level of T cell tolerance has been established centrally or peripherally. Despite this constraint, tumor/self Ags are shared between cancer patients, and hence, from a practical standpoint, are attractive candidates for vaccine construction. In several studies, immunization of mice with tumor/self Ag-encoding pDNA [18, 24–27] or self-replicating mRNA [7] has been shown to overcome tolerance, conferring tumor protection. However, when used as therapy, the responses to tumor/self Ag pDNA vaccines were variable. For example, established B16 melanomas were not rejected following the administration of a protective vaccine [26], although benefit was obtained 10 days after B16 implantation by co-immunization with xenogeneic Ag and GM-CSF pDNAs [18]. The effect of vaccinating with tumor/self Ag mRNA in cancer-bearing mice has not been examined. To investigate this question, we used the TRAMP mouse, a transgenic model of autochthonous prostate carcinoma (CaP) [28, 29] that has a number of useful features: (1) the oncogenic SV40 large T Ag (TAg) is a defined tumor/self Ag; (2) CaPs arise predictably and progress slowly, allowing for tumor-immune system interaction prior to vaccination; (3) CaP growth has been controlled with immunotherapy [30, 31]; and (4) TAg-specific CTL can be generated in male mice by immunization [30]. With OVA mRNA vaccination, we found that tumor-bearing TRAMP mice were capable of mounting CTL responses equivalent to that of wild-type (wt) controls, suggesting intact mechanisms of Ag presentation, and T cell priming and effector function. Nonetheless, TRAMP mice did not respond to a GM-CSF-augmented, tumor/self Ag TAg mRNA vaccine. This failure occurred at the thymic level, as a single adoptive transfer (AT) of naïve wt splenocytes prior to the first immunization led to TAg-specific CTL activity, lymphoid infiltration of the tumor, and reduced CaP grade and weight. These results suggest that a nonreplicating TAA mRNA vaccine, while efficient at activating high avidity CD8+ T cells, even in hosts chronically bearing spontaneous tumors, is unable to prime CTL recognizing subdominant epitopes derived from a tumor/self Ag. Thus, for this form of nucleic acid immunization to be effective in therapeutic settings where levels of tolerance are high, additional strategies for enhancing vaccine immunogenicity, or complementary means of restoring the high avidity, tumor-reactive T cell repertoire, may be ultimately necessary.
Materials and methods
Mice
C57BL/6 (B6) mice were obtained at 4–6 weeks of age from Charles River Laboratories (Wilmington, MA). Male TRAMP mice were F1 crosses of B6 and transgenic breeders (C57BL/6-TgN(TRAMP) 8,247 Ng) purchased from Jackson Laboratories (Bar Harbor, ME). To genotype TRAMP mice, DNA was extracted with phenol-chloroform from distal tail segments incubated overnight in HB buffer (50 mM Tris–HCl, 50 mM EDTA, 100 mM NaCl, pH 8.0) containing 1% SDS and 0.77 mg/ml proteinase K. The 0.6 kb TAg fragment was amplified by PCR using the primers: rat probasin-forward (F) - 5′-CCG GTC GAC CGG AAG CTT CCA CAA GTG CAT TTA-3′; TAg-reverse (R) − 5′-CTC CTT TCA AGA CCT AGA AGG TCC A-3′. Murine β-casein (0.5 kb fragment) served as a positive control. Thermocycling conditions consisted of 94°C denaturation for 15 s, 55°C annealing for 30 s, 72°C elongation for 30 s (30 cycles). TRAMP mice were monitored for tumors by palpation, and were euthanized when tumors caused weight loss or morbidity. Experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee, and adhered to published principles of laboratory animal care (NIH publication No. 85-23, revised 1985).
Cell lines and reagents
EL-4, E.G7, F10.9, F10.9-OVA, F10.9-3.1 and BLK SV cells were grown in Dulbecco’s Modified Eagle Medium supplemented with 10% FBS, 5×10−5 M 2-ME, 2 mM l-glutamine, 25 mM HEPES buffer, 1 mM Na pyruvate, 1 mM amino acids, and penicillin/streptomycin. Selection medium for OVA cDNA (E.G7; F10.9-OVA) and empty vector (F10.9–3.1) transfectants contained 400–800 μg/ml G418. The H2-Kb-restricted peptides OVA257–264 (SIINFEKL); mutant connexin3752–59 (FEQNTAQP; referred to as MUT-1) [32]; and SV40 TAg404–411 (VVYDFLKC) [33] were purchased from Research Genetics (Birmingham, AL). Human interleukin (IL)-2 was obtained from Genzyme (Cambridge, MA). Cationic liposomes included DOTAP and DOSPER (Roche, Mannheim, DE), and DOTAP-DOPE (Sigma, St. Louis, MO). 5-(and-6)-carboxyfluoroscein diacetate, succinimidyl ester (CFSE) was obtained from Molecular Probes (Eugene, OR).
Cloning of SV40 TAg
Full-length TAg cDNA was produced by reverse transcription (PowerScript RT; Clontech Laboratories, Inc., Palo Alto, CA) of RNA isolated from CaP tissue, and PCR amplification using high-fidelity KlenTaq polymerase (Clontech) with the primers: SV-TAg-F: 5′-CTT GAA GTC GAC GCC ACC GAT GGA TAA AGT TTT AAA CAG AGA GG-3′; SV-TAg-R: 5′-GCC CGT GAA TTC TTT ATG TTT CAG GTT CAG G-3′. The forward primer contained Kozak (5′-GCC ACC-3′) and initiation codon sequences. Thermocycling conditions consisted of 95°C denaturation for 15 s, 65°C annealing for 30 s, 68°C elongation for 30 s (35 cycles). The PCR product was cloned into a modified pSP73 transcription vector (Promega, Madison, WI) designated pSP73’, containing the multiple cloning site derived from pGEM4Z. The pSP73’-[TAg]-A64 plasmid was purified from DH5α Competent Cells (Life Technologies, Gaithersburg, MD) using a QIAFilter MaxiPrep kit (Qiagen, Valencia, CA), and stored in TE buffer (1 mg/ml). The sequence of the clone was determined by the Duke Sequencing Facility and confirmed by alignment analysis using MacVector software (Accelrys, Princeton, NJ).
RNA preparation and vaccination
The plasmids pGEM4Z-[OVA]-A64, pGEM4Z-[green fluorescent protein (GFP)]-A64, pGEM4Z-[muGM-CSF]-A64, pGEM4Z-[muActin]-A64 (all previously generated in our laboratory), and pSP73’-[TAg]-A64 were linearized with Spe I (New England Biolabs, Beverly, MA), and transcribed using an AmpliCap T7 High Yield Message Maker kit (Epicentre Technologies, Madison, WI) according to the manufacturer’s directions. Template DNA was removed by DNase I, and purified mRNA was dissolved in RNase-free water at 1 mg/ml. To verify mRNA integrity, samples were diluted in formamide-formaldehyde-glycerol loading buffer and electrophoresed on a pre-equilibrated, 1.2% agarose-formaldehyde gel. Human IL-2 and murine CD80 mRNAs were kindly provided by C. Adlakha. For vaccination, mRNA was added at 1:2 or 1:4 w:w ratio to cationic liposomes diluted in PBS in 96-well, U-bottomed plates, mixed by pipetting, and incubated for 10 min at 22°C to allow complex formation. For intradermal (ID) administration, mice were anesthetized with 1.2% tribromoethanol, and 25–40 μl mRNA-liposomes were injected into each pinna. Vaccine volumes for intravenous (IV) and subcutaneous (SQ) administration ranged from 80 to 160 μl.
Generation and peptide loading of bone marrow-derived DCs (BMDCs)
Erythrocyte-depleted B6 bone marrow cells were suspended at 106 cells/ml in RPMI 1640 medium containing 5% FBS, 5×10−5 M 2-ME, 2 mM L-glutamine, 10 mM HEPES buffer, 1 mM Na pyruvate, 1 mM amino acids, penicillin/streptomycin, and 15 ng/ml murine GM-CSF in 6-well plates, and incubated at 37°C under 5% CO2. On day 3, medium was replaced, and on day 7, loosely adherent, immature BMDCs were re-plated in fresh medium and harvested 2 days later. BMDCs were pulsed with peptides on either day 8 or 9 of culture, and resuspended in PBS at 2.5–5×107 cells/ml for injection.
Cytotoxicity assays
To measure CTL responses, 107 lymph node (LN) cells or erythrocyte-depleted splenocytes that were harvested 7 to 30 days postvaccination were co-cultured with 106 irradiated (200 Gy) Ag-expressing stimulator cells in 5 ml R-10 medium (RPMI-1640 supplemented with 10% FBS, 5×10−5 M 2-ME, 2 mM L-glutamine, 25 mM HEPES buffer, 1 mM Na pyruvate, 1 mM amino acids, penicillin/streptomycin, and 5 U/ml huIL-2) in a 6-well plate for 5–6 days at 37°C. Following purification over a Histopaque 1.083 gradient (Sigma), serial dilutions of effectors were incubated in triplicate with europium diethylenetriamine pentaacetate-labeled target cells (104) in 200 μl R-10 in 96-well, V-bottomed plates. After 4 h, supernatant europium content was assayed by time-resolved fluorescence (Delta fluorometer, Wallac, Gaithersburg, MD). Spontaneous and total releases were determined by the addition of medium or 1% Triton-X, respectively, to wells containing targets alone. The mean spontaneous release in all experiments was 9.6% (range 4.8–20.8%). Cytolytic activity was calculated according to the formula: % lysis = (experimental release − spontaneous release)/(total release − spontaneous release) × 100. In vivo CTL responses were measured as previously described [40]. Briefly, vaccinated mice were injected IV with an equal mixture of unpulsed, CFSElo-labeled (0.5 μM) or OVA peptide-pulsed (10 μg/ml) CFSEhI-labeled (5.0 μM) nonadherent splenocytes (6×107) IV; 22 h later, mandibular LNs were harvested and analyzed for differential target loss by flow cytometry.
OVA-expressing tumor protection/therapy studies
The lowest tumor cell inoculum allowing rapid, consistent SQ growth was determined in pilot titration experiments for E.G7 (2×107) and F10.9-OVA (1×105), and OVA expression was verified with the RF.3370 T cell hybridoma that recognizes H2-Kb-bound SIINFEKL [34]. Tumor growth was recorded 2–5 times per week by measuring bisecting diameters with calipers, and either mean diameter (d=[a + b]/2) or volume (v=[a 2 × b]/2, where a = short axis, b = long axis) was calculated.
TRAMP immunotherapy studies
Male 18-week-old (age range 116–132 days) TRAMP (n=5) and wt (n=2) mice were assigned to vaccine groups by random selection of ear tag numbers. Mice were immunized IV six times over a 10-week-period with either PBS, or GFP or TAg mRNA. Two groups of TRAMP mice were injected intraperitoneally with 5×107 nonadherent splenocytes from male nontransgenic littermates 3 days prior to the first vaccination (TRAMP/AT mice). Three weeks after the last injection, mice were sacrificed for CTL assay, and gross and histopathologic examination. Mice that were euthanized or died prior to the end of the study were included in analysis only if death was related to tumor-related causes. En bloc-resected urogenital tracts (UGT), and isolated prostates and/or prostatic tumors, were weighed, formaldehyde-fixed, and processed by the Duke University Immunohistochemistry Research Laboratory. Two 5 μM sections (200 μM apart) were stained with hematoxylin and eosin (H&E), and then areas of peak severity were scored in blinded fashion by one investigator (PRH) at 10× on a 1 to 5 scale, modified slightly from published criteria [35]: 1: normal; 2: prostatic intraepithelial neoplasia (PIN), with “cross-bridging”, cribriform structures, and increased apoptotic and mitotic figures; 3: well-differentiated carcinoma, similar to PIN, but also including large nuclei, prominent nucleoli, basement membrane invasion, and thick periductal stromal layers; 4: moderately differentiated carcinoma, with duct remnants lacking open lumens and often invading adjacent tissues; and 5: poorly differentiated carcinoma—sheets of anaplastic cells with no glandular structures. To reduce the possibility of bias, coded histology slides from three experiments were combined prior to reading.
Statistical analyses
In OVA experiments, ANOVA, with either Tukey’s or Dunnett’s multiple comparisons posttests, was used to compare means between groups, using Prism 4.0 (GraphPad Software, Inc., San Diego, CA). In TRAMP experiments, vaccine effects on net UGT and tumor weights were evaluated using generalized least-squares analysis, and effects on histologic grade were assessed using both generalized least-squares analysis and the Kruskal-Wallis test, using SAS software (SAS Institute, Cary, NC), In all analyses, the level of significance was set at P<0.05, and error bars represent standard deviation.
Results
Priming of specific CTL responses by ID injection of liposome-encapsulated mRNA
Vaccines were prepared by complexing IVT mRNAs with the cationic liposome, DOTAP [36]. We first verified that mRNA was translated in vivo, by injecting GFP mRNA intradermally into the pinna, and detecting green fluorescence at the site 24 h later by microscopy (not shown). When mice were injected in the same fashion with OVA mRNA, specific CTL could be demonstrated in the draining LN 7 days after the second immunization (Fig. 1a). The lack of response in GFP mRNA-vaccinated mice indicated that responses were primed in vivo, and not during in vitro stimulation. Vaccination with mRNA stimulated a more potent response, with less nonspecific killing, than that elicited by ID injection of BMDCs pulsed with 5 ng/ml OVA peptide. A cytolytic response was also evident in vivo 6 days after a single mRNA immunization (Fig. 1b), similar to that given by peptide-pulsed BMDCs.
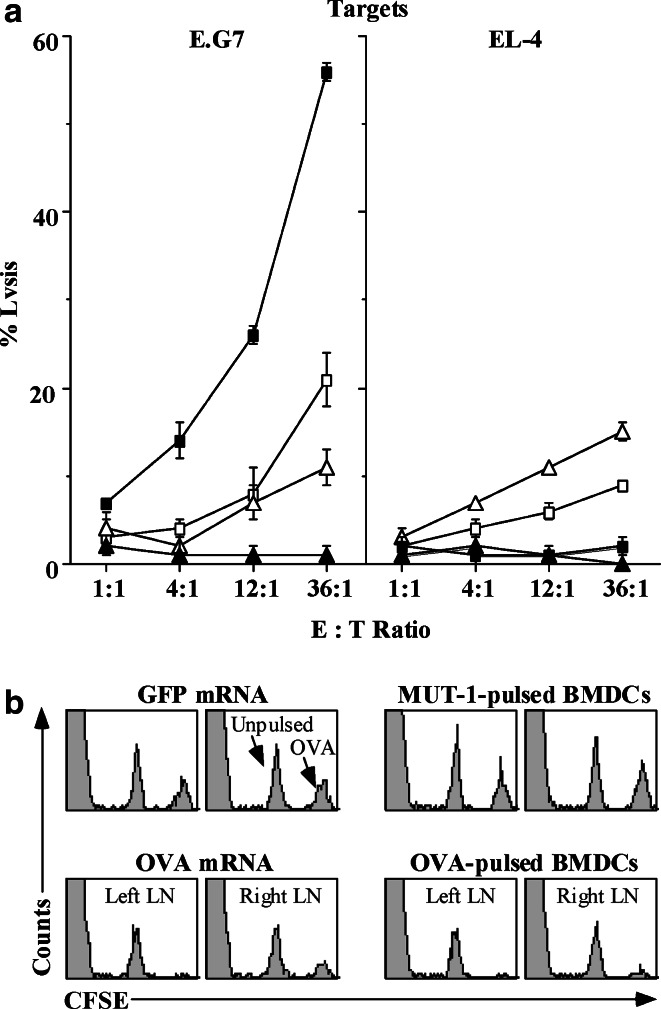
Fig. 1.
Injection of liposome-encapsulated OVA mRNA elicits specific CTL responses detectable in vitro and in vivo. a B6 mice (n=3/group) received 2 ID injections in the pinnae, 14 days apart, of 5 μg OVA (filled square) or GFP (filled triangle) IVT mRNA complexed 1:2 with DOTAP (1:2 RNA:liposome w:w ratio), or 7.5×104 BMDCs pulsed with OVA (open square) or control MUT-1 (open triangle) peptide. Seven days postinjection (PI), mandibular LN cells were stimulated in vitro with E.G7 for 5 days, and then assayed for CTL activity as described in “Materials and methods”. The results represent three experiments. b B6 mice received a single injection of 10 μg OVA or GFP IVT mRNA complexed 1:2 with DOTAP, or 105 BMDCs pulsed with OVA or MUT-1 peptide, in each pinna. Five days PI, mice were adoptively transferred IV with a 50:50 mixture of unpulsed and OVA257–264-pulsed syngeneic splenocytes, distinguished by CFSE intensity. Mandibular LN cells harvested 22 h later were analyzed for CFSE+ events by flow cytometry
Effects of mRNA vaccine-elicited CTL responses on tumor growth in vivo
We then determined whether OVA-specific CTL primed by mRNA vaccination would protect mice from challenge with the OVA-expressing tumor, E.G7. As shown in Fig. 2a, mice immunized twice with OVA mRNA were completely protected (no palpable tumors before day 10), while two mice that received only one injection of OVA mRNA developed small tumors. Protection experiments using an immunogenic tumor such as E.G7, which regresses spontaneously by 20 days after SQ challenge, are not especially stringent. Thus, we also tested the efficacy of mRNA vaccination using an OVA-transfected clone (F10.9-OVA) of the murine melanoma B16. F10.9-OVA cells express low levels of class I molecules, are resistant to CTL lysis in vitro, and grow progressively in B6 mice. Figure 2b shows that therapeutic immunization begun 3 days after tumor challenge with liposome-encapsulated OVA mRNA significantly slowed, but did not completely prevent, the growth of F10.9-OVA tumors.
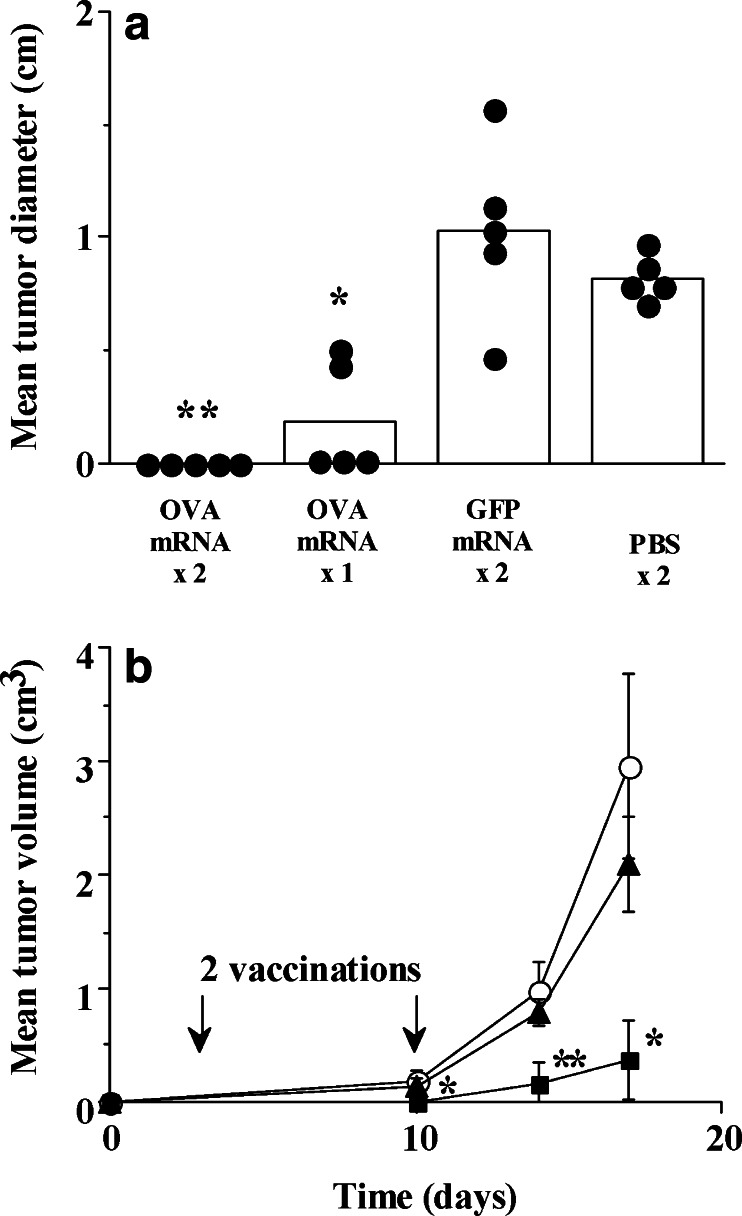
Fig. 2.
Intradermal vaccination with liposome-encapsulated OVA mRNA protects against E.G7 tumor challenge, and delays the growth of established, OVA-expressing melanomas. a B6 mice (n=5/group) received 1 or 2 ID injections in the pinnae, 14 days apart, of OVA or GFP mRNA (5 μg) complexed 1:2 with DOTAP, or PBS; 7 days PI, 2×107 E.G7 cells suspended in 200 μl PBS were injected SQ interscapularly. Tumor diameters were calculated as the average of bisecting measurements recorded 10 days after challenge. Individual mice are represented by filled circles; bars indicate the group mean. Protection from tumor growth with OVA mRNA was significant (*, P<0.01; **, P<0.001), although there was no difference between groups receiving either 1 or 2 injections. b B6 mice (n=5/group) were implanted SQ on day 0 with 105 F10.9-OVA cells over the right flank; 3 days and 10 days after challenge, mice were immunized (arrows) with 5 μg OVA (filled square) or GFP (filled triangle) IVT RNA complexed with DOTAP-DOPE (1:4), or PBS (open circle). Long- and short-tumor axes were measured on the indicated days, and mean tumor volumes were subsequently calculated. Inhibition of tumor growth by OVA mRNA vaccination was significant at all measured time points (*, P<0.05; **, P<0.001)
Optimal parameters for consistently effective mRNA vaccination
Over multiple experiments, we observed that the magnitude of CTL responses to mRNA vaccination was variable, and occasionally, only weak or negligible activity was elicited. We first examined whether alternative cationic liposomes could improve responses, comparing the efficacy of DOTAP to two other commercially available agents—DOSPER and DOTAP-DOPE—in generating OVA-specific CTL. The best response was obtained with DOTAP-DOPE at a 1:4 (w:w) ratio of mRNA to liposome (not shown). We also considered the possibility that periodic vaccine failure may have been due to the inconsistent distribution of the mRNA-liposome solution in the pinna, and therefore examined other routes of administration, comparing splenic CTL activity following ID, IV, and SQ injections of OVA mRNA. As seen in Fig. 3a, there was no response to SQ immunization, while IV injection elicited a vigorous CTL response. Data from several experiments suggested that ID and IV routes were equivalent in potency; however, because ID injection sometimes failed to prime CTL, and required anesthesia, subsequent experiments were performed with IV immunization. Using this route, we then re-evaluated the same liposomes. Similar to previous findings, no response was seen in mRNA-DOSPER-vaccinated mice, and CTL activity generated with DOTAP-DOPE was considerably stronger than that elicited with DOTAP alone (Fig. 3b). Consequently, in succeeding experiments, all vaccines were prepared with DOTAP-DOPE. The minimum dose of this optimized vaccine was determined, and as shown in Fig. 3c, CTL responses were detected following a single injection of 1 μg of liposome-encapsulated mRNA.
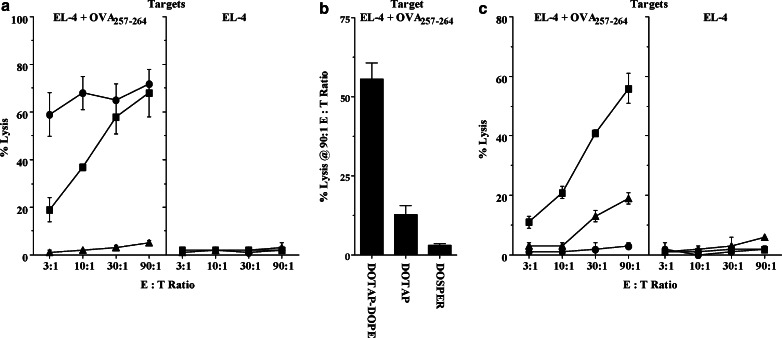
Fig. 3.
The efficacy of mRNA vaccination in priming CTL depends on the route of injection, the cationic liposome, and RNA dose. a B6 mice (n=2/group) received 2 vaccinations of 3 μg OVA RNA complexed 1:4 with DOTAP-DOPE, 14 days apart, by either ID (pinna; filled square), IV (tail vein; filled circle), or SQ (tail base; filled triangle) routes. The results represent 3 experiments. b B6 mice (n=2/group) were vaccinated IV once with 3 μg OVA IVT RNA complexed 1:4 with either DOTAP-DOPE, DOTAP, or DOSPER liposomes. The graph represents the % lysis of OVA peptide-pulsed EL-4 cells at the highest effector : target (E:T) ratio assayed (90:1); the lysis of unpulsed EL-4 control targets was ≤1% in all groups. c B6 mice (n=2/group) were vaccinated IV once with a 3/1 (filled square), 1/3 (filled triangle), or 0.3/3.7 (filled circle) μg mixture of OVA/actin IVT RNAs, complexed 1:4 with DOTAP-DOPE. Murine actin mRNA, which is not immunogenic, was used as a “spacer” to insure that all mice received the same quantity of RNA (4 μg) and cationic liposomes (16 μg). The results represent 2 experiments. In a, splenocytes were harvested 7 days PI and stimulated with E.G7 for 5 days; in both b and c, splenocytes were harvested 11 days PI and stimulated with OVA257–264-pulsed EL-4 cells for 5 days. All were assayed for CTL activity as previously described
Effects of murine GM-CSF mRNA on CTL priming
Co-administration of pDNAs encoding Ag and various cytokines has been shown to potentiate T cell responses, by altering the immune environment at the injection site and draining LN. In analogous fashion, we screened several candidate adjuvants by CTL assay to determine whether inclusion of mRNAs encoding molecules that attract DCs (GM-CSF), increase co-stimulation signal density (CD80), or promote T cell proliferation (IL-2), would enhance vaccine-elicited responses (Fig. 4a). Consistent with previous reports documenting the adjuvant effect of GM-CSF as protein, inclusion of this cytokine mRNA significantly strengthened CTL activity induced by a single injection of Ag mRNA. The enhancement produced by GM-CSF co-administration appeared dose dependent (Fig. 4b). In addition to improving short-term effector activity, mice co-immunized with OVA and GM-CSF mRNAs also exhibited a more durable CTL response at 30 days (Fig. 4c), indicating enhanced generation of memory CD8+ T cells.
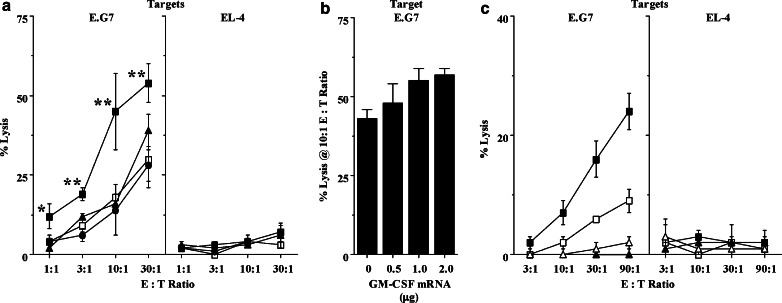
Fig. 4.
The inclusion of GM-CSF mRNA enhances the immunogenicity of an OVA mRNA vaccine. a B6 mice (n=2/group) were vaccinated IV once with 3 μg OVA mixed with either 1 μg GFP (negative control; open square), CD80 (filled triangle), IL-2 (filled circle), or GM-CSF (filled square) mRNA. Translation of candidate adjuvant mRNAs was verified by in vitro cell transfection and ELISA of culture supernatants (GM-CSF, IL-2), or surface staining and flow cytometry (CD80) (not shown). Eight days PI, splenocytes were stimulated in vitro with E.G7 for 5 days prior to CTL assay. At all E:T ratios, only GM-CSF mRNA significantly (*, P<0.05; **, P<0.01) enhanced CTL responses. The results represent three experiments. b B6 mice (n=2/group) were vaccinated IV once with 2 μg OVA mixed with either no or 0.5, 1, or 2 μg GM-CSF mRNA, as indicated. The graph represents the % lysis of E.G7 at an E:T ratio of 10:1. As in previous experiments, additional actin mRNA was added to insure that all mice received the same total quantity of RNA (5 μg). Splenocytes were harvested 8 days PI, stimulated with E.G7 cells for 6 days, and subsequently assayed for CTL activity. Lysis of control EL-4 targets (not shown) was ≤1% in all groups. c B6 mice (n=2/group) were vaccinated IV once with either 3 μg OVA mixed with 1 μg GM-CSF (filled square) or actin (open square) IVT mRNA, or 3 μg GFP mixed with 1 μg GM-CSF (filled triangle) or actin (open triangle) IVT mRNA. Thirty days PI, splenocytes were stimulated for 5 days with E.G7, and assayed for CTL activity. In all experiments in Fig. 4, mRNA was complexed with DOTAP:DOPE at a 1:4 ratio
Immunization of TRAMP mice with a GM-CSF-augmented, TAg mRNA vaccine
The induction of tumor-protective CTL suggested that mRNA vaccination might be useful as cancer immunotherapy, and we wished to test this method in a more realistic setting. Specifically, we examined whether therapeutic immunization of TRAMP mice with liposome-encapsulated mRNA encoding the model tumor/self Ag TAg would elicit specific CTL responses and inhibit CaP progression. TRAMP mice develop prostatic epithelial hyperplasia by 12 weeks of age, and invasive carcinoma by 24 weeks [29]. On the basis of this bracketing, we began immunization at 18 weeks of age, when most males are expected to have low-grade, organ-confined CaP. Baseline phenotypes in several 16- and 18-week-old TRAMP mice showed grossly normal prostates with PIN (mean tumor grade = 2 to 2.5 [normal = 1]—not shown). We evaluated antitumor responses in TRAMP mice—defined as a reduction in CaP histologic grade and weight—vaccinated with either 5 μg TAg or GFP (control) mRNA. Injection of PBS alone was used as an additional control for the possible nonspecific effects of mRNA or liposome administration. As a positive control, we vaccinated TRAMP mice that had received splenocytes from TAg-naïve, wt littermates prior to the first TAg mRNA injection. We had established in a pilot experiment that TAg vaccination of splenocyte-recipient mice generated specific CTL, and significantly decreased tumor mass and grade (not shown). A second group of TRAMP/AT mice were vaccinated with GFP mRNA to evaluate the effect of transfer alone. We also evaluated CTL in TRAMP mice, comparing responses with those of wt littermates receiving identical vaccines. For this assay, additional OVA mRNA-vaccinated groups were used to verify the T cell immunocompetence of CaP-bearing mice. Because the presence of an Ag-expressing tumor may be insufficient to maintain vaccine-primed CTL activity [37], vaccination was repeated approximately every 12 days through termination of the study at 31 weeks of age.
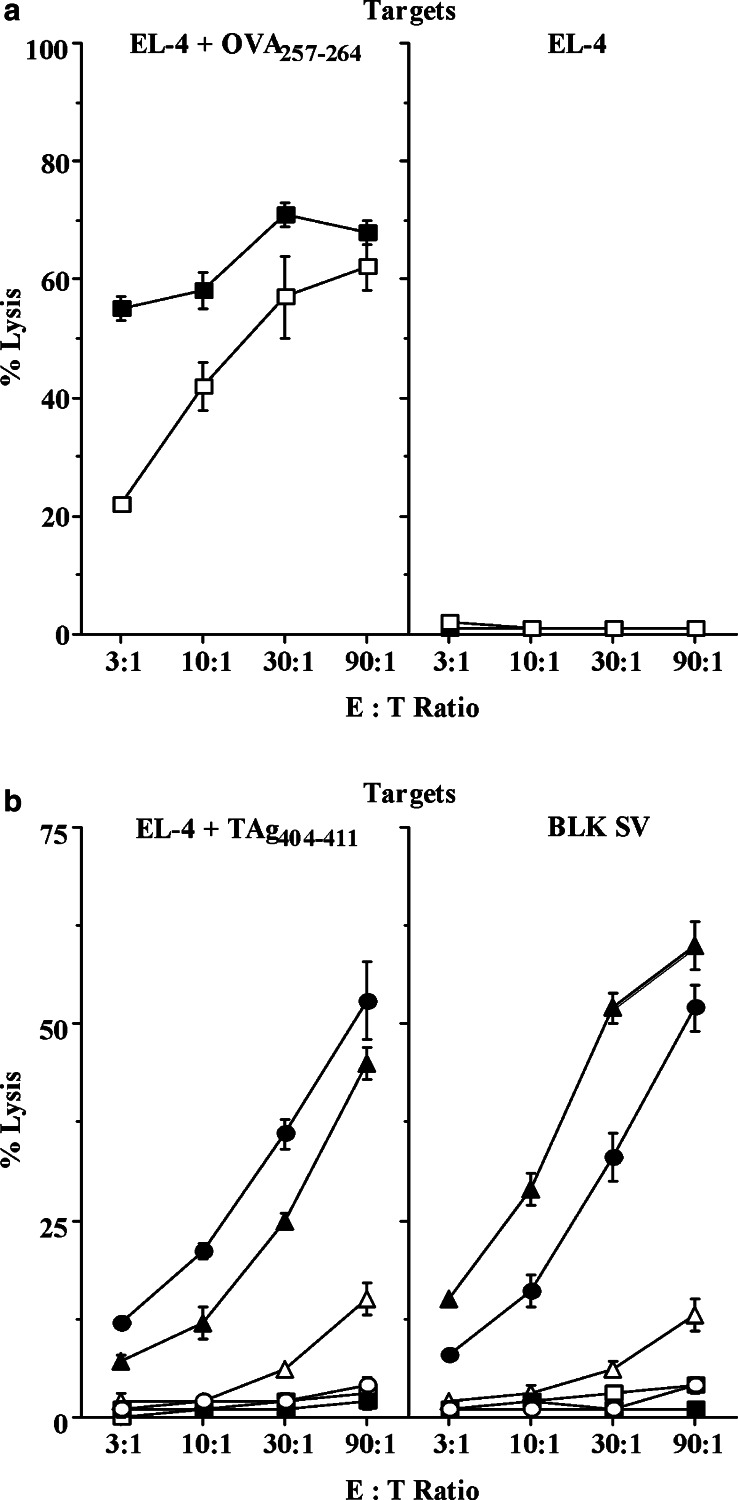
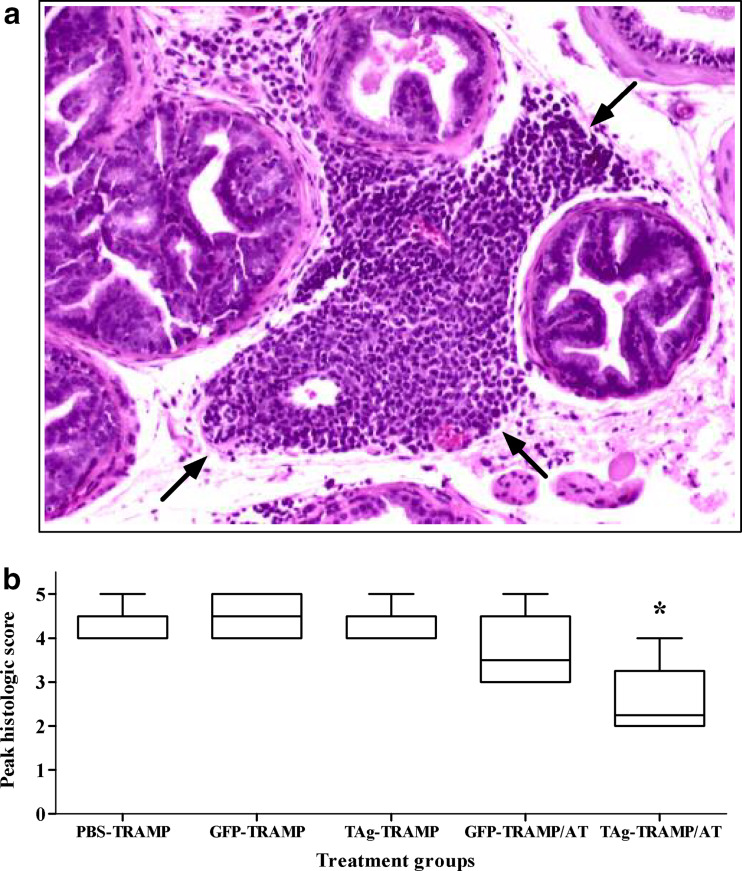
As seen in Fig. 5a, mRNA immunization was equally as efficient in generating OVA-specific CTL responses in tumor-bearing TRAMP mice as in nontransgenic male littermates. The TAg mRNA vaccine was also immunogenic, demonstrated by the killing of TAg404–411peptide-pulsed EL-4 cells by splenocytes from immunized wt mice (Fig. 5b). Responses against this immunodominant epitope were absent in adult TRAMP mice, as previously observed [30, 38]. Immunization with full-length TAg mRNA was predicted to prime CTL responses against subdominant epitopes, detectable in vitro as lysis of TAg-transformed BLK SV cells, and in vivo as inhibition of tumor growth. Nonetheless, neither CTL activity (Fig. 5b) nor antitumor effects (Fig. 6, Table 1) were seen in TRAMP mice repeatedly immunized with TAg and GM-CSF mRNAs, indicating that the vaccine was unable to overcome tolerance to this tumor/self Ag. This unresponsiveness was not due to loss of tumor antigenicity, or intrinsic resistance to CTL killing, as TAg mRNA vaccination of TRAMP/AT mice was associated with inflammatory infiltrates in prostatic interductal spaces (Fig. 6a), a reduction in tumor grade (Fig. 6b), and decreased UGT and tumor weight (Table 1).
Fig. 5.
TRAMP mice respond normally to OVA mRNA vaccination, but immunization with TAg mRNA is insufficient to overcome tolerance to this tumor/self Ag. a TRAMP (filled square) and B6 mice (open square) (n=2/group) were vaccinated with OVA mRNA, and CTL activity assayed against OVA257–264-pulsed or unpulsed EL-4 targets. b TRAMP mice (n=5/group; represented by squares) were vaccinated with GFP (open square) or TAg (filled square) mRNAs; TRAMP/AT mice (n=5/group; represented by triangles) were vaccinated with GFP (open triangle) or TAg (filled triangle) mRNAs; and nontransgenic littermates (n=2/group; represented by circles) were vaccinated with GFP (open circle) or TAg (filled circle) mRNAs. TRAMP/AT mice received a single injection of splenocytes (5×107) from wt littermates 3 days before the first immunization. CTL activity was assayed against TAg404–411-pulsed or unpulsed, Tag-transformed BLK SV targets. Lysis of unpulsed EL-4 targets (not shown) by all groups was ≤3% at all E:T ratios. In both a and b, mice were vaccinated IV six times with Ag (5 μg) and GM-CSF (1 μg) mRNAs, complexed 1:4 with DOTAP:DOPE. Twenty-two days PI, splenocytes were stimulated for 6 days with BLK SV fibroblasts pulsed with either 1 μg/ml OVA257–264 peptide a or 1 μM TAg404–411 peptide b before testing for CTL activity. The results represent two experiments
Fig. 6.
a, Vaccination of TRAMP/AT mice with tumor/self Ag mRNA is associated with inflammatory changes in CaP tissue. Arrows indicate lymphoid infiltrates in the prostatic interductal spaces of a 31-week-old TRAMP mouse immunized with TAg and GM-CSF mRNAs following AT of naïve splenocytes. Sections (5 μm) were stained with H & E, and photographed through an Olympus BX40 microscope (10×). b, Repetitive immunization with a TAg mRNA vaccine does not inhibit the progression of CaP in unreconstituted TRAMP mice. Peak scores were generated and analyzed as described in the “Materials and Methods”. The grade in the TAg-vaccinated TRAMP/AT group was significantly lower (*, P<0.05) than that in the 3 groups of negative controls: PBS-injected TRAMP mice, or GFP mRNA-vaccinated TRAMP or TRAMP/AT mice. The results represent two experiments
Table 1.
Effect of mRNA vaccination on net UGT and CaP weights in TRAMP mice
| Mice | TRAMP | TRAMP/AT | |||
|---|---|---|---|---|---|
| Vaccination | PBS | GFP | TAg | GFP | TAg |
| UGT weight (g) | 1.51±1.00 | 2.34±2.79 | 1.88±0.88 | 4.04±6.06 | 0.32±0.46 |
| 1.44 | 0.99 | 2.21 | 1.44 | 0.18 | |
| CaP weight (g) | 1.21±0.79 | 1.98±2.95 | 1.11±0.81 | 3.32±6.39 | 0.14±0.26 |
| 1.13 | 0.53 | 0.92 | 0.15 | 0.02 | |
The mean (±standard deviation; top) and median (bottom) net weights of the UGT and CaP of 31-week-old TRAMP and splenocyte-recipient TRAMP/AT mice injected IV 6 times at 12-day intervals with the indicated GM-CSF-augmented mRNA vaccines, or PBS negative control. The mean prostate weight in age-matched, vaccinated, nontransgenic mice (n=6) was 0.099 g (range 0.078–0.118). The results represent two experiments
Discussion
In this study we investigated the feasibility of vaccinating with nonreplicating IVT mRNA as cancer immunotherapy. This approach may be less hazardous than immunization with TAA-encoding pDNA, which poses a risk of malignant transformation, either due to mutagenesis (from random insertion or homologous recombination), or from prolonged oncogene expression. For injection, mRNA was encapsulated in cationic liposomes, which are a convenient, reproducible means of delivering nucleic acids into cells, comparable to physical methods such as electroporation or gold particle bombardment. In vivo, liposomes are neither toxic nor antigenic, allowing safe and repeated administration. We found that liposomes composed of a 1:1 mixture of DOTAP and the neutral phospholipid DOPE were much more effective in priming CTL than DOTAP alone, which is consistent with the action of DOPE in protecting nucleic acids from hydrolysis [40], and facilitating the destabilization of endosomes [41]. In these experiments, IV injection of mRNA-liposome complexes yielded the most consistent CTL responses, which may reflect rapid clearance from circulation by hepatic and splenic Ag-presenting cells (APCs) [42].
We also investigated the effects of adding mRNA encoding immunologically active molecules to Ag mRNA to strengthen the specific cytotoxic response. The activities of the three adjuvants tested (GM-CSF, CD80, and IL-2) span the major phases of CTL priming—Ag acquisition and presentation; APC-T cell interaction; and effector proliferation; and when included as pDNA in vaccines, have been shown to enhance T cell activity [43–46]. We found that the mixture of OVA and GM-CSF mRNAs, but not CD80 or IL-2 mRNAs, enhanced the CTL response. The superior adjuvant properties of GM-CSF were first demonstrated in a gene-modified tumor vaccine (GMTV) model [47]. Delivery of this cytokine in the vaccine may be optimal, as the risk of toxicity associated with systemic administration is diminished, and GM-CSF can operate in paracrine fashion [48]. In fact, for pDNA vaccination, immune responses were only enhanced when GM-CSF was injected at the same site as the Ag-encoding plasmid [43]. In addition to pDNA and GMTVs, GM-CSF has been co-delivered with tumor Ag in various forms, such as transfected “bystander” cells, protein, Ag-GM-CSF fusion proteins, and recombinant virus. Immune enhancement by co-immunization with GM-CSF and Ag mRNAs has not been previously demonstrated. Schirrmacher et al. [9] found that co-injection of GM-CSF mRNA did not augment CTL responses elicited by a pDNA vaccine. These contrasting findings may reflect the use of self-replicating RNA, or other particulars of their model, as GM-CSF pDNA similarly had no enhancing effect.
This study also examined the efficacy of a GM-CSF-augmented mRNA vaccine encoding a tumor/self Ag in the treatment for a spontaneous tumor. As previously mentioned, one approach to the practical production of vaccines for clinical immunotherapy of solid tumors is the use of patient-shared Ags. Generally, these Ags are either nonmutated, over-expressed global self proteins, or tumor/self Ags whose expression is restricted to the neoplastic and parent tissue. T cell activity against such Ags can be demonstrated in polyepitopic antitumor responses. For example, we have previously shown that DCs transfected with prostate tumor RNA generated CTL in vitro against telomerase reverse transcriptase and prostate-specific Ag (PSA) [49]. Interestingly, numerous murine studies, with a few exceptions [18, 50], have demonstrated that tumor/self Ag-reactive CTL primed by vaccination can mediate tumor rejection without causing autoimmune disease [3, 25, 51–54]. Tolerance to such Ags appears to be a more substantial obstacle to their use; however, reproductive-origin malignancies may uniquely provide a permissive environment for active immunization against tumor/self Ags, since hormone-dependent expression of differentiation proteins is restricted to a period late in ontogeny (i.e., at sexual maturity). Thus, human PSA-transgenic mice, expressing the transgene only in prostatic epithelium, mount PSA-specific CTL responses of the same magnitude as nontransgenic mice [55]. Similarly, high-avidity, PSA-specific CTL activity can be generated from T cells of both women and men [56]. Nonetheless, even when autoreactive T cells are available at sufficient precursor frequencies, the ability of a given tumor/self Ag vaccine to overcome tolerance is largely an empirical question.
In this study, repeated vaccination of TRAMP mice with TAg mRNA failed to induce CTL activity against immunodominant or subdominant epitopes. Given the ability of tumor-bearing TRAMP mice to respond normally to OVA mRNA vaccination, and to retain constitutively primed (GFP mRNA-vaccinated TRAMP/AT mice), TAg-specific CTL long-term (>3 months), the lack of TAg404–411-reactivity likely represents the loss of the cognate repertoire at the thymocyte level. Although TAg expression in TRAMP mice originally was described as prostate-restricted, Zheng et al. [57] recently reported the androgen-independent expression of TAg (and nontransgenic, prostate-specific probasin) in the TRAMP thymus, and clonal deletion of H2-Kk -restricted, TAg559–576-specific, transgenic T cells in double-transgenic TRAMP F1 hybrid mice. The lack of response to subdominant epitopes observed here similarly could represent central loss, or instead, result from the inactivation of specific CD8+ T cells in the periphery. Consistent with the latter possibility, immunization of TRAMP hybrid mice with a GM-CSF-secreting GMTV was only successful in delaying tumor progression when administered with concomitant cytotoxic T lymphocyte Ag (CTLA)-4 blockade [31]. This combination of treatment induced prostatitis in wt controls, suggesting that the response was directed at a nontransgenic tumor/self Ag. On the other hand, reports demonstrating CTL responses against TAg-transformed cells following the priming of TRAMP mice with fibroblasts or peptides suggests that active, recruitable CD8+ T cells are available [30, 38], although the effects of such immunization on tumor growth were not ascertained. It could be argued that our inability to detect cytolytic responses directed against subdominant epitopes in TAg-vaccinated TRAMP mice was due to the limited sensitivity of the 4-h CTL assay, rather than vaccine failure. In the more meaningful experimental readout, however—control of CaP progression over the 3-month study period—no response was seen. Moreover, TAg-reactive CTL were seen in GFP mRNA-vaccinated TRAMP/AT mice without evidence of an antitumor effect, indicating that the in vitro assay had a greater sensitivity in detecting specific cytotoxic responses. The latter observation also demonstrates that CTLs primed by tumor presence alone were unable to constrain neoplastic growth. This failure suggests the possibility that TRAMP tumors, while maintaining antigenicity, could express low levels of TAg-derived epitopes, and thus, remain resistant to killing by low-avidity CTL. Because we were specifically monitoring TRAMP mice for therapeutic benefit, such CTL responses, if elicited by mRNA vaccination, would have been undetectable.
In the TRAMP/AT group, immunization with tumor/self Ag mRNA was able to boost TAg404–411-reactive CTL activity to wt levels and to inhibit tumor growth. This data suggests that this form of cancer vaccination may be useful in clinical settings where a competent, tumor-reactive T cell repertoire is available; for example, with the use of shared viral Ags, or with the use of tumor/self Ags following AT of ex vivo-expanded tumor-infiltrating lymphocytes or engineered T cells [58, 59]. Our findings are similar to those of Eck et al. [54] who reported that influenza hemagglutinin (HA)-transgenic HA104 mice were tolerant of an HA-expressing mammary carcinoma, despite prior HA immunization, unless first adoptively transferred with naïve, syngeneic lymphocytes.
In summary, we found that immunization with cationic liposome-encapsulated mRNA was a safe, reproducible means of priming CTL against nonself TAAs, and that the vaccine immunogenicity could be enhanced by inclusion of GM-CSF mRNA. However, the vaccination of tumor-bearing mice with tumor/self Ag mRNA was ineffective in overcoming tolerance. When the repertoire recognizing an immunodominant epitope was replenished by bulk transfer of naïve T cells, mRNA vaccination was able to amplify CTL responses and delay progression of the primary tumor. For cancer patients, this limitation may obligate the restoration of a high-avidity, autoreactive T cell population prior to initiating treatment with nucleic acid vaccines encoding tumor/self Ags.
Acknowledgments
We are grateful to Duane Mitchell, Brenda Faiola, Carmen Wong, Charu Adlakha, Justin Hart, and Catherine McLaughlin. We also thank Erning Li for help with statistical analysis, and Xu Lin, Barbara Foster, and Norman Greenberg for their assistance with the TRAMP mouse model.
Abbreviations
- Ag
Antigen
- APCs
Antigen-presenting cells
- AT
Adoptive transfer
- BMDC
Bone marrow-derived dendritic cells
- CaP
Prostate carcinoma
- CTL
Cytotoxic T lymphocyte
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- E:T
Effector: target
- GFP
Green fluorescent protein
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GMTV
Gene-modified tumor vaccine
- HA
Hemagglutinin
- ID
Intradermal
- IL
Interleukin
- IV
Intravenous
- IVT
In vitro transcribed
- OVA
Ovalbumin
- pDNA
Plasmid DNA
- PI
Postinjection
- PIN
Prostatic intraepithelial neoplasia
- PSA
Prostate-specific antigen
- SQ
Subcutaneous
- TAA
Tumor-associated antigen
- TAg
SV40 large T antigen
- TRAMP
Transgenic adenocarcinoma of mouse prostate
- wt
Wild type
Footnotes
Eli Gilboa was supported in part by National Institutes of Health grants RO1-CA-85307 and RO1-CA-89536.
References
- 1.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 2.van Stipdonk MJB, Hardenberg G, Bijker M, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 3.Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 4.Boczkowski D, Nair SK, Synder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Krishnan S, Lenzen G, Magne R, Gomard E, Guillet J-G, Levy J-P, Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 6.Hoerr I, Obst R, Rammensee H-G, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30:1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W-Z, Hoon DSB, Huang SKS, Fujii S, Hashimoto K, Morishita R, Kaneda Y. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum Gene Ther. 1999;10:2719–2724. doi: 10.1089/10430349950016762. [DOI] [PubMed] [Google Scholar]
- 8.Ying H, Zaks TZ, Wang R-F, Irvine KR, Kammula US, Marincola FM, Leitner WW, Restifo NP. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5:823–827. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schirrmacher V, Forg P, Dalemans W, Chlichlia K, Zeng Y, Fournier P, von Hoegen P. Intra-pinna anti-tumor vaccination with self-replicating infectious RNA or with DNA encoding a model tumor antigen and a cytokine. Gene Ther. 2000;7:1137–1147. doi: 10.1038/sj.gt.3301220. [DOI] [PubMed] [Google Scholar]
- 10.Colmenero P, Liljestrom P, Jondal M. Induction of P815 tumor immunity by recombinant Semliki Forest virus expressing the P1A gene. Gene Ther. 1999;6:1728–1733. doi: 10.1038/sj.gt.3301004. [DOI] [PubMed] [Google Scholar]
- 11.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC. Enhancement of sindbis virus self-replicating RNA vaccine potency by linkage of herpes simplex virus type 1 VP22 protein to antigen. J Virol. 2001;75:2368–2376. doi: 10.1128/JVI.75.5.2368-2376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii N, Fukushima J, Kaneko T, Okada E, Tani K, Tanaka S-I, Hamajima K, Xin K-Q, Kawamoto S, Koff WC. Cationic liposomes are a strong adjuvant for a DNA vaccine of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1997;13:1421–1427. doi: 10.1089/aid.1997.13.1421. [DOI] [PubMed] [Google Scholar]
- 13.Li X-L, Boyanapalli M, Weihua X, Kalvakolanu DV, Hassel BA. Induction of interferon synthesis and activation of interferon-stimulated genes by liposomal transfection reagents. J Interferon Cytokine Res. 1998;18:947–952. doi: 10.1089/jir.1998.18.947. [DOI] [PubMed] [Google Scholar]
- 14.Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/S1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 16.Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 17.Charo J, Ciupitu AM, Le Chevalier De Preville A, Trivedi P, Klein G, Hinkula J, Kiessling R. A long-term memory obtained by genetic immunization results in full protection from a mammary adenocarcinoma expressing an EBV gene. J Immunol. 1999;163:5913–5919. [PubMed] [Google Scholar]
- 18.Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, Lewis JJ, Houghton AN. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1711–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich EI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 20.Bronte V, Chappelli DB, Appoloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony stimulating factor by tumors inhibits CD8+ T cell response by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5278–5283. [PMC free article] [PubMed] [Google Scholar]
- 21.Mizoguchi H, O’Shea JJ, Longo DL, Loefller CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 22.Kolenko V, Wang Q, Riedy MC, O’Shea J, Ritz J, Cathcart MK, Rayman P, Tubbs R, Edinger M, Novick A, et al. Tumor-induced suppression of T lymphocyte proliferation coincides with inhibition of Jak3 expression and IL-2 receptor signaling: role of soluble products from human renal cell carcinomas. J Immunol. 1997;159:3057–3067. [PubMed] [Google Scholar]
- 23.Correa MR, Ochoa AC, Ghosh P, Mizoguchi H, Harvey L, Longo DL. Sequential development of structural and functional alterations in T cells from tumor-bearing mice. J Immunol. 1997;158:5292–5296. [PubMed] [Google Scholar]
- 24.Dyall R, Bowne WB, Weber LW, LeMaoult J, Szabo P, Moroi Y, Piskun G, Lewis JJ, Houghton AN, Nikolic-Zugic J. Heteroclitic immunization induces tumor immunity. J Exp Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi A, Oka Y, Ogawa H, Elisseeva OA, Li H, Kawasaki K, Aozasa K, Kishimoto T, Udaka K, Sugiyama H. Cytotoxic T-lymphocyte responses elicited to Wilms’ tumor gene WT1 product by DNA vaccination. J Clin Immunol. 2000;20:195–202. doi: 10.1023/A:1006637529995. [DOI] [PubMed] [Google Scholar]
- 26.Bronte V, Apolloni E, Ronca R, Zamboni P, Overwijk WW, Surman DR, Restifo NP, Zanovello P. Genetic vaccination with “self” tyrosinase-related protein 2 causes melanoma eradication but not vitilligo. Canc Res. 2000;60:253–258. [PMC free article] [PubMed] [Google Scholar]
- 27.Tuting T, Bambotto A, De Leo A, Lotze MT, Robbins PD, Storkus WJ. Induction of tumor antigen-specific immunity using plasmid DNA immunization in mice. Canc Gene Ther. 1999;6:73–80. doi: 10.1038/sj.cgt.7700020. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg NM, DeMayo F, Finegold MH, Medina D, Tilley WD, Aspinal JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MH, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Canc Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 30.Granziero L, Krajewski S, Farness P, Yuan L, Courtney MK, Jackson MR, Peterson PA, Vitiello A. Adoptive immunotherapy prevents prostate cancer in a transgenic animal model. Eur J Immunol. 1999;29:1127–1138. doi: 10.1002/(SICI)1521-4141(199904)29:04<1127::AID-IMMU1127>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Canc Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 32.Mandleboim O, Berke G, Fridkin M, Feldman M, Eisenstein M, Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 33.Mylin LM, Deckhut AM, Bonneau RH, Kierstead TD, Tevethia MJ, Simmons DT, Tevethia SS. Cytotoxic T lymphoctye escape variants, induced mutations, and synthetic peptides define a dominant H2-Kb-restricted determinant in simian virus 40 tumor antigen. Virology. 1995;208:159–17 2. doi: 10.1006/viro.1995.1139. [DOI] [PubMed] [Google Scholar]
- 34.Rock KL, Rothstein L, Gamble S. Generation of class I MHC-restricted T-T hybridomas. J Immunol. 1990;145:804–811. [PubMed] [Google Scholar]
- 35.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Canc Res. 1997;57:4687–491. [PubMed] [Google Scholar]
- 36.Lu D, Benjamin R, Kim M, Conry RM, Curiel DT. Optimization of methods to achieve mRNA-mediated transfection of tumor cells in vitro and in vivo employing cationic liposome vectors. Canc Gene Ther. 1994;1:245–252. [PubMed] [Google Scholar]
- 37.Speiser DE, Miranda R, Zakarian A, Bachman MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossmann ME, Davila E, Celis E. Avoiding tolerance against prostatic antigens with subdominant peptide epitopes. J Immunother. 2001;24:237–241. doi: 10.1097/00002371-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Schell TD, Knowles BB, Tevethia SS. Sequential loss of cytotoxic T lymphocyte responses to simian virus 40 large T antigen epitopes in T antigen transgenic mice developing osteosarcomas. Canc Res. 2000;60:3002–3012. [PubMed] [Google Scholar]
- 40.Mounkes LC, Zhong W, Cipres-Palacin G, Heath TD, Debs RJ. Proteoglycans mediate cationic liposome-DNA complex-based gene delivery in vitro and in vivo. J Biol Chem. 1998;273:26164–26170. doi: 10.1074/jbc.273.40.26164. [DOI] [PubMed] [Google Scholar]
- 41.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochem Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 42.Segal AW, Wills EJ, Richmond JE, Slavin G, Black CDV, Gregoriadis G. Morphological observations on the cellular and subcellular destination of intravenously administered liposomes. Brit J Exp Path. 1974;55:320–326. [PMC free article] [PubMed] [Google Scholar]
- 43.Haddad D, Ramprakash J, Sedegah M, Charoenvit Y, Baumgartner T, Kumar S, Hoffman SL, Weiss WR. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J Immunol. 2000;165:3772–3781. doi: 10.4049/jimmunol.165.7.3772. [DOI] [PubMed] [Google Scholar]
- 44.Bowne WB, Wolchok JD, Hawkins WG, Srinivasan R, Gregor P, Blachere NE, Moroi Y, Engelhorn ME, Houghton AN, Lewis JJ. Injection of DNA encoding granulocyte-macrophage colony-stimulating factor recruits dendritic cells for immune adjuvant effects. Cytokines Cell Mol Ther. 1999;5:217–225. [PubMed] [Google Scholar]
- 45.Kim JJ, Trivedi NN, Nottingham LK, Morrison L, Tsai A, Hu Y, Mahalingam S, Dang K, Ahn L, Doyle NK, et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Kim JJ, Bagarazzi ML, Trivedi N, Hu Y, Kazahaya K, Wilson DM, Ciccarelli R, Chattergoon MA, Dang K, Mahalingam S, et al. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotech. 1997;15:641–646. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 47.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soo Hoo W, Lundeen KA, Kohrumel JR, Pham N-L, Brostoff SW, Bartholomew RM, Carlo DJ. Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J Immunol. 1999;162:7343–7349. [PubMed] [Google Scholar]
- 49.Heiser A, Maurice MA, Yancey DR, Wu NZ, Dahm P, Pruitt SK, Boczkowski D, Nair SK, Ballo MS, Gilboa E, et al. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J Immunol. 2001;166:2593–2960. doi: 10.4049/jimmunol.166.5.2953. [DOI] [PubMed] [Google Scholar]
- 50.Ludewig B, Ochsenbein AF, Odermatt F, Paulin D, Hengartner H, Zinkernagel RM. Immunotherapy with dendritic cells directed against tumor antigen shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795–803. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Canc Res. 1999;59:676–683. [PubMed] [Google Scholar]
- 52.Limmer A, Sacher T, Alferink J, Kretschmar M, Schonrich G, Nichterlein T, Arnold B, Hammerling GJ. Failure to induce organ-specific autoimmunity by breaking of tolerance: importance of the microenvironment. Eur J Immunol. 1998;28:2395–2406. doi: 10.1002/(SICI)1521-4141(199808)28:08<2395::AID-IMMU2395>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 53.Morgan DJ, Kreuwel HTC, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 54.Eck SC, Turka LA. Adoptive transfer enables tumor rejection targeted against a self-antigen without the induction of autoimmunity. Canc Res. 2001;61:3077–3083. [PubMed] [Google Scholar]
- 55.Wei C, Willis RA, Tilton BR, Looney RJ, Lord EM, Barth RK, Frelinger JG. Tissue-specific expression of the human prostate-specific antigen gene in transgenic mice: implications for tolerance and immunotherapy. Proc Natl Acad Sci USA. 1997;94:6369–6374. doi: 10.1073/pnas.94.12.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heiser A, Dahm P, Yancey DR, Maurice MA, Boczkowski D, Nair SK, Gilboa E, Vieweg J. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J Immunol. 2000;164:5508–5514. doi: 10.4049/jimmunol.164.10.5508. [DOI] [PubMed] [Google Scholar]
- 57.Zheng X, Gao J-X, Zhang H, Geiger TL, Liu Y, Zheng P. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens overexpressed in solid tumors. J Immunol. 2002;169:4761–4769. doi: 10.4049/jimmunol.169.9.4761. [DOI] [PubMed] [Google Scholar]
- 58.Morgan RA, Dudley ME, Yu YYL, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2:957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]